Abstract
Objective
Daptomycin, a cyclic lipopeptide that exhibits rapid, concentration-dependent bactericidal activity in vitro against a broad spectrum of Gram-positive pathogens, has now, since 2003, been approved in more than 70 countries and regions to treat skin and soft-tissue infections (SSTIs). The purpose of this meta-analysis was to compare the safety and efficacy of daptomycin with other antibiotics, especially with vancomycin which has long been considered the standard therapy for complicated SSTIs.
Design
Meta-analysis of randomised controlled trials (RCTs).
Data sources
We thoroughly searched PubMed, EMBASE, Cochrane Central to identify relevant RCTs. Six RCTs with a total of 1710 patients were included in this meta-analysis.
Results
The results demonstrated that the efficacy of daptomycin was at par with or maybe better than other first-line antibiotics for treating SSTIs as shown by the OR for clinical success (OR=1.05, 95% CI 0.84 to 1.31, p=0.65, I2=0%); daptomycin versus vancomycin subgroup (OR=1.19, 95% CI 0.77 to 1.83, p=0.43, I2=0%); overall microbiological success (OR=1.05, 95% CI 0.61 to 1.79, p=0.86, I2=42%); microbiological success of daptomycin versus comparators for Staphylococcus aureus (SA, OR=1.05, 95% CI 0.61 to 2.60, p=0.53, I2=47%), for methicillin-resistant S. aureus (OR=0.90, 95% CI 0.77 to 1.06, p=0.20, I2=56%). However, daptomycin tended to have a similar treatment-related adverse events (AEs) incidence in comparison with other antibiotics (OR=1.06, 95% CI 0.71 to 1.59, p=0.76, I2=41%). The trend showed that daptomycin might cause less discontinuation due to AEs and death compared with other first-line antibiotics (OR=0.71, 95% CI 0.46 to 1.10, p=0.12, I2=11%). Significantly more patients in the daptomyicn group had creatine phosphokinase elevation than those in the control group; however, it could be reversed when the therapy ended (OR=1.95, 95% CI 1.04 to 3.65, p=0.04, I2=0).
Conclusions
This meta-analysis demonstrated that the safety and efficacy of daptomycin was not inferior to that of other first-line drugs, and daptomycin tended to exhibit superior efficacy when compared with vancomycin or with comparators for SA infections; nevertheless, more high-quality RCTs are needed to draw a more credible conclusion.
Keywords: Epidemiology
Strengths and limitations of this study.
Skin and soft-tissue infections (SSTIs) are some of the most common infections, usually caused by Gram-positive bacteria and are closely related to ageing and obesity. Vancomycin and linezolid are the first-line antimicrobial agents for Gram-positive infections, but recently cases of drug resistance have been repeatedly reported.
Daptomycin, a cyclic lipopeptide, was approved 10 years ago in the USA and is now cleared in more than 70 countries to treat Gram-positive pathogens. Until now, drug resistance of daptomycin remains rare.
This is the first meta-analysis of randomised controlled trials of daptomcyin for SSTIs. To the best of our knowledge, this is also the first time daptomycin's potential myotoxicity was confirmed by meta-analysis. Comparative subgroup analyses of daptomycin and vancomycin were conducted to determine the drug's rate of clinical success; the same was carried out for daptomycin versus comparators, in relation to treating Staphylococcus aureus, to determine its microbiological success.
Introduction
Skin and soft-tissue infections (SSTIs) are among some of the most common infections, usually with mild-to-moderate severity. Distressingly, the incidence of SSTIs has rapidly increased in the USA in the community-acquired (CA)-methicillin-resistant Staphylococcus aureus (MRSA) era, which appears to disproportionately affect certain populations.1 SSTIs are usually caused by purulent pathogenic bacteria which invade the epidermis, dermis and subcutaneous tissue.2 SSTIs have a wide range, from superficially localised skin infection to deep-seated necrotising soft-tissue infection which is severe enough to cause disability of extremities or even death. Owing to their different clinical characteristics, SSTIs were divided into uncomplicated SSTIs and complicated SSTIs (cSSTIs). cSSTIs were defined as specific sources of infection or opportunistically pathogenic situations such as trauma, cancer, chemotherapy which were accompanied by impairment of skin barrier function or decreased-immune function.3
For hospitalised patients with cSSTI, besides surgical debridement and broad-spectrum antibiotics, empirical therapy for MRSA should be considered. Antibiotic options include vancomycin, linezolid, daptomycin, telavancin and clindamycin and 7–14 days of therapy are recommended.4 5 The majority of CA-SSTIs in western countries were caused by S. aureus (SA) and β-haemolytic streptococci.2 6 SA is also the main pathogen of hospital-acquired SSTIs, where MRSA exists in high proportions.3 7
Vancomycin has been regarded as the mainstay of parenteral therapy for MRSA infections for decades. Recently, however, its minimum inhibitory concentrations (MICs) in MRSA have been increasing, and linezolid resistance has been reported likewise.8 In the fight against MRSA, daptomycin, a cyclic lipopeptide, that exhibits rapid, concentration-dependent bactericidal activity in vitro against a broad spectrum of Gram-positive pathogens is now approved in more than 70 countries and regions.9 10 Analyses of daptomycin treatment outcomes showed that treatment with daptomycin has resulted in a high clinical success rate for a wide range of Gram-positive infections, such as cSSTIs at the dosage of 4 mg/kg/day11 or for S. aureus bacteraemia (SAB) and right-sided infective endocarditis (IE) at the dosage of 6 mg/kg/day.12
Linezolid can cause anaemia, thrombocytopaenia and gastrointestinal side effects, especially with prolonged therapeutical usage.13 The main side effect of vancomycin is nephrotoxicity, and teicoplanin can cause fever.14 Daptomycin is a comparably safer antibiotic, with myotoxicity being the most relevant side effect and this can be reversed when the therapy ends.15 With drug resistance an urgent problem, new antibiotics are needed to treat infectious diseases, and daptomycin might become such an alternative agent, especially when standard therapies do not work. Comparator drugs in this review refer to vancomycin (mainly), semisynthetic penicillins (SSPs) and teicoplanin, which were used as counterparts for daptomycin in the control group in included studies.
