Abstract
Rendering populations of vectors of diseases incapable of transmitting pathogens through genetic methods has long been a goal of vector geneticists. We outline a method to achieve this goal that does not involve introduction of any new genetic variants to the target population. Rather we propose that shifting the frequencies of naturally occurring alleles that confer refractoriness to transmission can reduce transmission below a sustainable level. The program employs methods successfully used in plant and animal breeding. Because no artificially constructed genetically modified organisms (GMOs) are introduced into the environment, the method is minimally controversial. We use Aedes aegypti and dengue virus (DENV) for illustrative purposes, but point out the proposed program is generally applicable to vector-borne disease control.
Keywords: vector control, vector-borne diseases, GMOs, genetic modification
Background
Plant and animal breeders have been genetically modifying economically important crops and animals for centuries. The results have been nothing short of transforming including a Green Revolution that sustains a human population much beyond what was once thought to be the natural carrying capacity of the Earth. Despite more than 50 years of discussions of control of vector-borne diseases using various methods for genetically modifying vector populations, little true progress has been forthcoming. Many contemporary proposals are based on molecular genetic technologies that are often described as “genetic engineering” (see Glossary). In all this excitement, it seems the tried and true proven methods of the plant and animal breeders have been overlooked.
The success of selective breeding from natural populations relies on genetic variance for the trait being selected. A now-outdated typological concept of populations argued against the success of such endeavors. In this view, species are monolithic units with characteristic essential traits that do not vary within the species. This stems from the classic genetic concept of a wild type, with all mutant variants being deviants from the idealized normal. Modern population thinking discarded this essentialist concept of species and replaced it with a view that natural populations are replete with naturally occurring genetic variation segregating for a large number of traits. Forty years ago, Lewontin [1] documented that attempting selection for any trait in Drosophila, genetic variance was found in natural populations for that trait. To our knowledge, the same is true for arthropod vectors. The vector control program outlined here relies on lessons learned from two established fields: selective breeding and evolutionary genetics.
The Program
The program for deriving vectors with reduced capacity for disease transmission to use in release programs is summarized in Figures 1 and 2. We illustrate the program for dengue virus (DENV) and its major vector, Aedes aegypti. This vector is one of the easiest mosquitoes to breed in the laboratory as well as one of the most amenable vectors to test for competence in transmission of a human pathogen of major importance. The principles we discuss are applicable to many other combinations of vector and pathogen with, of course, modification taking into consideration particulars of the system.
Figure 1.
Schematic of proposed selection schemes. Sample of ~200 females from the target populations are tested for competence. In Scheme I, mass selection, the 10% with lowest competence are chosen for the next generation. Two hundred offspring from these 10% are tested for competence, and the 10% least competent allowed to produce the population intended for release. Scheme II is family selection. The amount of competence testing is the same for each scheme. We illustrate only two generations of selection to avoid inbreeding and adaptation to laboratory conditions. If more than two generations of selection are needed, one should start with more than 200 from the field to avoid inbreeding. Alternatively, replicate parallel selected lines beginning with different field samples can be mixed to reduce inbreeding. Numbers suggested are simply examples, and the schemes can be modified to accommodate particulars of a given combination of vector and pathogen.
