Abstract
Precise wiring of the nervous system depends on coordinating the action of conserved families of proteins that direct axons to their appropriate targets. Slit-Robo repulsion and Netrin-DCC (Frazzled) attraction must be tightly regulated to control midline axon guidance in vertebrates and invertebrates, but the mechanism mediating this regulation is poorly defined. Here we show that the Fra receptor has two genetically separable functions in regulating midline guidance in Drosophila. First, Fra mediates canonical chemoattraction in response to Netrin, and second, it functions independently of Netrin to activate commissureless transcription, allowing attraction to be coupled to the down-regulation of repulsion in pre-crossing commissural axons.
Establishing precise midline circuitry is essential to control rhythmic and locomotor behaviors (1, 2). Conserved signals that regulate axon guidance at the midline include attractive cues such as Netrins, and repulsive cues such as Slits, Semaphorins and Ephrins (3, 4). In Drosophila, Netrin attracts many commissural axons to the midline through activation of the Frazzled (Fra)/DCC (Deleted in Colorectal Cancer) receptor (5–8), while the repellant Slit and its receptor Roundabout (Robo) prevent commissural axons from re-crossing (9, 10). Commissureless (Comm) controls midline crossing by negatively regulating surface levels of Robo on pre-crossing commissural axons (11–13). Comm is expressed transiently in commissural neurons as their axons traverse the midline, where it sorts Robo to endosomes (12). Once across the midline, comm expression is extinguished, resulting in increased levels of Robo on the growth cone. How comm expression is spatially and temporally regulated to gate midline crossing is unknown.
While characterizing the structural requirements for Fra-mediated axon attraction, we observed that neuronal expression of a dominant negative form of Fra (FraΔC) leads to a dose-dependent “commissureless” phenotype (14). Searching for candidate genes that modify this phenotype, we found that removing one copy of comm enhances the midline crossing defects caused by expressing UASFraΔC (fig. S1), suggesting a role for Fra in regulating Comm during midline guidance. Consistent with this idea, removing one copy of comm in hypomorphic fra mutants increases the commissural defects as shown by thin or missing commissures in many segments, as well as an increased frequency of non-crossing defects in a subset of commissural neurons: the eagle neurons (Fig. 1 and Table S1). Similar genetic interactions are also observed using additional alleles of both fra and comm (fig. S2 and Table S1). These dose-dependent genetic interactions suggest that fra and comm may function in the same pathway to control commissural axon guidance.
Fig. 1. Genetic interaction between fra and comm.
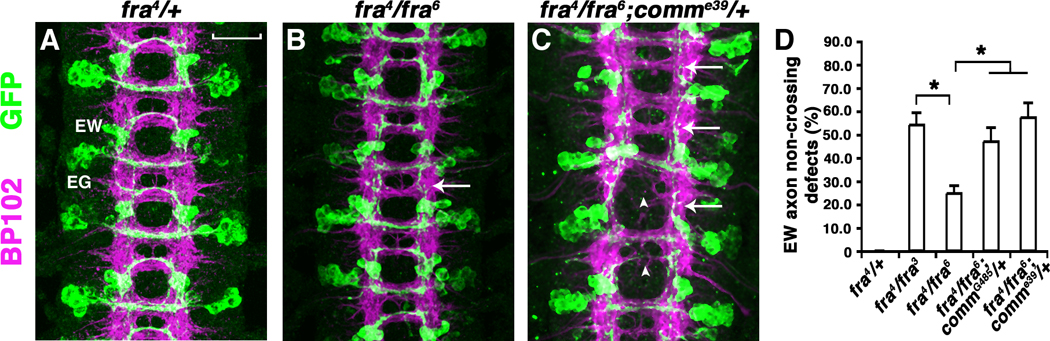
(A to C) Stage 16 eglGal4::UASTau-MycGFP embryos stained with MAb-BP102 (magenta) to display all CNS axons and anti-GFP (green) to visualize the eagle neurons. Anterior is up. (A) In fra4/+ or fra4/+; comme39/+ embryos, EW and EG neurons (white labels) project their axons across the midline in almost every segment. (B) fra4/fra6 mutants have normal commissure formation and a mild EW axon non-crossing defect (arrow). (C) Compared to fra4/fra6, fra4/fra6; comme39/+ embryos have missing and thin commissures in many segments (arrowheads) and many EW axons also fail to cross the midline (arrows). (D) Quantification of EW axon non-crossing defects. Error bars represent standard error of the mean. Asterisks denote p < 0.02 in a Student’s t test. Scale bar in (A), 20 microns.
