Abstract
Reduced density of glial cells and low levels of some astrocyte proteins have been described in the orbitofrontal cortex (OFC) in depression and alcoholism, two disorders often comorbid. These regressive changes may also involve the communication between astrocytes via gap junctions and hemichannels, which play important regulatory roles in neurotransmission. We determined levels and morphological immunostaining parameters of connexin 43 (Cx43), the main protein subunit of astrocyte gap junctions/hemichannels, in the OFC of subjects with depression, alcoholism or comorbid depression/alcoholism as compared to non-psychiatric subjects. Postmortem brain samples from 23 subjects with major depressive disorder (MDD), 16 with alcohol dependence, 13 with comorbid MDD and alcohol dependence, and 20 psychiatrically-normal comparison subjects were processed for western blots to determine Cx43 levels. Area fraction of Cx43 immunoreactivity, and density and average size of immunoreactive puncta were measured in histological sections. There was a significant, larger than 60 percent decrease in Cx43 level in the three psychiatric groups as compared to controls. Area fraction of immunoreactivity and immunoreactive punctum size were reduced in all psychiatric groups, but Cx43-immunoreactive puncta density was reduced only in alcohol-dependent subjects. Among psychiatric subjects, no difference in Cx43 levels or immunostaining was found between suicides and non-suicides. The present data suggest that dysfunction of the OFC is accompanied by reduction in the levels of gap junction protein Cx43 in depression and alcoholism, and reduction in density of Cx43 immunoreactive puncta only in alcoholism, pointing to altered gap junction or hemichannel-based communication in the pathophysiology of those disorders.
Keywords: Alcoholism, major depressive disorder, prefrontal cortex, postmortem, immunohistochemistry, gap junctions
OBJECTIVES OF THE STUDY AND BACKGROUND
Depression and alcoholism are often co-morbid, the presence of one of these disorders increasing the risk for the other and its severity (Gilman and Abraham, 2001; Greenfield et al., 1998; Hasin and Grant, 2002; Kranzler et al., 1996; Regier et al., 1990). Despite clear differences in diagnostic criteria and pathology, both disorders also share impediments in decision-making, emotional control and adequate planning, all functions crucially involving the prefrontal cerebral cortex (PFC) (Diekhof et al., 2008; O’Doherty, 2011). These impediments are associated with the neurophysiological and cellular pathology in the PFC that must underpin behavioral and emotional pathology in both major depressive disorder (MDD) (Drevets, 2007; Kringelbach and Rolls, 2004; Price and Drevets, 2012; Rajkowska, 2000) and alcohol-dependence (Dao-Castellana et al., 1998; Flatscher-Bader and Wilce, 2008; Miguel-Hidalgo et al., 2002; Sullivan et al., 2000).
Even in the absence of gross morphological abnormalities, disturbances in glial cells, and not just in neurons, may crucially mediate neural dysfunction in psychiatric disorders (Banasr et al., 2008; Rajkowska and Miguel-Hidalgo, 2007). In both depression and alcoholism the number or packing density of glial cells, including astrocytes, and the expression of astrocyte markers are reduced in the dorsolateral and orbitofrontal cortices, both subdivisions of the PFC (Khundakar and Thomas, 2009; Miguel-Hidalgo, 2009). Nevertheless, alcoholism and major depression can differ in specific aspects of astrocyte involvement (Miguel-Hidalgo and Rajkowska, 2003; Miguel-Hidalgo et al., 2010), such as the levels of astrocytic glutamate transporters or glutamine synthetase (Miguel-Hidalgo et al., 2010).
Some of the neuronal support functions of astrocytes (Haydon and Carmignoto, 2006; Volterra and Meldolesi, 2005) strongly depend on astrocytes’ ability to communicate with each other and with other cells through gap junctions and hemichannels (Cotrina and Nedergaard, 2012; Figiel et al., 2007; Giaume et al., 1997; Rouach et al., 2002), mainly composed of the protein connexin 43 (Cx43), with a smaller involvement of connexin 30 (Cx30). Thus, deficient expression or modifications of Cx43 could contribute to prefrontal physiopathology. Recently, we showed that alcohol preference and intake increased after gap junction blockade in the PFC of rats (Miguel-Hidalgo et al., 2009). In addition, in alcoholism, gap junction communication and Cx43 processing may be directly affected by ethanol exposure (Wentlandt et al., 2004). Likewise, PFC astrocyte pathology in depression may involve gap junction alterations. Recent studies show reduced levels of Cx43 and Cx30 mRNA in the PFC of subjects with psychiatric diagnoses who died by suicide (Ernst et al., 2011) and low Cx43 mRNA in the locus coeruleus in MDD (Bernard et al., 2011)
The goal of the present study was to ascertain whether major depression and alcoholism are correlated with significant variations in the levels and tissue distribution of Cx43 immunoreactivity in the orbitofrontal cortex (OFC). The OFC is a PFC subdivision heavily involved in the regulation of emotion and decision-making, which are dysfunctional in both alcoholism and major depression (Austin et al., 2001; Dom et al., 2005; Drevets, 2007; Volkow and Fowler, 2000). In addition, structural and functional neuroimaging studies have revealed significant abnormalities in the OFC in depression and alcoholism (Dom et al., 2005; Drevets, 2007; Schulte et al., 2010).
MATERIALS AND METHODS
The protocol for tissue collection was approved by the Institutional Review Boards of the University Hospitals of Cleveland and the University of Mississippi Medical Center. Written informed consent was obtained from legal next-of-kin for informant-based retrospective diagnostic interviews. Postmortem brain tissues were collected at autopsy at the Cuyahoga County Coroner’s Office. Cases with evidence of neurological injury or disorder, prolonged agonal states or coma were excluded.
Retrospective diagnoses according to the Diagnostic and Statistical Manual of Mental Disorders (4th ed.) (DSM-IV) (APA, 1994) followed next-of–kin interviews using the Structured Clinical Interview for DSM Axis I Disorders modified for third-person reporting (First et al., 1995). Interview notes and clinical histories were reviewed independently by two licensed mental health clinicians to reach consensus diagnoses. Urine and blood collected at autopsy were examined by the coroner for presence of psychoactive substances.