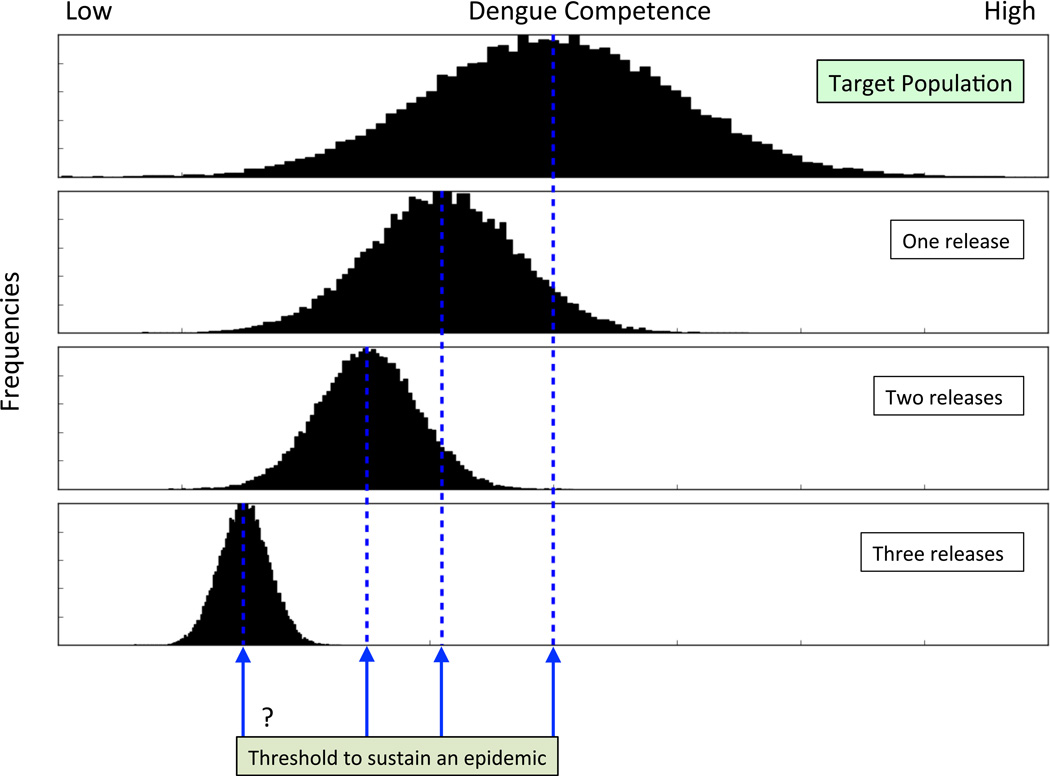
Figure 2.
Hypothetical target population. One release may not move the mean (dotted lines) sufficiently to the left to affect or eliminate transmission. Subsequent releases would move the mean further. A threshold, though not known initially, would be crossed at some point with subsequent interruption of transmission.
First, one identifies a population of the vector that will be the target for modification. For Ae. aegypti, this might be an isolated village, town, or island. A sample of females from the target population is taken and tested in the laboratory for competence to transmit DENV. We suggest a tested sample be about 200 to be adequate. Ideally, this would be a sample of mated adult females from the target population, although this may be difficult to obtain. A sample of several hundred larvae from numerous larval sites could be collected, adults allowed to emerge and randomly mate among themselves, providing the sample of adult females. Given the common experience encountered with reluctance of many mosquito species of field-collected females to take blood meals in the laboratory, an excess of 200 females, for example, 400, may require exposure to a blood meal containing DENV.
Some measure of vector competence is used such that the amount of virus detected in the head of each female following appropriate extrinsic incubation at a temperature reflective of the conditions experienced by the target population, for example, fluctuating temperature within a natural range [2]. A continuous phenotype measure such as provided by RT-PCR specific for the DENV genome in the heads of exposed females is proposed. This is an easily obtained measure presumably closely tied to actual transmission potential as it measures the level of virus in the salivary glands. For illustrative purposes, suppose the resulting distribution of competence in the tested sample is approximately normal (Figure 1). Many biological traits are approximately normally distributed, that is, have a continuous distribution with the same mean, mode, and median. In reality, the distribution of competence for DENV in a population of Ae. aegypti may be bi-modal rather than normal; we consider this possibility later. The normal distribution is assumed as a more general expectation for a quantitative trait such as vector competence.
In addition to challenging the females with DENV, eggs are collected from each female tested allowing controlled breeding. The breeding program has two goals: minimize vector competence and minimize inbreeding. In order to achieve these, the offspring of females at the low end of the distribution are collected. The compromise is between taking the extreme low competence females yet maintaining genetic diversity. We suggest that females with the lowest competence – the bottom 10% to 20% – from a sample of 200 is reasonable. This represents 80 genomes taken from the field population with two from the female and two from the male with whom it mated.
Figure 1 illustrates two schemes (Box 1). Scheme I is simple mass selection that may be appropriate to vectors that are not amenable to maintaining single female lines in the laboratory. Scheme II, family selection, is possible with Ae. aegypti because lines from the offspring of a single female can be established and maintained in the laboratory Family-based selection is more effective than mass selection for most quantitative traits [3].
Box 1. Spin offs and future avenues.
Can the proposed scheme aid in identifying the actual causative genetic basis of vector competence?
The proposed schemes are “blind” in the sense that no attempt is made to identify precisely the nature, number, or chromosome location of genes affecting competence. Initially in plant and animal breeding this was also the case. With modern genetic analytical tools, it is possible to more clearly delineate exactly what genetic changes are being induced. Two advances make this possible: detailed sets of genomic markers (e.g., SNPs) and DNA sequences of complete genomes. It is conceivable that if the selected release populations are genotyped for thousands of SNPs, those consistently identified as increasing or decreasing in frequency when selecting for refractoriness mark regions of the genome containing a causative gene. Identifying the position of these associated SNPs on the complete genome would allow identification of candidate genes affecting competence. If the program were carried out in a number of geographic populations one could get information on whether the same genes affecting competence are variable in all populations or whether populations vary in segregating factors affecting competence. Similarly, if different serotypes of DENV (or multiple genotypes of other pathogens) were used, it could be determined whether genes affecting competence for one pathogen genotype also affects other pathogen genotypes.