The purpose of this meta-analysis was to compare the safety and efficacy of daptomycin with other antibiotics to treat SSTIs, such as vancomycin or SSPs. The safety end points were treatment-related adverse events (AEs), discontinuation due to AEs and all-cause mortality and creatine phosphokinase (CPK) elevation. The efficacy end points were clinical success and microbiological success at the test-of-cure (TOC) visit.
Methods
Data sources
PubMed (up to September 2013), EMBASE (up to September 2013) and Cochrane Central (Issue 9 of 12 September 2013) were searched to find relevant clinical trials with a prespecified search strategy, which was revised appropriately through databases. Trials other than randomised controlled trial (RCT) were eliminated from consideration. Search terms included ‘daptomycin’, ‘cubicin’, ‘lipopeptide’, ‘SSRIs’, ‘cellulitis’, ‘wounds infection’, ‘abscess’ and ‘erysipelas’, and they were combined by patients, intervention, control, and outcomes (PICOs) principle. No language restriction settled in the searching process. Statistical experts were consulted to make the search strategy and emails were sent to corresponding authors of relevant studies and pharmaceutical companies to gather information about any ongoing RCTs related to daptomycin.
Study selection
Two authors (SZW and ZHT) independently searched and examined the relevant literatures, and scanned the title and abstract of every retrieved article to determine which of them required further assessment. Full articles were obtained when the information given in the titles and abstracts implied that the study included a prospective design research for the purpose of comparing daptomycin with vancomycin or other antibiotics (with or without cointerventions). When disagreements occurred, they were discussed thoroughly to reach a consensus. The inclusion criteria were: (1) any RCTs that compared daptomycin with other antibiotics in treating SSTIs, (2) included patients were of any age, any gender, had a complicated skin and skin-structure infection (SSSI) requiring intravenous antibiotic treatment, (3) daptomycin intravenous infusion with any dosage; comparator antibiotics intravenous infusion with any dosage. Cointerventions that targeted confirmed or probable infections with Gram-negative aerobic and anaerobic pathogens were also permitted.
Qualitative assessment
The methodological quality of the RCTs included in this review was independently evaluated by two authors (SWZ and ZHT), using the Jadad scale,16 which evaluates randomisation and blinding. If the methodology revealed that the study applied appropriate randomisation and blinding procedures, two scores were given to randomisation and two scores to blinding. If it only mentioned about randomisation or blinding but no detail was elucidated, one score was deducted accordingly. If information about attrition was thoroughly elucidated, one score was given. Thus, the score ranges were from 0 to 5 and a trial with a score higher than 2 was considered a trial of high methodological quality.
Data extraction
Two review authors (SWZ and ZHT) independently extracted data with a prespecified data extraction form specifically designed for this review. The data extraction form included the following detailed information: (1) year of publication, clinical settings, (2) the number of intention-to-treat (ITT) and clinically evaluable (CE) patients, (3) descriptions of dose, route and timing of daptomycin and other antibiotics, (4) clinical success, microbiological success, treatment-related AEs, discontinuation due to AEs and all-cause mortality and CPK elevation cases. If missing data were detected in the trial reports, the corresponding authors were contacted to request for information. If this was not successful, ITT analyses were conducted for all dichotomous outcomes (eg, clinical success, microbiological success, treatment-related AEs, all-cause mortality).
Analysed outcomes
The primary outcomes of this review were clinical success and microbiological success. Outcomes were judged by clinical and microbiological evaluations performed at the baseline (within 72 h before receipt of the first dose of study drug) and at the TOC visit (6–20 days after receipt of the last dose). Clinical success was defined as the test subjects exhibiting biological indicators that no further antibiotic therapy was required at the TOC visit. Microbiological success was defined as the eradication of pathogen (present at admission but absent from culture at the TOC visit) or the presumed eradication of pathogen (no material available for culture but the patient was deemed as cured or improved by the study investigator at the TOC visit). Secondary outcomes were proportions of patients with treatment-related AEs, discontinuation due to AEs and all-cause mortality, and cases of CPK elevation.
Data analysis and statistical methods
Data analyses of this review were performed by Review Manager 5.2 (Version: 5.2.6, Cochrane Collabration, UK). Clinical heterogeneity was assessed in the population, methodology, and in the intervention and outcome measures of each study to evaluate whether pooling of results was feasible. Heterogeneity assessment was performed using the χ2 test, where a p value less than 0.1 was considered as the significance set. A funnel plot was applied to check for publication bias and I2 was applied to estimate the total variation attributed to heterogeneity among studies.17 Values of I2 less than 25% were deemed to have low heterogeneity, and a fixed-effect model for meta-analysis was then used. Values of I2 between 25% and 75% were considered to represent moderate levels of heterogeneity, and a random effects model was then utilised. Values of I2 higher than 75% indicated high levels of heterogeneity, in which case no meta-analysis was performed. All statistical tests were two-sided and a p value less than 0.05 was considered statistically significant.
Results
Study selection process
Flow diagram in figure 1 shows the whole scanning and selection process. A total of 310 articles were retrieved by means of electronic database searches. After deleting duplicates, 293 articles were retained to read the title and abstract. Full text of 23 articles was then obtained for further review after scanning. Additionally, emails were sent to Aastrazeneca China which is in charge of marketing daptomycin in China. We were informed that the phase-3 clinical trial of daptomycin for SSTIs was completed in China, yet so far no data have been published. Finally, 6 of the 23 articles met the inclusion criteria.
Figure 1.
