Abstract
Streptococcus suis is an important pathogen causing economic problems in the pig industry. Moreover, it is a zoonotic agent causing severe infections to people in close contact with infected pigs or pork-derived products. Although considered sporadic in the past, human S. suis infections have been reported during the last 45 years, with two large outbreaks recorded in China. In fact, the number of reported human cases has significantly increased in recent years. In this review, we present the worldwide distribution of serotypes and sequence types (STs), as determined by multilocus sequence typing, for pigs (between 2002 and 2013) and humans (between 1968 and 2013). The methods employed for S. suis identification and typing, the current epidemiological knowledge regarding serotypes and STs and the zoonotic potential of S. suis are discussed. Increased awareness of S. suis in both human and veterinary diagnostic laboratories and further establishment of typing methods will contribute to our knowledge of this pathogen, especially in regions where complete and/or recent data is lacking. More research is required to understand differences in virulence that occur among S. suis strains and if these differences can be associated with specific serotypes or STs.
Keywords: pig pathogen, sequence types, serotypes, Streptococcus suis , zoonotic agent
INTRODUCTION
Streptococcus suis is one of the most important pathogens in the porcine industry causing septicemia, meningitis and many other infections.1 In addition, it is an emerging zoonotic agent responsible for septicemia with or without septic shock, meningitis and other less common infections in humans.1 During the last decade, the number of reported human cases due to S. suis has dramatically increased, and while most sporadic human cases of infection appear to be due to close occupational contact with pigs/pork products, particularly in Western countries (farmers, veterinarians, butchers, food processing workers, etc.), two epidemics were recorded in China in 1998 and 2005.1 As of 2006, the number of human cases reported in Asia has increased.2,3 In fact, in some Asian countries, the general population is at risk.2 However, an update on the distribution of the different serotypes and sequence types (STs), as determined by multilocus sequence typing (MLST), of strains responsible for infections in both pigs and humans from around the world have not been recently compiled. Yet, knowledge of this distribution is necessary in order to not only understand the current situation regarding S. suis, but also to evaluate areas where knowledge is lacking. This review covers the global distribution of the S. suis serotypes and STs responsible for infections reported in pigs from 1 January 2002 to 31 December 2013. A complete review on serotypes from humans since the first description in 1968 was also carried out. However, data of the STs from human cases are available only since 2002. Complete reviews have already covered the different virulence factors implicated in the pathogenesis of the infection caused by this important pathogen,4,5 and this will not be further addressed in this review.
Brief description of the general aspects of infection in pigs and human
The natural habitat of S. suis is the upper respiratory tract of pigs, more particularly the tonsils and nasal cavities, but also the genital and digestive tracts.6 With almost 100% of pig farms worldwide having carrier animals, S. suis is one of the most important bacterial pig pathogens. Transmission of S. suis among animals is considered to be mainly through the respiratory route.6 Of the various manifestations of the disease, septicemia and meningitis are by far the most striking features, but endocarditis, pneumonia and arthritis can also be observed.7 Nevertheless, in peracute cases of infection, pigs are often found dead with no premonitory signs of disease.7 Presumptive diagnosis of infection in pigs is usually based on clinical signs and macroscopic lesions.8 Confirmation of the infection is mandatory and must be achieved by isolation and characterization of the pathogen.8
Since first described in Denmark in 1968,9 over 1600 human cases of S. suis infection have been reported with many more probably never diagnosed or misdiagnosed. S. suis is the most common cause of adult meningitis in Vietnam, the second most common in Thailand and the third most frequent cause of community-acquired bacterial meningitis in Hong Kong.10,11,12 Different from pigs, the main route of entry of S. suis in humans is thought to be through contact of cutaneous lesions, most usually on the hands and arms, with contaminated animals, carcasses or meat.3 However, this situation seems to be different in some Asian countries where the oral route is taken into consideration since many cases of infection have been reported after ingestion of contaminated raw pork products.13 In Western countries, infections in humans most usually occur sporadically.1 After an incubation period that ranges from a few hours to days,13 S. suis usually produces meningitis in humans, but is also responsible for cases of endocarditis, pneumonia, peritonitis, arthritis and other less common diseases usually related to generalized septicemia.14,15 In addition, peracute infections with shock and a high mortality rate have been described, particularly in the case of the streptococcal toxic shock-like syndrome (STSLS) closely associated with the 2005 Chinese epidemic, but also observed independently of this outbreak.16
IDENTIFICATION OF S. suis
S. suis is an encapsulated gram-positive bacterial coccus that occurs singly, frequently in pairs, or occasionally in short chains. The organism grows well in aerobiosis, but growth is enhanced by microaerophilic conditions. The majority of strains are alpha-hemolytic on bovine and sheep blood agar plates after 24 h of incubation at 37°C.
Pig and human strains isolated from clinical cases of infection
For clinical cases in pigs, isolation and identification of strains is relatively easy since successful identification can be achieved by a minimum of biochemical tests and confirmed by serotyping (see below).17 However, the use of rapid multi-test biochemical kits may be misleading as some strains of S. suis can be misidentified.18 Although S. suis field isolates readily grow on media used for culturing meningitis-causing bacteria and veterinary diagnostic laboratories easily identify this pathogen, many human diagnostic laboratories are less aware of this pathogen and may misidentify it as enterococci, Streptococcus pneumoniae, Streptococcus bovis, viridans group streptococci (e.g., Streptococcus anginosus and Streptococcus vestibularis) or even Listeria monocytogenes.14,18,19,20,21,22 In many cases, the initial gram stain presumptive diagnosis of the cerebrospinal fluid specimen is pneumococcal meningitis. This confusion may have led to the misdiagnosis of S. suis infections in the past. Many cases were diagnosed retrospectively after the isolates were initially misidentified.23 More recently, polymerase chain reaction (PCR) tests have been used to directly detect S. suis DNA from cerebrospinal fluid samples with a sensitivity considerably higher than direct culture, especially if antibiotics have been used.2 However, most PCR tests used for humans detect serotype 2 strains only and will not detect infections caused by other S. suis serotypes (see below). Instead of the serotype 2 PCR test, it is advisable to use other validated PCR tests that allow the detection of the S. suis species (see below).
Strains isolated from clinically healthy pigs
S. suis is a normal inhabitant of the oral cavity of pigs. The biochemical identification of isolates recovered from clinically healthy pigs (mainly from tonsil samples) is difficult to achieve due to the presence of other streptococci that are part of the normal oral microflora and that are phenotypically similar to S. suis. For this reason, molecular biology techniques have been developed during the last decade to allow the detection and identification of S. suis strains, such as a PCR assay targeting the specific house-keeping gene encoding for the glutamate dehydrogenase (gdh).24 Although widely used, the gdh PCR test can sometimes fail to correctly identify certain S. suis isolates.25 Using this test, it has also been reported that there was a risk of misidentifying Streptococcus gallolyticus as S. suis.26
SEROTYPING
Definition of serotypes
Serotyping, which should be a part of the routine identification of S. suis strains recovered from diseased pigs and humans, will provide further confirmation regarding the pathogen's identity. A total of 35 serotypes of S. suis have been described and are defined based on the antigenicity of their capsular polysaccharide (CPS).6 Evidence accumulated throughout the years has demonstrated a high level of genetic diversity in the S. suis species, as shown by sequence analysis of the 16S rRNA and cpn60 genes: these two methods suggested that serotypes 32 and 34 were divergent, being clustered together at a significant distance from the other serotypes. This led to the suggestion that these two serotypes might be reclassified as a different species (Streptococcus orisratti).27,28 However, field strains belonging to these serotypes have been isolated from diseased pigs in recent years in Canada and China.25,29,30 More recently, further studies have suggested that the reference strains of additional serotypes (20, 22, 26, and 33) do not belong to the S. suis species.31 As such, there is an urgent need for a consensus in defining whether a serotype belongs to S. suis or not.32
In order to understand the important roles of the CPS in serotyping and in the interactions of S. suis with the host, knowledge regarding the specific structure that confers capsular properties to each individual serotype is necessary. Van Calsteren et al.33,34 have described the composition and structures of the S. suis serotypes 2 and 14 CPS. Still, the chemical composition and structure of the CPS of the serotypes other than 2 and 14 are presently unknown.
Serological methods
Proper serological typing, which is one of the most important features of the S. suis infection diagnosis, must be performed using either a co-agglutination test, capillary precipitation test or with Neufeld's capsular reaction using reference antisera.35,36 Details on how to perform the co-agglutination test have already been published.37 This test is preferred by many laboratories, especially in North America.17 Some serotypes cross-react, indicating the presence of common antigenic determinants. This cross-reaction is probably due to similar or closely related structural features of the CPS. To date, the following cross-reactions have been described: serotype 1/2 with serotypes 1 and 2, serotypes 6 and 16, serotypes 2 and 22, and serotypes 1 and 14.35,38 In some cases, absorption is recommended in order to obtain monospecific antisera.17 However, levels of cross-reactions for some serotypes vary with field strains (Gottschalk M, unpublished data, 2014).
Serotyping by multiplex PCR
The idea of molecular serotyping by PCR amplification of serotype specific cps genes using either a simplex or multiplex PCR is attractive due to the fact that animals are not used for serum production, ease of development and effectiveness. Early studies reported the use of assays targeting some serotypes.39,40 Liu et al.41 reported a new protocol using four multiplex PCR assays allowing the detection of the 33 serotypes of S. suis (serotypes 1 to 31, 33 and 1/2), but not those related to S. orisratti (serotypes 32 and 34). Strains reacting in serology with more than one serotype could be confirmed using this technique. More recently, Okura et al. developed a PCR for all 35 described serotypes of S. suis.42 Regardless of the PCR test used, a major disadvantage is the fact that the serotypes 2 and 14 cannot be distinguished from serotypes 1/2 and 1, respectively, since both of these serotype pairs do not possess unique cps genes.43 This represents a major problem for pig isolates since serotypes 1, 1/2, 2 and 14 are commonly isolated in swine.3,10 The use of specific antisera is mandatory for such strains. For this reason, only serologically confirmed isolates of serotypes 2 and 14 recovered from pigs were considered in the present review. Although it is also necessary to confirm isolates from humans, it may represent a less important drawback since serotype 1/2 has never been isolated from humans and serotype 1 has been reported in only three non-serologically confirmed cases (at least with both serotypes 1 and 14 antisera), which is important considering these serotypes cross-react (see above).44,45 For this reason, isolates from humans reported in the literature as being serotype 2 or 14 based only on PCR reactions (although not serologically confirmed) will be considered as such in the present review.
‘Serotyping' by biochemical identification
In the past, some reported cases of S. suis infection in humans have been attributed to serotype 2 based on the biochemical analyses obtained using the rapid multi-test commercial kits. While many of these kits claim to differentiate between serotype 1 and 2 strains based on sugar fermentation, there is still no evidence of a correlation between a specific serotype and its biochemical properties.17,20,46 As a result, some human cases have been reported as serotype 2, while others were reported as serotype 1, but since the serotypes of these strains have not been confirmed using antisera (and not even by PCR), their serotypes are herein reported as ‘unconfirmed by reference antisera or PCR tests'.
Non-typable strains
Some S. suis isolates do not agglutinate with any of the typing antisera directed against the 35 serotypes and are identified as non-typable isolates.29 Non-typable S. suis strains may correspond to either truly encapsulated strains that belong to novel, not yet described, encapsulated serotypes, or to non-encapsulated strains, which are impossible to serotype using the serological methods based on CPS antigens. The proportion of non-typable isolates varies between studies depending on the number of serotypes detected using antisera.
Using antisera against all 35 serotypes, Gottschalk et al.25,47 demonstrated that 89% of non-typable S. suis strains presented high surface hydrophobicity, suggesting that they were poorly or non-encapsulated. This was confirmed using transmission electron microscopy, demonstrating that highly hydrophobic strains (74%–93%) were non-encapsulated.25 More than 40% of these non-encapsulated strains have been recently shown to belong to known serotypes using a multiplex PCR for all 35 serotypes.42 It is also difficult to be certain if these strains were already non-encapsulated when causing disease, or if they lost their CPS during isolation and culture. It has been previously reported that 34% of isolates belonging to serotype 1/2 or 2 recovered from cases of endocarditis in Japan were non-encapsulated due to deletions and insertions in the genes of the CPS locus.48 It was concluded that although the CPS is considered an important virulence factor for S. suis, loss of capsular production might be beneficial to S. suis in the course of infective endocarditis. In fact, non-encapsulated strains were shown to possess not only high adhesion properties to mammalian cells, but also a capacity to form biofilms.47 Since the sites of isolation of these non-typable strains were similar to those of the most important serotypes (meninges/brain, joints, heart and lungs), their potential virulence capacity should not be disregarded.