Can the proposed scheme provide candidates for use in GMO control strategies?
The identification of naturally occurring genes that control resistance to vector competence also provides new genes that could be considered for GMO control strategies. Here we consider only their use in release strategies of non-GMOs into natural populations to shift gene frequencies. Other strategies would be possible using these same genes in concert with technologies that facilitate the spread of genes in nature, that is, transgenesis and paratransgenesis.
Will the knowledge of naturally occurring vector competence genes provide new strategies for vector-borne disease control?
The identification of specific naturally occurring vector competence genes will allow understanding of the functions of these genes in natural populations. It is likely that these genes have pleiotropic effects on the phenotypes of vectors with an impact on vector fitness in the absence of the pathogen [24]. The information on function will provide the ability to assess the features of the environment of the population that influence the frequencies of these genes. It may be possible to alter some features of the vector environment that will facilitate the shift in vector competence gene frequencies to the desired goal of providing refractory vector populations. As one example, if temperature affected the fitness of carriers of a competence related gene, releases could be timed to coincide with the temperature at which fitness was maximized.
Family selection is effective for deriving resistant and susceptible Ae. aegypti for two human arboviruses, DENV and yellow fever (YFV). This includes single female lines differing greatly in DENV competence [4–5] and similar lines with competence for YFV [6– 7]. While these studies derived one or a few lines resistant to pathogen infection, such lines will necessarily be inbred. This is why in our scheme (Figure 1) we propose deriving several such lines, for instance, 20. The ten lines where the tested offspring are most resistant would be chosen, that is, the 10/20 lines where the offspring reflect most closely the mother phenotype. For release, offspring from all ten lines would mate randomly for one or two generations to re-establish the approximate level and constitution of genetic variation in the target population. This represents a mixture of 40 genomes from the target populations and thus not inbred. This maximizes the chances the release strain is competitive in the target environment.
Mass selection, Scheme I, is appropriate for vectors not amenable to family selection, for example, species where swarm mating is required. It is somewhat less effective especially for traits with low heritability where there is weaker association of phenotype and genotype. Nevertheless, it is effective if practiced over multiple generations as has been done for Anopheles gambiae and Plasmodium [8]. However, as with the family selection, the minimum number of generations of selection required should be applied and careful consideration of maintaining as closely as possible the level and nature of genetic variation in the target population. If more than two generations of laboratory selection are required to obtain a sufficiently refractory population, one strategy would be to mass select multiple independent strains from the starting target population and randomly interbreed to generate the release strain.
In the case of DENV and likely with other pathogens, the distribution of competence of the vector is not normal [9]. Rather, a certain percentage of the female Ae. aegypti in a population show no infection upon initial challenge with DENV while the remaining fraction of the population displays an approximate normal distribution (Figure 3). In this case, one would take females falling into the zero category in the initial challenge and derive the release population. While not showing competence with the initial challenge, offspring of such females may well display some degree of competence depending on the strength of the additive genetic variants relative to environment-induced variation. One or two further generations would select for true-breeding refractory mosquitoes, especially if they displayed the bi-modal distribution in each laboratory generation of selection. Again, if more than two generations of laboratory selection are required, mixing of parallel independent selection projects is necessary.
Figure 3.
Hypothetical case where phenotype is not a normal distribution. In this example, possibly mimicking DENV [9], a portion of females from the field target population show no DENV in the head after extrinsic incubation, while the rest form a continuum. Selection can be applied to the phenotypically non-competent fraction. Releases can be continued until the target population falls below the threshold for continued transmission.
Selection for quantitative traits is enhanced by marker-assisted selection [3, 10]. This is particularly true for traits where phenotype is determined for only one sex; for vector-borne diseases, generally only females are relevant for transmission. Male mates (no phenotype known) can be selected based on genetic relatedness to females of known refractory phenotype. If genetic markers such as microsatellites or single nucleotide polymorphisms (SNPs) are available for the vector, selection with these would be more effective. The selection program can be modified depending on the nature of the vector, the genetic tools available, the type of assay for vector competence, the nature of the underlying genetic variation, etc.