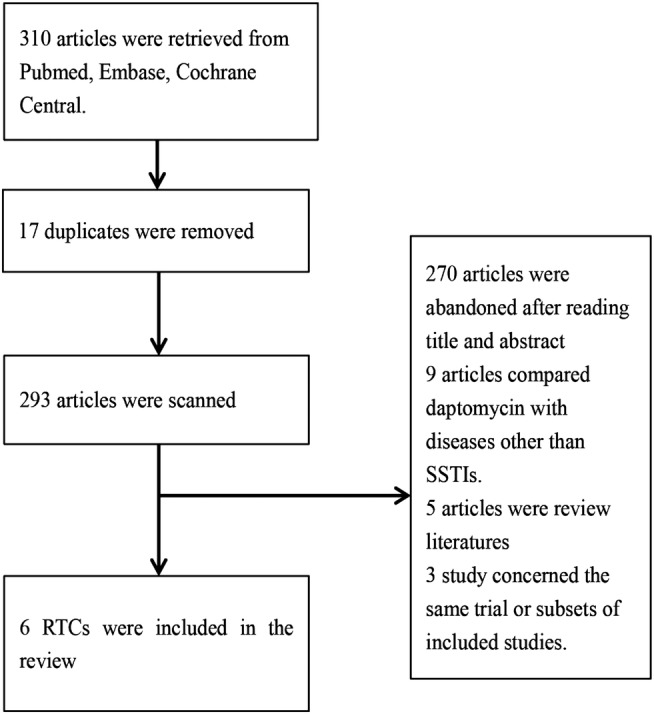
Study flow diagram for relevant randomised controlled trials.
The main characteristics of the six RCTs (type of study design, Jadad score, characteristics of patients, dose and treatment duration of studied drugs, ITT population, CE population) included in this meta-analysis are presented in table 1. All of the six studies were multicentre trials.18–23 The total number of patients included in the trials was 1710. Only adults were enrolled in the included trials, and one trial only aimed at elder patients aged at least 65 years.18 In terms of methodology, all the six included trials were deemed eligible, with a Jadad score ≥2. Allocation concealment was not thoroughly stated in all the six included trials. Funnel plots were performed to check publication bias (SE of log OR plotted against OR). The six studies were neither participant blinded nor personnel blinded. First, overall clinical success analysis was performed on the ITT and CE populations; second, microbiological success was analysed for the microbiologically evaluable population; third, AEs were analysed for the ITT population. Note that 36 patients with MRSA not identified as the causative pathogen (of these, 33 were receiving daptomycin and 3 were receiving vancomycin) were excluded from the efficacy analysis of MITT-MRSA population in one study.19 Four of the six included studies were phase-3 trials,18–20 23 one was a phase-2 trial22 and one was a phase-4 trial.21 Vancomycin was the only comparator drug used in two trials.19 21 In one trial, the comparator drugs were vancomycin and teicoplanin.20 In two trials, the comparator drugs were vancomycin and SSPs.18 23 The infecting organism was confirmed as not MRSA in patients randomised to vancomycin (control); investigators were permitted to switch therapy to an SSP in one study.18 ITT patients of all the six studies were designated to receive intravenous therapy, but patients could be switched to oral treatment in three trials if they already had had at least 4 or 5 days of intravenous therapy and had demonstrated clear clinical improvement.18 22 23 Daptomycin, at a dosage of 4 mg/kg/day, was administered in five trials; the same drug at a dosage of 10 mg/kg/day was administered in another study.22 In all the six trials, comparator drugs were administered according to the standard of care. The efficacy and safety end points were similar across the six included studies.
Table 1.
Main characteristics of the studies included in the meta-analysis
| Group |
Population |
||||||
|---|---|---|---|---|---|---|---|
| Reference | Design | Jadad score | Patients characteristics | Daptomycin (dose, treatment duration) | Comparator (type, dose, treatment duration) | ITT, n (daptomycin vs comparator) | CE, n (daptomycin vs comparator) |
| Konychev et al18 | Multicentre Evaluator-Blinded RCT | 3 | N=120, patients aged ≥65 years with cSSTIs | 4 or 6 mg/kg over 30 min once daily for 5–14 or 10–28 days with bacteraemia | SSP 2 g every 6 h or every 4 h for PTs with bacteraemia; vancomycin 1 g q12 h for 5–14 or 10–28 days with bacteraemia | 120 (81 vs 39) | 103 (73 vs 30) |
| Aikawa et al19 | Multicentre Evaluator-Blinded RCT | 2 | N=101, PTs aged ≥20 years, SSTIs, MRSA confirmed within 3 days | 4 mg/kg over 30 min once daily, for 7–14 days | Vancomycin 1 g over at least 60 min, twice daily, 7–14 days | 111 (88 vs 22) | 74 (55 vs 19) |
| Quist et al20 | Multicentre Evaluator-Blinded RCT | 3 | N=194, adults requiring intravenous antimicrobial treatment for cSSTIs | Daptomycin 4 mg/kg intravenously once daily | Vancomycin 1 g intravenously twice daily; teicoplanin 400 mg intravenously once daily | 189 (97 vs 92) | 108 (58 vs 47) |
| Pertel et al21 | Multicentre Evaluator-Blinded RCT | 2 | N=103, patients ≥18 years, cellulitis or erysipelas intravenous antibiotic therapy | Daptomycin 4 mg/kg intravenously once daily for 7–14 days | Vancomycin was administered intravenously according to standard of care for 7–14 days | 103 (51 vs 52) | 101 (50 vs 51) |
| Katz et al22 | Multicentre Evaluator-Blinded RCT | 3 | N=100, PTs ≥18 years with cSSSI requiring intravenous antibiotic treatment | Daptomycin 10 mg/kg intravenously q24 h for 4 days | Vancomycin 1 g intravenously q12 h for up to 14 days | 96 (48 vs 48) | 79 (39 vs 39) |
| Arbeit et al23 | Multicentre Evaluator-Blinded RCT | 2 | N=1092, patients were aged 18–85 years | Daptomycin 4 mg/kg intravenously once daily for 7–14 days | Penicillinase-resistant penicillin 4–12 g intravenously four times a day or vancomycin,1 g intravenously q12 h by 60-min infusion | 1092 (534 vs 558) | 1002 (446 vs 456) |
Jadad score ranges from 0 to 5, score higher than 2 was considered as trial of high methodological quality.