MULTILOCUS SEQUENCE TYPING
Being a pathogen capable of causing sporadic cases of infection and epidemics in both pigs and humans, the global surveillance of S. suis is very important in order to better understand the epidemiology of this bacterial species.49 Though different methods based on DNA have been used for the surveillance of S. suis, these methods are effective for short-term epidemiology only as they are based on non-characterized genomic differences between isolates.49 In contrast, MLST distinguishes a large number of genotypes while using genetic variations that accumulate very slowly, in housekeeping genes, and has allowed global and long-term epidemiology for many important meningitis-causing bacteria by determining the STs present within a population.49,50
In 2002, King et al.51 established a model of MLST for S. suis using seven different house-keeping genes (cpn60, dpr, recA, aroA, thrA, gki and mutS). Since being established, this MLST model has been used by multiple laboratories throughout the world to determine the STs of S. suis strains isolated from pig and human cases of infection. When used alongside serotyping, MLST allows gathering further information about the genetic diversity of the S. suis strains within the different serotypes. The popularity of this typing method for S. suis continues to increase due to the ease in carrying out the technique (PCR and sequencing) and due to the fact that the data are transferrable and comparable from one laboratory to another. Thanks to the S. suis MLST Database, the global distribution of the different S. suis STs can be shared and compared. When the MLST data take into consideration the serotypes, it is important that a trustable and complete serotyping system has been applied to the field strains; otherwise, the final data are difficult to interpret. More recently, studies have begun combining data obtained from MLST with the presence or absence of different S. suis virulence-associated markers at the gene and protein levels including the suilysin (SLY, encoded by the sly gene), muramidase-released protein (MRP, encoded by the mrp gene), extracellular factor (EF, encoded by the epf gene) and different pili in order to compare STs data with phenotypic characteristics.52,53
METHODOLOGY USED IN THE PRESENT REVIEW
In the present review, literature searches were completed using the following databases: Medline (PubMed; http://www.ncbi.nlm.nih.gov/pubmed/), Web of Science (http://thomsonreuters.com/web-of-science-core-collection/) and Science Direct (http://www.sciencedirect.com/). Relevant articles in English were used, while references in other languages were considered when available. MLST results were obtained from the S. suis MLST Database (http://ssuis.mlst.net/). Regarding the distribution of serotypes, the results reported for clinical cases in pigs correspond only to those where serotyping was carried out using antisera and not PCR, while for human cases, reports where serotyping was performed by either antisera or PCR (for serotypes 2 and 14) are both reviewed. This consideration is primarily based on the fact that serotype 1 and serotype 1/2 isolates have not yet been recovered (or confirmed) from human cases of infection. It is important to note that when the correlation between STs and serotypes are reported in this review, it is impossible to confirm the methodology used for the serotyping for many cases.51 We decided to take the data into consideration, especially for those included in the S. suis MLST Database, since the number of cases used for this review would otherwise have been highly limited. For the distribution of STs, the ST complexes represent hyperinvasive lineages to which two or more STs belong as a result of genetic proximity and are based on the MLST phylogeny diagram presented by Lachance et al.54 STs considered to be unrelated to another ST according to this phylogeny diagram are identified as being ‘unrelated' to any known ST complex. With the exception of the serotype distribution of S. suis human cases, which comprises all cases reported since first described in 1968, distribution of serotypes for clinical cases in pigs and distribution of all STs are based on data published between 1 January 2002 to 31 December 2013.
WORLDWIDE DISTRIBUTION OF SEROTYPES
Diseased pigs
In order to fully appreciate the prevalence of human infections and the zoonotic potential of S. suis, one must understand the situation at the farm level since transmission from diseased pigs or pork-derived products is a prerequisite for infection of humans. The data compiled from studies since 1 January 2002 are shown in Table 1.
Table 1. Worldwide distribution of serotypes for reported clinical S. suis cases of infection in pigs by country from 1 January 2002 to 31 December 2013.
| Country | Clinical cases | Predominant serotypesa (frequency in %) | Reference | ||
|---|---|---|---|---|---|
| Worldwide | 4711 | 2 (27.9%) | 9 (19.4%) | 3 (15.9%) | |
| North America | 3162 (67.1%) | 2 (24.3%) | 3 (21.0%) | 1/2 (13.0%) | |
| Canada | 3 065 | 2 | 3 | 1/2 | 25, 29 |
| United States | 97 | 3 | 2 | 7 | 55 |
| South America | 125 (2.7%) | 2 (57.6%) | 1/2 (9.6%) | 14 (8.8%) | |
| Brazil | 125 | 2 | 1/2 | 14 | 56,57 |
| Asia | 659 (14.0%) | 2 (44.2%) | 3 (12.4%) | 4 (5.6%) | |
| Mainland China | 639 | 2 | 3 | 4 | 30,58–60 |
| South Korea | 20 | 3 | 4 | 2, 8, 22 | 61 |
| Europe | 765 (16.2%) | 9 (61.0%) | 2 (18.4%) | 7 (6.7%) | |
| Netherlands | 99 | 9 | 2 | 7 | 62 |
| Spain | 666 | 9 | 2 | 7 | 63–65 |
Only the three most predominant serotypes, which were identified by co-agglutination (or an equivalent method using reference antisera), are shown in this table.
While S. suis is part of the normal microflora of pigs and many studies have focused on carriage in healthy pigs, these studies were not included in this review since the serotype distribution in healthy carriers greatly differs from that of clinical strains, with serotype 2 being much less frequently isolated.65,66,67,68,69,70,71 In addition, since S. suis is a normal inhabitant of the upper respiratory tract,6 most of these studies identified only a small part of the real population of each carrier. Finally, in the case where species-specific PCR tests were not used, many of the ‘untypable' strains detected probably did not belong to the S. suis species. The contribution of these commensal strains to the risk posed to humans in close-contact with pigs or with pork-derived products remains to be further studied14,72,73 and seems to occur particularly in immunocompromised patients. The situation may be different when animals are not really ‘healthy carriers' but rather convalescent animals, carrying in their tonsils virulent strains. However, this situation is almost impossible to define in field studies.
During the last 12 years, more than 4500 serologically confirmed strains recovered from diseased pigs have been reported (Table 1). Globally, the predominant S. suis serotypes isolated from clinical cases in pigs are, in decreasing order, serotypes 2, 9, 3, 1/2 and 7, along with 15.5% of the strains being non-typable by serotyping. However, there is a clear geographical effect on the distribution of serotypes (see below) and these figures are influenced by the number of published studies.
Almost 70% of studies on worldwide isolates recovered from diseased pigs are from North America (Table 1). Nevertheless, this is not an indication of a higher number of cases but simply of a higher number of published reports and studies. In fact, 97% of North American data are from Canada and the rest from the United States, with no data from Mexico. In North America, serotype 2 is the most prevalent in Canada, while in the United States, it is serotype 3. Meanwhile, the second most prevalent serotype in Canada is serotype 3 and is serotype 2 in the United States. However, only slight differences in percentages have been observed in both countries, demonstrating a similar distribution when the data are combined: both serotypes 2 and 3 are the two most prevalent serotypes isolated from clinical pig cases in North America with 24.3% and 21.0% of prevalence, respectively, followed by serotypes 1/2, 8 and 7. Similar distributions of serotypes in Canada and the United States may be explained by a fluid movement of animals between the two countries.
In South America, only two studies have been published, both from Brazil, which report serotype 2 as being the most prevalent with a mean of 57.6% of cases, followed by, in decreasing order of prevalence, serotypes 1/2, 14, 7 and 9 (Table 1). Interestingly, although no clinical cases in pigs have been published, human cases have been reported in other South American countries (see below).74,75,76,77 On the other hand, no human cases have yet been reported in Brazil.
In Asia, where the vast majority of human cases have been reported (see below), studies on clinical cases in pigs account for only 14.0% of the data available and are provided from China and South Korea only. The most prevalent serotypes in infected pigs are, in decreasing order of prevalence, serotypes 2, 3, 4, 7 and 8 (Table 1). The South Korean study surprisingly reported serotypes 3 and 4 as being predominant, followed by serotype 2 at a low prevalence of only 8.3%. This anomaly may be due to the fact that the paper reported a relatively low number of cases (only 20 isolates) from animals with acute polyserositis only.61 Even more disturbing is the fact that the information available regarding the status of S. suis infections in pigs in Asia is the most scarce of the three continents, while this is the continent where cases of zoonosis occur the most frequently in the general population. Although many hospitals and microbiologists in both Thailand and Vietnam are aware of S. suis infections when it comes to diagnosis,2 the four veterinary studies published from these countries investigated healthy or slaughterhouse pigs only.69,70,78,79 Similarly, there have been 10 human S. suis cases reported in Japan between 1994 and 2009, but no complete study on the distribution of isolates from clinically ill pigs including all serotypes has been recently published. In fact, data from the last epidemiological studies in Japan date back to between 1987 and 1991, more than 20 years ago, which predate the human cases reported in this country and illustrate the necessity to gather data in order to increase our knowledge of the current epidemiological situation.80 In Hong Kong, where S. suis has been reported as the third most common culture-confirmed cause of community-acquired bacterial meningitis, there have been 47 human cases from 1983 to 2001 and 21 from 2003 to 2005,12 yet there is a complete absence of epidemiological data regarding S. suis in pigs, with the exception of two studies investigating the prevalence of S. suis in pork meat sold in wet markets.81,82 In Singapore, the Philippines, Laos and Cambodia, where human cases have occurred during the last decade (Table 2), there are no data available on the epidemiology of S. suis infections in pigs.
Table 2. Worldwide distribution of reported clinical S. suis cases of infection in humans by country until 31 December 2013.
| Reported cases | Confirmed serotypesa | Unconfirmedc or unknown serotypesd | ||||
|---|---|---|---|---|---|---|
| Country | 2 | 14 | Othersb | Reference | ||
| Worldwide | 1642 | 1227 (74.7%) | 33 (2.0%) | 5 (0.3%) | 377 (23.0%) | |
| North America | 8 (0.5%) | 7 (87.5%) | 1 (12.5%) | 0 | 0 | |
| Canada | 5 | 4 | 1 | — | — | 7,20,94,95 |
| United States | 3 | 3 | — | — | — | 96–98 |
| South America | 9 (0.5%) | 2 (22.2%) | 0 | 1 (11.1%) | 6 (66.7%) | |
| Argentina | 4 | 1 | — | 1 | 2 | 74,77,99,100 |
| Chile | 4 | — | — | — | 4 | 75,76 |
| French Guiana | 1 | 1 | — | — | — | 101 |
| Asia | 1481 (90.2%) | 1133 (76.5%) | 29 (2.0%) | 3 (0.2%) | 316 (21.3%) | |
| Cambodia | 13 | 13 | — | — | — | 102 |
| Mainland China | 245 | 245 | — | — | — | 16,103–107 |
| Hong Kong | 69 | 53 | — | — | 16 | 12,82,108–113 |
| Japan | 11 | 10 | — | — | 1 | 114–118 |
| Laos | 1 | — | — | — | 1 | Unpubl.e |
| Philippines (United States) | 1 | — | — | — | 1 | 119 |
| Singapore | 3 | — | — | — | 3 | 120–122 |
| South Korea | 4 | — | — | — | 4 | 123–126 |
| Taiwan | 7 | 2 | 2 | — | 3 | 21,22,127 |
| Thailand | 553 | 292 | 21 | 2 | 238 | 11,13,23,26,45,73,128–143 |
| Vietnam | 574 | 518 | 6 | 1 | 49 | 10,72,144–149 |
| Europe | 140 (8.5%) | 84 (60.0%) | 3 (2.1%) | 1 (0.7%) | 52 (37.1%) | |
| Austria | 1 | — | — | — | 1 | 150 |
| Belgium | 4 | 3 | — | — | 1 | 151–154 |
| Croatia | 2 | — | — | — | 2 | 44 |
| Denmark | 8 | 6 | 1 | — | 1 | 9,155–159 |
| France | 19 | 8 | — | — | 11 | 160–177 |
| Germany | 9 | 8 | — | — | 1 | 19,92,178–184 |
| Greece | 2 | — | — | — | 2 | 185,186 |
| Ireland | 1 | — | — | — | 1 | 187 |
| Italy | 3 | 2 | — | — | 1 | 188–190 |
| Netherlands | 51 | 39 | 1 | 1 | 10 | 14,191–199 |
| Poland | 1 | — | — | — | 1 | 200 |
| Portugal | 1 | — | — | — | 1 | 201 |
| Serbia | 5 | — | — | — | 5 | 202 |
| Spain | 13 | 4 | — | — | 9 | 203–214 |
| Sweden | 1 | 1 | — | — | — | 215 |
| United Kingdom | 19 | 13 | 1 | — | 5 | 216–230 |
| Oceania | 4 (0.2%) | 1 (25.0%) | 0 | 0 | 3 (75.0%) | |
| Australia | 3 | — | — | — | 3 | 231,232 |
| New Zealand | 1 | 1 | — | — | — | 91 |
Serotypes were identified by co-agglutination (or an equivalent method using reference antisera) or by PCR specific reaction for serotype 2 (and 1/2) or for serotype 14 (and 1).
Serotypes other than serotypes 2 and 14. See main text and Table 3 for details.
Serotypes were determined based on biochemical identification so are reported as ‘unconfirmed'.
Strain serotype was not mentioned in the publication and is thus considered as ‘unknown'.
Wertheim H, unpublished data (2014).