The OFC was dissected from the locks of PFC, then it was frozen, and stored at −80 °C, and the postmortem interval (PMI; time between death and freezing of tissue) noted. In each subject, brain pH was determined from frozen tissue. This study included the following groups of subjects: comparison subjects without diagnosis of a psychiatric disorder (COMP, n=20), subjects with alcohol-dependence diagnosis alone (ALC, n=16), subjects with major depressive disorder (MDD) alone (n=23), and subjects with both MDD and alcoholism (MDA, n=13). The MDD group included 17 subjects with an antidepressant prescription within the last month of life, 6 subjects that had not received antidepressant medication within the last month of life, and 2 had medication for only 4 days before death. In the MDA group, 4 subjects had no antidepressant prescription. Responsiveness to medication was estimated before laboratory experiments, with only two cases estimated after experiments. Estimates were made according to clinical impressions from antemortem information and next-of-kin testimony. In the ALC group, ethanol was detected in the blood of 11 subjects, with eight at or over the legal limit (0.08 g/dL). In the MDA group, ethanol was detected in three subjects, all over the legal limit. Table 1 presents a summary of demographic and medical descriptors.
Table 1.
Summary of descriptive variables for the subject groups
| DESCRIPTIVES\GROUP | COMPARISON | ALC | MDD (no alco.) | MDA (comorbid alco.) |
|---|---|---|---|---|
| AGE | 49.20±11.10 | 47.81±8.56 | 50.00±16.45 | 52.31±14.43 |
| GENDER | 12M, 8F | 13M, 3F | 15M, 8F | 9M, 4F |
| Causes of death | 17CV, 1BP, 1PN, 1MT | 6SUIC, 8CV, 1APX, 1EG | 18SUIC, 1HK, 1GW, 3CV | 6SUIC, 6CV, 1PTE |
| ETHNICITY | 14C, 6AA | 15C, 1AA | 20C, 2AA, 1A | 13C |
| PMI | 22.063±6.73 | 22.06±10.18 | 24.08±7.36 | 26.29±6.54 |
| pH | 6.50±0.33 | 6.70±0.18 | 6.55±0.21 | 6.62±0.23 |
| ONSET (depr) | N/A | N/A | 38.65±17.23 | 32.69±12.13 |
| DURATION (depr) | N/A | N/A | 11.15±14.82 | 14.21±10.56 |
| ONSET (alco) | N/A | 22.12±5.37 | N/A | 24.38±9.92 |
| DURATION (alco) | N/A | 25.75±10.57 | N/A | 23.67±13.27 |
Abbreviations: AA=African American, A=East Asian, APX=asphyxia, ALC=Alcohol dependent subjects, alco=alcohol dependence, BP=bronchopneumonia, C=Caucasian, CV=cardiovascular disease, depr= major depression, EG=esophagitis, F=female, GW= gunshot wound, HK=hyperkalemia, M=male, N/A=not applicable, MDD=major depressive disorder, MT=multitrauma in car accident, PMI=postmortem interval (time from death to freezing of brain samples), PN=pancreatitis, PTE=pulmonary thromboembolism, SUIC=suicide. AGE, ONSET and DURATION are given in years, and PMI in hours. Numerical values for AGE, PMI, pH, ONSET and DURATION are given as mean±standard deviation.
Brodmann’s area 47 was identified in the left OFC (Uylings et al. 2010), and frozen 50 μm- and 20 μm-thick sections were collected for use in western blot-based protein measurements and immunohistochemistry, respectively.
Western blotting
Sections were sampled with a punch (0.5 mm diameter) that included all cortical layers but not white matter. These samples were collected in vials, stored at −80°C and later homogenized in 0.01M Tris-HCl containing 1% SDS, 2 mM EDTA, and protease inhibitor. The homogenate was centrifuged at 4°C and 12,000g for 30 min. Supernatant containing 25 μg of protein was applied in duplicate to wells in 10% Bis-Tris precast gels in the NuPAGE Bis-Tris Electrophoretic System (Invitrogen, Carlsbad, California). After electrophoresis, gels were transferred to PVDF membranes, later incubated with anti-Cx43 mouse monoclonal antibody (clone 2/Connexin-43, from BD Transduction Laboratories, cat. no. 610061) diluted 1:500, washed and incubated with an alkaline phosphatase-conjugated secondary antibody. This monoclonal antibody was developed against a peptide containing amino acids 252–270 of rat Cx43 and specifically binds Cx43 in tissue from humans and other vertebrate species (Asrih et al., 2012; Corinne et al., 2010; Labovsky et al., 2010). Membrane chemiluminescent bands were digitized for further analysis. Membranes were stripped of antibodies, incubated with anti-β-actin (Sigma-Aldrich) and chemiluminescence images collected. Each membrane contained subjects from the four groups. One of the lanes per blot was reserved for the same control subject across all blots. This lane was used as a reference value to normalize measurements.
Immunohistochemistry
20 μm-thick sections were mounted on slides, washed five minutes in PBS, fixed 30 minutes in 4% paraformaldehyde and washed in Tris-HCl (pH 7.6) saline solution (TBS). After overnight incubation with anti-Cx43 (clone 2/Connexin-43, BD Transduction Laboratories, cat. no. 610061) diluted 1:500, sections were washed in TBS, incubated with biotinylated secondary antibody, washed, and processed using the ABC method (Vector Laboratories, Burlingame, CA) with VIP chromogen (Vector Laboratories, Burlingame, CA). In each experiment sections from all groups were processed together using common incubating and chromogen solutions. Due to tissue availability there were no data of Cx43 puncta density and area fraction from three MDD and one MDA subjects.
Packing density of Cx43-immunoreactive puncta
Immunohistochemistry resulted in strong staining of Cx43-immunoreactive puncta (immunoreactive patches sharply defined against the lightly stained tissue background). We assessed abundance of Cx43 puncta by determining their packing density in the gray matter. This density was calculated in three sections of the OFC evenly separated by 400 μm using StereoInvestigator software (Microbrightfield, version 8). In each section a sample was defined by an outline containing all cortical layers and excluding the white matter. The outline was 2 mm wide (tangentially to the brain surface). Within each outline a series of 3-dimensional counting boxes (15X15X8 μm) was defined by systematic random sampling (with a X100 objective). Within the box, all immunoreactive puncta were counted according to the optical disector (Williams and Rakic, 1988). In each section the total number of puncta counted was divided into the cumulative volume of all the counting frames sampled to obtain packing density. The values from the three sections were averaged to establish the packing density for each subject, and use it for statistical analyses.
Estimation of immunoreactive punctum size
An estimate of average size of single immunoreactive puncta for each subject was obtained by applying the nucleator stereological probe (Gundersen, 1988) to each of the counted Cx43-immunoreactive puncta, with an oil immersion X100 objective of 1.4 numerical aperture, using Stereo Investigator (version 11.0, Microbrightfield, Inc.). To implement the nucleator, the center of each immunoreactive punctum was defined at the confluence of the major and minor axes of the punctum. The average coefficients of error for immunoreactive punctum size were 0.038 for MDD, 0.041 for ALC, and 0.049 for comparison subjects.