CE, clinically evaluable; cSSSI, complicated skin and skin- structure infection; cSSTI, complicated skin and soft-tissue infection; ITT, intention-to-treat; MRSA, methicillin-resistant Staphylococcus aureus; PT, prothrombin time; RCT, randomised controlled trial.
Clinical success
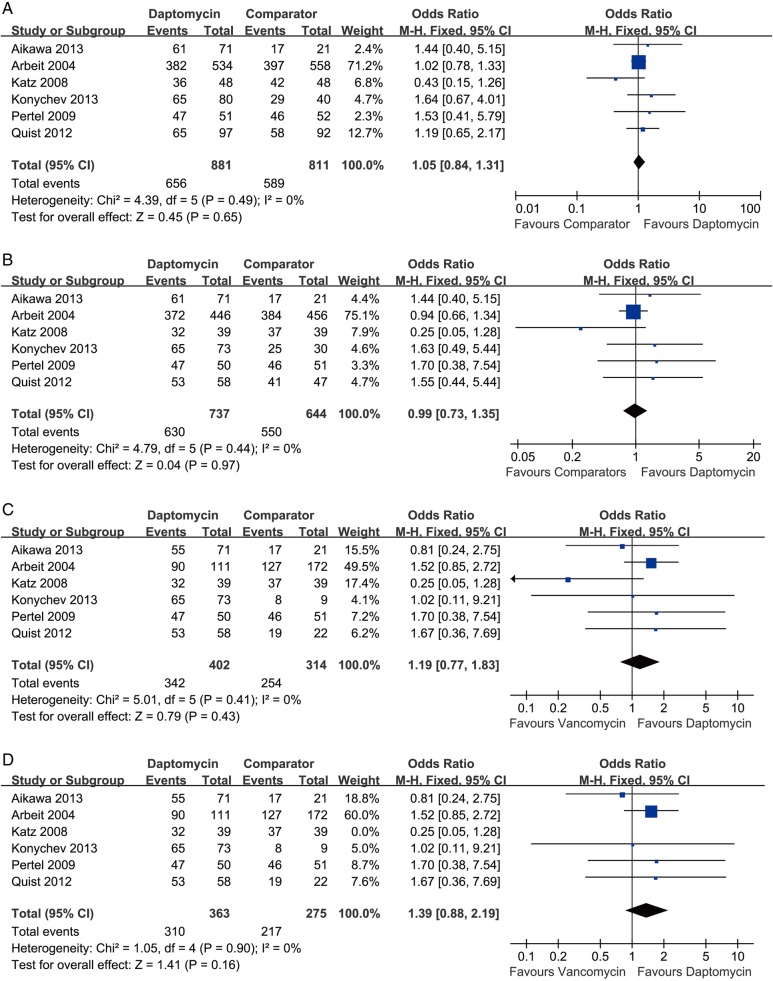
Clinical success rate analysis was performed on the ITT population (all randomised patients with an SSSI who received ≥1 dose of study medication) and CE population (all patients in the ITT population who met protocol-specified inclusion or exclusion criteria relating to the prespecified assessments and to the absence of confounding factors, including completion of the required visits) as well. The pooling result of the ITT population showed that the clinical success rate of daptomycin was similar to that of comparator drugs at the TOC visit (six RCTs, 1674 patients, OR=1.05, 95% CI 0.84 to 1.31, p=0.65, I2=0; figure 2A). Pooling the result of CE population also demonstrated that no difference existed in the clinical success rate between daptomycin and other antibiotics for treating SSTIs (six RCTs, 1381 patients, OR=0.99, 95% CI 0.73 to 1.35, p=0.97, I2=0; figure 2B).
Figure 2.
Meta-analysis of clinical success compares daptomycin with comparator drugs for SSTIs: (A) clinical success (ITT population), (B) clinical success (CE population), (C) daptomycin versus vancomycin for clinical success (CE population). (D) Daptomycin versus vancomycin for clinical success (CE population, excluded the study by Katz et al). The vertical line suggests no difference between daptomycin and comparator drugs. The size of each square represents the proportion of information given by each trial. CE, clinically evaluable; ITT, intention to treat ; SSTI, skin and soft-tissue infection.
In terms of daptomycin versus vancomycin subgroup, the clinical success rate of daptomycin was higher (not significantly) than that of vancomycin (six RCTs, 716 patients, OR=1.19, 95% CI 0.77 to 1.83, p=0.43, I2=0; figure 2C). Briefly, 342 of 402 patients in the daptomycin group and 254 of 314 patients in the vancomycin group achieved clinical success at the TOC visit. Since the study of Katz et al used a higher dosage than the other included studies, after its exclusion, the pooling result showed a trend favouring daptomycin (five RCTs, 638 patients, OR=1.39, 95% CI 0.88 to 2.19, p=0.16, I2=0; figure 2D).