How could Fra regulate the function of Comm? Because comm mRNA is up-regulated in commissural neurons as their axons cross the midline and DCC has been shown to mediate Netrin-induced axon outgrowth and turning through activation of the MAP Kinase and Calcineurin/NFAT signaling cascades (15, 16), we tested whether Fra regulates comm mRNA expression. Examination of comm mRNA in fra mutant nerve cords by real time PCR reveals a 12-fold reduction of total comm mRNA relative to wild type (fig. S3). To analyze comm mRNA expression with single cell resolution, we focused on the eagle neurons. At stage 14 in wild-type or fra/+ embryos, when the Eagle axons are crossing the midline, they have high comm RNA expression in their cell bodies (Fig. 2, A–C). However, in fra mutants comm mRNA is reduced in both the EW and EG neurons (Fig. 2, fig. S4, and S5). comm mRNA reduction in fra mutants is unlikely to be secondary to the failure of these axons to cross the midline, since a similar reduction is observed in EWs that have normal trajectories (Fig. 2). This implies that crossing the midline is not sufficient to induce comm transcription. Furthermore, the down-regulation of comm mRNA is likely a reflection of reduced transcription, rather than reduced mRNA stability, since we detect a similar reduction of comm pre-mRNA expression using a comm intron probe for hybridization (fig. S6). Finally, comm mRNA reduction in fra mutants is specific to commissural neurons since comm mRNA expression in the midline glia is not affected (Fig. 2).
Fig. 2. Fra is required cell-autonomously for comm mRNA expression.
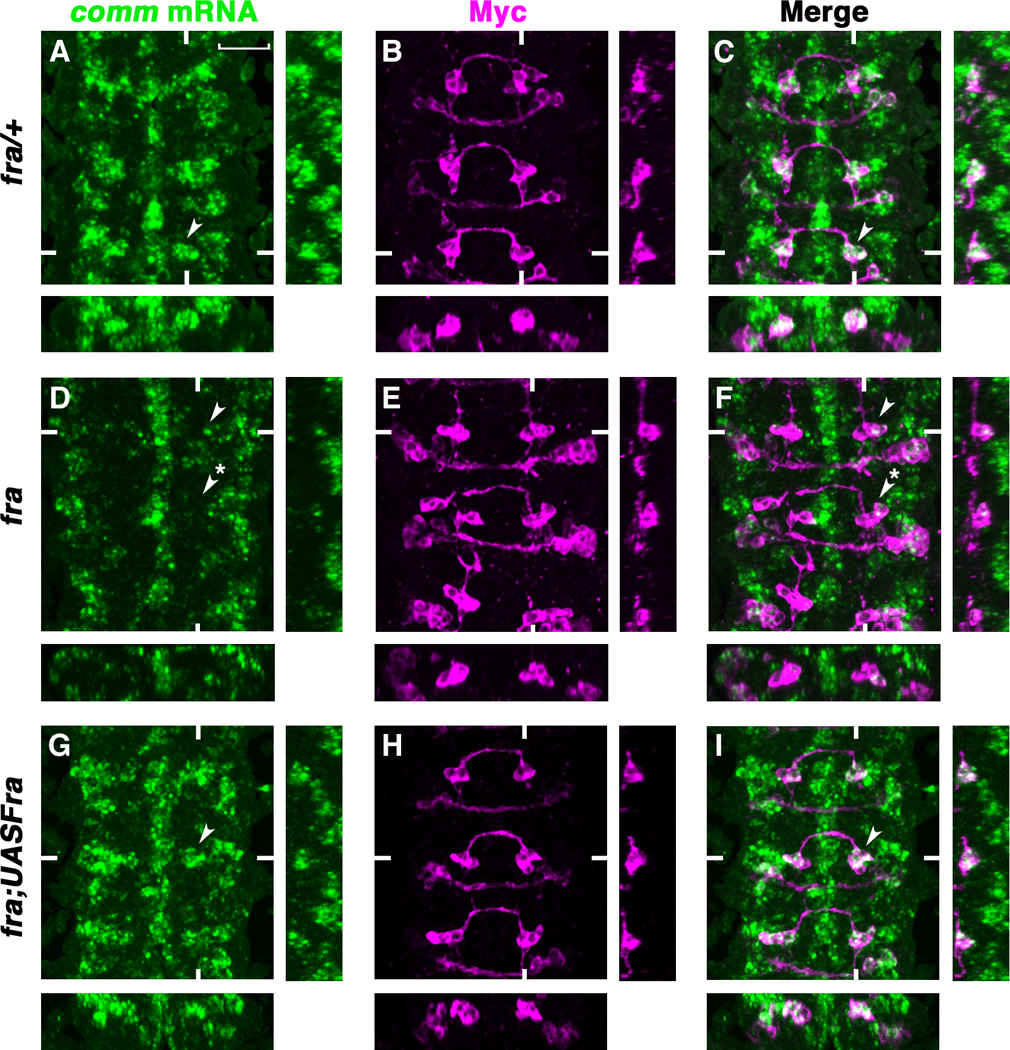