Area fraction of Cx43 immunoreactivity
The area fraction of immunoreactivity was measured using ImageJ (version 1.44o, by Wayne Rasband, National Institutes of Health). Area fraction was measured in three optical fields that were randomly selected across the cortical layers. Pictures were taken at 20x magnification with a Nikon E600 microscope using a fixed intensity of microscopic illumination and constant condenser settings. The optical field of the picture was then segmented into the non-immunoreactive background (determined in a portion of the tissue under the microscope with no visible puncta and uniform background) and Cx43 immunoreactive structures. Immunoreactivity was defined as 20 optical density points over background optical density (Miguel-Hidalgo et al., 2010). Optical density was measured on a 0 (brightest) to 255 (darkest) scale. Area fraction was obtained for each optical field by dividing the area occupied by Cx43 immunoreactivity greater than background by the total area of the field.
Statistics
Cx43 protein levels, packing density of Cx43-immunoreactive puncta, punctum size and area fraction for each group were compared using analysis of variance (ANOVA). There were no significant differences between groups in age at the time death, postmortem interval (PMI), brain pH, and brain weight. The relationship between Cx43-related variables and age, or with duration and onset of disease were assessed by Pearson correlational analyses. Posthoc pairwise univariate comparisons were performed only following a significant ANOVA (p<0.05) using the Bonferroni adjustment for multiple comparisons. Results are presented as mean ± standard error of the mean unless otherwise indicated.
RESULTS
Cx43 protein levels from western blots
The levels of Cx43 protein were significantly lower in all three groups with a psychiatric diagnosis as compared to non-psychiatric controls (ANOVA F(3,68)=11.35, p<0.001) (Fig. 1A, B). The reduction was about 60% in alcohol-dependent (p<0.002), 77% in MDD (p<0.001), and 70% in MDA (p<0.001) subjects. Consideration of death by suicide as an additional grouping factor in a 2-way ANOVA did not show a significant effect of suicide (F(1,65)=0.645, p=0.421) or an interaction between suicide and diagnostic groups (F(2,65)=0.418, p=0.660), but still showed a difference between psychiatric diagnoses (F=6.66, p=0.001). The level of Cx43-immunoreactivity in each of the suicide (1.085±0.475, p=0.002 versus controls) and non-suicide (1.264±0.632, p=0.022 versus controls) psychiatric groups was significantly lower than that in non-psychiatric controls (3.295±0.380).
Figure 1.
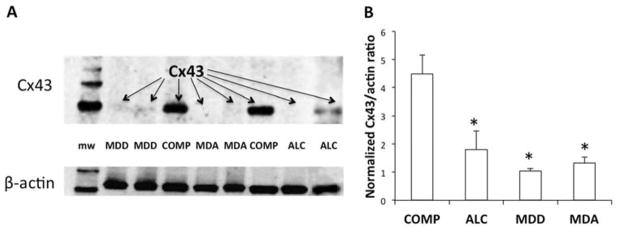
Quantification of connexin 43 (Cx43) levels in the OFC of non-psychiatric comparison subjects (COMP), subjects with alcohol dependence (ALC), subjects with major depressive disorder (MDD), and subjects with comorbid major depressive disorder and alcohol dependence (MDA). A) Representative western blot with subjects from the four diagnostic groups. The lane in the left shows bands at known molecular weights (mw) from a commercial standard. B) Bar graph representing the normalized levels of Cx43 in COMP (n=20), ALC (n=16), MDD (n=23) and MDA subjects (n=13). Whiskers represent the standard error of the mean. *p<0.002 relative to non-psychiatric comparison group (ANOVA F=3.68, p < 0.001). mw = molecular weight marker.
Packing density of Cx43 immunopositive puncta and area fraction of immunoreactivity
Using the same antibody as in western blots we detected highly contrasted immunoreactive puncta of variable size (Figs. 2, 3) in the gray matter of the OFC. There was a significant effect of diagnosis on the packing density of Cx43-immunolabeled puncta (ANOVA, F(3, 64)=3.173, p=0.03) with the highest average in comparison subjects. The density of puncta in the ALC subjects was significantly lower than in COMP subjects (univariate contrast p<0.019), but not different in MDD (p=0.842) or MDA (p=1.00) subjects (Fig 4A). When using suicide and psychiatric diagnosis as factors in a two-way ANOVA, there was a trend for a lower density of Cx43 puncta in subjects that died by suicide as compared to non-suicides (suicide F(1,62)= 3.144, p=0.081). There was a statistical trend for an interaction between diagnostic group and suicide (p<0.066), mainly because puncta density in suicide MDA subjects (1.182±0.944 punctaX106/mm3) was markedly lower than in non-suicide MDA subjects (4.013±0.798 puncta X106/mm3).
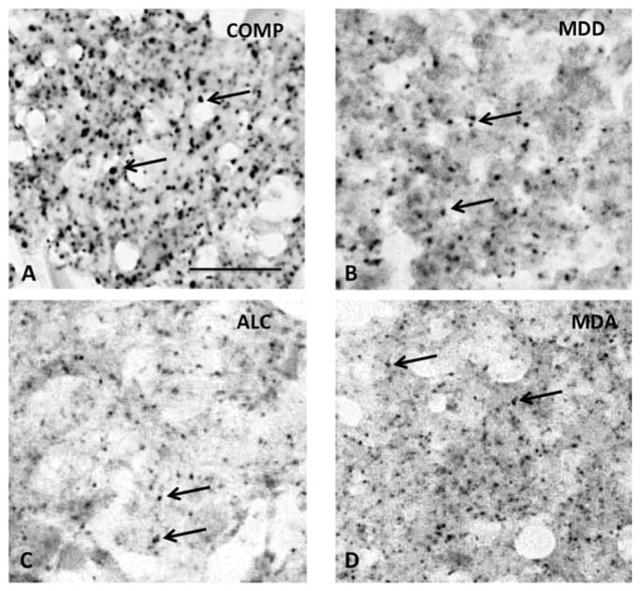
Figure 2.