Microbiological success
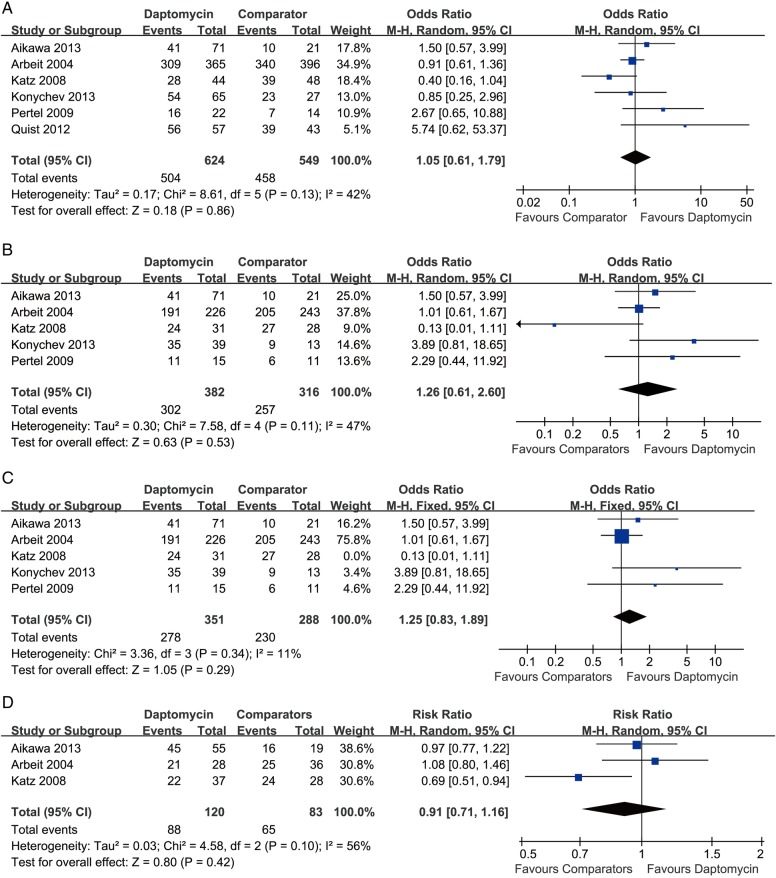
Microbiological success rate analysis was performed on microbiologically evaluable patients (all patients in the CE population who had a causative Gram-positive organism isolated at baseline); the pooling result showed that the microbiological success rate of daptomycin was similar to that of comparator drugs (six RCTs, 1173 patients, OR=1.05, 95% CI 0.61 to 1.79, p=0.86, I2=42%; figure 3A). In brief, 504 of 624 patients in the daptomycin group and 458 of 549 patients in the control group achieved microbiological success.
Figure 3.
Meta-analysis of microbiological success compares daptomycin with comparator drugs for SSTIs based on the microbiologically evaluable population: (A) overall microbiological success, (B) microbiological success for Staphylococcus aureus. (C) Microbiological success for S. aureus (excluded Katz et al's study). (D) Microbiological success for MRSA. The vertical line suggests no difference between daptomycin and comparator drugs. The size of each square represents the proportion of information given by each trial. MRSA, methicillin-resistant Staphylococcus aureus; SSTI, skin and soft-tissue infection.
The data of SA subgroup were not extractable in the study by Quist et al.20 In terms of the microbiological success rate for SA (methicillin susceptible and methicillin resistant), the pooling result demonstrated that no significant difference existed between daptomycin and comparator drugs (five RCTs, 698 patients, OR=1.59, 95% CI 0.61 to 2.60, p=0.53, I2=47%; figure 3B). After the exclusion of the study by Katz et al, the overall heterogeneity dropped, but the result remained unchanged (four RCTs, 639 patients, OR=1.25, 95% CI 0.83 to 1.89, p=0.29, I2=11%; figure 3C). For MRSA infections, data were successfully extracted from three studies; the overall heterogeneity was expectedly high, under which circumstance random model was applied, and the result showed that the success rate of daptomycin was slightly lower than that of comparator drugs (three RCTs, 203 patients, OR=0.91, 95% CI 0.77 to 1.06, p=0.10, I2=56%; figure 3D).
AEs outcomes and mortality outcomes
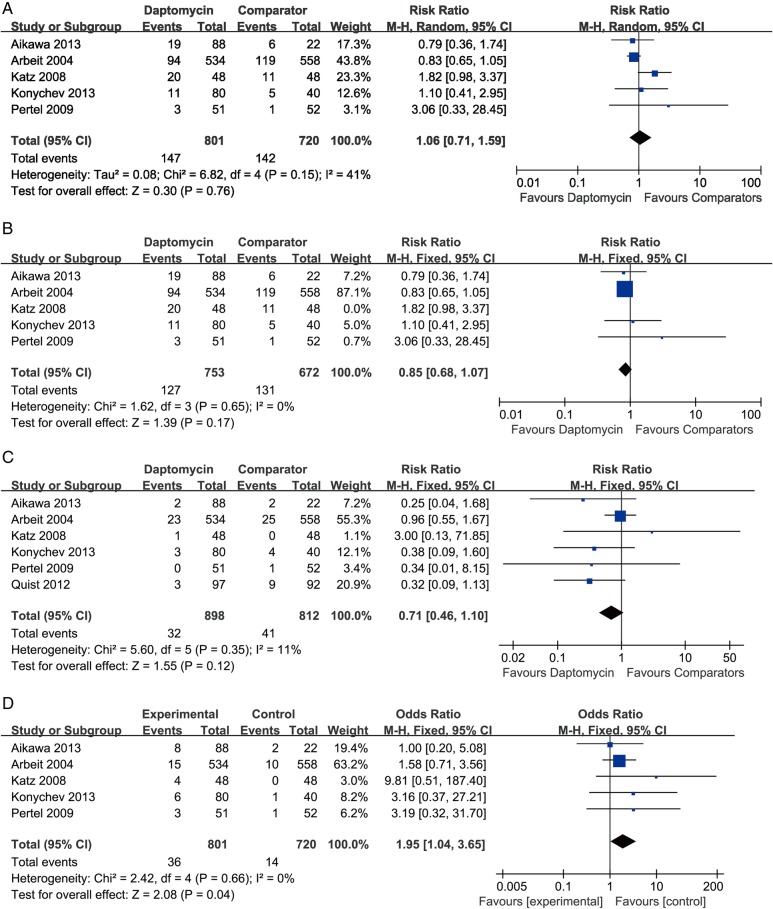
In terms of treatment-related AEs, the study by Quist et al20 was excluded from the pooling result because no information was given about whether AEs were treatment related or not. No significant difference was detected between daptomycin and comparator drugs (five studies, 1521 patients, OR=1.06, 95% CI 0.71 to 1.59, p=0.76, I2=41%; figure 4A). After the study by Katz et al was excluded, there was a dramatic decline in heterogeneity, and the result changed to favour daptomycin (four RCTs, 1425 patients, OR=0.85, 95% CI 0.68 to 1.07, p=0.17, I2=0; figure 4B).
Figure 4.