Important pig producing European countries, such as Denmark, Belgium, France, Germany, Italy and the United Kingdom, have not recently reported the distribution of serotypes recovered from clinical cases in pigs: the last reports from these countries regarded strains isolated between 1990 and 2000.66,83 Before the year 2000, serotype 2 was the most common serotype recovered in Italy, France and Spain, whereas serotype 9 was more frequently found in the Netherlands, Germany and Belgium.76 This lack of information is of high importance. The only two countries with more recent data are Spain and the Netherlands. In fact, in Spain, serotype 2 is no longer the most prevalent serotype, but the second behind serotype 9, followed by serotypes 7, 8 and 3.63,64,65 In the Netherlands, between 2002 and 2007, serotype 9 was still the most prevalent, followed by serotypes 2, 7, 1 and 4.62 Although serotype 9 is the most prevalent serotype in Spain and in the Netherlands and was also prevalent in many European countries before the year 2000, no human cases associated with this serotype have yet been reported. Taken together, these two European countries provide a relatively similar serotype distribution with serotypes 9, 2, 7, 8 and 3 in order of importance (Table 1). In studies previous to 2002, serotype 1 also appeared to be prevalent in countries such as Belgium and the United Kingdom;76 however, it is not clear if this is the current situation. The situation is similar in Denmark where only serotype 7 isolates were characterized during the last twelve years and where the last serological study regarded isolates recovered in 1995–1996.84,85 For the time period covered in this review, two reports, from France and Italy, have been published, although the data from none of these was considered since one of them used serotyping by PCR, detecting serotypes 1 (and 14), 2 (and 1/2), 7 and 9 only and, thus, did not meet the inclusion criterion (see above), while the other studied the distribution of serotypes in healthy animals.67,86 Interestingly, human cases have emerged in other European countries in many of which few or no studies on S. suis isolation from diseased pigs have been conducted. In Serbia, a recent prevalence study was published using isolates from swab samples taken from dead animals, clinically healthy pigs, slaughterhouse pig carcasses and butchers' knives, but it was impossible to associate corresponding serotypes to the diseased animals only.87 In Croatia, only one study pertaining to the antimicrobial susceptibility of S. suis type 2 isolates was published,88 but no epidemiological studies on the distribution of serotypes is available. Moreover, in Greece and Poland, where human cases have been reported, there are still no pig data available. As such, there is an urgent need to evaluate fresh data on the prevalence of S. suis in clinical isolates from pigs in Europe where many countries are among the more important pig producers in the world.
Finally, there is also a lack of relatively recent serotyping data from Australia and New Zealand. In Australia, where three human cases have been recently reported, the studies concerning serotype distribution in pigs predate these human cases, thus showing that there were once active surveillance studies in this country.89,90 One human case of S. suis was also reported in New Zealand,91 but no recent studies regarding isolates recovered from diseased pigs have been published in this country.
In summary, there are countries where the serotype distribution of field strains recovered from diseased pigs has been systematically undertaken during the last 12 years, generating data that may influence any analysis of the worldwide distribution of serotypes. There is an urgent need for new data from European countries on the serotype distributions of S. suis strains isolated from diseased pigs, especially considering the fact that currently available vaccines are bacterins, which are supposed to confer serotype-specific protection. Some countries with an important pig production, such as Brazil, have few studies on pig disease and no human cases declared. Other countries commonly report human cases, but data from diseased animals are almost absent.
Human cases
One of the goals of this review was to compile the serotype distribution of S. suis infections in humans, which has not been completed since 1989,92 and to update the total number of cases from recent reviews on human cases, all of which are excellent comprehensive reviews regarding the clinical features of S. suis infections in humans.3,18,93 In the present study, and differently from clinical pig isolates, serotype 2 and 14 isolates were considered to be as such even if identified by PCR, which is unable to differentiate them from serotypes 1/2 and 1, respectively. This consideration is primarily based on the fact that serotype 1 and serotype 1/2 isolates have not yet been recovered (or confirmed) from human cases. A total of 1642 cases have been reported worldwide as of 31 December 2013 (Table 2).
Of these cases, serotype 2 was the most frequently reported with 74.7%, followed by serotype 14 with 2.0%. It is important to note that 262 (21.5%) serotype 2 strains and two (6.0%) serotype 14 strains were identified by PCR only. The most striking aspect regarding human cases of infection is that 377 cases (23.0%) of all reported cases either do not specify the serotype in the case report or employed a method for serotype identification that was judged inadequate (‘biochemical serotyping'). Even though many of these reports claim to be caused by serotype 2, which is probably true, serotyping, or at the very least PCR, should be performed if the strains are still available in order to increase our knowledge of S. suis infections in humans. Meanwhile, the remaining five human cases of infection were caused by the following serotypes: 4,14 5,133 16,146 2177 and 24.133 Since 2011, human S. suis cases of infection have been reported for the first time in Cambodia, Chile, French Guiana, Poland and South Korea.
The vast majority of human cases have occurred in Asia, which account for more than 90% of all reported cases, particularly in Vietnam, Thailand and China. These three countries alone account for 83.6% of all cases worldwide. However, in China, almost all cases were described during the 1998 and 2005 outbreaks.16,105 In East and Southeast Asia, S. suis zoonosis should be considered endemic due in part to the high density of pigs, relatively high number of backyard-type production farms, slaughtering practices with the use of few preventive measures, presence of wet markets and consumption of ill pigs and/or consumption of uncooked or undercooked pork products.3 In Thailand, most of the reported cases were caused by serotype 2, while serotype 14 is the second highest, the latter accounting for 21 cases of meningitis and sepsis out of a total of 530 cases. However, infections by other serotypes, such as a serotype 5 peritonitis and a serotype 24 sepsis, have also been reported. In Vietnam, where S. suis is now the most frequent cause of adult bacterial meningitis,10 most cases are also due to serotype 2, six cases of meningitis were caused by serotype 14, and one case of peritonitis by serotype 16. The diversity of infections caused by serotypes other than serotype 2 could be explained by the awareness of diagnosticians to S. suis infections. Consequently, this could also explain why Vietnam and Thailand have the largest number of reported cases and a higher probability of encountering atypical cases on a more regular basis. It is also interesting that the patients who suffered infections by serotypes 5, 16 and 24 were also suffering from cirrhosis: a likely explanation is that these infections were the result of immunocompromisation. The compilation of human cases in China includes sporadic cases and the two S. suis serotype 2 epidemics, the first in Jiangsu province in 1998, where 14 deaths out of 25 cases occurred,16 and the second in Sichuan province in 2005, where 38 deaths out of 215 cases were reported. This second epidemic remains the largest outbreak of S. suis in humans.105 These epidemics were unprecedented for S. suis infections in humans considering the high incidence of systemic disease, the low number of cases of meningitis, and the high rate of mortality observed.1 The patients presented cases consisting of either sepsis, meningitis, or STSLS, based on the presence of the following symptoms: sudden onset of high fever, diarrhea, hypotension, blood spots and petechial, clear erythematous blanching rash and dysfunction of multiple organs, such as acute respiratory distress syndrome, liver and heart failure, disseminated intravascular co-agulation, and acute renal failure.1 Even though China has the largest pig production in the world and is the site of the 1998 and 2005 outbreaks that attracted much attention from the scientific community, the number of reported cases is considerably lower than in Thailand and Vietnam. One possible explanation is that dishes containing raw pork and blood, very popular in Thailand and Vietnam, are uncommon in China. In fact, since the last outbreak in 2005, only four other sporadic human cases were reported in mainland China.107,113
Human cases were also reported in Cambodia, Hong Kong, Japan, Laos, Singapore, South Korea and Taiwan. It is of interest to note that in Hong Kong, S. suis serotype 2 was reported as the third most frequent cause of adult bacterial meningitis.12,108 There is also a human case of infection from the Philippines, although it was not officially reported in this country. In fact, even though the infection was diagnosed as S. suis serotype 2 meningitis and treated in the United States, the patient had just returned from a seven-month vacation in the Philippines where he had consumed raw pork, making this an Asian case and considered as such in this study.119
The second continent where most human infections have been described is Europe, accounting for 8.5% of all reported human cases (Table 2), with approximately 71.4% of all European human cases occurring in countries with a highly developed pig industry: the Netherlands, United Kingdom, France and Spain. Cases were also reported in Austria, Belgium, Croatia, Denmark, Germany, Greece, Ireland, Italy, Poland, Portugal, Serbia and Sweden. Surprisingly, no cases have yet been reported in Russia, a country with an increased developing pig production. Human cases of S. suis infection were reported for the first time in Europe, with the first ever case in Denmark, in 1968. It is interesting to note that all cases reported before 1983 were from Western Europe.9,108 Only two countries consider S. suis infections in humans as an industrial disease: the United Kingdom and France since 1983 and 1995, respectively.18 This recognition led to legislation and regulations which may have contributed to reducing the number of cases in both of these countries, with the last human case in the United Kingdom being reported in 2001. In France, although few cases have been reported since 1995, most were related to wild boar hunters. It is known that wild boars also carry S. suis.163 Consequently, hunters should be aware of the necessary precautions when preparing the carcasses, which present the greatest risk.176,177 Most human cases of infection contracted from wild boars were serotype 2, with two cases of meningitis caused by serotypes 4 and 14 in the Netherlands, one case of meningitis by serotype 14 in Denmark, and one case of septicemia by serotype 14 in the United Kingdom.14,158,226
Although being the continent with the highest number of S. suis infection reports from diseased pigs (Table 1), only a few sporadic cases of S. suis infection in humans have been reported in North America. As previously mentioned, the isolation rate of S. suis serotype 2 from diseased pigs is considerable lower than that of Europe or Asia. The lower number of pig cases of disease due to this serotype may be a reason for the lower number of human cases of infection. It has been suggested that S. suis strains of serotype 2 from Canada and the United States present lower virulence properties than those from Europe and Asia.54 For these two countries, confirmed human cases were all serotype 2, with the exception of one serotype 14 case of meningitis in Canada.95 In the United States, two of the cases were from the continent while the third was from Hawaii, an island in the Pacific Ocean importing pigs from Asia. While the case in Hawaii is considered American (unlike the case from the Philippines), it would perhaps be more appropriate to compare the features of this infection with those from Asia. In fact, the strain recovered from Hawaii presents phenotypic characteristics typical of Asian strains (Gottschalk M, unpublished data, 2014).
Sporadic cases were also reported elsewhere throughout the world, such as those in South America (Argentina, Chile and French Guiana), and Oceania (Australia and New Zealand), but when combined, they only account for 0.8% of all reported cases. As was shown by Wertheim et al.,3 sporadic cases of S. suis infections in humans are encountered in most regions where pig rearing is important, with notable exceptions being Mexico and Brazil. In the case of Mexico, even though one study evaluated upper respiratory tract colonization of slaughterhouse workers233 (see below), no official reports on clinical pig cases or human infections are available, which is alarming, knowing full well that in previous studies, clinical strains of S. suis isolated from pig and human cases were included.83,234 Despite the lack of available reports, this infection is very common in Mexican farms (Gottschalk M, unpublished data, 2014). As such, action is required in order to remedy the troubling situation in this country which has well-developed veterinary diagnostic laboratories. It remains to be seen if the absence of human cases is due to a lack of awareness of S. suis as a zoonotic pathogen in these regions when diagnosing bacterial infections, or if these strains are of lower virulence, as with some strains from Canada and the United States.
Table 3 shows the frequency of the clinical manifestations of infection by confirmed serotype, illustrating that the serotypes 2 and 14 are involved in similar proportions in meningitis (50%–70%) and septicemia (20%–25%). Septic shock was defined as a category comprising sepsis, septicemia, bacteremia and/or STSLS, while meningitis comprises all meningeal-related symptoms. Serotype 2 infections also represented 2.9% of the cases where other afflictions where diagnosed such as endocarditis, septic arthritis, pneumonia, peritonitis, pulmonary edema, myocarditis and other infections. Serotypes 4 and 21 were isolated from cases of meningitis, serotypes 5 and 16 from cases of peritonitis, and serotype 24 from a case of sepsis.
Table 3. Clinical manifestations of reported clinical S. suis cases of infection in humans by serotypes confirmed by co-agglutination.
| Confirmed serotypes | Reported cases | Clinical manifestations | |||
|---|---|---|---|---|---|
| Meningitis | Septic shocka | Otherb | Unknownc | ||
| 2 | 867 | 590 (68.1%) | 233 (26.9%) | 25 (2.9%) | 19 (2.2%) |
| 14 | 28 | 14 (50.0%) | 6 (21.4%) | — | 8 (28.6%) |
| 4 | 1 | 1 | — | — | — |
| 5 | 1 | — | — | 1 | — |
| 16 | 1 | — | — | 1 | — |
| 21 | 1 | 1 | — | — | — |
| 24 | 1 | — | 1 | — | — |
Septic shock includes bacteremia, sepsis, septicemia and streptococcal toxic shock-like syndrome cases.
Other clinical manifestations such as endocarditis, septic arthritis, pneumonia, peritonitis, pulmonary edema, myocarditis, etc.
Clinical manifestations were either not specified or could not be associated with a particular serotype.