High power micrographs of Cx43 immunoreactivity in the cortical gray matter of the OFC COMP (A), ALC (B), MDD (C), and MDA (C) subjects. All pictures centered in lower cortical layer III. Arrows in all micrographs point to representative Cx43 immunoreactive puncta. Calibration bar in A is 25 μm and valid for all micrographs. Abbreviations, arrows and dimensions are as in figure 1
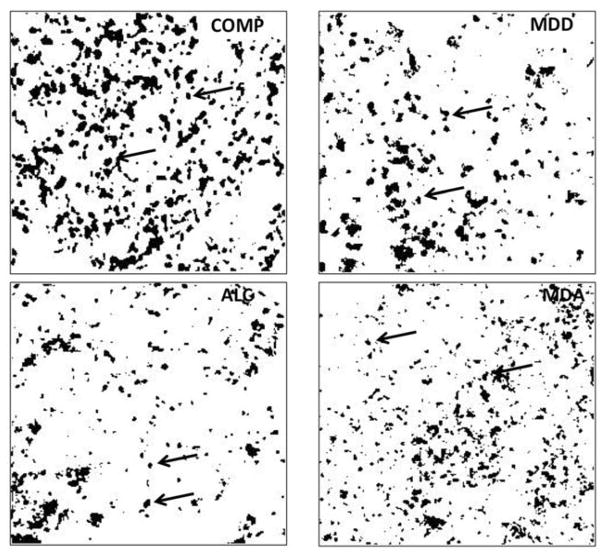
Figure 3.
Binary rendering of the four micrographs in Fig. 2 using Image J software and applying the same procedure to each micrograph to establish a threshold and differentiate Cx43 immunoreactive structures (black spots) from non-immunoreactive background (white background). Abbreviations, arrows and dimensions are as in figure 1.
Figure 4.
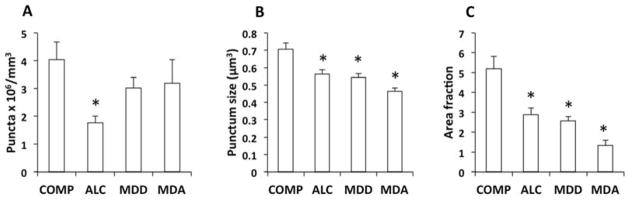
Packing density of Cx43-immunoreactive puncta (A), average size (volume) of discrete immunoreactive puncta (B) and area fraction of Cx43 immunoreactivity (C) in the gray matter of the OFC of COMP, ALC, MDD and MDA subjects. A) Bar graph of average packing density of Cx43-immunoreactive puncta in the four diagnostic groups; only the intensely dark, highly contrasting puncta were counted to determine density of puncta. *p = 0.019 relative to COMP subjects (ANOVA, F=3.173, p=0.03). B) Average size of individual immunoreactive puncta as estimated with the nucleator probe. *p < 0.005 relative to COMP subjects. (ANOVA, F = 11.66, p<0.005). C) Bar graph of average area fraction of Cx43-immunoreactivity in the cortical gray matter. *p < 0.003 relative to COMP subjects (ANOVA, F = 12.8, p<0.001). Other abbreviations as in figure 1.
Size of Cx43 immunoreactive puncta
The average volume of individual Cx43 immunoreactive puncta (punctum size) as estimated by the nucleator stereological probe was significantly different between diagnostic groups (F(3, 68)=11.66, p<0.005). Punctum size was smaller in each of the three psychiatric groups than in the COMP group, with the smallest average size found in the MDA subjects (Fig. 4B).
Area fraction of Cx43 immunoreactivity
Area fraction of Cx43 immunoreactivity was significantly different between groups (ANOVA, F(3,67)=12.8, p<0.001) and pairwise contrasts showed that area fraction in each of the diseased groups (MDD p<0.003, ALC p<0.001, MDA, p<0.001) was significantly lower that in the comparison group (Figs. 3, 4C). Two-way ANOVA with psychiatric diagnosis and suicide as grouping factors showed no significant effect of suicide (F(2,64)=0.487, p=0.488) or suicide by psychiatric diagnosis interaction (F(1, 64)=0.260, p=0.772).
Correlation with age at the time of death, duration and onset of disorder
Age at the time of death was negatively correlated with the levels of Cx43 protein either when considering all subjects together (r=−0.241, p=0.041) or in non-psychiatric comparison subjects (r=−0.519, p=0.019) but was not correlated with age in the diseased groups.
In the psychiatric groups, we analyzed the relationship between levels of protein and the estimated duration and age of onset of alcohol-dependence or major depressive disorder. There were no significant correlations between duration of alcohol dependence or depression and levels of Cx43 in any of the groups or when all alcohol-dependent (ALC+MDA) or all subjects with depression (MDA+MDD) were grouped together. Only the level of Cx43 in the MDA group showed a significant negative correlation with the age at onset of alcoholism (r=−0.628, p=0.016).
In controls and in MDD subjects there was a significant positive correlation between density of Cx43 puncta and age (control r=0.513, p=0.015; MDD r=0.469, p=0.037), that is, opposite to the negative correlation between levels of Cx43 and age in controls. There was no significant correlation between the density of puncta and age in ALC or MDA subjects. There was no correlation between average punctum size and the age at the time of death in any of the diagnostic groups. We also examined the relationship of puncta density and punctum size to the estimated age at onset or duration of alcoholism and depression in the three psychiatric groups. The age at onset of alcoholism in the MDA group was significantly and positively correlated with the density of Cx43 immunoreactive puncta (r=0.588, p=0.044). There were no significant correlations between puncta density and duration or onset of disorder in the other groups or with duration of depression in MDA. Neither duration nor onset of depression or alcoholism was pcorrelated with punctum size in any of the psychiatric groups.
Influence of treatment with antidepressants
MDD and MDA subjects were combined (MDD+MDA) and the resulting cohort was divided into subjects that had antidepressant prescription in the last month of life (Adep) and Adep-untreated subjects. There were no differences in Cx43 levels between treated and untreated subjects (Fig. 5, top panel). Dividing the Adep cohort into those that responded to ADep and those unresponsive did not reveal any difference in their Cx43 levels either (Fig. 5, bottom panel). The density of Cx43 immunoreactive puncta did not differ either according to Adep treatment or to response to treatment (Fig. 6A,B) while the density remained significantly lower in ALC subjects than in COMP subjects in either case (Fig. 6A,B). Punctum size was significantly lower in the psychiatric groups resulting from separating Adep treated and non-treated subjects as compared to the COMP group, but was not influenced by antidepressant treatment or the response to it (Fig. 6C,D).