Meta-analysis of AEs compares daptomycin with comparator drugs for SSTIs based on the ITT population: (A) treatment-related adverse events, (B) treatment-related adverse events (excluded the study by Katz et al), (C) discontinuation due to AEs and all-cause mortality, (D) CPK elevations regarded as adverse events. Vertical line suggests no difference between daptomycin and comparator drugs. The size of each square represents the proportion of information given by each trial. AE, adverse event; CPK, creatine phosphokinase; ITT, intension to treat; SSTI, skin and soft-tissue infection.
Discontinuation due to AEs and all-cause mortality during treatment was rare in the six included studies. No deaths were reported in three studies,18 21 22 while another study reported discontinuation due to AEs and death combined.20 On account of the above reasons, discontinuation due to AEs and all-cause mortality were pooled together. With a total of 1710 patients enrolled in the analysis, the pooling result suggested that no significant difference existed between daptomycin and comparator drugs (six RCTs, 1710 patients, OR=0.76, 95% CI 0.46 to 1.10, p=0.12, I2=11%; figure 4C). A comparison of CPK elevations considered as AEs between daptomycin and comparator drugs yielded that significantly more patients had CPK elevation in the daptomycin group than in the comparator drugs group (five RCTs, 1521 patients, OR=1.95, 95% CI 1.04 to 3.65, p=0.04, I2=0; figure 4D).
Discussion
This is an updated meta-analysis based on the study by Bliziotis et al,24 which compares efficacy and safety of daptomycin with vancomycin and other antibiotics for treating SSTIs. There were some clear limitations found in the previous meta-analysis. First, it enrolled only four trials, in which three were RCTs, including one RCT which was found to have considerable heterogeneity in our analyses due to a high dose of daptomycin with a short duration. In addition, there was one historically controlled trial (not randomised) which was excluded in this review.25 Besides the previous three RCTs, three more RCTs were considered to be eligible in terms of clinical homogeneity. Daptomycin was approved by the Food and Drug Administration (FDA) in September 2003, for treatment of complicated SSTIs because of the drug-resistant urgency. However, there were only a handful RCTs available, and a distinct lack of high-quality meta-analysis yielding high-level clinical evidence.
The results of this review indicate that daptomycin was as effective and safe as other drugs in treating SSTIs. The clinical success rate of daptomycin in the ITT population (OR=1.05, 95% CI 0.84 to 1.31, p=0.65, I2=0) and CE population (OR=0.99, 95% CI 0.73 to 1.35, p=0.97, I2=0) was equivalent to that of other drugs used to treat SSTIs. Of note, in the study by Katz et al,22 a high dose (10 mg/kg/day) intake of daptomycin with a short-treatment duration (4 days) led to reduced clinical and microbiological success rate of daptomycin, when compared with comparator drugs. This shortened therapy duration could possibly have undermined the efficacy of daptomycin and brought about some clinical heterogeneity, resulting in statistical heterogeneity in our data analyses. The microbiological success rate of daptomycin was also similar to that of other first-line drugs (OR=1.05, 95% CI 0.61 to 1.79, p=0.86, I2=42%). SA was the main pathogen for SSTIs; the microbiological success rate for SA showed no significant difference between the two groups (OR=1.26, 95% CI 0.61 to 2.60, p=0.53, I2=47%). However, after the exclusion of the study by Katz et al which used a different dosage, the heterogeneity declined, and the result tended to favour daptomycin (OR=1.25, 95% CI 0.83 to 1.89, p=0.86, I2=11%). With MRSA as the most common drug-resistant pathogen in SSTIs, the pooling result of the success rate of daptomycin versus comparators indicated that no significant difference existed between the groups (OR=0.90, 95% CI 0.77 to 1.06, p=0.20, I2=56%). Only 203 patients were enrolled in the MRSA subgroup analysis, while simultaneously the heterogeneity was high; thus, the result should be interpreted prudently. That the included studies were conducted in diverse countries at different times, and that there was a lack of uniformity in epidemiological characteristics for each trial should have some confounding impacts on the final results. Not all the included studies reported duration of treatment; however, the study by Arbeit et al23 found that significantly more patients in the daptomycin group than patients in the comparator drugs group needed only 4–7 days of treatment; two other included studies found no significant difference existed between the two groups in terms of duration of treatment.18 21 Furthermore, there were no significant difference between daptomycin and comparator drugs in terms of treatment-related AEs (OR=1.06, 95% CI 0.71 to 1.59, p=0.76, I2=41%). However, after the study by Katz et al was excluded, daptomycin tended to have less treatment-related AEs (OR=0.85, 95% CI 0.68 to 1.07, p=0.86, p=0.17, I2=0) and less patients associated with discontinuation or death (OR=0.71, 95% CI 0.46 to 1.10, p=0.12, I2=11%). Daptomycin was reported to have potential muscle toxicity,15 as a result, CPK was closely monitored in the included studies during the treatment process. This close monitoring revealed that CPK elevation occurred more frequently in daptomycin-treated patients (OR=1.95, 95% CI 1.04 to 3.65, p=0.04, I2=0), but on most occasions, it declined to normal levels during or after the therapy. Therefore, one may conclude that daptomycin might be a safer and more efficacious drug to use, in comparison with other comparator drugs in the matters of microbiological success, treatment-related AEs, discontinuation or death. Of note, in the study by Aikawa et al,19 1 of the 88 patients in the daptomycin group experienced anaphylactic shock, which was resolved 4 days after discontinuation of drug treatment. Therefore, despite the safety of daptomycin being satisfying, clinicians should be cautious about administering it to patients of hypersensitivity.
Infectious Diseases of America recommended that vancomycin be used for empirical therapy in clinical settings with an increased prevalence of MRSA; for institutions with preponderant MRSA isolates that have vancomycin MIC values >2 mg/mL, alternative agents such as daptomycin should be used.26 An antimicrobial resistance surveillance in China had also documented that SA and Escherichia coli were the most common multidrug-resistant pathogens for which linezolid, tigecycline, daptomycin and vancomycin provided the best antimicrobial coverage.27 Vancomycin was also the first-line drug to treat MRSA infections in hospitalised children. So comparing the efficacy of daptomycin with vancomycin is necessary and useful since it could provide helpful data to clinicians. The daptomycin versus vancomycin subgroup analysis of our review found that daptomycin tended to exhibit higher clinical success rate in comparison with vancomycin (OR=1.19, 95% CI 0.77 to 1.83, p=0.43, I2=0). Excluding the study by Katz et al, the pooling that resulted favoured daptomycin even further (OR=1.39, 95% CI 0.88 to 2.19, p=0.16, I2=0).