(A to I) Stage 14 eglGal4::UASTau-MycGFP embryos double-labeled with RNA in situ probes for comm (green) and anti-Myc (magenta) to visualize the eagle neurons. Anterior is up. Confocal sections of the EWs are shown. White hash marks indicate the positions of the XZ and YZ sections. (A to C) comm mRNA expression in the EWs of fra/+ embryos (arrowheads). (D to F) comm mRNA is reduced in the EWs of fra4/fra3 mutants (arrowhead, EW with crossing defect; starred arrowhead, EW that projects normally). (G to I) Expressing UASFra-Myc in the eagle neurons of fra mutants rescues comm mRNA expression in the EWs (G and I arrowheads). Scale bar in (A), 20 microns.
Fra has non cell-autonomous functions (17, 18), so we tested whether Fra is required exclusively in commissural neurons to control comm transcription. Expressing a UASFra-Myc transgene in the eagle neurons of fra mutants not only rescues the guidance defects of EWs as previously reported(14), but also rescues comm mRNA expression (Fig. 2, G to I and fig. S4). comm mRNA expression is also recovered in the few EWs (1.8%) that are not rescued and comm mRNA levels are normally regulated when the EW axons are prevented from crossing the midline by mis-expressing the Robo receptor, indicating that crossing the midline is not necessary to induce comm expression (fig. S4 and S7). During axon migration, growth cones of ipisilateral neurons extend long filopodia that reach all the way across the midline (19), suggesting that even when commissural axons extend ipsilaterally they could still have access to midline signals.
In contrast to wild type Fra, expression of FraΔC in fra mutants does not rescue comm mRNA expression (fig. S8). In fact, expressing UASFraΔC in the eagle neurons of wild type animals results in a decrease in comm expression in EWs; an observation consistent with FraΔC’s function as a dominant negative (fig. S9). Altogether these results support a cell autonomous requirement for Fra to activate comm transcription in commissural neurons as they cross the midline, and furthermore this effect is dependent on an intact cytoplasmic domain.