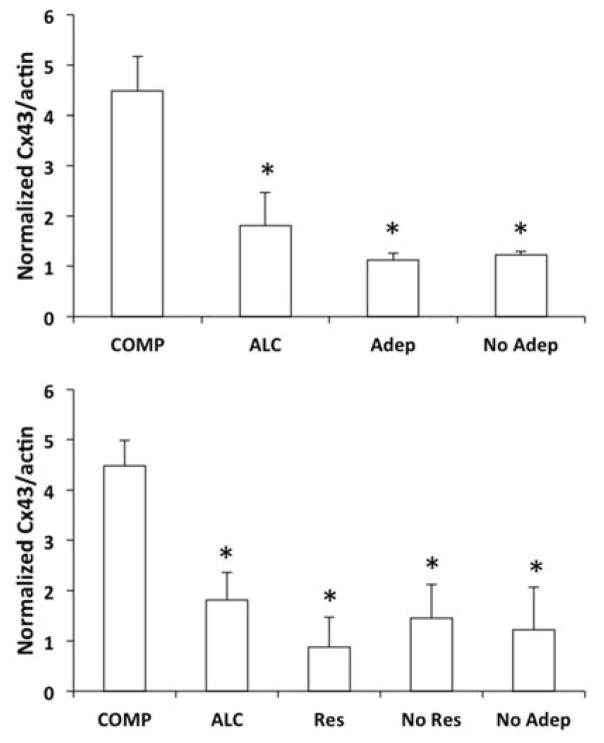
Figure 5.
Bar graphs representing differences in the Western blot-base levels of Cx43 in the OFC of non-psychiatric comparison (COMP), alcohol dependent (ALC, without depression), and subjects with major depression (Adep, NoAdep). In the top panel major depression subjects treated with a current prescription of antidepressant (Adep) were separated from those without antidepressants (NoAdep) (ANOVA F(3,68)=11.255, p<0.001). In the bottom panel Adep subjects were further subdivided into responders to the antidepressant treatment (Res) and non-responders (No Res) F(4,67)=8.49, p<0.001). Note a significantly lower level of Cx43 in all groups diagnosed with major depression or alcoholism regardless of antidepressant treatment (with *p<0.002 in the top panel and *p<0.003 in the bottom panel, against COMP subjects).
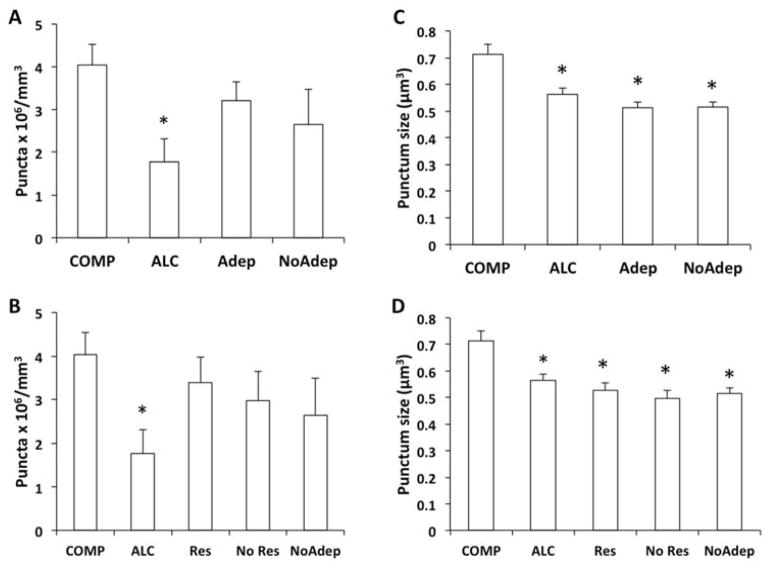
Figure 6.
Bar graphs representing differences in the packing density and average size of Cx43 immunoreactive puncta in the OFC of non-psychiatric comparison (COMP), alcohol dependent (ALC, without depression), and major depression (Adep, NoAdep). In A and C subjects with major depression treated with a current prescription of antidepressant (Adep) were separated from those without antidepressants (NoAdep). In B and D Adep subjects were further subdivided into responders to the antidepressant treatment (Res) and non-responders (No Res). Only the density of immunoreactive puncta in ALC subjects remained significantly different from that in COMP subjects (*pairwise comparisons, p=0.017 in A, p=0.032 in B). There was no difference among psychiatric groups in punctum size due to treatment or response to it (C, D) but each of these groups was significantly different from COMP subjects (*p<0.005).
DISCUSSION
The present study revealed that Cx43 immunoreactivity levels in the OFC (Brodmann’s area 47) are significantly reduced in subjects with alcohol dependence, major depressive disorder or comorbid depression plus alcohol dependence relative to non-psychiatric comparison subjects. This reduction in Cx43 was consistently observed whether measured in western blots or as an area fraction of Cx43 immunoreactivity in OFC sections. In addition, the average size of individual immunoreactive Cx43 puncta in the three diseased groups was lower than in the comparison subjects. Only in alcohol dependent subjects (without depression) there was a significantly lower packing density of Cx43 immunoreactive puncta than in comparison subjects.
Six subunits of Cx43 form a connexon with a central channel that spans the cell membrane. Connexons assemble to form gap junctions between adjacent cells, these junctions being grouped into larger aggregates or plaques, and serving as channels for calcium and organic molecules smaller than 1000 Da. Cx43 also forms aggregates of hemichannels, which are connexons not coupled to connexons in adjacent cells. Hemichannels have been related to release of ATP or glutamate from astrocytes (Bruzzone and Ressot, 1997). The gap junctions and hemichannel aggregates result in sharply contrasting immunoreactive puncta as confirmed by electron-microscopy (Nagy et al., 1996; Yamamoto et al., 1990). In alcoholism, both lower density of identifiable puncta and smaller punctum size would be a reflection of low Cx43 levels and would be consistent with diminished aggregation and membrane targeting of Cx43 into gap junctions or hemichannels. In depression, in the absence of reduced density of puncta, the main factor affecting lower Cx43 levels and area fraction of Cx43 immunoreactivity would have been the smaller size of Cx43 immunoreactive puncta, suggesting that different mechanisms influencing the distribution of Cx43 into membrane gap junctions or hemichannels may be at play in depression and alcoholism.
Density and size changes of Cx43 connexon aggregates may have direct functional consequences for astrocyte intercellular communication because those changes affect gap junction- and hemichannel-based permeability (Brokamp et al., 2012; Bukauskas et al., 2000). Reduction in the density or size of hemichannel aggregates caused by insufficient astrocytic tissue coverage in depression might play a role in the involvement of reduced ATP signaling in depression, because hemichannels allow the release of ATP, which acts onto receptors in astrocytes and neurons to regulate synaptic transmission (Bennett et al., 2003). Deficient gap junction communication between astrocytes has been also hypothesized as a contributor to brain dysfunction in depression (Mitterauer, 2010). The possibility of such functional alterations in MDD is further supported by recent postmortem research documenting a decrease in Cx43 mRNA levels in MDD but not in bipolar disorder (Bernard et al., 2011). An important qualification to the present data and to the so far discussed functional interpretation of Cx43 reductions is that the antibody used in this study may not have detected forms of Cx43 with differential posttranslational processing, and that these alternative forms may have been more prevalent in the diseased groups and have an important role in possible functional alterations.