Why reported strains isolated from human cases are almost exclusively serotypes 2 and 14 is puzzling. While the serotypes 3, 9 and 1/2 are among the most highly prevalent in diseased pigs after serotype 2, at least in North America, no human infections due to these serotypes have yet been reported in any country. These differences in virulence between pig and human isolates may be partially due to serotype-specific CPS structural features, for which the CPS structures are still unknown, except for serotypes 2 and 14, and/or due to other serotype-specific bacterial factors involved in the pathogenesis. We could speculate that the serotypes 2 and 14 are more virulent than the other serotypes based on the observation that three out of the five cases caused by other serotypes (5, 16 and 24) occurred in patients suffering from cirrhosis, which in turn may have immunocompromised them and made them susceptible to serotypes uncommon in human infections.133,146 These differences could also be explained by varying serotype-specific colonization abilities. However, many cases caused by serotypes 2 and 14 have also been described in individuals with predisposing conditions. More research is required in order to understand how differences in virulence of S. suis strains occurs and if these differences can be associated with specific serotypes.
Subclinically infected humans: zoonotic potential to workers?
Subclinically infected pigs are carriers of S. suis mainly in the tonsils.6 However, this information is not clearly available for humans. Only a handful of studies have investigated S. suis subclinical infections in humans as displayed in Table 4, either by serology (antibodies) or by bacterial detection.
Table 4. Studies exploring human carriage and exposure to S. suis in risk groups.
| Study (reference) | Technique employed/target | Risk groups | Positive carriage or exposure (positive/tested) | Serotypes found |
|---|---|---|---|---|
| Serological | ||||
| New Zealand, 1989235 | Indirect ELISA/serotype 2 whole bacteria | Pig farmers | 15/70 (21.4%) | — |
| Meat inspectors | 11/107 (10.3%) | |||
| Dairy farmers | 9/96 (9.4%) | |||
| Netherlands, 1999236 | Western blot/serotype 2 MRP/EF | Veterinarians | MRP: 6/100 (6.0%) | — |
| EF: 2/100 (2.0%) | ||||
| Pig farmers | MRP: 2/190 (1.1%) | |||
| EF: 1/190 (0.5%) | ||||
| United States, 2008237 | Indirect ELISA/serotype 2 whole bacteria | Pig workers | 7/73 (9.6%) | — |
| Upper respiratory tract colonization (isolation) | ||||
| Italy, 1989238 | Tonsil swabs | Slaughterhouse workers | 2/10 (20%) | 2 |
| Mexico, 2001233 | Tonsil swabs | Slaughterhouse workers | 4/69 (5.8%) | 2, 27, NTa |
| Germany, 2002181 | Pharyngeal swabs | Slaughtering and meat processing workers | 7/132 (5.3%) | 2 |
Non-typable strain (NT).
Three of these were serological studies, with two using an indirect enzyme-linked immunosorbent assay (ELISA) with S. suis serotype 2 whole bacteria (formalin-inactivated or not) as antigen (Table 4). In New Zealand, the authors examined sera from 70 pig farmers, 96 dairy farmers, 107 meat inspectors and 16 veterinary students.235 Twenty-one percent of pig farmers, 10% of meat inspectors and 9% of dairy farmers (most of them also raising pigs on their farms) were positive for ‘anti-S. suis serotype 2 antibodies'. It is impossible to be certain if these titers were specific for serotype 2 since cross-reacting antibodies with other S. suis serotypes and/or with other bacterial species were probably detected. Antibody titers were associated with longer occupational exposure for both pig farming and meat inspection. In the United States, using a similar antigen, sera from 73 pig-exposed and 67 non-pig-exposed adults from Iowa were titrated. Ten percent of pig-exposed workers were found to be positive compared to only one positive individual (1.5%) from the non-pig-exposed group.237 Once again, the authors concluded that positive titers were associated with either working with pigs or living on a pig farm for more than 10 years, and also found that study participants who worked with both finishing and nursery pigs had 8.8 times the odds of having a positive titer when compared to non-exposed participants. The third serological study, from the Netherlands, is interesting since the authors chose to perform Western blot analysis, with higher specificity, targeting two virulence-related markers of S. suis serotype 2 that are widely present among virulent strains in that country, the MRP and EF proteins, in sera from 102 veterinarians and 191 pig farmers.236 Results showed that 6% of veterinarians were positive for anti-MRP and 2% were positive for anti-EF, while 1% of pig farmers were positive for anti-MRP and 0.5% were positive for anti-EF. It is important to note that these antigens have not been reported to be present in bacterial species other than S. suis. These three serological studies, while the titers obtained cannot be directly compared, illustrate the zoonotic potential of S. suis based on the presence of antibodies possibly generated through long-term exposure and/or exposure-related subclinical infections. It should be noted that the presence of antibodies does not guarantee a carriage status for positive individuals.
In addition to these serological studies, three studies have directly focused on carriage in the upper respiratory tract of humans by trying to isolate S. suis from pharyngeal or tonsil swabs of pig-exposed workers (Table 4). In Italy, 10 volunteer slaughterhouse workers were swabbed and two were found to be positive for S. suis serotype 2.238 In Mexico, the tonsils from 69 slaughterhouse workers were swabbed and four were found to be positive.233 Of the four strains isolated, one was serotype 2, another was serotype 27 and two were non-typable by serotyping. The virulence of these four strains was also evaluated in mice and it was determined that three of them were mildly virulent (30%–80% mortality) and one of the non-typable strains was highly virulent (70%–90% mortality). Finally, in Germany, the tonsils from 132 meat workers (from either a slaughterhouse or meat processing factory) and 140 controls were swabbed and seven workers were found to be positive carriers of S. suis serotype 2, while none of the controls showed positive results. For the seven workers found to be positive carriers, four were retested 3 weeks later and were confirmed to still be positive carriers, indicating that the bacteria probably remained in the tonsils of healthy individuals for a relatively long period of time, which was also shown to be the case in pigs.6 However, since these individuals were continuously exposed to pork products, repeated recolonization could not be ruled out.2 All of the positive workers were employed for an average of 9.9 years ranging from three to 21 years. These three studies regarding the nasopharyngeal carriage all report positive samples in pig-related meat workers and none in the population without occupational exposure to pig. It is also very important to note that of the 13 strains isolated from healthy workers, 84.6% were serotype 2.
Taken together, these studies illustrate the zoonotic potential through long-term exposure, either by demonstrating the presence of antibodies towards S. suis or by isolating S. suis from tonsils or pharynx in pig-exposed workers. It must be noted that the positive rates in the studies of carriers of the upper respiratory tract are probably underestimated since detection of the pathogen was achieved using isolation methods, which are considered as having low sensitivity. Studies using molecular techniques (species-specific PCR) would probably present a significantly higher sensitivity. Exposure to S. suis may lead to subclinical infections, induction of antibodies, serious disease, as was the case in the human cases discussed previously, or just an insignificant and transitory atypical colonization of the mucosal membranes within the respiratory route.110,181 The annual risk of developing meningitis due to S. suis in the Netherlands was estimated to be 3.5/100 000 for slaughterhouse workers, 2.7/100 000 for pig breeders and 1.2/100 000 for butchers.14 Compared to the non-exposed general population (0.002/100 000), the risk for slaughterhouse workers is 1500 times higher. In the United Kingdom, the risk for butchers is even higher.239 The annual incidence for the occupational group (direct contact with pigs) in Hong Kong was 32/100 000, 350 times higher than that of the general population (0.09/100 000) and 30 times higher than the homologous group in the Netherlands.82 In addition, a less dramatic difference between the occupational group and the general population was observed in Hong Kong (350 times higher in Hong Kong versus 1500 times in the Netherlands). All these differences may be due, at least in part, to a closer contact between people and pork carcasses in Asia.2 Also, a case–control study conducted in Vietnam from 2006 to 2009 showed that eating high risk dishes common in Vietnam, such as fresh (Tiẽt Canh) or undercooked blood, tonsils, tongue, stomach, intestines and uterus from pigs within 2 weeks prior to admission was the most significant risk factor for S. suis infection, followed by exposure to pigs, pork products and preparation of pork in the presence of skin lesions within two weeks prior to admission.72 The authors also investigated the presence of S. suis in pork by-products collected from slaughterhouses and wet/retail markets in Vietnam, and found an 11% contamination rate with S. suis serotype 2 in internal organ samples. Unfortunately, they were unable to demonstrate S. suis carriage rates in 1022 healthy individuals or patients without S. suis infection using a PCR targeting S. suis serotype 2 and 1/2. A PCR for the detection of S. suis may have been more sensitive and could possibly have identified potential carriers of other serotypes.233 The authors suggested that their results, which include six positive rectal swabs from patients, strengthen the hypothesis that the gastrointestinal tract may be a route of entry for S. suis, which is in agreement with cases following the ingestion of raw pork meat. Similarly, data from Thailand suggest a high incidence rate (6.2/100 000) of S. suis infection in the general population in 2010, mostly related to consumption of raw pork products.130
WORLDWIDE DISTRIBUTION OF SEQUENCE TYPES
Global distribution of sequence types
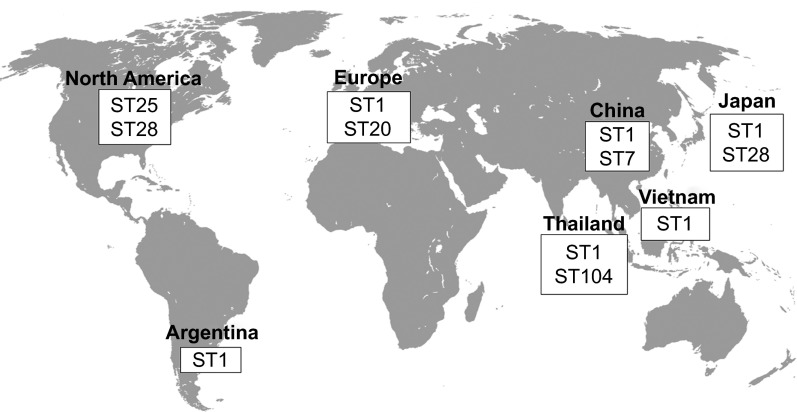
As presented in Figure 1, different serotype 2 STs predominate in different regions of the world. The ST1 is mostly associated with disease in both pigs and humans in Europe (though the ST20 is important in the Netherlands), Asia (Cambodia, mainland China, Hong Kong, Japan, Thailand and Vietnam) and Argentina (Tables 5 and 6). Meanwhile, the ST7, responsible for the 1998 and 2005 epidemics, is mostly endemic to mainland China (Tables 5 and 6). North American cases greatly vary from those in Eurasia, with most strains being either ST25 or ST28, two STs also recovered in Thailand and Japan, respectively (Tables 5 and 6). Finally, the ST101 to 104 are endemic to Thailand and appear to be more and more commonly isolated from human cases, especially the ST104 (Table 6). As such, it can be easily observed that the current distribution of the S. suis serotype 2 STs greatly varies throughout the world, though data have only been available for a little over a decade, from only a few countries and mostly only for the serotype 2.
Figure 1.
Worldwide distribution of the most important S. suis serotype 2 sequence types isolated from both clinical pig and human cases of infection.
Table 5. Determined sequence types of reported clinical S. suis cases of infection in pigs from 1 January 2002 to 31 December 2013.
