Abstract
In this paper, we describe a San Francisco collaboration’s process to select optimal measures of linkage to care in response to the CDC’s Enhanced Comprehensive HIV Prevention Planning (ECHPP) program, and to understand the implications of measure selection and the challenges of accessing data sources to measure outcomes along the HIV care continuum.
Challenges identified are the variety of definitions of linkage to care and the non-integrative nature of the multiple data systems necessary to measure linkage to care and other continuum outcomes. The choice of linkage measures, which at the extremes is a choice between higher-resolution measures based on clinical visit data in a subset of patients vs. a lower resolution proxy measure based on surveillance data, has key implications. Choosing between the options needs to be informed by the primary use of the measure. For representing trends in overall performance and response to interventions more generalizable measures based on surveillance data are optimal. For identifying barriers to linkage to care for specific populations and potential intervention targets within the linkage process, higher-resolution measures of linkage that include clinical, laboratory, and social work visit information are optimal.
Cataloging the different data systems along the continuum and observations of challenges of data sharing between systems highlighted the promise of integrated data management systems that span HIV surveillance and care systems. Such integrated data management systems would have the ability to support detailed investigation and would provide simplified data to match newly developed, cross-agency HHS measures of HIV care continuum outcomes.
INTRODUCTION
In July 2010, the National HIV/AIDS Strategy (NHAS) established specific goals for the United States’ response to the HIV epidemic, including reductions in new infections; improvements in access to high-quality care and improved health outcomes among people living with the disease; and reductions in HIV-related health disparities.1 These objectives align with scientific research highlighting the critical role of prompt HIV diagnosis, linkage to care, and initiation of antiretroviral therapy. Since the introduction of the NHAS and its detailed implementation plan, significant progress has been made toward achieving the strategy’s goals, including a more coordinated national response by HIV/AIDS programs across multiple federal agencies. On July 15, 2013, the White House Office of National HIV/AIDS Policy (ONAP) introduced the Accelerating Improvements in HIV Prevention and Care in the United States through the HIV Care Continuum initiative, which builds on the NHAS to improve outcomes along the continuum from HIV diagnosis to successful retention in HIV care (i.e., the “HIV care continuum”).2
The Centers for Disease Control and Prevention (CDC) Enhanced Comprehensive HIV Prevention Planning (ECHPP) initiative for the 12 US jurisdictions most affected by HIV is a central part of the response to the NHAS.3 This program involves the local planning and subsequent implementation of a combination of 14 required HIV-prevention interventions and several optional components. The ECHPP initiative holds the promise of significantly advancing our understanding of the barriers and facilitators to comprehensive HIV prevention and treatment, and evaluating the initiative is crucial to elucidating best practices for realizing the goals of the NHAS. National Institutes of Health (NIH) supported ECHPP evaluation efforts by supplementing the Centers for AIDS Research (CFAR) to enhance collaborations between NIH-funded clinical and behavioral investigators and local public health department officials implementing and evaluating the ECHPP initiative.
In this paper, we describe our current collaboration’s process to select optimal measures of linkage to care in response to the ECHPP program, and to understand the implications of measure selection and the challenges of accessing and utilizing multiple data sources to measure outcomes along the HIV care continuum.
THE SAN FRANCISCO CFAR ECHPP COLLABORATION
San Francisco has a long history of collaborative efforts across local community based organizations, community advisory boards and planning councils, academic research institutions, clinical care providers, and branches of the San Francisco Department of Public Health (SFDPH).4–7 The ECHPP San Francisco effort built upon this existing network by including additional investigators with clinical and behavioral expertise in measuring linkage to care from the UCSF Center for AIDS Prevention Studies (CAPS), a behaviorally focused, NIMH-sponsored national AIDS research center; the UCSF–Gladstone Institute of Virology and Immunology Center for AIDS Research (CFAR), a NIAID-funded center for basic and clinical research; and HIV care providers at San Francisco General Hospital’s Positive Health Program.