Changes in density of Cx43 immunoreactive puncta, size of individual puncta and Cx43 levels in alcoholism suggest reduced gap junctional or hemichannel-based communication, which would be consistent with our study in rats (Miguel-Hidalgo et al., 2009), where infusion of gap junction blocker 18-α-glycyrrhetinic acid (AGA) into the rat PFC resulted in a transient increase in the preference and intake for a 10% ethanol solution. An increase in alcohol drinking was also detected when astrocytes were damaged by infusion of a glial toxin, although AGA did not result in depletion of GFAP-positive astrocytes (Miguel-Hidalgo et al., 2009). In fact, persistent decrease in gap junction permeability could arise from direct and indirect actions of alcohol on Cx43 expression (Wentlandt et al., 2004), and other in vitro and in vivo experiments have demonstrated alcohol-induced reduction in the expression of Cx43 proteins in neural precursor cells (Sun et al., 2009), smooth muscle cells and liver epithelial cells (Xiong et al., 2009), and in cardiac muscle (Huang et al., 2013).
Since most MDA subjects and many of the MDD subjects had been treated with antidepressants (Adeps) within the last month of life it could be argued that Adeps may have increased the density of gap junction aggregates (though not their size) as compared to ALC subjects. However, when combining all subjects with depression (MDD+MDA) and then assessing them according to Adep treatment there was no significant difference in packing density of Cx43 puncta between Adep-treated and non-treated subjects. Experiments in rodents reveal that repeated antidepressant treatment increases Cx43 protein levels in the prefrontal cortex (Fatemi et al., 2008). Further duly powered in vitro and in vivo experiments should help clarify whether Adep actions might be partly mediated by changes in Cx43 expression or trafficking (Segretain and Falk, 2004).
Ernst et al. (2011) reported lower levels of mRNA for Cx43 in the PFC of subjects dying by suicide (Ernst et al., 2011). However, in the present study there was no significant difference in Cx43 immunodetection parameters between suicide and non-suicide psychiatric subjects. The apparent discrepancy with Ernst et al. (Ernst et al., 2011) may partly rest on the fact that, unlike their non-psychiatric controls, all subjects in their Canadian suicide cohort had a psychiatric diagnosis. The authors also point that there was no difference in Cx43 mRNA levels between subjects with or without depression in the suicide group. However, if depression and substance abuse were a main cause for lower expression of Cx43 mRNA, one would not expect to find psychiatric diagnosis effects within their suicide group. It may be worth to point as well that Ernst et al. (2011) measured mRNA for all subjects in their cohorts, while in our study all subjects were analyzed for immunoreactivity of the Cx43 protein. Cx43 mRNA levels are not necessarily correlated with the levels of immunoreactive protein (Xiong et al., 2009). Given the very low levels of protein detected in some subjects it is also possible that disease-related changes in Cx43 immunoreactivity are partly due to disorder-specific variations in posttranslational modifications.
Animal experiments by Ernst et al. (2011) further appeared to rule out that alcohol exposure results in reduced Cx43 mRNA levels. By contrast, we found that alcoholics had significantly lower Cx43 protein levels and distribution as compared to controls. Nevertheless, several other studies (Huang et al., 2013; Sun et al., 2009; Wentlandt et al., 2004; Xiong et al., 2009) support the ability of alcohol to reduce the expression of Cx43 protein even when levels of Cx43 mRNA remain unaltered. Whether reduced Cx43 immunoreactivity in the postmortem area 47 of the OFC is consistent with mechanisms operating in the in vitro experiments remains to be determined.
Difference in the prefrontal regions studied may also explain discrepancies between studies. Ernst et al. (2011) sampled the dorsolateral prefrontal cortex (dlPFC) defined as comprising Brodmann’s areas 9, 10, 11, 44a, 45, 46, and 47, which includes some areas (areas 11 and 47) usually assigned to the OFC (Kringelbach and Rolls, 2004; Uylings et al., 2010). In all, their region is remarkably more heterogeneous that the restricted area 47 in our study. Nonetheless, it cannot be ruled out that reduced detection of Cx43 is related to increased suicide risk, which is well known to be higher in subjects with depression or alcohol dependence.
It is important to stress that the psychiatric groups in the present study contained a high proportion of subjects dying by suicide. If this fact is considered with enhanced association of suicidal ideation in depression and alcoholism even in subjects that died of other causes (Garlow et al., 2008; Gonzalez, 2012; Joiner et al., 2005) it may result in a significant association between suicidality and low Cx43 levels. Such an association could not be unequivocally explored in the present study. In addition, the direction of a causal relationship between the terms of this association (suicidality and Cx43 or gap junction function) remains to be determined. The aim of including subjects dying by suicide in the psychiatric groups was to compare subjects dying by suicide with those dying by other causes in each of the diagnostic groups. Depression is a well-known risk factor for suicide (Joiner et al., 2005). In comparison to depression, less attention has been paid to the fact that alcohol abuse, particularly during drinking episodes, is also a high risk for suicide even in subjects without a diagnosis of depression (Pirkola et al., 2000; Roy and Linnoila, 1986). Thus, suicide subjects in the ALC group would have permitted a first approximation of a possible association of low Cx43 levels with suicide not related to the clinical diagnosis of depression. However, the present study did not find a significant correlation between suicide and Cx43 levels in ALC subjects.
The present study also revealed an age-dependent decrease in Cx43 levels in COMP subjects, although the density of immunoreactive puncta was positively correlated with age in controls and MDD subjects, suggesting that the synthesis of Cx43 and its trafficking to the cell membrane to form gap junctions (immunoreactive puncta) may be differentially affected by age. Alternatively, but not exclusively, a greater density of puncta despite declining Cx43 synthesis, may result from a slower turnover of Cx43 from the membrane combined with an increase in gliosis features in the aging brain of control and MDD subjects (Miguel-Hidalgo et al., 2000; Si et al., 2004). Further studies should examine if age-dependent disturbances in Cx43 synthesis and turnover are related to an increased degree of gliosis in the OFC.