| Country | Serotypesa | ST | ST complex | Number of cases | Reference |
|---|---|---|---|---|---|
| North America | |||||
| Canada | 2 | 1 | 1 | 2 | 240 |
| 25 | 25 | 28 | 52,240 | ||
| 28 | 28 | 18 | 52 | ||
| 145 | 25 | 1 | MLST Database | ||
| 23 | 80 | Unrelated | 1 | 51 | |
| 24 | 68 | Unrelated | 1 | 51 | |
| 25 | 69 | Unrelated | 1 | 51 | |
| 27 | 72 | Unrelated | 1 | 51 | |
| 28 | 75 | Unrelated | 1 | 51 | |
| 29 | 92 | Unrelated | 1 | 51 | |
| 30 | 77 | Unrelated | 1 | 51 | |
| 31 | 70 | Unrelated | 1 | 51 | |
| Not specified | 13 | 13/149 | Not specified | 51 | |
| United States | 2 | 1 | 1 | 3 | 52 |
| 25 | 25 | 28 | 52 | ||
| 28 | 28 | 33 | 52 | ||
| Europe | |||||
| Denmark | 4 | 54 | 53/54 | 1 | 51 |
| 5 | 53 | 53/54 | 1 | 51 | |
| 6 | 55 | Unrelated | 1 | 51 | |
| 8 | 87 | 87 | 1 | 51 | |
| 9 | 82 | Unrelated | 1 | 51,240 | |
| 10 | 78 | Unrelated | 1 | 51 | |
| 11 | 91 | Unrelated | 1 | 51 | |
| 12 | 74 | Unrelated | 1 | 51 | |
| 13 | 71 | Unrelated | 1 | 51,241 | |
| 16 | 73 | Unrelated | 1 | 51 | |
| Finland | 2 | 37 | Unrelated | 1 | 51 |
| Not specified | 27 | 27 | Not specified | 51 | |
| 38 | Unrelated | 1 | 51 | ||
| France | 2 | 1 | 1 | 4 | 242 |
| 140 | Unrelated | 1 | MLST Database | ||
| 141 | 1 | 1 | MLST Database | ||
| 142 | 28 | 1 | MLST Database | ||
| 143 | 1 | 1 | MLST Database | ||
| 144 | 1 | 1 | MLST Database | ||
| 229 | 25 | 1 | MLST Database | ||
| 230 | 25 | 1 | MLST Database | ||
| 14 | 231 | Unrelated | 1 | MLST Database | |
| 15 | 81 | Unrelated | 1 | MLST Database | |
| Germany | 1/2 | 100 | Unrelated | 1 | 243 |
| 2 | 1 | 1 | 5 | 243 | |
| 25 | 25 | 1 | 243 | ||
| 28 | 28 | 2 | 243 | ||
| 97 | Unrelated | 1 | 243 | ||
| 3 | 95 | Unrelated | 1 | 243 | |
| 7 | 29 | 29 | 7 | 240,243 | |
| 89 | 16 | 1 | 243 | ||
| 9 | 93 | Unrelated | 1 | 243 | |
| 96 | Unrelated | 1 | 243 | ||
| 98 | 16 | 2 | 243 | ||
| 99 | 16 | 1 | 243 | ||
| Italy | 2 | 1 | 1 | 1 | 86 |
| 9 | 138 | Unrelated | 1 | MLST Database | |
| Netherlands | 1 | 1 | 1 | 4 | 62,240 |
| 13 | 13/149 | 2 | 51,62 | ||
| 149 | 13/149 | 1 | 62 | ||
| 156 | 1 | 1 | 62 | ||
| 1/2 | 1 | 1 | 1 | 62,240 | |
| 2 | 1 | 1 | 21 | 62,240 | |
| 20 | 147 | 19 | 62 | ||
| 29 | 29 | 1 | 62 | ||
| 134 | 1 | 1 | MLST Database | ||
| 146 | 1 | 1 | MLST Database | ||
| 3 | 15 | 16 | 1 | 62 | |
| 35 | 27 | 1 | 51 | ||
| 4 | 17 | 147 | 3 | 62 | |
| 7 | 29 | 29 | 5 | 62 | |
| 135 | 29 | 1 | MLST Database | ||
| 136 | 16 | 1 | 62 | ||
| 150 | Unrelated | 1 | 62 | ||
| 153 | Unrelated | 1 | MLST Database | ||
| 218 | Unrelated | 1 | 62 | ||
| 8 | 87 | 87 | 1 | 62 | |
| 198 | 16 | 1 | 62 | ||
| 9 | 1 | 1 | 2 | 62,240 | |
| 15 | 16 | 1 | 62 | ||
| 16 | 16 | 38 | 62 | ||
| 136 | 16 | 2 | 62 | ||
| 148 | 1 | 1 | 62 | ||
| 151 | 16 | 1 | 62 | ||
| 152 | 16 | 1 | MLST Database | ||
| 154 | 16 | 1 | MLST Database | ||
| 155 | 16 | 1 | 62 | ||
| 182 | Unrelated | 1 | 62 | ||
| 184 | Unrelated | 1 | MLST Database | ||
| 189 | Unrelated | 1 | 62 | ||
| 220 | Unrelated | 1 | 62 | ||
| 15 | 81 | Unrelated | 1 | 51 | |
| Not specified | 183 | Unrelated | 1 | MLST Database | |
| Spain | 1 | 1 | 1 | 2 | 244MLST Database |
| 1/2 | 1 | 1 | 2 | 244MLST Database | |
| 28 | 28 | 1 | MLST Database | ||
| 64 | 94 | 1 | 51 | ||
| 2 | 1 | 1 | 31 | 244 | |
| 5 | 1 | 1 | 51 | ||
| 27 | 27 | 1 | MLST Database | ||
| 28 | 28 | 1 | MLST Database | ||
| 86 | 1 | 1 | MLST Database | ||
| 124 | 1 | 1 | 244 | ||
| 3 | 14 | 147 | 1 | 51 | |
| 15 | 16 | 1 | 51 | ||
| 27 | 27 | 1 | MLST Database | ||
| 89 | 16 | 1 | 51 | ||
| 4 | 16 | 16 | 1 | 51,240 | |
| 5 | 17 | 147 | 1 | 51 | |
| 9 | 16 | 16 | 2 | 51,240 | |
| 59 | 123 | 1 | 51 | ||
| 123 | 123 | 8 | 244 | ||
| 125 | 123 | 9 | 244 | ||
| 361 | Unrelated | 1 | MLST Database | ||
| 367 | Unrelated | 1 | MLST Database | ||
| 14 | 1 | 1 | 1 | 244MLST Database | |
| 15 | 65 | Unrelated | 2 | 51 | |
| 27 | 65 | Unrelated | 2 | 51 | |
| United Kingdom | 1 | 1 | 1 | 5 | 240,242MLST Database |
| 12 | 11 | 1 | 51 | ||
| 13 | 13/149 | 1 | 51 | ||
| 1 or 14 | 10 | 1 | 1 | 51 | |
| 2 | 1 | 1 | 37 | 240,242MLST Database | |
| 2 | 1 | 5 | 51 | ||
| 9 | 1 | 1 | 51 | ||
| 25 | 25 | 7 | MLST Database | ||
| 28 | 28 | 5 | MLST Database | ||
| 29 | 29 | 1 | MLST Database | ||
| 30 | 28 | 1 | MLST Database | ||
| 3 | 27 | 27 | 2 | 51 | |
| 29 | 29 | 1 | MLST Database | ||
| 31 | 28 | 2 | 51 | ||
| 33 | 27 | 1 | 51 | ||
| 42 | Unrelated | 1 | 51 | ||
| 4 | 23 | 87 | 3 | 51 | |
| 5 | 39 | Unrelated | 1 | MLST Database | |
| 44 | Unrelated | 1 | 51 | ||
| 7 | 29 | 29 | 6 | MLST Database | |
| 34 | 225 | 1 | 51 | ||
| 83 | 29 | 1 | 51 | ||
| 8 | 1 | 1 | 1 | MLST Database | |
| 9 | 46 | Unrelated | 1 | 51 | |
| 48 | Unrelated | 2 | 51 | ||
| 50 | Unrelated | 1 | 51 | ||
| 11 | 88 | Unrelated | 1 | 51 | |
| 14 | 1 | 1 | 14 | MLST Database | |
| 15 | 43 | 43/52 | 1 | 51 | |
| 45 | Unrelated | 1 | 51 | ||
| 49 | Unrelated | 1 | 51 | ||
| 52 | 43/52 | 1 | 51 | ||
| 16 | 41 | Unrelated | 2 | 51MLST Database | |
| 47 | Unrelated | 1 | 51 | ||
| 28 | 21 | 87 | 1 | 51 | |
| Not specified | 40 | Unrelated | 1 | MLST Database | |
| 51 | Unrelated | 1 | MLST Database | ||
| 90 | Unrelated | 1 | MLST Database | ||
| Asia | |||||
| Mainland China | 2 | 1 | 1 | 49 | 59,245,246 |
| 7 | 7 | 216 | 59,241,242,245–247 | ||
| 25 | 25 | 1 | 246 | ||
| 28 | 28 | 31 | 59,245,246 | ||
| 86 | 1 | 1 | 245 | ||
| 117 | 27 | 27 | MLST database | ||
| 162 | 28 | 1 | 245 | ||
| 223 | 1 | 1 | MLST Database | ||
| 228 | 7 | 1 | MLST Database | ||
| 242 | 1 | 1 | MLST Database | ||
| 244 | 7 | 1 | MLST Database | ||
| 245 | 28 | 1 | MLST Database | ||
| 289 | 1 | 1 | 59 | ||
| 290 | Unrelated | 1 | MLST Database | ||
| 352 | Unrelated | 1 | MLST Database | ||
| 353 | Unrelated | 1 | MLST Database | ||
| 354 | Unrelated | 1 | MLST Database | ||
| 355 | Unrelated | 1 | MLST Database | ||
| 418 | Unrelated | 1 | MLST Database | ||
| 419 | Unrelated | 1 | MLST Database | ||
| 3 | 224 | Unrelated | 1 | MLST Database | |
| 7 | 129 | 29 | 1 | MLST Database | |
| 225 | 225 | 1 | MLST Database | ||
| 335 | Unrelated | 1 | MLST Database | ||
| 420 | Unrelated | 1 | MLST Database | ||
| 9 | 222 | Unrelated | 1 | MLST Database | |
| 226 | 226/227 | 1 | MLST Database | ||
| 227 | 226/227 | 1 | MLST Database | ||
| 239 | 239/241 | 1 | MLST Database | ||
| 241 | 239/241 | 1 | MLST Database | ||
| 417 | Unrelated | 1 | MLST Database | ||
| 11 | 260 | Unrelated | 1 | MLST Database | |
| 263 | Unrelated | 1 | MLST Database | ||
| 13 | 262 | Unrelated | 1 | MLST Database | |
| 27 | 258 | Unrelated | 1 | MLST Database | |
| 31 | 261 | 27 | 1 | MLST Database | |
| 265 | 27 | 1 | MLST Database | ||
| Not specified | 29 | 29 | 1 | 59 | |
| 118 | Unrelated | 1 | 59 | ||
| 156 | 1 | 2 | 59 | ||
| 264 | Unrelated | 1 | MLST Database | ||
| 266 | Unrelated | 1 | MLST Database | ||
| 267 | Unrelated | 1 | MLST Database | ||
| 303 | Unrelated | 1 | MLST Database | ||
| 383 | Unrelated | 2 | MLST Database | ||
| 421 | Unrelated | 1 | MLST Database | ||
| 422 | Unrelated | 1 | MLST Database | ||
| Japan | 1 | 1 | 1 | 1 | 53,248 |
| 2 | 1 | 1 | 5 | 53,248 | |
| 28 | 28 | 48 | 53,248 | ||
| 324 | 28 | 1 | 248 | ||
| 3 | 108 | 94 | 1 | 53 | |
| 117 | 27 | 1 | 53 | ||
| 7 | 29 | 29 | 1 | 53 | |
| 118 | Unrelated | 1 | 53 | ||
| 11 | 108 | 94 | 1 | 53 | |
| Vietnam | 9 | 390 | Unrelated | 1 | MLST Database |
Serotypes were identified by co-agglutination (or an equivalent method using reference antisera), by PCR or, sometimes, by undefined methods.
Table 6. Determined sequence types of reported clinical S. suis cases of infection in humans from 1 January 2002 to 31 December 2013.
| Country | Serotypea | ST | ST complex | Number of cases | Reference |
|---|---|---|---|---|---|
| North America | |||||
| Canada | 2 | 25 | 25 | 3 | 53,242 |
| 14 | 6 | 1 | 1 | 51 | |
| United States | 2 | 1 | 1 | 1 | 53 |
| 25 | 25 | 1 | 98 | ||
| South America | |||||
| Argentina | 2 | 1 | 1 | 1 | Unpubl.b |
| French Guiana | 2 | 1 | 1 | 1 | 101 |
| Europe | |||||
| France | 2 | 20 | 147 | 2 | 53,242 |
| Italy | 2 | 1 | 1 | 1 | 86 |
| 134 | 1 | 2 | 86,188 | ||
| Netherlands | 2 | 1 | 1 | 14 | 62,240 |
| 20 | 147 | 11 | 62,240 | ||
| 134 | 1 | 1 | 62 | ||
| 146 | 1 | 1 | 62 | ||
| 14 | 6 | 1 | 1 | 51 | |
| Spain | 2 | 3 | 1 | 1 | 204 |
| United Kingdom | 2 | 1 | 1 | 1 | 53 |
| 14 | 2 | 1 | 1 | MLST Database | |
| Asia | |||||
| Cambodia | 2 | 1 | 1 | 13 | 102 |
| Mainland China | 2 | 1 | 1 | 11 | 242,247 |
| 7 | 7 | 210 | 242,240,247 | ||
| 14 | 1 | 1 | 1 | 241 | |
| Hong Kong | 2 | 1 | 1 | 14 | 51,249 |
| 9 | 1 | 12 | 51,249 | ||
| 25 | 25 | 1 | 249 | ||
| Japan | 2 | 1 | 1 | 7 | 53,116 |
| 28 | 28 | 1 | 116 | ||
| Thailand | 2 | 1 | 1 | 123 | 53 |
| 25 | 25 | 17 | 53,129 | ||
| 28 | 28 | 4 | 53,129 | ||
| 101 | 225 | 1 | 128 | ||
| 102 | 25 | 2 | 53 | ||
| 103 | 25 | 6 | 53,129 | ||
| 104 | 225 | 45 | 53 | ||
| 126 | 1 | 3 | 129 | ||
| 5 | 181 | Unrelated | 1 | 133 | |
| 14 | 11 | 11 | 1 | 53 | |
| 105 | 1 | 19 | 130,132 | ||
| 127 | 1 | 1 | 132 | ||
| 24 | 221 | 221/234 | 1 | 133 | |
| Vietnam | 2 | 1 | 1 | 56 | 10,240 |
| 107 | 1 | 1 | 10 | ||
| 14 | 105 | 1 | 1 | 10 | |
| 16 | 106 | Unrelated | 1 | 146 |
Serotypes were identified by co-agglutination (or an equivalent method using reference antisera) or by PCR. However, it is impossible to distinguish between serotypes 1 and 14 and serotypes 1/2 and 2 by PCR, so serotypes remain to be confirmed.
Gottschalk M, unpublished data (2014).