To test whether Fra is sufficient to induce comm mRNA expression, we over-expressed Fra in a subset of ipsilateral neurons, the apterous (Ap) neurons. In wild type embryos, the Ap neurons do not express comm. Only stochastic expression of comm can be detected at late stages in these neurons (stage 16 and 17) (Fig. 3, A and C arrows) (12). Over-expressing a UASFra-myc transgene in the Ap neurons frequently induces ectopic comm mRNA expression (16% of hemi-segments contain Ap neurons that express comm, n = 160 hemi-segments) (Fig. 3, D and F arrows). In addition Fra expression causes the Ap axons to cross the midline in many segments (35%, n=18) (Fig. 3E asterisks). Therefore, Fra is both necessary and sufficient for comm mRNA expression in subsets of neurons in vivo.
Fig. 3. Fra is sufficient to induce comm mRNA expression.
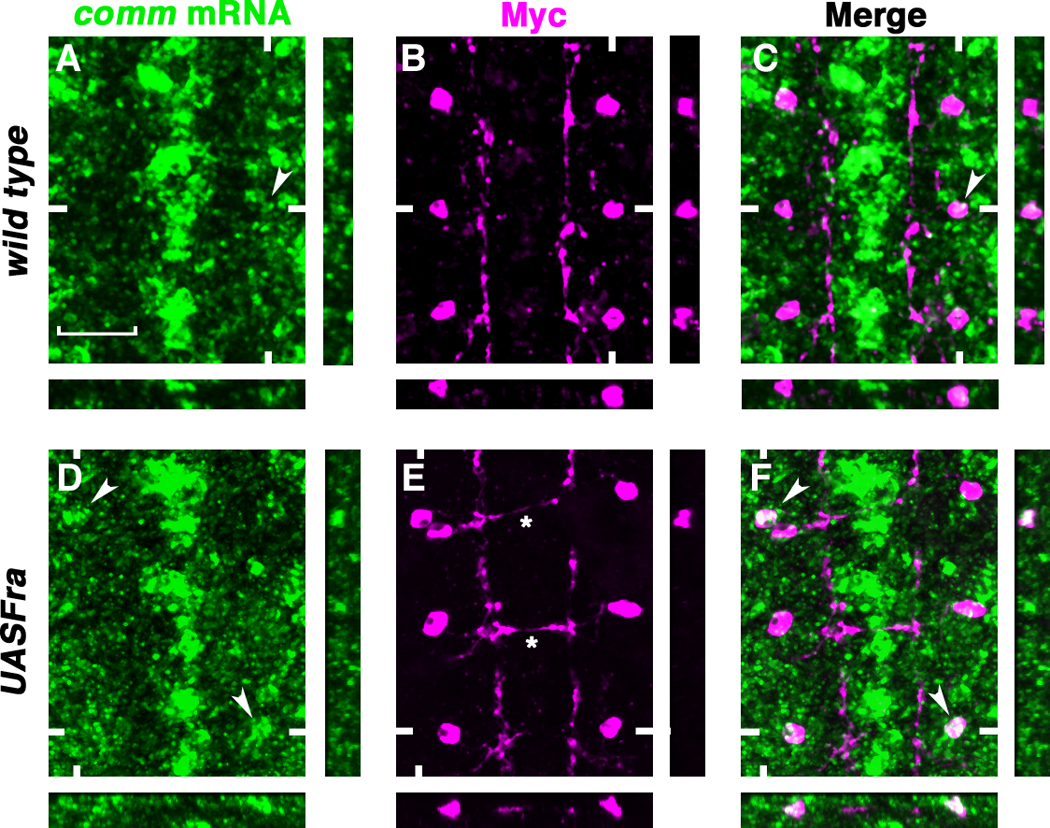
(A to F) Stage 16 aptGal4::UASTau-MycGFP embryos double-labeled with RNA in situ probes for comm (green) and anti-Myc (magenta) to visualize the Ap neurons. Anterior is up. Confocal sections of the Aps are shown. White hash marks indicate the positions of the XZ and YZ sections. (A to C) Stochastic comm mRNA expression in the Ap neurons (arrowheads). (D to F) Expressing UASFra-Myc in the Ap neurons induces comm mRNA expression frequently [arrowheads in (D) and (F)] and leads to ectopic midline crossing in many segments [asterisks in (E)]. Scale bar in (A), 20 microns.