In summary, the present data suggest that dysfunction of the OFC in depression and alcoholism is accompanied by a reduction in Cx43 and a diminution of Cx43-immunoreactive gap junction or hemichannel aggregates. Lower Cx43 in depression was not paralleled by reduced density of Cx43 immunoreactive puncta, but there was significant reduction of Cx43-immunoreactive puncta in alcoholism suggesting that some functional disturbances in Cx43-immunoreactive gap junctions may be specific to the manifestations of each of those disorders.
Acknowledgments
We gratefully acknowledge the assistance of Dr. James C Overholser, Dr. George Jurjus and Lisa Konick in establishing the psychiatric diagnoses and collecting tissues. We thank the Cuyahoga County Coroner’s office, Cleveland, OH, and the next-of-kin of our subjects for their participation and support.
Role of funding source: Funding for this study was provided by NIH grants MH82297 and P30 GM103328. The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributors: Author Jose J. Miguel-Hidalgo designed the study, wrote the protocols and wrote the first draft of the article. Author Barbara A. Wilson, performed the western blot experiments and edited the article. Authors Syed Hussain and Ashish Meshram performed immunohistochemistry, collected morphometric data and contributed to the methods section. Author Grazyna Rajkowska contributed to the correct anatomical identification of brain areas and to the discussion. Author Craig A. Stockmeier contributed to the final draft of the discussion and introduction, and to the selection of postmortem material according to postmortem psychological diagnosis. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asrih M, Lerch R, Papageorgiou I, Pellieux C, Montessuit C. Differential regulation of stimulated glucose transport by free fatty acids and PPARα or -δ agonists in cardiac myocytes. American Journal of Physiology - Endocrinology and Metabolism. 2012;302:E872–E884. doi: 10.1152/ajpendo.00427.2011. [DOI] [PubMed] [Google Scholar]
- Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. British Journal of Psychiatry. 2001;178:200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Molecular Psychiatry. 2008;15:501–511. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MV, Contreras JE, Bukauskas FF, Saez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends in Neurosciences. 2003;26:610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, Schatzberg AF, Myers RM, Akil H, Watson SJ. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Molecular Psychiatry. 2011;16:634–646. doi: 10.1038/mp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokamp C, Todd J, Montemagno C, Wendell D. Electrophysiology of Single and Aggregate Cx43 Hemichannels. PLoS One. 2012;7:e47775. doi: 10.1371/journal.pone.0047775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R, Ressot C. Connexins, gap junctions and cell-cell signalling in the nervous system. The European Journal of Neuroscience. 1997;9:1–6. doi: 10.1111/j.1460-9568.1997.tb01346.x. [DOI] [PubMed] [Google Scholar]
- Bukauskas FF, Jordan K, Bukauskiene A, Bennett MVL, Lampe PD, Laird DW, Verselis VK. Clustering of connexin 43–enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Proceedings of the National Academy of Sciences. 2000;97:2556–2561. doi: 10.1073/pnas.050588497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corinne N, Carla H, Joseph PS. Interaction of connexin 43 and protein kinase C-delta during FGF2 signaling. BMC Biochemistry. 2010;11:14–14. doi: 10.1186/1471-2091-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Nedergaard M. Brain connexins in demyelinating diseases: Therapeutic potential of glial targets. Brain Research. 2012;1487:61–68. doi: 10.1016/j.brainres.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Feldman L, Barrucand D, Rancurel G, Féline A, Syrota A. Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychological Medicine. 1998;28:1039–1048. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Falkai P, Gruber O. Functional neuroimaging of reward processing and decision-making: a review of aberrant motivational and affective processing in addiction and mood disorders. Brain Research Reviews. 2008;59:164–184. doi: 10.1016/j.brainresrev.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Dom G, Sabbe B, Hulstijn W, van den Brink W. Substance use disorders and the orbitofrontal cortex: systematic review of behavioural decision-making and neuroimaging studies. British Journal of Psychiatry. 2005;187:209–220. doi: 10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Orbitofrontal cortex function and structure in depression. Annals of the New York Academy of Sciences. 2007;1121:499–527. doi: 10.1196/annals.1401.029. [DOI] [PubMed] [Google Scholar]
- Ernst C, Nagy C, Kim S, Yang JP, Deng X, Hellstrom IC, Choi KH, Gershenfeld H, Meaney MJ, Turecki G. Dysfunction of astrocyte connexins 30 and 43 in dorsal lateral prefrontal cortex of suicide completers. Biological Psychiatry. 2011;70:312–319. doi: 10.1016/j.biopsych.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Pandian T, Braun NN, Haug K. Chronic psychotropic drug treatment causes differential expression of connexin 43 and GFAP in frontal cortex of rats. Schizophrenia Research. 2008;104:127–134. doi: 10.1016/j.schres.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Figiel M, Allritz C, Lehmann C, Engele J. Gap junctional control of glial glutamate transporter expression. Molecular and Cellular Neuroscience. 2007;35:130–137. doi: 10.1016/j.mcn.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Flatscher-Bader T, Wilce PA. Impact of alcohol abuse on protein expression of midkine and excitatory amino acid transporter 1 in the human prefrontal cortex. Alcoholism, Clinical and Experimental Research. 2008;32:1849–1858. doi: 10.1111/j.1530-0277.2008.00754.x. [DOI] [PubMed] [Google Scholar]
- Garlow SJ, Rosenberg J, Moore JD, Haas AP, Koestner B, Hendin H, Nemeroff CB. Depression, desperation, and suicidal ideation in college students: results from the American Foundation for Suicide Prevention College Screening Project at Emory University. Depression and Anxiety. 2008;25:482–488. doi: 10.1002/da.20321. [DOI] [PubMed] [Google Scholar]
- Giaume C, Tabernero A, Medina JM. Metabolic trafficking through astrocytic gap junctions. Glia. 1997;21:114–123. [PubMed] [Google Scholar]