Nevertheless, there is an important issue regarding the serotypes attributed to the different strains typed by MLST. Many of the studies, including most of the strains from the S. suis MLST Database, do not specify the method used for serotyping. In other cases, such as the study of King et al.,51 the authors did not necessarily confirm the serotypes of the strains used. As explained above, the use of PCR for serotyping of serotypes 2 and 14 strains is an inappropriate method, especially for clinical pig cases, even though cases serotyped using this method were still considered for this part of the review. As such, in the cases where more than one serotype was identified for a single ST, it is possible that the serotypes were misidentified and remain to be confirmed using reference antisera. Hence, it is important to keep in mind these discrepancies when analyzing the distribution of STs, particularly for the clinical pig cases. On the other hand, if confirmed, it would be extremely interesting to study strains of different serotypes sharing the same ST, since capsular switching has not been clearly demonstrated for this pathogen.
Diseased pigs
In contrast to the serotype distribution of diseased pigs (the section on ‘Diseased pigs' under ‘WORLDWIDE DISTRIBUTION OF SEROTYPES'), results where the serotype was identified by either serological methods, PCR or unidentified methods and where the ST was determined using the MLST method described by King et al.,51 were taken into consideration for the worldwide distribution of the STs of clinical cases in pigs.
In North America, the majority of MLST studies conducted on S. suis strains isolated from diseased pigs have been serotype 2 (Table 5). It was determined that 44% of North American strains are ST25, 51% ST28 and only 5% are ST1.52 In Canada, the proportions of ST25 and ST28 are similar to 54% and 46%, respectively, but in the United States, 75% of strains were shown to be ST28 and only 10% ST25, while the remaining 15% are ST1.52 As with North American S. suis strains, the majority of European studies have been conducted using serotype 2 strains (Table 5). Most of these studies have demonstrated that ST1 strains are predominately isolated from diseased pigs in the Netherlands, Spain and the United Kingdom.51,62,244 King et al. had already associated the serotype 2 ST1 strains with invasive infections.51 Nevertheless, many strains of serotype 9 have also been recovered from diseased pigs and typed.62,244 In both the Netherlands and Spain, serotype 9 isolates were identified as belonging to the ST16, where they represent 43% of strains in the Netherlands.62 Unlike some countries in Europe, where the serotype 9 is as important as the serotype 2, most S. suis strains isolated from diseased pigs in Asia are serotype 2, representing 90% of cases in mainland China.59 However, as mentioned above, there are relatively few reports of isolation from diseased pigs in Asia. Of these serotype 2 cases, the predominant STs are ST1, ST7 and ST28 (Table 5). In mainland China, Chen et al.59 demonstrated that 22% of serotype 2 strains are ST1 and 77% are ST7. Meanwhile, the few ST28 strains recovered in that country were mostly associated with cases of pneumonia.246 Regarding Japan, ST1 and ST28 strains isolated from cases of endocarditis in diseased pigs account for 8% and 76% of serotype 2 strains, respectively.248
Human cases
With 97% of all serotype confirmed human cases of S. suis infection due to serotype 2, the determination of the STs responsible for these cases becomes highly important (Table 6). Globally, ST1 strains have been described as mostly responsible for S. suis serotype 2 human cases, particularly in South America, Europe and Asia, but also one case in North America.10,62,86,129,242,249 Nevertheless, multiple other STs have been described worldwide, though these appear to be endemic to certain geographical regions. For example, the ST20 is important in the Netherlands and France but not in the rest of Europe.62 ST7 strains, to which belong the strains responsible for the 1998 and 2005 Chinese epidemics, were isolated from human patients only in mainland China and Hong Kong.242,249 Meanwhile, ST25 and ST28 strains have been particularly associated with human cases in North America and Japan, respectively.53,116,242 The situation is particular in Thailand, where ST1 and ST104 strains are predominant, causing mainly meningitis and non-meningitis cases, respectively.53,128,129 A few cases of ST25, ST28, ST101, ST102 and ST103 have also been described.129 Interestingly, ST101–104 are so far endemic to Thailand only.
Though S. suis serotype 14 infections are less frequent in humans than serotype 2 cases, representing 2% of all the serotype-confirmed cases, the number of human infections caused by this serotype appears to be increasing. The ST105 is prevalent in Southeast Asia, particularly in Vietnam and Thailand. In the latter country, 92% of human serotype 14 cases are caused by this ST (Table 6).10,132
Only three human S. suis cases, other than those caused by serotypes 2 and 14, have been typed by MLST (Table 6), and all three were described as newly identified STs. Serotype 5, 16 and 24 human cases of infection were identified as ST181, ST106 and ST221, respectively.133,146 Interestingly, no data have yet been published on the possible presence of these three newly identified STs for human isolates in diseased pigs.
Association between pig and human sequence types
Albeit no study has yet associated STs of strains isolated from human cases with those from diseased pigs in the same geographical region, it would seem reasonable to suggest that this association exists, particularly for the serotype 2, as most human strains appear to originate from contact with either pigs or pork by-products. In North America, where serotype 2 ST25 and ST28 strains are predominately isolated from diseased pigs, it is of no surprise that human ST25 cases were identified.52,98,242 Interestingly, no cases due to ST28 have been diagnosed in humans. It has been suggested that ST25 strains from pigs are more virulent than their ST28 counterparts,52 which may explain this situation. Moreover, it is interesting to note that even though ST1 strains account for only 5% of isolates from diseased pigs, a human case caused by an ST1 strain was reported in the United States.96 It is possible that ST1 strains were imported from pigs from Europe and Asia. Being more virulent than their ST25 and ST28 counterparts, it may be hypothesized that a higher number of cases in pigs due to ST1 strains will appear in the near future in North America. Furthermore, similar STs have been described in some Asian countries for both diseased pigs and humans. For example, in Japan, ST1 and ST28 strains have been isolated from both species, while ST1 and ST7 strains have been identified for human and pig isolates in mainland China.53,59,116,128,241,242,248 The situation is similar in Europe where serotype 2 ST1 clonal complex strains are predominately isolated from diseased pigs in Spain, Italy, the Netherlands and the United Kingdom and where most human cases have also been typed as belonging to this complex.51,62,86,204,240,244
This association between pig and human strains within a geographical region, though not definitive and currently only reflecting the situation for the serotype 2, confirms the results obtained by Chatellier et al.250 whereby using randomly amplified polymorphic DNA, they concluded that strains isolated from pigs and humans could not be genotypically distinguished and were similar.
Association between sequence types and virulence markers
Presently, the most popular virulence markers used in association with STs are the SLY (sly), MRP (mrp) and EF (epf). It is important to note that although the genotypes of strains belonging to other serotypes have been reported, these factors are mainly associated with serotype 2 strains.
Serotype 2 ST1 strains recovered from both clinical pig and human cases have for the most part been genotyped/phenotyped as sly+mrp+epf+/SLY+MRP+EF+, regardless of the geographic origin (whether it be mainland China, Japan, North America or Spain for both diseased pigs and human cases), which is identical to the serotype 2 ST7 strains isolated from diseased pigs and humans in mainland China.52,242,244,245,246,248 Nevertheless, other important genetic differences vary between ST1 and ST7 strains including the presence of a 89K pathogenicity island in ST7 strains.251 These ST1 complex strains interestingly differ from not only the human serotype 2 ST104 strains of Thailand which are sly+mrp-epf- but also from the human serotype 2 ST20 strains recovered in the Netherlands that were epf-.62,129,242 Also of interest is a human case from Spain where the serotype 2 strain isolated was typed as being a ST3, and presented a large variant of the mrp, identified as mrp* (which has a higher molecular weight), though being sly+epf+.204,252 In Europe, it was determined that strains isolated from diseased pigs belonging to the ST16 complex differ from the ST1 complex strains in being mrp* rather than mrp, while in Spain, the endemic ST123 and ST125 are both mrp- and epf-.244 As for ST25 strains isolated from diseased pigs in North America, these were identified as being SLY-MRP-EF-, while the ST28 isolated from North America, mainland China and Japan were sly-mrp+epf- or SLY-MRP+EF-.52,246,248 Though currently not as widely used as the above mentioned virulence markers, different pili (srtB, srtC, srtD, srtF and srtG) have also been associated with different STs. It was identified that ST1 strains isolated from both diseased pigs and human cases of serotype 2 infections in Japan and Thailand were srtBCD+ and srtF+ but srtG-.53 Meanwhile North American ST25 strains isolated from diseased pigs and human cases were srtF- and srtG+ and ST28 strains isolated from diseased pigs and human cases from North America and Japan were srtF+ and srtG+.52,248
It remains difficult to be certain of the association between STs and virulence markers as being universal, but it appears to be representative of populations within a region and may be a useful diagnostic tool with methods identifying the genotypic or phenotypic presence or absence of the different virulence markers.
CONCLUDING REMARKS
S. suis serotype 2 remains the most isolated serotype and the one most associated with disease in both pigs and humans in most parts of the world. Due to its endemic status in Southeast Asia and the two epidemics it caused in Jiangsu (1998) and Sichuan (2005), China, characterized by STSLS and high mortality rates, this bacterium should no longer be considered merely an important pig pathogen, but also an important and emerging zoonotic agent. Many avenues of research remain regarding the definition of a ‘true' S. suis serotype and the adoption and use of a complete serotyping system. In this regard, the recent availability of new complete PCR serotyping systems will allow any laboratory in the world to serotype S. suis isolates without the need of specific antibodies. The identification of this pathogen by medical and veterinary laboratories significantly varies among countries, and in only a few regions both are well developed. In some important pig producing countries in the Americas, such as Canada, the United States, Mexico and Brazil, diagnostic laboratories in human medicine should be more aware of the zoonotic potential of S. suis due to occupational exposure to pigs and/or pork-derived products. The scientific community should request that the specific serotype of each reported case in humans be identified using acceptable techniques to keep useful epidemiological data. On the other hand, in some countries in Asia where human cases are routinely reported, the number of studies provided by diagnostic laboratories working in veterinary medicine should be significantly increased, since there are almost no data regarding strains recovered from diseased animals. The same applies to European countries which are important pig producers and from which no data regarding the distribution of serotypes from diseased animals were reported in the last 12 years, hampering the use of species-specific protective bacterins.
Though the MLST data currently available for S. suis strains isolated from both diseased pigs and human cases of infection come from different countries throughout the world, there remains a long way to go before a complete picture of the current situation of S. suis can be obtained. Yet, this picture is necessary as it could hint at both dominating and emerging STs and could possibly help identify epidemic strains quickly and categorize the zoonotic potential of specific STs. With the dissemination of information about STs across the internet, particularly thanks to the S. suis MLST Database, it would be important to set up a complete and reliable serotype identification of the different strains added. Although this database is a great tool that facilitates the sharing of knowledge, the lack of many details pertaining to each strain such as the serotype, method used for serotyping, health status of the host, and host itself, limit the full potential of the STs as was demonstrated in this review where many strains could not be included. Furthermore, in the absence of identification of the serotyping method, it is impossible to confirm if the serotype indicated is correct. Nevertheless, the global distribution of S. suis, particularly serotype 2 strains, greatly varies, an aspect that could be useful in developing diagnostic and preventive tools specific for a particular geographical distribution. Despite the fact that studies have recently associated virulence markers and profiles with given STs, the biological significance of these associations still needs to be studied, as only a handful of studies have compared STs from different geographical origins. In fact, some STs found in North America and described as low virulent are frequently isolated from patients in Asia. There is an urgent need to compare virulence properties of strains of similar STs from different geographical origins. Finally, though still tentative, the possibility of associating strains from diseased pigs and human cases in a given region, as determined based on serotypes and STs, would provide further epidemiological support for the zoonotic potential of this pig pathogen, demonstrating the need for proper hygiene practises in order to reduce the risk of zoonotic infections. With more and more laboratories using a complete serotyping system and MLST as a tool for S. suis classification, we will one day have the complete picture regarding the S. suis distribution.
Acknowledgments
The research carried out on Streptococcus suis in our laboratories is funded by the Natural Sciences and Engineering Research Council of Canada (grant #154280 to Marcelo Gottschalk and 342150-07 to Mariela Segura) and by China–Canada Joint Health Research Initiative financed by Canadian Institutes of Health Research and the National Natural Science Foundation of China (812111251). This publication made use of the Multilocus Sequence Typing Website (http://www.mlst.net) at Imperial College London developed by David Aanensen and funded by the Welcome Trust.