Daptomycin is mainly metabolised by kidneys. Aikawa et al demonstrated that in patients with mild-to-moderate renal impairment, when compared with patients having normal renal function, clearance of daptomycin was not markedly different. Furthermore, 6 mg/kg of daptomycin once daily was found to be safe for extended dialysis patients, which simultaneously could lower the substantial risk of underdosing of daptomycin.28 In hospitalised children with cSSTIs, vancomycin, clindamycin and linezolid were recommended for treatment, whereas daptomycin was not mentioned.4 Nevertheless, daptomycin therapy demonstrated clinical improvement for invasive Gram-positive bacterial infections in children,29 but the clearance of daptomycin in infants and 2–6 year-old children was higher than that of adolescents and adults. As a result, in order to achieve efficacious exposures, this younger group might need a higher dosage of daptomycin.30 Vancomycin, however, has potential renal toxicity which limits its usage in patients with renal impairment, and for these patients daptomycin might be an eligible alternative agent. In recent years, vancomycin-resistant SA infection cases have been repeatedly reported in the USA31; for these, daptomycin with an efficacy equivalent to vancomycin could be used as an eligible alternative treatment. Of note, Aikawa et al19 found a trend that along with the increment of MICs of daptomycin, the clinical success rate declined gradually. In spite of that, up till now, non-susceptibility to daptomycin remains rare.32 Recently, one meta-analysis demonstrated that compared with vancomycin, linezolid had superior efficacy for MRSA infections.33 To the best of our knowledge, there was no RCT directly comparing linezolid with daptomycin for MRSA infections. What is more, cost-effectiveness analysis studies of daptomycin, vancomycin and linezolid for MRSA-related cSSTIs found that daptomycin and linezolid were potentially more cost effective than vancomycin; however, daptomycin had no advantage when compared with linezolid.34 35 RCTs about daptomycin aimed at other diseases also proved daptomycin was safe and effective in treating issues like prosthetic joint infection,36 or SAB and IE at a dosage of 6 mg/kg/day.12 Note that age was a risk factor for SSTIs since the average age of all patients exceeded 40 years old in the included studies. The mean or median body weight index in four trials (all exceeded 25 kg/m2) also revealed that obesity is also a risk factor.18 19 21 22 Additionally, diabetes mellitus, peripheral vascular disease and immunocompromise present the usual comorbid conditions for SSTI.21–23 Wound infections were common in surgical departments and surgical intensive care unit, and accounted for nearly 41% of the total patients in four included studies. Although the efficacy and safety data were not charted for specific type of SSTI in every included trial, the high proportion of wound infections in the included studies is adequate to exhibit the safety and efficacy of daptomycin for these.
There are several limitations of our meta-analysis. First of all, none of the six included RCTs were participant blinded or personnel blinded, thus, performance bias was unpredictable. Furthermore, the study by Arbeit et al had dominant influence on overall clinical success rate analysis of ITT and CE populations, as it weighed more than 70% in these two analyses. Additionally, too few of our data analyses reached statistical significance, which led to insufficient credibility to draw conclusions for some potentially disputable issues.
Conclusions
On the basis of our analyses, suffice it to say that daptomycin does not have an inferior efficacy and has safety equivalent to comparator drugs, especially when compared with vancomycin which has been considered as the standard therapy for cSSTIs. Based on the present evidence, daptomycin is a promising new agent for Gram-positive infections like SSTIs, and more high-quality RCTs are expected to explore its potentiality.
Supplementary Material
Acknowledgments
The authors would like to thank Professor Tomoko Yoshinari and Ricardo Chaves, who were the corresponding authors of two included studies. In the data extraction process, several emails were sent to them to deal with problems involving some of the data. Their patient replies provided valuable help. The authors also thank Eileen Mcintyre of Cubist Pharmaceutical, to whom email inquiries were sent regarding any ongoing clinical trials of daptomycin for skin and soft-tissue infections. She wrote to Aastrazeneca China which is in charge of selling daptomycin in China concerning our inquiry and the latter proved to be another helpful source of information. Finally, the authors thank Helen Cadogan, English teacher and editor for the editorial assistance.