Since Netrins are the ligands for DCC to activate downstream gene transcription during vertebrate axon outgrowth and turning, we tested whether Netrins are required for comm transcription. Unexpectedly, there is no reduction of comm mRNA in the eagle neurons of netAB mutants compared to netAB/+ siblings (Fig. 4 and fig. S8). Even in the EWs that fail to cross the midline, comm mRNA is expressed normally, again arguing that midline crossing is not required to induce comm transcription (Fig. 4, D and F arrows). In addition, expressing either a UASMyr-Fra-Myc transgene that removes the entire extracellular domain of Fra (and therefore its ability to bind Netrin) or a UASFraΔP1ΔP2ΔP3-Myc transgene can also rescue comm mRNA expression (fig. S8). Accordingly, the midline crossing defects of the EW axons in these embryos are partially rescued, resulting in a milder phenotype (Table S1). The conserved cytoplasmic P3 motif of Fra is required for Netrin-mediated attraction (14). Therefore, FraΔP1ΔP2ΔP3 loses its chemoattractive function, but still retains the ability to activate comm transcription. These results support the idea that Netrins are not the ligands for Fra to induce comm transcription, and indicate that chemoattraction and the regulation of comm expression are controlled by distinct regions of the Fra cytoplasmic domain. Moreover, the transcriptional activation of comm appears to be independent of any of the conserved P motifs.
Fig. 4. Netrins are not required for comm mRNA expression.
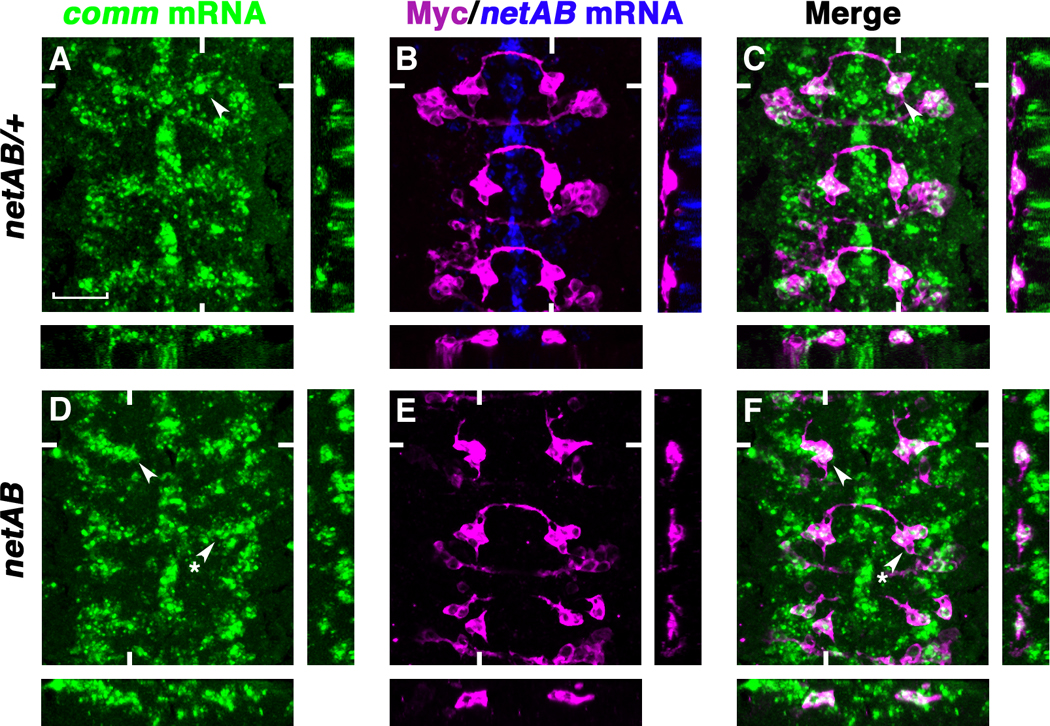
(A to F) Stage 14 eglGal4::UASTau-MycGFP embryos triple-labeled with RNA in situ probes for comm (green) and netrinAB (blue), and anti-Myc (magenta) to visualize the eagle neurons. Anterior is up. Confocal sections of the EWs are shown. White hash marks indicate the positions of the XZ and YZ sections. (A to C) comm mRNA expression in the EWs of netAB/+ embryos (arrowheads). (D to F) netAB mutants have normal levels of comm mRNA expression (arrowheads indicate an EW that has crossing defects and starred arrowheads indicate an EW that projects normally). Scale bar in (A), 20 microns.