References
- Gottschalk M, Segura M, Xu J. Streptococcus suis infections in humans: the Chinese experience and the situation in North America. Anim Health Res Rev. 2007;8:29–45. doi: 10.1017/S1466252307001247. [DOI] [PubMed] [Google Scholar]
- Gottschalk M, Xu J, Lecours MP, Grenier D, Fittipaldi N, Segura M. Streptococcus suis infections in humans: what is the prognosis for Western countries? (Part II) Clin Microbiol Newslett. 2010;32:97–102. [Google Scholar]
- Wertheim HF, Nghia HD, Taylor W, Schultsz C. Streptococcus suis: an emerging human pathogen. Clin Infect Dis. 2009;48:617–625. doi: 10.1086/596763. [DOI] [PubMed] [Google Scholar]
- Fittipaldi N, Segura M, Grenier D, Gottschalk M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol. 2012;7:259–279. doi: 10.2217/fmb.11.149. [DOI] [PubMed] [Google Scholar]
- Baums CG, Valentin-Weigand P. Surface-associated and secreted factors of Streptococcus suis in epidemiology, pathogenesis and vaccine development. Anim Health Res Rev. 2009;10:65–83. doi: 10.1017/S146625230999003X. [DOI] [PubMed] [Google Scholar]
- Higgins R, Gottschalk M.Streptococcal diseasesIn: Straw BE, D'Allaire S, Mengeling WL, Taylor DJ (eds.)Diseases of swine Ames, IA; Iowa State University Press; 2006769–783. [Google Scholar]
- Sanford SE, Tilker ME. Streptococcus suis type II-associated diseases in swine: observations of a one-year study. J Am Vet Med Assoc. 1982;181:673–676. [PubMed] [Google Scholar]
- Staats JJ, Feder I, Okwumabua O, Chengappa MM. Streptococcus suis: past and present. Vet Res Commun. 1997;21:381–407. doi: 10.1023/a:1005870317757. [DOI] [PubMed] [Google Scholar]
- Perch B, Kristjansen P, Skadhauge K. Group R streptococci pathogenic for man. Two cases of meningitis and one fatal case of sepsis. Acta Pathol Microbiol Scand. 1968;74:69–76. [PubMed] [Google Scholar]
- Mai NT, Hoa NT, Nga TV, et al. Streptococcus suis meningitis in adults in Vietnam. Clin Infect Dis. 2008;46:659–667. doi: 10.1086/527385. [DOI] [PubMed] [Google Scholar]
- Suankratay C, Intalapaporn P, Nunthapisud P, Arunyingmongkol K, Wilde H. Streptococcus suis meningitis in Thailand. Southeast Asian J Trop Med Public Health. 2004;35:868–876. [PubMed] [Google Scholar]
- Hui AC, Ng KC, Tong PY, et al. Bacterial meningitis in Hong Kong: 10-years' experience. Clin Neurol Neurosurg. 2005;107:366–370. doi: 10.1016/j.clineuro.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Fongcom A, Pruksakorn S, Mongkol R, Tharavichitkul P, Yoonim N. Streptococcus suis infection in northern Thailand. J Med Assoc Thai. 2001;84:1502–1508. [PubMed] [Google Scholar]
- Arends JP, Zanen HC. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis. 1988;10:131–137. doi: 10.1093/clinids/10.1.131. [DOI] [PubMed] [Google Scholar]
- Huang YT, Teng LJ, Ho SW, Hsueh PR. Streptococcus suis infection. J Microbiol Immunol Infect. 2005;38:306–313. [PubMed] [Google Scholar]
- Tang J, Wang C, Feng Y, et al. Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med. 2006;3:e151. doi: 10.1371/journal.pmed.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins R, Gottschalk M. An update on Streptococcus suis identification. J Vet Diagn Invest. 1990;2:249–252. doi: 10.1177/104063879000200324. [DOI] [PubMed] [Google Scholar]
- Gottschalk M, Xu J, Calzas C, Segura M. Streptococcus suis: a new emerging or an old neglected zoonotic pathogen. Future Microbiol. 2010;5:371–391. doi: 10.2217/fmb.10.2. [DOI] [PubMed] [Google Scholar]
- Lutticken R, Temme N, Hahn G, Bartelheimer EW. Meningitis caused by Streptococcus suis: case report and review of the literature. Infection. 1986;14:181–185. doi: 10.1007/BF01645260. [DOI] [PubMed] [Google Scholar]
- Michaud S, Duperval R, Higgins R. Streptococcus suis meningitis: first case reported in Quebec. Can J Infect Dis. 1996;7:329–331. doi: 10.1155/1996/354693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Lee SS, Wann SR, Huang TS, Chen YS, Liu YC. Streptococcus suis meningitis with ventriculoperitoneal shunt infection and spondylodiscitis. J Formos Med Assoc. 2005;104:948–950. [PubMed] [Google Scholar]
- Yen MY, Liu YC, Wang JH, Chen YS, Wang YH, Cheng DL. Streptococcus suis meningitis complicated with permanent perceptive deafness: report of a case. J Formos Med Assoc. 1994;93:349–351. [PubMed] [Google Scholar]
- Donsakul K, Dejthevaporn C, Witoonpanich R. Streptococcus suis infection: clinical features and diagnostic pitfalls. Southeast Asian J Trop Med Public Health. 2003;34:154–158. [PubMed] [Google Scholar]
- Okwumabua O, O'Connor M, Shull E. A polymerase chain reaction (PCR) assay specific for Streptococcus suis based on the gene encoding the glutamate dehydrogenase. FEMS Microbiol Lett. 2003;218:79–84. doi: 10.1111/j.1574-6968.2003.tb11501.x. [DOI] [PubMed] [Google Scholar]
- Gottschalk M, Lacouture S, Bonifait L, Roy D, Fittipaldi N, Grenier D. Characterization of Streptococcus suis isolates recovered between 2008 and 2011 from diseased pigs in Quebec, Canada. Vet Microbiol. 2013;162:819–825. doi: 10.1016/j.vetmic.2012.10.028. [DOI] [PubMed] [Google Scholar]
- Tien le HT, Sugiyama N, Duangsonk K, Tharavichitkul P, Osawa R. Phenotypic and PCR-based identification of bacterial strains isolated from patients with suspected Streptococcus suis infection in northern Thailand. Jpn J Infect Dis. 2012;65:171–174. [PubMed] [Google Scholar]
- Hill JE, Gottschalk M, Brousseau R, Harel J, Hemmingsen SM, Goh SH. Biochemical analysis, cpn60 and 16S rDNA sequence data indicate that Streptococcus suis serotypes 32 and 34, isolated from pigs, are Streptococcus orisratti. Vet Microbiol. 2005;107:63–69. doi: 10.1016/j.vetmic.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Brousseau R, Hill JE, Prefontaine G, Goh SH, Harel J, Hemmingsen SM. Streptococcus suis serotypes characterized by analysis of chaperonin 60 gene sequences. Appl Environ Microbiol. 2001;67:4828–4833. doi: 10.1128/AEM.67.10.4828-4833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier S, Lacouture S, Gottschalk M. Distribution of Streptococcus suis capsular types from 2001 to 2007. Can Vet J. 2008;49:461–462. [PMC free article] [PubMed] [Google Scholar]
- Wang K, Sun X, Lu C. Development of rapid serotype-specific PCR assays for eight serotypes of Streptococcus suis. J Clin Microbiol. 2012;50:3329–3334. doi: 10.1128/JCM.01584-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien le HT, Nishibori T, Nishitani Y, Nomoto R, Osawa R. Reappraisal of the taxonomy of Streptococcus suis serotypes 20, 22, 26, and 33 based on DNA–DNA homology and sodA and recN phylogenies. Vet Microbiol. 2013;162:842–849. doi: 10.1016/j.vetmic.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Segura M, Zheng H, Greeff Ad, et al. Latest developments on Streptococcus suis: an emerging zoonotic pathogen: part 1. Future Microbiol. 2014;9:441–444. doi: 10.2217/fmb.14.14. [DOI] [PubMed] [Google Scholar]
- Van Calsteren MR, Gagnon F, Lacouture S, Fittipaldi N, Gottschalk M. Structure determination of Streptococcus suis serotype 2 capsular polysaccharide. Biochem Cell Biol. 2010;88:513–525. doi: 10.1139/o09-170. [DOI] [PubMed] [Google Scholar]
- Van Calsteren MR, Gagnon F, Calzas C, et al. Structure determination of Streptococcus suis serotype 14 capsular polysaccharide. Biochem Cell Biol. 2013;91:49–58. doi: 10.1139/bcb-2012-0036. [DOI] [PubMed] [Google Scholar]
- Gottschalk M, Higgins R, Jacques M, Mittal KR, Henrichsen J. Description of 14 new capsular types of Streptococcus suis. J Clin Microbiol. 1989;27:2633–2636. doi: 10.1128/jcm.27.12.2633-2636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk M, Higgins R, Jacques M, Beaudoin M, Henrichsen J. Isolation and characterization of Streptococcus suis capsular types 9–22. J Vet Diagn Invest. 1991;3:60–65. doi: 10.1177/104063879100300113. [DOI] [PubMed] [Google Scholar]
- Gottschalk M, Higgins R, Boudreau M. Use of polyvalent coagglutination reagents for serotyping of Streptococcus suis. J Clin Microbiol. 1993;31:2192–2194. doi: 10.1128/jcm.31.8.2192-2194.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perch B, Kjems E, Slot P, Pedersen KB. Biochemical and serological properties of R, S, and RS streptococci. Acta Pathol Microbiol Scand B. 1981;89:167–171. doi: 10.1111/j.1699-0463.1981.tb00171_89b.x. [DOI] [PubMed] [Google Scholar]
- Wisselink HJ, Joosten JJ, Smith HE. Multiplex PCR assays for simultaneous detection of six major serotypes and two virulence-associated phenotypes of Streptococcus suis in tonsillar specimens from pigs. J Clin Microbiol. 2002;40:2922–2929. doi: 10.1128/JCM.40.8.2922-2929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois C, Bougeard S, Gottschalk M, Kobisch M. Multiplex PCR assay for detection of Streptococcus suis species and serotypes 2 and 1/2 in tonsils of live and dead pigs. J Clin Microbiol. 2004;42:3169–3175. doi: 10.1128/JCM.42.7.3169-3175.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zheng H, Gottschalk M, et al. Development of multiplex PCR assays for the identification of the 33 serotypes of Streptococcus suis. PLoS One. 2013;8:e72070. doi: 10.1371/journal.pone.0072070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura M, Lachance C, Osaki M, et al. Development of a two-step multiplex PCR assays for typing of capsular polysaccharide synthesis gene clusters of Streptococcus suis. J Clin Microbiol. 2014;52:1714–1719. doi: 10.1128/JCM.03411-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura M, Takamatsu D, Maruyama F, et al. Genetic analysis of capsular polysaccharide synthesis gene clusters from all serotypes of Streptococcus suis: potential mechanisms for generation of capsular variation. Appl Environ Microbiol. 2013;79:2796–2806. doi: 10.1128/AEM.03742-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopic J, Paradzik MT, Pandak N. Streptococcus suis infection as a cause of severe illness: 2 cases from Croatia. Scand J Infect Dis. 2002;34:683–684. doi: 10.1080/00365540210147769. [DOI] [PubMed] [Google Scholar]
- Vilaichone RK, Vilaichone W, Nunthapisud P, Wilde H. Streptococcus suis infection in Thailand. J Med Assoc Thai. 2002;85:S109–S117. [PubMed] [Google Scholar]
- Prieto C, Garcia FJ, Suarez P, Imaz M, Castro JM. Biochemical traits and antimicrobial susceptibility of Streptococcus suis isolated from slaughtered pigs. Zentralbl Veterinarmed B. 1994;41:608–617. doi: 10.1111/j.1439-0450.1994.tb00271.x. [DOI] [PubMed] [Google Scholar]
- Bonifait L, Gottschalk M, Grenier D. Cell surface characteristics of nontypeable isolates of Streptococcus suis. FEMS Microbiol Lett. 2010;311:160–166. doi: 10.1111/j.1574-6968.2010.02086.x. [DOI] [PubMed] [Google Scholar]
- Lakkitjaroen N, Takamatsu D, Okura M, Sato M, Osaki M, Sekizaki T. Loss of capsule among Streptococcus suis isolates from porcine endocarditis and its biological significance. J Med Microbiol. 2011;60:1669–1676. doi: 10.1099/jmm.0.034686-0. [DOI] [PubMed] [Google Scholar]
- Brehony C, Jolley KA, Maiden MC. Multilocus sequence typing for global surveillance of meningococcal disease. FEMS Microbiol Rev. 2007;31:15–26. doi: 10.1111/j.1574-6976.2006.00056.x. [DOI] [PubMed] [Google Scholar]
- Maiden MC. Multilocus sequence typing of bacteria. Annu Rev Microbiol. 2006;60:561–588. doi: 10.1146/annurev.micro.59.030804.121325. [DOI] [PubMed] [Google Scholar]
- King SJ, Leigh JA, Heath PJ, et al. Development of a multilocus sequence typing scheme for the pig pathogen Streptococcus suis: identification of virulent clones and potential capsular serotype exchange. J Clin Microbiol. 2002;40:3671–3680. doi: 10.1128/JCM.40.10.3671-3680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fittipaldi N, Xu J, Lacouture S, et al. Lineage and virulence of Streptococcus suis serotype 2 isolates from North America. Emerg Infect Dis. 2011;17:2239–2244. doi: 10.3201/eid1712.110609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu D, Nishino H, Ishiji T, et al. Genetic organization and preferential distribution of putative pilus gene clusters in Streptococcus suis. Vet Microbiol. 2009;138:132–139. doi: 10.1016/j.vetmic.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Lachance C, Gottschalk M, Gerber PP, Lemire P, Xu J, Segura M. Exacerbated type II interferon response drives hypervirulence and toxic shock by an emergent epidemic strain of Streptococcus suis. Infect Immun. 2013;81:1928–1939. doi: 10.1128/IAI.01317-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fittipaldi N, Fuller TE, Teel JF, et al. Serotype distribution and production of muramidase-released protein, extracellular factor and suilysin by field strains of Streptococcus suis isolated in the United States. Vet Microbiol. 2009;139:310–317. doi: 10.1016/j.vetmic.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Martinez G, Pestana de Castro AF, Ribeiro Pagnani KJ, Nakazato G, Dias da Silveira W, Gottschalk M. Clonal distribution of an atypical MRP+, EF*, and suilysin+ phenotype of virulent Streptococcus suis serotype 2 strains in Brazil. Can J Vet Res. 2003;67:52–55. [PMC free article] [PubMed] [Google Scholar]