Footnotes
Contributors: SWZ and ZTH conceived this study, identified studies for inclusion and extracted data together. The English manuscript was written and revised by SWZ. Other authors made supportive contributions. All the authors read and approved the final manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Hersh AL, Chambers HF, Maselli JH, et al. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 2008;168:1585–91 [DOI] [PubMed] [Google Scholar]
- 2.Fung HB, Chang JY, Kuczynski S. A practical guide to the treatment of complicated skin and soft tissue infections. Drugs 2003;63:1459–80 [DOI] [PubMed] [Google Scholar]
- 3.Dryden MS. Complicated skin and soft tissue infection. J Antimicrob Chemother 2010;65(Suppl 3):iii35–44 [DOI] [PubMed] [Google Scholar]
- 4.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011;52:e18–55 [DOI] [PubMed] [Google Scholar]
- 5.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005;41:1373–406 [DOI] [PubMed] [Google Scholar]
- 6.Moet GJ, Jones RN, Biedenbach DJ, et al. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004). Diagn Microbiol Infect Dis 2007;57:7–13 [DOI] [PubMed] [Google Scholar]
- 7.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 2006;355:666–74 [DOI] [PubMed] [Google Scholar]
- 8.Gould IM, David MZ, Esposito S, et al. New insights into meticillin-resistant Staphylococcus aureus (MRSA) pathogenesis, treatment and resistance. Int J Antimicrob Agents 2012;39:96–104 [DOI] [PubMed] [Google Scholar]
- 9.Tally FP, DeBruin MF. Development of daptomycin for gram-positive infections. J Antimicrob Chemother 2000;46:523–6 [DOI] [PubMed] [Google Scholar]
- 10.Rybak MJ, Hershberger E, Moldovan T, et al. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against Staphylococci and Enterococci, including vancomycin- intermediate and -resistant strains. Antimicrob Agents Chemother 2000;44:1062–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghavan M, Linden PK. Newer treatment options for skin and soft tissue infections. Drugs 2004;64:1621–42 [DOI] [PubMed] [Google Scholar]
- 12.Fowler VG, Jr, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006;355:653–65 [DOI] [PubMed] [Google Scholar]
- 13.Kuter DJ, Tillotson GS. Hematologic effects of antimicrobials: focus on the oxazolidinone linezolid. Pharmacotherapy 2001;21:1010–13 [DOI] [PubMed] [Google Scholar]
- 14.Wood MJ. The comparative efficacy and safety of teicoplanin and vancomycin. J Antimicrob Chemother 1996;37:209–22 [DOI] [PubMed] [Google Scholar]
- 15.Oleson FB, Jr, Berman CL, Kirkpatrick JB, et al. Once-daily dosing in dogs optimizes daptomycin safety. Antimicrob Agents Chemother 2000;44:2948–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12 [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konychev A, Heep M, Moritz RK, et al. Safety and efficacy of daptomycin as first-line treatment for complicated skin and soft tissue infections in elderly patients: an open-label, multicentre, randomized phase IIIb trial. Drugs Aging 2013;30:829–36 [DOI] [PubMed] [Google Scholar]
- 19.Aikawa N, Kusachi S, Mikamo H, et al. Efficacy and safety of intravenous daptomycin in Japanese patients with skin and soft tissue infections. J Infect Chemother 2013;19:447–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quist SR, Fierlbeck G, Seaton RA, et al. Comparative randomised clinical trial against glycopeptides supports the use of daptomycin as first-line treatment of complicated skin and soft-tissue infections. Int J Antimicrob Agents 2012;39:90–1 [DOI] [PubMed] [Google Scholar]
- 21.Pertel PE, Eisenstein BI, Link AS, et al. The efficacy and safety of daptomycin vs. vancomycin for the treatment of cellulitis and erysipelas. Int J Clin Pract 2009;63:368–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz DE, Lindfield KC, Steenbergen JN, et al. A pilot study of high-dose short duration daptomycin for the treatment of patients with complicated skin and skin structure infections caused by gram-positive bacteria. Int J Clin Pract 2008;62:1455–64 [DOI] [PubMed] [Google Scholar]
- 23.Arbeit RD, Maki D, Tally FP, et al. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin Infect Dis 2004;38:1673–81 [DOI] [PubMed] [Google Scholar]
- 24.Bliziotis IA, Plessa E, Peppas G, et al. Daptomycin versus other antimicrobial agents for the treatment of skin and soft tissue infections: a meta-analysis. Ann Pharmacother 2010;44:97–106 [DOI] [PubMed] [Google Scholar]
- 25.Davis SL, McKinnon PS, Hall LM, et al. Daptomycin versus vancomycin for complicated skin and skin structure infections: clinical and economic outcomes. Pharmacotherapy 2007;27:1611–18 [DOI] [PubMed] [Google Scholar]
- 26.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009;49:1–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones RN, Castanheira M, Hu B, et al. Update of contemporary antimicrobial resistance rates across China: reference testing results for 12 medical centers (2011). Diagn Microbiol Infect Dis 2013;77:258–66 [DOI] [PubMed] [Google Scholar]
- 28.Kielstein JT, Eugbers C, Bode-Boeger SM, et al. Dosing of daptomycin in intensive care unit patients with acute kidney injury undergoing extended dialysis—a pharmacokinetic study. Nephrol Dial Transplant 2010;25:1537–41 [DOI] [PubMed] [Google Scholar]
- 29.Ardura MI, Mejias A, Katz KS, et al. Daptomycin therapy for invasive Gram-positive bacterial infections in children. Pediatr Infect Dis J 2007;26:1128–32 [DOI] [PubMed] [Google Scholar]
- 30.Cohen-Wolkowiez M, Watt KM, Hornik CP, et al. Pharmacokinetics and tolerability of single-dose daptomycin in young infants. Pediatr Infect Dis J 2012;31:935–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sievert DM, Rudrik JT, Patel JB, et al. Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin Infect Dis 2008;46:668–74 [DOI] [PubMed] [Google Scholar]
- 32.Sader HS, Flamm RK, Jones RN. Antimicrobial activity of daptomycin tested against Gram-positive pathogens collected in Europe, Latin America, and selected countries in the Asia-Pacific Region (2011). Diagn Microbiol Infect Dis 2013;75:417–22 [DOI] [PubMed] [Google Scholar]
- 33.An MM, Shen H, Zhang JD, et al. Linezolid versus vancomycin for meticillin-resistant Staphylococcus aureus infection: a meta-analysis of randomised controlled trials. Int J Antimicrob Agents 2013;41:426–33 [DOI] [PubMed] [Google Scholar]
- 34.Bounthavong M, Zargarzadeh A, Hsu DI, et al. Cost-effectiveness analysis of linezolid, daptomycin, and vancomycin in methicillin-resistant Staphylococcus aureus: complicated skin and skin structure infection using Bayesian methods for evidence synthesis. Value Health 2011;14:631–9 [DOI] [PubMed] [Google Scholar]
- 35.Stephens JM, Gao X, Patel DA, et al. Economic burden of inpatient and outpatient antibiotic treatment for methicillin-resistant Staphylococcus aureus complicated skin and soft-tissue infections: a comparison of linezolid, vancomycin, and daptomycin. Clinicoecon Outcomes Res 2013;5:447–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byren I, Rege S, Campanaro E, et al. Randomized controlled trial of the safety and efficacy of daptomycin versus standard-of-care therapy for management of patients with osteomyelitis associated with prosthetic devices undergoing two-stage revision arthroplasty. Antimicrob Agents Chemother 2012;56:5626–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.