Choice of pathogen strain
Pathogens are heterogeneous and it is important that any vector strain selected for disease control be refractory to the particular variety of pathogen affecting the targeted locality. In the case of DENV, there are four serotypes as well as considerable heterogeneity within each serotype [11]. This virus genetic heterogeneity, coupled with the genetic heterogeneity of Ae. aegypti populations, is important for understanding the efficiency of transmission of DENV [12]. For malaria, the pathogen heterogeneity is at the level of species, with additional variation within species(e.g., [13]). While less well-studied, doubtless the same holds for almost all vector-borne pathogens affecting humans.
Thus, it is important that the challenging strain of DENV is pertinent to the target area as outlined (Figure 1). This is easily accomplished by using a DENV strain derived directly from the target area. If more than one serotype is present, the release strain should be challenged to determine if there is cross-refractoriness or whether parallel selection for different serotypes is needed.
The proposed program allows for specificity on both the vector side and pathogen side. That is, the strain of mosquito is directly derived from the mosquito population resident in the target area, and the DENV strain that it has been selected to be refractory to, is that DENV strain affecting the target area.
Evidence of genetic variance for DENV competence
The above scheme relies on there being genetic variance in natural populations of Ae. aegypti for the ability to transmit DENV. Literature on Ae. aegypti variation for competence to DENV as well as YFV is extensive. Variation in Ae. aegypti susceptibility to infection is found within and between geographic populations for YFV [6,14] and DENV [4, 9, 15–21]. For example, Ae. aegypti susceptibility to infections with DENV-2 exhibits extensive variation [4]. Families created as single female lines varied from 0% to 58% susceptibility with several lines showing resistance to infection.
Means of introduction
As with any proposal to manipulate vector populations genetically, this scheme requires introducing laboratory-reared mosquitoes into the field. This could be achieved in a number of ways. Three factors are especially important to consider: (i) stage of mosquito introduced, that is, adult, pupae, larvae, or eggs; (ii) sex of introduced mosquitoes; and (iii) timing of introduction(s).
In the case of Ae. aegypti, we propose that the most effective manner would be to introduce large numbers during the time of seasonal lowest density or following an insecticide campaign. Timing release of a laboratory population of Ae. aegypti is simplified by the fact that eggs can be stored dormant for several months. Multiple temporally staggered releases of the same generation laboratory population could be accomplished by hatching aliquots of eggs.
With any release program involving laboratory rearing there is the possibility, perhaps probability, that the release strain is to some degree at a competitive disadvantage with the target field population. Our scheme maximizes the chance of this not being the case by: (i) minimizing inbreeding; (ii) minimizing the number of generations of laboratory rearing; and (ii) releasing a strain as genetically similar to the target populations as possible.
Multiple iterations of the program
It may well be that a single release along the lines proposed will only shift the gene frequencies in the target population partially toward the desired level of vector competence below which sustained transmission is not possible. In this case, additional rounds of selection and release should be a contingency (Figure 2). If subsequent derivation of release populations is undertaken, beginning selection over again with females directly from the partially shifted target population is important. The initial release population may carry only some genetic variance for pathogen competence, and repeatedly going back to the target population to begin new rounds of selection could capture additional genetic variance. In addition to capturing more of the genetic variants affecting competence, multiple sampling of the target population could randomize associations owing to linkage.
Genetic monitoring
Because the release population(s) is selected to have a different phenotype from the target population, in as much as the phenotype is at least partially genetically based, the release population will be genetically different from the target field population for some portion of the genome. By genotyping the release and target populations before release for variation at several thousand markers, as possible with a SNP chip, those loci linked to genes affecting DENV competence would differ in the release and target populations. While the release and target populations may not differ by fixed allelic differences at SNP loci, they are expected to differ in allele frequencies at several hundred loci (if say ~30,000 SNPs are assayed). Are the allelic frequencies of the target population shifting toward that of the release population, indicating incorporation of genes from the release strain into the target population? Having several hundred loci that differ between the release and target populations allows discrimination between random gene frequency fluctuations and true migration from release to target populations.
In addition to monitoring the success in the early stages after release(s), genetic monitoring for longer periods can aid in anticipating breakdown in the control. It is conceivable that after successfully shifting the pertinent gene frequencies to lower or eliminate transmission, over time the target population would begin to drift or be selected to revert to the initial state. Detecting this early would allow implementation of another round of selection and release before the target population completely reverted to previous competence levels. Genotyping the target population at various times after release allows monitoring the effectiveness and dynamics of genetically shifting the field population and anticipating need for additional intervention.