SAN FRANCISCO PREVENTION AND TREATMENT ENVIRONMENT
San Francisco, one of 12 ECHPP sites, has 15,705 living HIV/AIDS cases. This constitutes 13% of California’s living HIV/AIDS cases and 2% of living HIV/AIDS cases nationwide.8 The current San Francisco HIV Prevention Strategy is a comprehensive, multilevel combination prevention, care, and treatment approach.9 Preceding ECHPP, there have been efforts to expand coverage and frequency of HIV testing for MSM and other populations at risk for HIV and to institute earlier initiation of ART.10–12 San Francisco has implemented key evidence-based interventions utilizing antiretroviral drugs, including universal offer of antiretroviral treatment (UART),11 non-occupational post-exposure prophylaxis,13 and pre-exposure prophylaxis.14–18 Access to HIV care and antiretroviral therapy is high in San Francisco, with low-income or medically indigent individuals provided coverage through federal, state, and local programs including, Medi-Cal, the California Medicaid program); Ryan White; and Healthy San Francisco, a health coverage program for uninsured San Franciscans. In California, there is no waiting list for the AIDS Drug Assistance Program (ADAP).19 The history of the HIV epidemic in San Francisco has also resulted in a large network of experienced HIV care providers and service organizations. Due to these factors, San Francisco shows robust outcomes along the HIV continuum of care: low rates of unrecognized HIV infection (7.5% among MSM), prompt linkage to care (87% within 6 months of diagnosis), moderate levels of engagement and retention in care, good ART uptake, and relatively high population rates of virologic suppression.12, 20, 21
While San Francisco shows robust outcomes along the HIV continuum of care, there remains room for improvement, and significant disparities exist along the continuum of care for disadvantaged and marginalized populations. Selecting optimal measures for assessment of improvements along the continuum and decreasing disparities was an identified priority of the SF CFAR ECHPP Collaboration, with a particular focus on linkage to care.
SELECTING MEASURES OF LINKAGE TO CARE
To arrive at consensus for the optimal definitions and measurements relating to linkage to care, the ECHPP San Francisco research collaboration conducted a review of relevant literature supplemented by key informant interviews and informal discussions with national and local HIV experts, HIV care providers, and patients. Two concurrent federal processes also guided the collaboration in choosing among numerous approaches to define optimal measures for outcomes along the HIV continuum of care, and in particular, the optimal definition of linkage to care. First, the IOM report “Monitoring HIV Care in the United States: Indicators and Data Systems,” released March 15, 2012, identifies core indicators for use by the HHS to gauge the effects of the NHAS and Affordable Care Act (ACA) on improvements in HIV care and access to supportive services for people with HIV; it also highlights 12 data-collection systems that could be used to monitor the impact of the NHAS and ACA.22 Second, the Department of Health and Human Services (HHS) conducted an assessment of the numerous approaches to calculating outcomes along the HIV continuum of care and reached consensus on the set of 7 core, population-level indicators measuring diagnosis (including late diagnosis), linkage, retention, ART use, viral suppression, and housing status.23 This information was then used to inform a small-group meeting of collaboration partners for conceptual synthesis and refinement.
Linkage-to-Care Conceptual Processes and Definitions
For quantitative analyses, linkage must be operationalized as an easily abstractable and discrete point or set of points based on objective measures. However, the reality is much more complex; linkage is a process involving physical locations, patient perspectives and decisions, laboratory testing, and care provider decisions (Figure 1). Furthermore, measurement approaches may use clinical or surveillance data.
Figure 1.