Together these results suggest that to ensure midline crossing, Fra signaling has dual functions in commissural neurons: first it mediates Netrin-dependent axon attraction and second it leads to Netrin-independent activation of comm transcription. Comm, in turn, down-regulates Robo levels on commissural axons, allowing midline crossing (fig. S10). If this model is correct, the guidance defects observed in fra mutants should be due to a combination of the loss of attraction and a failure to activate comm transcription, and at least four genetic predictions can be made. First, fra mutants should have more severe EW commissural guidance defects than netAB mutants. Second, expressing UASComm transiently in commissural neurons should partially rescue the guidance defects in fra mutants and these partially rescued fra; UASComm mutant animals should have similar guidance defects to netAB mutants. Third, fra, robo double mutants should display the same severity of defects as fra; UASComm animals. Finally, expressing UASComm in commissural neurons of netAB mutants should have no effect on the midline crossing defects.
To test these predictions, we compared the EW axon guidance defects in the genotypes described above and a phenotypic analysis was performed blind to genotype (Fig. 5). As predicted, the EW guidance defects in fra mutants are significantly stronger than those in netAB mutants (Fig. 5, B, F and H; Table S1). Expressing UASComm in the eagle neurons of fra mutants partially rescues the EW guidance defects leading to a phenotype similar to that observed in netAB mutants (Fig. 5, C and I; Table S1). Similarly, the EW guidance defects in fra, robo double mutants are also less severe than fra single mutants (Fig, D and I; Table S1) (20). Finally, over-expression of UASComm in netAB mutants does not affect the guidance defects (Fig. 5, F and G; Table S1). These observations strongly support a Netrin-independent role for Fra in triggering comm transcription. Fra-dependent transcriptional regulation is unlikely to be the only mechanism to activate comm expression, since fra mutants have less severe commissural guidance defects than comm mutants.
Figure 5. Expression of Comm partially rescues guidance defects in fra mutants.
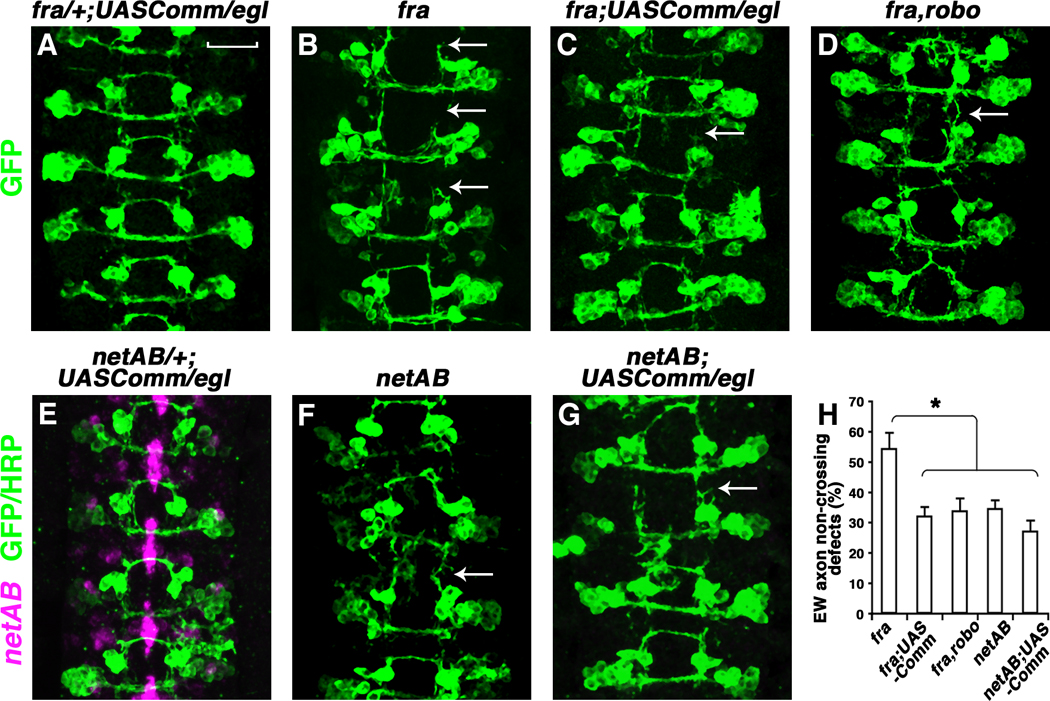
(A to G) Stage 16 eglGal4::UASTau-MycGFP embryos stained anti-GFP (green). Embryos in (E to G) were also labeled with RNA in situ probes for netrinAB (Magenta). Anterior is up. Over-expressing UASComm in the eagle neurons partially rescues the EW guidance defects in fra mutants [compare arrows in (B) and (C)], but not in netAB mutants [compare arrows in (F) and (G)]. The EW guidance defects in fra,robo mutants are also partially rescued [compare arrows in (B) and (D)]. Over-expressing UASComm in fra/+ or netAB/+ does not affect the trajectories of eagle neurons (A) and (E). (H) Quantification of EW axon non-crossing defect. Error bars represent standard error of the mean. Asterisk denotes p < 0.001 in a Student’s t test. Scale bar in (A), 20 microns.