Advantages of the program
The proposed program has several advantages over many of the other proposed genetic manipulation schemes: (i) no artificially constructed genetically modified organisms (GMOs) are involved. We propose to simply shift the frequencies of naturally occurring genes already present in the target population without introduction of ‘foreign’ genetic material into the environment. (ii) The release population is refractory to the specific pathogen genotype(s) affecting the residents in the target area. (iii) Chance of success is higher because the release population is derived directly from the target population with minimal laboratory manipulation. (iv) Chance of enduring after releases end is greater because the genetic changes induced are minor and simply shifts in frequencies of naturally occurring alleles. (v) The program is relatively inexpensive and thus could be repeated until the desired degree of shift in gene frequencies is reached. (vi) Genetically fingerprinting the released strain could allow monitoring of how effective the releases are in shifting gene frequencies. (vii) Any vector can be used if rearing in the laboratory and testing for competence are possible. There is no need for a priori knowledge of genetics of the species.Thus, our proposed program maximizes chances of sustained success in lowering transmission, while minimizing societal concerns.
General application
For illustrative purposes, we have couched our proposal in the specific case of Ae. aegypti transmitting DENV. In principal, the same approach can be used with many other vectorpathogen combinations. There are only three requirements: (i) ability to control breeding of the vector in captivity; (ii) ability to measure the phenotype, that is, the ability or inability of the vector to transmit the pathogen of interest; and (iii) genetic variance must exist in natural populations for the ability to transmit the pathogen.
Many vectors of serious human, as well as animal and plant, pathogens can be reared in captivity and phenotyped for efficiency of pathogen transmission (i and ii). Here we confine our discussion to (iii), which does not denigrate the importance of developing breeding methods for vectors and devising new methods to measure competence for transmission of pathogens.
The presence of genetic variance for vector competence in natural vector populations has been observed for many vectors and their pathogens (reviewed in [22–24]). In addition to DENV and YFV, Ae. aegypti has genetic variance for Chikungunya virus competence [25]. Vectors for other arboviruses, Culex tarsalis [26] and two Culex species [27], display genetic variation in transmission efficiency of western equine encephalitis virus and West Nile Virus, respectively. Anopheline vectors of malaria display genetic variation in ability to transmit various species of Plasmodium [8, 28, 29]. Genetic variance in the ability of mosquitoes to transmit filarial worms has also been demonstrated [30,31,32]. This is only a partial listing of studies of mosquito-borne pathogens. It seems safe to assume the same would hold for other vectors such as ticks, sand flies, tsetse, etc. Virtually all natural (field) populations of vectors tested for competence to transmit a variety of pathogens have displayed genetic variance for the trait.
Caveats and potential pitfalls
Several technical obstacles will require surmounting for the program to work. The facilities to rear sufficient mosquitoes to affect a field population would need to be available. Methods to induce females directly from the field to take blood meals and to oviposit large numbers of eggs must be improved. Obtaining and storing sufficient eggs are a challenge, particularly when trying to minimize the number of generations of laboratory rearing. Most of these difficulties could be handled with sufficient resources and imaginative thinking.
The most important unknowns that could lessen the effectiveness of the above approach are: (i) the behavior of the selected genotypes under natural conditions; (ii) fitness effects of genes affecting the ability of vectors to transmit pathogens; and (iii) the ability to spread the refractory genotype throughout the target population. Difficulties exist for predicting the phenotypes of vector competence genotypes influenced by environmental factors [24]; for example, the length of storage of Ae. aegypti eggs increased susceptibility of adult females to infection with DENV-2 [4]. It is also likely that genes influencing vector competence have pleiotropic effects and other functions that may influence fitness [24]. It will be important to understand the fitness consequences of selected genotypes under field conditions to assess whether they will increase in the target population, decrease due to fitness costs, or perhaps, because the released genotypes are already in the target population, behave as neutral variants. With knowledge of the environmental factors influencing competence genotypes in nature, manipulating these factors to increase the fitness of desired refractory genotypes may be possible. For example, if the fitness of the release strain is temperature sensitive, releases could occur when temperatures favor released mosquitoes. (Box 2)
Box 2. Outstanding issues and gaps in knowledge.
The release of any vector that transmits human diseases has multiple ethical and political implications. Will residents in a targeted area accept the release of biting arthropods that may transmit a disease? For many vectors, only females take blood meals and transmit diseases, so release of only males is one strategy. However, at present separating sexes before release is very laborious and would add to the cost of such programs. In any case, any release program requires transparency and education of the residents in the target area as well as their explicit cooperation and acceptance of the program.