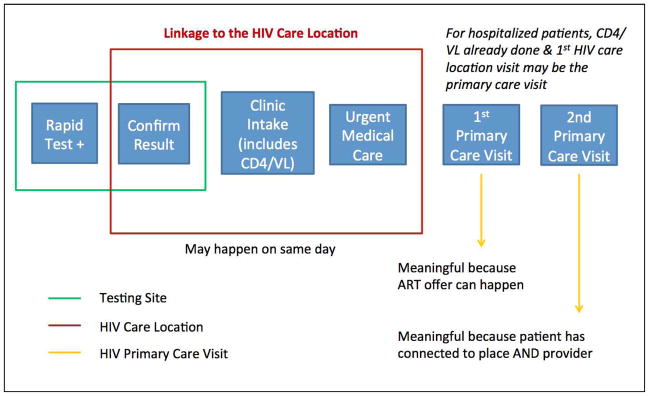
Linkage-to-Care Conceptual Framework36
After an initial preliminary positive diagnosis with a rapid test in a community-based HIV testing program, the linkage process to HIV primary care is initiated. At the care location, staff may order or disclose results of confirmatory HIV testing (for those diagnosed via rapid testing), if not done already. In order to promote faster linkage to HIV primary care, SFDPH implemented a change in policy allowing preliminarily positive clients to be seen at primary care clinics and have their confirmatory testing done there rather than requiring the client to return to the community-based testing site for the confirmatory test. At the intake visit, all patients generally receive staging laboratory tests, including CD4 and HIV viral load (VL). At high volume clinical sites, the intake usually happens with a social worker, nurse, or both. The first visit with a primary HIV care provider may or may not be on the same day as the intake. A second visit with the HIV provider is also important, as it suggests that the patient has made a connection both to the care location and to the provider. The HIV provider generally orders subsequent CD4 cell count and HIV viral load tests as part of a treatment plan. The offer and prescription of antiretroviral therapy often occur as early as the first HIV provider visit.
Based on this conceptual understanding, clinic-based definitions of linkage can include one HIV care location visit (during which the patient may or may not have been seen by a provider with prescribing privileges),24 one visit with a prescribing provider,25 or two visits with a prescribing provider.26 Varying time parameters have been used to further define these measures—for example, one visit with a prescribing provider within 30, 60, or 90 days.27, 28 The HHS recommendation for the linkage to care measure is the proportion of the persons who attended a routine HIV medical care visit within 3 months of HIV diagnosis over the number of persons with an HIV diagnosis in the 12-month measurement period.23
In contrast to definitions of linkage using data from clinical electronic medical records which give high-resolution detail (identifying intermediate steps in the linkage process), surveillance-based definitions of linkage to care use laboratory data (the CD4 and VL measurements) reported to the HIV surveillance system as a less-detailed proxy for an HIV primary care visit. Different measures used include one CD4 or VL measurement on a day other than the day of the test that diagnosed HIV infection,29 two CD4 or VL measurements within 6 or 12 months of the test that diagnosed HIV infection,30 or one CD4 or VL measurement within 3 months of the test that diagnosed HIV infection and another within the following 9 months.31,20 The surveillance measure assumes that having a CD4 or VL drawn indicates that the individual presented to an HIV care setting and was seen by a primary care doctor. However, as the conceptual framework reflects, the lab-based measure rather than a clinic-based measure of clinic attendance may not be as high-resolution, because lab values are a proxy measure only, and do not necessarily indicate that a visit with an HIV primary care provider has occurred.32–34
In summary, options identified for measuring linkage to care ranged from high-resolution clinical visit information available from electronic medical records, to proxy measures for clinical visits based on mandated lab reporting to the HIV/AIDS surveillance system.
Sources of Data for Measuring Linkage to Care
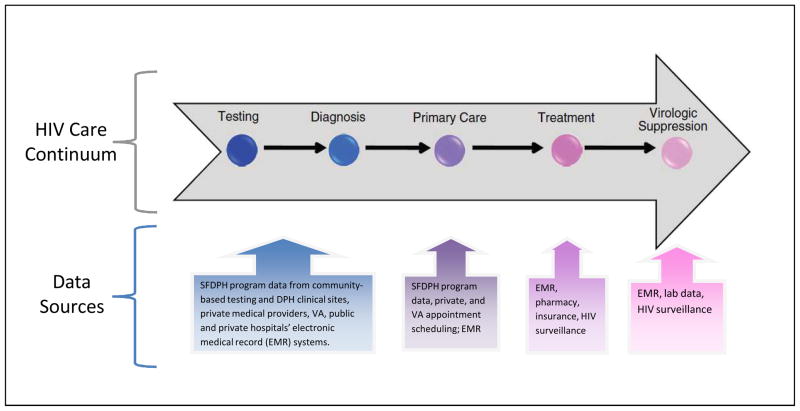
The quality and availability of data sources is an important structural constraint on choosing optimal measures for linkage to care. The SF CFAR ECHPP research collaboration mapped data sources along the HIV care continuum to understand the diversity and quality of data available for measuring linkage to care and other continuum outcomes. (Figure 2).
Figure 2.