Preventing conflicting signals at the midline from confusing navigating axons is fundamental to neuronal development. One mechanism that may allow axons to coordinate their responses to conflicting attractive and repulsive signals has been described in cultured Xenopus spinal neurons, where Slit induces a physical interaction between Robo and DCC/Fra (21). This direct receptor-receptor interaction silences Netrin attraction and this mechanism is proposed to prevent post-crossing commissural axons from re-crossing the midline (21). Here, we provide in vivo evidence supporting a distinct mechanism to regulate axon responses: two conserved guidance receptor signaling pathways (Fra and Robo) are coupled through a transcriptional event in pre-crossing commissural neurons to prevent premature repulsive responses, and therefore ensure midline crossing. Although transcriptional regulation by Netrin-DCC signaling is required for embryonic axon outgrowth and turning in vitro, it is less clear whether it is relevant in vivo. Furthermore, to our knowledge no transcriptional target gene(s) important for axon pathfinding has been identified. Here we show that Fra signaling triggers a transcriptional event in vivo, and identify a specific target gene- comm- a key regulator of repulsion at the Drosophila midline.
Surprisingly, Fra-mediated transcriptional activation is Netrin-independent, raising the question of whether there is an extrinsic midline signal required to activate Fra-dependent comm transcription. The spatial/temporal comm expression pattern is tightly associated with midline crossing, strongly suggesting the existence of such a midline signal. At first glance, our finding that Fra-induced comm transcription can be restored by expression of a myristolated Fra cytoplasmic domain seems inconsistent with this idea. While it may be tempting to conclude from this observation that the regulation of comm is strictly ligand independent, it is also possible (and in our view likely given the tight temporal window of comm expression) that the myrFracyto construct is either constitutively active or that it can associate with a co-receptor. Indeed, a similar construct when expressed in C. elegans leads to constitutive activity (22), and myristolated guidance receptor cytoplasmic domains have been shown to be competent to interact with co-receptors in a ligand-dependent manner (23, 24). Identifying the signals that trigger fra to activate comm transcription and determining how these events are restricted to commissural neurons are of high future priority.