A second question concerns heterogeneity of pathogens. In the case of dengue, four serotypes are known to exist with heterogeneity within each. In the scheme proposed here it is practical to select for resistance to transmission of only one strain of pathogen, most reasonably the strain of pathogen affecting the target area at the time the program is initiated. Would such a selected strain also be refractory to new strains of pathogen that might enter the targeted area? Could they be more competent for other pathogen strains? Or even other species of pathogen. For example, could dengue resistant mosquitoes be more competent for transmission of Chikungunya virus?
Quantitative mathematical modeling should also be a key part of any scheme of the sort proposed here. Two excellent examples for Aedes aegypti and dengue are [33,34]. Knowing the size of the target population would be crucial in determining the number of released vectors needed to significantly affect gene frequencies. Other ecological parameters such as longevity and dispersion are critical in the design of release programs.
The nature of the method to measure vector competence in the laboratory is another issue of some concern. How realistic is it to simply infect a host vector and then measure how the pathogen grows after some period of incubation? We suggested a simple practical proxy (amount of virus in the head of a mosquito, presumably representing virus in the salivary glands). To truly test “competence”, the infected vector should be allowed to feed on an animal susceptible to the pathogen or at least test for pathogen in saliva. This is impractical in most cases. Nevertheless, whatever practical proxy is used to determine competence should be carefully tested for realism under field conditions.
Concluding remarks
In attempts to continue improving crops and livestock, agricultural geneticists today are employing artificially constructed GMOs that have promise to produce a second saltation in improvements, while simultaneously engendering controversy. However, current use of these methods occurs subsequently to exhausting the tried and true, less controversial methods of selective breeding developed over centuries. From a practical standpoint as well as from ethical and political considerations, one could argue that the same approach is preferable for control of vector-borne diseases; artificially constructed vector GMOs should be employed only after exhaustion of non-GMO genetic methods of disease control. Vector geneticists seem to be skipping an important step when proposing direct use of genetically engineered GMOs.
To be sure, what we are proposing is not strictly analogous to plant and animal breeding. In deriving higher producing crops and more productive livestock, agricultural geneticists breed the selected populations in captivity. There is no need to release them back into the populations from which they were derived. The challenge in our program is to shift the frequencies of naturally occurring genetic variants in vector populations sufficiently to significantly reduce disease transmission. There is no theoretical reason this is not possible, although we do note caveats. At the very least, the proposed approach deserves serious consideration and can only be evaluated by attempts at implementation.
Highlights.
A strategy requiring no GMOs can be used to modify vectors to be incompetent to transmit pathogens.
This strategy is applicable to many vector-pathogen systems.
The strategy requires shifting existing allele frequencies present in natural populations.
Stands a better chance of success than many alternative proposals.
May target specific genetic strain of pathogen in the target locality.
Acknowledgments
Trudy Mackay, two reviewers and the editor kindly provided advice and helpful suggestions. Financial support for related work in the Powell lab is provided by NIH RO1 AI101112.
Glossary
- Arbovirus
an arthropod-borne virus causing a disease that is transmitted by an arthropod such as a mosquito, tick, sand fly, etc.
- Field population
see Natural population
- Family selection
Breeding for desired phenotypes based on families. Correlation between phenotypes of parents and offspring allow evaluation of the additive genetic variance controlling the trait, or how “true breeding” the trait is in a family. In contrast to mass selection.
- Genetic engineering
Term used to describe various forms of genetic manipulation of organisms usually based on gene cloning. May describe insertion of “foreign” genes into organisms, i.e., genes not naturally occurring in the species or strain.
- GMO
Genetically modified organism. Refers to organisms whose genetics has been altered by humans. Could apply to virtually all commercial crops and livestock that humans have modified over generations of selection. More often refers to modification based on molecular technologies like cloning of genes. In this case, the modification could involve inserting genes into an organism derived from the same or different species, or even a completely synthetic gene.
- Inbreeding
Close mating among relatives leading to increased homozygosity. Almost always associated with lowering fitness.
- Isofemale line
Isolated female line. A genetic strain or line of a species that had one female parent at its origin.
- Mass selection
Selection for a trait based on phenotype of a group of parents. Parents of next generation are simply mixed to form the next generation so no measure of specific correlation of parent-offspring is possible. Contrast to family selection.
- Natural population
Population not in artificial breeding or captivity. One might question the use of this term for invasive species closely associated with human habitats, not their ancestral habitats. However, it has become quite “natural” for such species to share human habitats. Here used synonymously with field population.