Mapping of Data Sources across the Continuum of Care
The arrow representing the continuum of care, for the purposes of visualization, simplifies the details in the steps that take place after a client is diagnosed at a community-based testing site and linked to and engaged in care with a primary care HIV doctor. These steps include assessing the client’s health insurance options (which may or may not be done by community-testing staff); determining availability of an appropriate provider; making an appointment; and ensuring that the client attends the visit, has a laboratory appointment, receives CD4 and VL screening along with HIV genotype and other key intake lab tests, possibly has a follow-up appointment, and is initiated on antiretroviral therapy. As the boxes below highlight, for each step along the continuum of care, there are numerous distinct, non-interoperable, proprietary data systems that contain the information necessary to calculate outcomes.
In San Francisco, individuals can be diagnosed with HIV in numerous settings, including community-based HIV testing programs, the municipal sexually transmitted disease clinic, public health department primary care clinics, private medical providers, public or private emergency rooms and hospitals, or the jail. Each of these locations has a different data system to record the HIV testing date and results. In general, they are supported by distinct sources of funding and have different data-reporting requirements. Also, HIV testing programs vary greatly in their practices for ensuring and confirming linkage to primary HIV care has occurred, currently, most programs rely mainly upon the public health department to do this. Further along the continuum of care, information for appointment dates may be in multiple data systems—for example, a clinic scheduling system may be distinct from the electronic medical record (EMR), may connect to it, or may be part of the same software program. Similarly, prescription data could be contained in the EMR, a pharmacy data system, or in insurance or other administrative data systems. The IOM recommended that ART information be added to the HIV/AIDS surveillance system, and in San Francisco, current ART and history of ART have been collected by active surveillance activities for many years preceding these IOM recommendations.10, 20 Lastly, viral load dates and values could be contained in the following distinct data systems: the EMR, a laboratory data system, and/or the HIV surveillance registry.
These examples illustrate a key issue that emerges from examination of the numerous data sources across the continuum of care: necessary information for calculation of outcome measures along the continuum is housed in multiple systems. These include systems that may be paper-based or electronic, public or private, and clinical or administrative. These distinct data systems in most cases cannot share information with each other, thereby producing significant challenges to systematic measurement across the HIV care continuum. In conversations with other ECHPP implementation sites, this situation is a generalized one across many jurisdictions nationwide. Solutions that are being attempted in SF and other jurisdictions include 1) allocating ad hoc or systematic manual extraction and collation of data across the disparate data sources utilizing significant human resources, and 2) implementation of a single integrated data-management system. The results of these efforts will be revealed in the coming years.
IMPLICATIONS OF MEASURE SELECTION
Taken together, the numerous approaches for measuring linkage to care, along with the diversity of data sources with varying quality and availability, present competing options. At one extreme, there is high-resolution clinical visit data on a subset of HIV-infected patients linked or failing to link to care. On the other extreme, there is a low-resolution proxy measure (CD4, VL) on a very high proportion of HIV-infected patients diagnosed and obtaining care in San Francisco. Choosing the high-resolution measure available only in a subset of individuals provides a more comprehensive understanding of the process of linkage to care at the expense of generalizability. This may be useful in specific sub-populations of interest to identify potential targets within the linkage process for linkage to care or other interventions. Choosing a lower resolution, widely available measure has the advantages of broad generalizability, relative ease of use, and ability to measure longitudinal trends. Additionally, surveillance-based data are easy to compare across jurisdictions.
Engaging Community Stakeholders in Measure Selection
The SFDPH engaged key community stakeholders to provide input and feedback regarding the optimal measures of ECHPP efforts, including linkage to care. The HIV Prevention Planning Council (HPPC), the jurisdictional HIV prevention planning group, was asked to review San Francisco’s options for measurement, taking into consideration the HHS proposed indicators. The HPPC addressed the competing priorities of lower resolution, more generalizable data on linkage, with higher resolution data on linkage on a smaller group of patients by endorsing the use of both approaches. The council noted that the best way to measure success of ECHPP and other prevention efforts at the population-level was to use a “HIV continuum of care” approach, using the data that is most consistently and routinely available for the entire jurisdiction (such as CD4 and VL data from the HIV/AIDS surveillance system) to calculate the outcomes. For the HHS proposed linkage to care measure, the HPPC realized that it currently could only be calculated for public health department clinical settings, as calculating these indicators on a jurisdiction-wide level would require integration of data (information about clinical visit dates) from numerous proprietary clinical systems. Thus, they recommended that SFDPH continue its efforts to measure linkage to care using clinical visit data among the subset of clients in the public health department clinical settings for whom that data was routinely available, for the purposes of monitoring as improving clinical and public health testing program quality.