Supplementary Material
Acknowledgments
This work was supported by a National Institutes of Health grant to G.J.B.
Footnotes
References and Notes
- 1.Goulding M, Pfaff SL. Curr Opin Neurobiol. 2005 Feb;15:14. doi: 10.1016/j.conb.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Ladle DR, Pecho-Vrieseling E, Arber S. Neuron. 2007 Oct 25;56:270. doi: 10.1016/j.neuron.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Garbe D, Bashaw G. Crit Rev Biochem Mol Biol. 2004 Sep-Dec;39:319. doi: 10.1080/10409230490906797. [DOI] [PubMed] [Google Scholar]
- 4.Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Annu Rev Neurosci. 2003;26:509. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell KJ, et al. Neuron. 1996;17:203. doi: 10.1016/s0896-6273(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 6.Kolodziej PA, et al. Cell. 1996;87:197. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- 7.Harris R, Sabatelli LM, Seeger MA. Neuron. 1996;17:217. doi: 10.1016/s0896-6273(00)80154-3. [DOI] [PubMed] [Google Scholar]
- 8.Brankatschk M, Dickson BJ. Nat Neurosci. 2006 Feb;9:188. doi: 10.1038/nn1625. [DOI] [PubMed] [Google Scholar]
- 9.Kidd T, Bland KS, Goodman CS. Cell. 1999;96:785. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 10.Kidd T, et al. Cell. 1998a;92:205. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- 11.Kidd T, Russell C, Goodman CS, Tear G. Neuron. 1998b;20:25. doi: 10.1016/s0896-6273(00)80431-6. [DOI] [PubMed] [Google Scholar]
- 12.Keleman K, et al. Cell. 2002 Aug 23;110:415. doi: 10.1016/s0092-8674(02)00901-7. [DOI] [PubMed] [Google Scholar]
- 13.Keleman K, Ribeiro C, Dickson BJ. Nat Neurosci. 2005 Feb;8:156. doi: 10.1038/nn1388. [DOI] [PubMed] [Google Scholar]
- 14.Garbe DS, O'Donnell M, Bashaw GJ. Development. 2007 Dec;134:4325. doi: 10.1242/dev.012872. [DOI] [PubMed] [Google Scholar]
- 15.Forcet C, et al. Nature. 2002 May 23;417:443. doi: 10.1038/nature748. [DOI] [PubMed] [Google Scholar]
- 16.Graef IA, et al. Cell. 2003 May 30;113:657. doi: 10.1016/s0092-8674(03)00390-8. [DOI] [PubMed] [Google Scholar]
- 17.Gong Q, Rangarajan R, Seeger M, Gaul U. Development. 1999 Apr;126:1451. doi: 10.1242/dev.126.7.1451. [DOI] [PubMed] [Google Scholar]
- 18.Hiramoto M, Hiromi Y, Giniger E, Hotta Y. Nature. 2000 Aug 24;406:886. doi: 10.1038/35022571. [DOI] [PubMed] [Google Scholar]
- 19.Murray MJ, Whitington PM. J Neurosci. 1999 Sep 15;19:7901. doi: 10.1523/JNEUROSCI.19-18-07901.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garbe DS, Bashaw GJ. J Neurosci. 2007 Mar 28;27:3584. doi: 10.1523/JNEUROSCI.0301-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein E, Tessier-Lavigne M. Science. 2001 Mar 9;291:1928. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- 22.Gitai Z, Yu TW, Lundquist EA, Tessier-Lavigne M, Bargmann CI. Neuron. 2003 Jan 9;37:53. doi: 10.1016/s0896-6273(02)01149-2. [DOI] [PubMed] [Google Scholar]
- 23.Hong K, et al. Cell. 1999;97:927. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 24.Stein E, Zou Y, Poo M, Tessier-Lavigne M. Science. 2001 Mar 9;291:1976. doi: 10.1126/science.1059391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