- Gilman SE, Abraham HD. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug and Alcohol Dependence. 2001;63:277–286. doi: 10.1016/s0376-8716(00)00216-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez VM. Association of solitary binge drinking and suicidal behavior among emerging adult college students. Psychology of Addictive Behaviors. 2012;26:609–614. doi: 10.1037/a0026916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Weiss RD, Muenz LR, Vagge LM, Kelly JF, Bello LR, Michael J. The effect of depression on return to drinking: a prospective study. Archives of General Psychiatry. 1998;55:259–265. doi: 10.1001/archpsyc.55.3.259. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Grant BF. Major depression in 6050 former drinkers: association with past alcohol dependence. Archives of General Psychiatry. 2002;59:794–800. doi: 10.1001/archpsyc.59.9.794. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiologycal Reviews. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Huang Q-Y, Li X-F, Liu S-P. Connexin 43 and Angiotensin II Alterations in Hearts of Rats Having Undergone an Acute Exposure to Alcohol. The American Journal of Forensic Medicine and Pathology. 2013;34:68–71. doi: 10.1097/PAF.0b013e31827bf67f. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Brown JS, Wingate LR. The Psychology and Neurobiology of Suicidal Behavior. Annual Review of Psychology. 2005;56:287–314. doi: 10.1146/annurev.psych.56.091103.070320. [DOI] [PubMed] [Google Scholar]
- Khundakar AA, Thomas AJ. Morphometric changes in early- and late-life major depressive disorder: evidence from postmortem studies. International Psychogeriatrics. 2009;21:844–854. doi: 10.1017/S104161020999007X. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Del Boca FK, Rounsaville BJ. Comorbid psychiatric diagnosis predicts three-year outcomes in alcoholics: a posttreatment natural history study. Journal of Studies On Alcohol. 1996;57:619–626. doi: 10.15288/jsa.1996.57.619. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Labovsky V, Hofer EL, Feldman L, Fernández Vallone V, García Rivello H, Bayes-Genis A, Hernando Insúa A, Levin MJ, Chasseing NA. Cardiomyogenic differentiation of human bone marrow mesenchymal cells: Role of cardiac extract from neonatal rat cardiomyocytes. Differentiation. 2010;79:93–101. doi: 10.1016/j.diff.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo J, Shoyama Y, Wanzo V. Infusion of gliotoxins or a gap junction blocker in the prelimbic cortex increases alcohol preference in Wistar rats. Journal of Psychopharmacology. 2009;23:551–558. doi: 10.1177/0269881108091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. Neuropathology of Depression, Alcoholism, and their Comorbidity in the Human Prefrontal Cortex. In: Sher L, editor. Comorbidity of Depression and Alcohol Use Disorders. New York: Nova Science Publishers, Inc; 2009. pp. 171–179. [Google Scholar]
- Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. GFAP-immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biological Psychiatry. 2000;48:860–872. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Rajkowska G. Comparison of prefrontal cell pathology between depression and alcohol dependence. Journal of Psychiatric Research. 2003;37:411–420. doi: 10.1016/s0022-3956(03)00049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Waltzer R, Whittom AA, Austin MC, Rajkowska G, Stockmeier CA. Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. Journal of Affective Disorders. 2010;127:230–240. doi: 10.1016/j.jad.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Wei J, Andrew M, Overholser JC, Jurjus G, Stockmeier CA, Rajkowska G. Glia pathology in the prefrontal cortex in alcohol dependence with and without depressive symptoms. Biological Psychiatry. 2002;52:1121–1133. doi: 10.1016/s0006-3223(02)01439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitterauer BJ. The syncytiopathy hypothesis of depression: downregulation of glial connexins may protract synaptic information processing and cause memory impairment. Medical Hypotheses. 2010;74:497–502. doi: 10.1016/j.mehy.2009.09.058. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Li W, Hertzberg EL, Marotta CA. Elevated connexin 43 immunoreactivity at sites of amyloid plaques in Alzheimer’s disease. Brain Research. 1996;717:173–178. doi: 10.1016/0006-8993(95)01526-4. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Contributions of the ventromedial prefrontal cortex to goal-directed action selection. Annals of the New York Academy of Sciences. 2011;1239:118–129. doi: 10.1111/j.1749-6632.2011.06290.x. [DOI] [PubMed] [Google Scholar]
- Pirkola SP, Isometsä ET, Heikkinen ME, Lönnqvist JK. Suicides of alcohol misusers and non-misusers in a nationwide population. Alcohol and Alcoholism. 2000;35:70–75. doi: 10.1093/alcalc/35.1.70. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends in Cognitive Science. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biological Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS and Neurological Disorders - Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Rouach N, Avignone E, Meme W, Koulakoff A, Venance L, Blomstrand F, Giaume C. Gap junctions and connexin expression in the normal and pathological central nervous system. Biology of the Cell. 2002;94:457–475. doi: 10.1016/s0248-4900(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Roy A, Linnoila M. Alcoholism and Suicide. Suicide and Life-Threatening Behavior. 1986;16:244–273. doi: 10.1111/j.1943-278x.1986.tb00354.x. [DOI] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Pfefferbaum A, Sullivan EV. Neurocircuitry of emotion and cognition in alcoholism: contributions from white matter fiber tractography. Dialogues in Clinical Neuroscience. 2010;12:554–560. doi: 10.31887/DCNS.2010.12.4/tschulte. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segretain D, Falk MM. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochimica et Biophysica Acta - Biomembranes. 2004;1662:3–21. doi: 10.1016/j.bbamem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Si X, Miguel-Hidalgo JJ, O’Dwyer G, Stockmeier CA, Rajkowska G. Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology. 2004;29:2088–2096. doi: 10.1038/sj.npp.1300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism, Clinical and Experimental Research. 2000;24:611–621. [PubMed] [Google Scholar]
- Sun T, Zhang D, Xie S, Wang Y, Feng Y, Xin H. Effect of ethanol on the expression of connexin 43 in primaru culture of neural precursor cells. Journal of Shandong University (Health Sciences) 2009;47:20–24. [Google Scholar]
- Uylings HB, Sanz-Arigita EJ, de Vos K, Pool CW, Evers P, Rajkowska G. 3-D cytoarchitectonic parcellation of human orbitofrontal cortex correlation with postmortem MRI. Psychiatry Research. 2010;183:1–20. doi: 10.1016/j.pscychresns.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nature Reviews Neuroscience. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Wentlandt K, Kushnir M, Naus CC, Carlen PL. Ethanol inhibits gap-junctional coupling between P19 cells. Alcoholism, Clinical and Experimental Research. 2004;28:1284–1290. doi: 10.1097/01.alc.0000139705.17646.ba. [DOI] [PubMed] [Google Scholar]
- Xiong L, Zhang JJ, Sun D, Liu H, Zhang L. Influence of chronic cerebral hypoperfusion on gap junction ultrastructure and expression of connexin (Cx) 36, Cx32, and Cx26 in hippocampus: experiment with rats. Zhonghua Yi Xue Za Zhi. 2009;89:1071–1074. [PubMed] [Google Scholar]
- Yamamoto T, Ochalski A, Hertzberg EL, Nagy JI. On the organization of astrocytic gap junctions in rat brain as suggested by LM and EM immunohistochemistry of connexin 43 expression. Journal of Comparative Neurology. 1990;302:853–883. doi: 10.1002/cne.903020414. [DOI] [PubMed] [Google Scholar]