MEASURING LINKAGE TO CARE IN SAN FRANCISCO
Currently, the SFDPH is reporting the HHS recommended 3-month linkage to care measure utilizing routinely collected surveillance data.8 In 2010 and 2011, eighty-five percent of all newly diagnosed San Francisco HIV cases were linked to care within three months of diagnosis. Among individuals analyzed in 2009–2010, linkage to care rates were worse among the following groups: men who have sex with men who also inject drugs, persons without an identified transmission risk, and persons without health insurance or whose insurance status was unknown (compared to reference groups without those characteristics).20 In addition, the SFDPH continues to not only measure but also ensure linkage to care occurs for the subset of newly diagnosed HIV patients diagnosed by SFDPH funded community sites as well as those seen in the SFDPH clinical system through a variety of human resource–intensive methods including review and collation of data from different systems, communication by phone and in person with clients and medical providers to both verify and support linkage for these clients.
DISCUSSION
CFAR supported efforts to evaluate local NHAS implementation through ECHPP focused on improving outcomes along the HIV continuum of care. At a local level in San Francisco, implementation of procedures to measure continuum outcomes has revealed some challenges, particularly for linkage to care following HIV diagnosis. Chief among these challenges are the variety of possible definitions of linkage to care and the non-integrative nature of the data systems necessary to measure linkage to care. The choice of linkage to care measures, which at the extremes is a choice between higher-resolution measures based on clinical visit data in a subset of patients vs. a lower resolution proxy measure based on surveillance data in a larger, more generalizable population, has key implications. Choosing between the options for the optimal measure needs to be informed by the primary purpose for which the measure will be utilized. For representing trends in overall performance and response to interventions such as ECHPP, more generalizable measures based on surveillance data covering the widest number of HIV infected persons in the jurisdiction are optimal. For identifying barriers to linkage to care for specific populations and potential intervention targets within the linkage process, higher-resolution measures of linkage to care that include clinical, laboratory, social work and benefits visit information are likely to be optimal.
Cataloging the different data systems along the continuum and observations around the challenges of data sharing between systems highlighted the promise of an integrated data management system that spans HIV surveillance and care systems. Such integrated data management systems would have the ability to support detailed investigation and measurement of the complex process of linkage to care and would provide simplified data to match newly developed, cross-agency HHS measures of HIV care continuum outcomes.23 Having such a system in place would facilitate moving beyond measuring these outcomes, to an improved capacity for real-time public health interventions to improve these outcomes. 35 Potential approaches to address these challenges are already underway at the local level. For example, the San Francisco public health department has been funded by the CDC to implement an integrated data system in parallel with the implementation of new electronic health record systems in its clinics. As efforts are made to develop integrated data systems that promote high levels of data security and protect confidentiality, we will better be able to evaluate NHAS and the implementation of the Affordable Care Act at the local level, and to use these data to obtain optimal cross-agency measures of the HIV care continuum to enhance our national public health HIV prevention efforts.
Acknowledgments
Source of Funding: Supported by supplemental funds to the NIH-funded DC Developmental Center for AIDS Research (DC D-CFAR, Grant number 5P30AI087714) for the Enhanced Comprehensive HIV Prevention Planning Initiative (CFAR ECHPP Initiative).
Footnotes
Conflicts of Interest
None of the authors has any conflict of interest to declare.
The opinions expressed are those of the authors and not of the San Francisco Department of Public Health or the UCSF-Gladstone Center for AIDS Research.