- Single nucleotide polymorphism (SNP)
a site in the DNA sequence where more than one nucleotide appears in natural populations.
- SNP chip
A tool for efficiently determining the genotype of 10s of thousands of SNPs from a single organism.
- Vector competence
The intrinsic permissiveness of an arthropod species or population to become infected with a pathogen, allow replication of the pathogen in the arthropod, and transmit the pathogen to another animal or plant host.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lewontin RC. The Genetic Basis of Evolutionary Change. New York: Columbia University Press; 1974. [Google Scholar]
- 2.Lambrechts L, et al. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl. Acad. Sci. USA. 2011;108:7460–7465. doi: 10.1073/pnas.1101377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. 4th edition. England: Pearson Education Limited, Essex; 1996. [Google Scholar]
- 4.Tardieux I, et al. Analysis of inheritance of oral susceptibility of Aedes aegypti (Diptera: Culicidae) dengue-2 virus using isofemale lines. J. Med. Entomol. 1991;28:518–521. doi: 10.1093/jmedent/28.4.518. [DOI] [PubMed] [Google Scholar]
- 5.Bennett KE, et al. Quantitative trait loci that control Dengue-2 virus dissemination in the mosquito Aedes aegypti. Genetics. 2005;170:185–194. doi: 10.1534/genetics.104.035634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallis GP, et al. Selection for susceptibility and refractoriness of Aedes aegypti to oral infection with yellow fever virus. Am.J. Trop. Med. Hyg. 1985;34:1225–1231. doi: 10.4269/ajtmh.1985.34.1225. [DOI] [PubMed] [Google Scholar]
- 7.Miller BR, Mitchell CJ. Genetic selection of a flavivirus-refractory strain of the yellow fever mosquito Aedes aegypti. Am. J. Trop. Med. Hyg. 1991;45:399–407. doi: 10.4269/ajtmh.1991.45.399. [DOI] [PubMed] [Google Scholar]
- 8.Collins FH, et al. Genetic selection of Plasmodium-refractory strain of the malaia vector Anopheles gambiae. Science. 1986;234:607–610. doi: 10.1126/science.3532325. [DOI] [PubMed] [Google Scholar]
- 9.Bennett KE, et al. Variation in vector competence for dengue 2 virus among 24 collections of Aedes aegypti from Mexico and the United States. Am. J. Trop. Med. Hyg. 2002;67:85–92. doi: 10.4269/ajtmh.2002.67.85. [DOI] [PubMed] [Google Scholar]
- 10.Lande R, Thompson R. Efficiency of marker-assisted selection in the improvement of quantitative traits. Genetics. 1990;124:743–756. doi: 10.1093/genetics/124.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Worobey MA, et al. Widespread intra-serotype recombination in natural populations of the dengue virus. Proc. Natl. Acad. Sci. (USA) 1999;96:7352–7357. doi: 10.1073/pnas.96.13.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambrechts LC, et al. Genetic specificity and potential for local adaptation between dengue viruses and mosquito vectors. BMC Evolutionary Biology. 2009;9:160. doi: 10.1186/1471-2148-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruce MC, et al. Genetic diversity and dynamics of Plasmodium falciparum and P. vivax populations in multiply infected children with asymptomatic infections in Papua New Guinea. Parasitology. 2000;121:257–272. doi: 10.1017/s0031182099006356. [DOI] [PubMed] [Google Scholar]
- 14.Tabachnick WJ, et al. Oral infection of Aedes aegypti with yellow fever virus: Geographic variation and genetic considerations. Am. J. Trop. Med. Hyg. 1982;34:1219–1224. doi: 10.4269/ajtmh.1985.34.1219. [DOI] [PubMed] [Google Scholar]
- 15.Sylla M, et al. Gene flow, subspecies composition, and dengue virus-2 susceptibility among Aedes aegypti collections in Senegal. PLoS Neglected Trop. Dis. 2009;3:e408. doi: 10.1371/journal.pntd.0000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diallo M, et al. Vector competence of Aedes aegypti populations from Senegal for sylvatic and epidemic dengue 2 virus isolated in West Africa. Trans. R. Soc. Trop. Med. Hyg. 2008;102:493–498. doi: 10.1016/j.trstmh.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Knox TB, et al. Enhanced vector competence of Aedes aegypti (Diptera: Culicidae) from the Torres Strait compared with mainland Australia for dengue 2 and 4 viruses. J. Med. Entomol. 2003;40:950–956. doi: 10.1603/0022-2585-40.6.950. [DOI] [PubMed] [Google Scholar]