References
- 1.White House Office of National AIDS Policy. National HIV/AIDS Strategy for the United States. Jul, 2010. [Google Scholar]
- 2.Executive Office of the President. Accelerating Improvements in HIV Prevention and Care in the United States Through the HIV Care Continuum Initiative. Federal Register. 2013:43055–43059. 78 FR 43055. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Enhanced Comprehensive HIV Prevention Planning and Implementation for Metropolitan Statistical Areas Most Affected by HIV/AIDS. http://www.cdc.gov/hiv/prevention/demonstration/echpp/
- 4.Dilley JW. Implications for the San Francisco model of care. AIDS Care. 1990;2(4):349–352. doi: 10.1080/09540129008257751. [DOI] [PubMed] [Google Scholar]
- 5.Silverman M. AIDS care: the San Francisco model. J Ambul Care Manage. 1988 May;11(2):14–18. doi: 10.1097/00004479-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Silverman M. Frontline: The Age of AIDS. http://www.pbs.org/wgbh/pages/frontline/aids/interviews/silverman.html.
- 7.Volberding P. Life Before the Lifeboat. http://vimeo.com/26935033.
- 8.San Francisco Department of Public Health. HIV/AIDS Epidemiology Annual Report 2012. HIV Epidemiology Section. 2013 http://www.sfdph.org/dph/files/reports/RptsHIVAIDS/AnnualReport2012.pdf.
- 9.San Francisco Department of Public Health. San Francisco HIV Prevention Strategy, 2012–2016: An Integrated Citywide Approach. 2012. [Google Scholar]
- 10.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5(6):e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geng EH, Hare CB, Kahn JO, et al. The effect of a “universal antiretroviral therapy” recommendation on HIV RNA levels among HIV-infected patients entering care with a CD4 count greater than 500/muL in a public health setting. Clin Infect Dis. 2012 Dec;55(12):1690–1697. doi: 10.1093/cid/cis750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raymond HF, Chen YH, Ick T, et al. A new trend in the HIV epidemic among men who have sex with men, San Francisco, 2004–2011. J Acquir Immune Defic Syndr. 2013 Apr 15;62(5):584–589. doi: 10.1097/QAI.0b013e318285febf. [DOI] [PubMed] [Google Scholar]
- 13.Kahn JO, Martin JN, Roland ME, et al. Feasibility of postexposure prophylaxis (PEP) against human immunodeficiency virus infection after sexual or injection drug use exposure: the San Francisco PEP Study. J Infect Dis. 2001 Mar 1;183(5):707–714. doi: 10.1086/318829. [DOI] [PubMed] [Google Scholar]
- 14.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec 30;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.HPTN 069: Phase II Randomized, Double-Blind, Study of Safety and Tolerability of Maraviroc, Maraviroc + Emtricitabine, Maraviroc + Tenofovir or Tenofovir + Emtricitabine for Preexposure Prophylaxis to Prevent HIV Transmission in At-Risk Men Who Have Sex with Men and in At-Risk Women. http://www.hptn.org/research_studies/hptn069.asp.
- 16.What Is iPrEx OLE? http://www.iprexole.com/1pages/aboutus/aboutus-whatisiprexole.php.
- 17.Highleyman L. Truvada PrEP Demonstration Project Debuts in San Francisco. 2012 HIVandHepatitis.com.