- 18.Paupy C, et al. Variation over space and time of Aedes aegypti in Phnom Penh (Cambodia): genetic structure and oral susceptibility to a dengue virus. Genet. Res. 2003;82:171–182. doi: 10.1017/s0016672303006463. [DOI] [PubMed] [Google Scholar]
- 19.Tardieux I, et al. Variation among strains of Aedes aegypti in susceptibility to oral infection with dengue type 2. Am. J. Trop. Med. Hyg. 1990;43:308–313. doi: 10.4269/ajtmh.1990.43.308. [DOI] [PubMed] [Google Scholar]
- 20.Gubler DJ, et al. Variation in susceptibility to oral infection with dengue viruses among geographic strains of Aedes aegypti. Am. J. Trop. Med. Hyg. 1979;28:1045–1052. doi: 10.4269/ajtmh.1979.28.1045. [DOI] [PubMed] [Google Scholar]
- 21.Gubler DJ, Rosen L. Variation among geographic strains of Aedes albopictus in susceptibility to infection with dengue viruses. Am. J. Trop. Med. Hyg. 1976;25:318–325. doi: 10.4269/ajtmh.1976.25.318. [DOI] [PubMed] [Google Scholar]
- 22.Beerntsen BT, et al. Genetics of mosquito vector competence. Microbiol. Mol. Biol. Rev. 2000;64:115–137. doi: 10.1128/mmbr.64.1.115-137.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black WC, IV, et al. Flavivirus susceptibility in Aedes aegypti. Arch. Med. Res. 2002;33:379–388. doi: 10.1016/s0188-4409(02)00373-9. [DOI] [PubMed] [Google Scholar]
- 24.Tabachnick WJ. Nature, nurture and evolution of intra-species variation in mosquito arbovirus transmission competence. Int. J. Environ. Res. Public Health. 2013;10:249–277. doi: 10.3390/ijerph10010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mourya DT, et al. Inheritance of oral susceptibility of Aedes aegypti to chikungunya virus. Am. J. Trop. Med. Hyg. 1994;51:295–300. doi: 10.4269/ajtmh.1994.51.295. [DOI] [PubMed] [Google Scholar]
- 26.Hardy JL, et al. Selection of a strain of Culex tarsalis highly resistant to infection following ingestion of western equine encephalomyelitis virus. Am.J. Trop. Med. Hyg. 1978;27:313–331. doi: 10.4269/ajtmh.1978.27.313. [DOI] [PubMed] [Google Scholar]
- 27.Kilpatrick AM, et al. Spatial and temporal variation in vector competence of Culex pipiens and Cx. restuans mosquitoes for West Nile virus. Am. J. Trop. Med. Hyg. 2010;83:607–613. doi: 10.4269/ajtmh.2010.10-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng LB, et al. Quantitative trait loci for refractoriness of Anopheles gambiae to Plasmodium cynomolgi B. Science. 1997;276:425–428. doi: 10.1126/science.276.5311.425. [DOI] [PubMed] [Google Scholar]
- 29.Niare O, et al. Genetic loci affecting resistance to human malaria parasites in a West African mosquito vector population. Science. 2002;298:213–216. doi: 10.1126/science.1073420. [DOI] [PubMed] [Google Scholar]
- 30.Bartholomay LC, Christensen BM. Vector-parasite interactions in mosquito-borne filariasis. World Class Parasites. 1984;5:9–19. [Google Scholar]
- 31.Severson DW, et al. Chromosomal mapping of two loci affecting filarial worm susceptibility in Aedes aegypti. Insect Mole. Biol. 1994;3:67–72. doi: 10.1111/j.1365-2583.1994.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 32.Beerntsen BT, et al. Aedes aegypti: a quantitative trait locus (QTL) influencing filarial worm intensity is linked to QTL for susceptibility to other mosquito-borne pathogens. Experimental Parasitol. 1995;81:355–362. doi: 10.1006/expr.1995.1126. [DOI] [PubMed] [Google Scholar]
- 33.Robert MA, et al. A reduce and replace strategy for suppressing vector-borne diseases: Insights from a deterministic model. PLoS One. 2013;8:e73233. doi: 10.1371/journal.pone.0073233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okamoto KW, et al. A reduce and replace strategy for suppressing vectorborne diseases: Insings from a stochastic spatial model. PLoS One. 2013;8:e81860. doi: 10.1371/journal.pone.0081860. [DOI] [PMC free article] [PubMed] [Google Scholar]