- 18.Cohen SE, Liu AY, Bernstein KT, Philip S. Preparing for HIV pre-exposure prophylaxis: lessons learned from post-exposure prophylaxis. Am J Prev Med. 2013 Jan;44(1 Suppl 2):S80–85. doi: 10.1016/j.amepre.2012.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Henry J. Kaiser Family Foundation. AIDS Drug Assistance Programs (ADAPs) with Waiting Lists or Other Cost-Containment Strategies, as of September 2012. http://kff.org/hivaids/state-indicator/adap-waiting-list-cost-containment/
- 20.Muthulingam D, Chin J, Hsu L, Scheer S, Schwarcz S. Disparities in engagement in care and viral suppression among persons with HIV. J Acquir Immune Defic Syndr. 2013 May 1;63(1):112–119. doi: 10.1097/QAI.0b013e3182894555. [DOI] [PubMed] [Google Scholar]
- 21.Das M, Chu P, Santos GM. Success of Test and Treat in San Francisco? Reduced Time to Virologic Suppression, Decreased Community Viral Load, and Fewer New HIV Infections, 2004 to 2009. Paper presented at: 18th Conference on Retroviruses and Opportunistic Infections; February 27–March 2, 2011; Boston. 2011. [Google Scholar]
- 22.Institute of Medicine. Monitoring HIV Care in the United States: Indicators and Data Systems. 2012. [PubMed] [Google Scholar]
- 23.Valdiserri RO, Forsyth AD, Yakovchenko V, Koh H. Public Health Rep. 2013. Measuring What Matters: Development of Standard HIV Core Indicators Across the U.S. Department of Health and Human Services; p. 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christopoulos KA, Kaplan B, Dowdy D, et al. Testing and linkage to care outcomes for a clinician-initiated rapid HIV testing program in an urban emergency department. AIDS patient care and STDs. 2011 Jul;25(7):439–444. doi: 10.1089/apc.2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christopoulos KA, Schackman BR, Lee G, Green RA, Morrison EA. Results from a New York City emergency department rapid HIV testing program. J Acquir Immune Defic Syndr. 2010 Mar 1;53(3):420–422. doi: 10.1097/QAI.0b013e3181b7220f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatia R, Hartman C, Kallen MA, Graham J, Giordano TP. Persons newly diagnosed with HIV infection are at high risk for depression and poor linkage to care: results from the Steps Study. AIDS and behavior. 2011 Aug;15(6):1161–1170. doi: 10.1007/s10461-010-9778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothman RE, Kelen GD, Harvey L, et al. Factors associated with no or delayed linkage to care in newly diagnosed human immunodeficiency virus (HIV)-1-infected patients identified by emergency department-based rapid HIV screening programs in two urban EDs. Acad Emerg Med. 2012 May;19(5):497–503. doi: 10.1111/j.1553-2712.2012.01351.x. [DOI] [PubMed] [Google Scholar]
- 28.Reed JB, Hanson D, McNaghten AD, et al. HIV testing factors associated with delayed entry into HIV medical care among HIV-infected persons from eighteen states, United States, 2000–2004. AIDS Patient Care STDS. 2009 Sep;23(9):765–773. doi: 10.1089/apc.2008.0213. [DOI] [PubMed] [Google Scholar]
- 29.Zetola NM, Bernstein K, Ahrens K, et al. Using surveillance data to monitor entry into care of newly diagnosed HIV-infected persons: San Francisco, 2006–2007. BMC Public Health. 2009;9:17. doi: 10.1186/1471-2458-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertolli J, Shouse RL, Beer L, et al. Using HIV surveillance data to monitor missed opportunities for linkage and engagement in HIV medical care. The open AIDS journal. 2012;6:131–141. doi: 10.2174/1874613601206010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dombrowski JC, Kent JB, Buskin SE, Stekler JD, Golden MR. Population-based metrics for the timing of HIV diagnosis, engagement in HIV care, and virologic suppression. AIDS. 2012 Jan 2;26(1):77–86. doi: 10.1097/QAD.0b013e32834dcee9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horberg MA, Hurley LB, Silverberg MJ, Klein DB, Quesenberry CP, Mugavero MJ. Missed Office Visits and Risk of Mortality Among HIV-Infected Subjects in a Large Healthcare System in the United States. Aids Patient Care STDS. 2013 Jul 19; doi: 10.1089/apc.2013.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozak M, Zinski A, Leeper C, Willig JH, Mugavero MJ. Late diagnosis, delayed presentation and late presentation in HIV: proposed definitions, methodological considerations and health implications. Antivir Ther. 2013;18(1):17–23. doi: 10.3851/IMP2534. [DOI] [PubMed] [Google Scholar]
- 34.Mugavero MJ, Amico KR, Westfall AO, et al. Early retention in HIV care and viral load suppression: implications for a test and treat approach to HIV prevention. J Acquir Immune Defic Syndr. 2011 Jan 1;59(1):86–93. doi: 10.1097/QAI.0b013e318236f7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das M. Beyond measuring what matters to managing what matters. Public Health Rep. 2013;128(September-October):360–363. doi: 10.1177/003335491312800505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christopoulos KA, Charlebois ED. Enhancing HIV Prevention in San Francisco. First National CFAR/APC ECHPP Conference; Washington, DC. 2012. [Google Scholar]