- Costa AT, Lobato FC, Abreu VL, Assis RA, Reis R, Uzal FA. Serotyping and evaluation of the virulence in mice of Streptococcus suis strains isolated from diseased pigs. Rev Inst Med Trop Sao Paulo. 2005;47:113–115. doi: 10.1590/s0036-46652005000200012. [DOI] [PubMed] [Google Scholar]
- Wei Z, Li R, Zhang A, et al. Characterization of Streptococcus suis isolates from the diseased pigs in China between 2003 and 2007. Vet Microbiol. 2009;137:196–201. doi: 10.1016/j.vetmic.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Chen L, Song Y, Wei Z, He H, Zhang A, Jin M. Antimicrobial susceptibility, tetracycline and erythromycin resistance genes, and multilocus sequence typing of Streptococcus suis isolates from diseased pigs in China. J Vet Med Sci. 2013;75:583–587. doi: 10.1292/jvms.12-0279. [DOI] [PubMed] [Google Scholar]
- Li LL, Liao XP, Sun J, et al. Antimicrobial resistance, serotypes, and virulence factors of Streptococcus suis isolates from diseased pigs. Foodborne Pathog Dis. 2012;9:583–588. doi: 10.1089/fpd.2011.1106. [DOI] [PubMed] [Google Scholar]
- Kim D, Han K, Oh Y, et al. Distribution of capsular serotypes and virulence markers of Streptococcus suis isolated from pigs with polyserositis in Korea. Can J Vet Res. 2010;74:314–316. [PMC free article] [PubMed] [Google Scholar]
- Schultsz C, Jansen E, Keijzers W, et al. Differences in the population structure of invasive Streptococcus suis strains isolated from pigs and from humans in The Netherlands. PLoS One. 2012;7:e33854. doi: 10.1371/journal.pone.0033854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela AI, Goyache J, Tarradas C, et al. Analysis of genetic diversity of Streptococcus suis clinical isolates from pigs in Spain by pulsed-field gel electrophoresis. J Clin Microbiol. 2003;41:2498–2502. doi: 10.1128/JCM.41.6.2498-2502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarradas C, Perea A, Vela AI, et al. Distribution of serotypes of Streptococcus suis isolated from diseased pigs in Spain. Vet Rec. 2004;154:665–666. doi: 10.1136/vr.154.21.665. [DOI] [PubMed] [Google Scholar]
- Luque I, Blume V, Borge C, et al. Genetic analysis of Streptococcus suis isolates recovered from diseased and healthy carrier pigs at different stages of production on a pig farm. Vet J. 2010;186:396–398. doi: 10.1016/j.tvjl.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Wisselink HJ, Smith HE, Stockhofe-Zurwieden N, Peperkamp K, Vecht U. Distribution of capsular types and production of muramidase-released protein (MRP) and extracellular factor (EF) of Streptococcus suis strains isolated from diseased pigs in seven European countries. Vet Microbiol. 2000;74:237–248. doi: 10.1016/s0378-1135(00)00188-7. [DOI] [PubMed] [Google Scholar]
- Marois C, Le Devendec L, Gottschalk M, Kobisch M. Detection and molecular typing of Streptococcus suis in tonsils from live pigs in France. Can J Vet Res. 2007;71:14–22. [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zhang W, Li X, et al. Characterization of Streptococcus suis isolates from slaughter swine. Curr Microbiol. 2013;66:344–349. doi: 10.1007/s00284-012-0275-4. [DOI] [PubMed] [Google Scholar]
- Ngo TH, Tran TB, Tran TT, et al. Slaughterhouse pigs are a major reservoir of Streptococcus suis serotype 2 capable of causing human infection in southern Vietnam. PLoS One. 2011;6:e17943. doi: 10.1371/journal.pone.0017943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdsin A, Dejsirilert S, Akeda Y, et al. Fifteen Streptococcus suis serotypes identified by multiplex PCR. J Med Microbiol. 2012;61:1669–1672. doi: 10.1099/jmm.0.048587-0. [DOI] [PubMed] [Google Scholar]
- Zhang CP, Ning YB, Zhang ZQ, et al. [Distributions of pathogenic capsular types and in vitro antimicrobial susceptibility of different serotypes of Streptococcus suis isolated from clinically healthy sows from 10 provinces in China.] Zhonghua Liu Xing Bing Xue Za Zhi 200930235–238.Chinese. [PubMed] [Google Scholar]
- Nghia HD, Tu le TP, Wolbers M, et al. Risk factors of Streptococcus suis infection in Vietnam. A case-control study. PLoS One. 2011;6:e17604. doi: 10.1371/journal.pone.0017604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilaichone RK, Mahachai V, Nunthapisud P. Streptococcus suis peritonitis: case report. J Med Assoc Thai. 2000;83:1274–1277. [PubMed] [Google Scholar]
- Lopreto C, Lopardo HA, Bardi MC, Gottschalk M.[Primary Streptococcus suis meningitis: first case in humans described in Latin America.] Enferm Infecc Microbiol Clin 200523110Spanish. [DOI] [PubMed] [Google Scholar]
- Koch E, Fuentes G, Carvajal R, et al. [Streptococcus suis meningitis in pig farmers: report of first two cases in Chile.] Rev Chilena Infectol 201330557–561.Spanish. [DOI] [PubMed] [Google Scholar]
- Alarcon P, Araya P, Aguayo C, et al. [Laboratory confirmation of Streptococcus suis in Chile.] Rev Chilena Infectol 201330539–540.Spanish. [DOI] [PubMed] [Google Scholar]
- Callejo R, Prieto M, Salamone F, Auger J, Goyette-Desjardins G, Gottschalk M. Atypical Streptococcus suis in man, Argentina, 2013. Emerg Infect Dis. 2014;20:500–502. doi: 10.3201/eid2003.131148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padungtod P, Tharavichitkul P, Junya S, et al. Incidence and presence of virulence factors of Streptococcus suis infection in slaughtered pigs from Chiang Mai, Thailand. Southeast Asian J Trop Med Public Health. 2010;41:1454–1461. [PubMed] [Google Scholar]
- Hoa NT, Chieu TT, Do Dung S, et al. Streptococcus suis and porcine reproductive and respiratory syndrome, Vietnam. Emerg Infect Dis. 2013;19:331–333. doi: 10.3201/eid1902.120470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Sugimoto C, Nakazawa M, Morozumi T, Kashiwazaki M. The epidemiological studies of Streptococcus suis infections in Japan from 1987 to 1991. J Vet Med Sci. 1993;55:623–626. doi: 10.1292/jvms.55.623. [DOI] [PubMed] [Google Scholar]
- Ip M, Fung KS, Chi F, et al. Streptococcus suis in Hong Kong. Diagn Microbiol Infect Dis. 2007;57:15–20. doi: 10.1016/j.diagmicrobio.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Ma E, Chung PH, So T, et al. Streptococcus suis infection in Hong Kong: an emerging infectious disease. Epidemiol Infect. 2008;136:1691–1697. doi: 10.1017/S0950268808000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie J, Morvan H, Berthelot-Herault F, et al. Antimicrobial susceptibility of Streptococcus suis isolated from swine in France and from humans in different countries between 1996 and 2000. J Antimicrob Chemother. 2002;50:201–209. doi: 10.1093/jac/dkf099. [DOI] [PubMed] [Google Scholar]
- Tian Y, Aarestrup FM, Lu CP. Characterization of Streptococcus suis serotype 7 isolates from diseased pigs in Denmark. Vet Microbiol. 2004;103:55–62. doi: 10.1016/j.vetmic.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Aarestrup FM, Jorsal SE, Jensen NE. Serological characterization and antimicrobial susceptibility of Streptococcus suis isolates from diagnostic samples in Denmark during 1995 and 1996. Vet Microbiol. 1998;60:59–66. doi: 10.1016/s0378-1135(98)00147-3. [DOI] [PubMed] [Google Scholar]
- Princivalli MS, Palmieri C, Magi G, et al. Genetic diversity of Streptococcus suis clinical isolates from pigs and humans in Italy (2003–2007) Euro Surveill. 2009;14 pii:19310. doi: 10.2807/ese.14.33.19310-en. [DOI] [PubMed] [Google Scholar]
- Stanojkovic A, Asanin R, Misic D, Asanin J, Stanojkovic-Sebic A. The presence and serological types of Streptococcus suis strains isolated from pigs originating from some farms in Serbia. Fresen Environ Bull. 2012;21:3558–3561. [Google Scholar]
- Seol B, Kelneric Z, Hajsig D, Madic J, Naglic T. Susceptibility to antimicrobial agents of Streptococcus suis capsular type 2 strains isolated from pigs. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1996;283:328–331. [PubMed] [Google Scholar]
- Buddle JR, Jones JE, Pass DA, Robertson J. The isolation of Streptococcus suis type II from a pig with meningitis. Aust Vet J. 1981;57:437–438. doi: 10.1111/j.1751-0813.1981.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Mwaniki CG, Robertson ID, Trott DJ, Atyeo RF, Lee BJ, Hampson DJ. Clonal analysis and virulence of Australian isolates of Streptococcus suis type 2. Epidemiol Infect. 1994;113:321–334. doi: 10.1017/s095026880005175x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie AS, Bremner DA, Wong PY, North JD, Robertson ID. Streptococcus suis bacteraemia. N Z Med J. 1987;100:677–678. [PubMed] [Google Scholar]
- Kohler W, Queisser H, Kunter E, Sawitzki R, Frach G.[Type 2 Streptococcus suis (R-Streptococci) as pathogens of occupational diseases. Report of a case and a review of the literature.] Z Gesamte Inn Med 198944144–148.German. [PubMed] [Google Scholar]
- Lun ZR, Wang QP, Chen XG, Li AX, Zhu XQ. Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect Dis. 2007;7:201–209. doi: 10.1016/S1473-3099(07)70001-4. [DOI] [PubMed] [Google Scholar]
- Trottier S, Higgins R, Brochu G, Gottschalk M. A case of human endocarditis due to Streptococcus suis in North America. Rev Infect Dis. 1991;13:1251–1252. doi: 10.1093/clinids/13.6.1251. [DOI] [PubMed] [Google Scholar]
- Haleis A, Alfa M, Gottschalk M, Bernard K, Ronald A, Manickam K. Meningitis caused by Streptococcus suis serotype 14, North America. Emerg Infect Dis. 2009;15:350–352. doi: 10.3201/eid1502.080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenburg KS, Sentochnik DE, Zadoks RN. Human Streptococcus suis meningitis in the United States. N Engl J Med. 2006;354:1325. doi: 10.1056/NEJMc053089. [DOI] [PubMed] [Google Scholar]
- Fittipaldi N, Collis T, Prothero B, Gottschalk M. Streptococcus suis meningitis, Hawaii. Emerg Infect Dis. 2009;15:2067–2069. doi: 10.3201/eid1512.090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler HN, Brown P, Rovira A, et al. Streptococcus suis meningitis in swine worker, Minnesota, USA. Emerg Infect Dis. 2013;19:330–331. doi: 10.3201/eid1902.120918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel A, Manias V, Busquets N, Sniadowsky S, Anzardi J, Mendez Ede L.[Streptococcus suis meningitis in an immunocompetent patient.] Rev Argent Microbiol 200840158–160.Spanish. [PubMed] [Google Scholar]
- Nunez JM, Marcotullio M, Rojas A, Acuna L, Caceres M, Mochi S.[First case of meningitis by Streptococcus suis in the norwest area of Argentina.] Rev Chilena Infectol 201330554–556.Spanish. [DOI] [PubMed] [Google Scholar]
- Demar M, Belzunce C, Simonnet C, et al. Streptococcus suis meningitis and bacteremia in man, French Guiana. Emerg Infect Dis. 2013;19:1545–1546. doi: 10.3201/eid1909.121872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlieghe E.The microbiological spectrum of invasive bacterial infections in Cambodian adults and implications for standard treatment guidelinesMD thesis, University of Leuven, Leuven, Belgium, 2014
- Cheng AF, Oo KT, Li EK, French GL. Septic arthritis caused by Streptococcus suis serotype 2. J Infect. 1987;14:237–241. doi: 10.1016/s0163-4453(87)93485-2. [DOI] [PubMed] [Google Scholar]
- Hu X, Zhu F, Wang H, et al. [Studies on human streptococcal infectious syndrome caused by infected pigs.] Zhonghua Yu Fang Yi Xue Za Zhi 200034150–152.Chinese. [PubMed] [Google Scholar]
- Yu H, Jing H, Chen Z, et al. Human Streptococcus suis outbreak, Sichuan, China. Emerg Infect Dis. 2006;12:914–920. doi: 10.3201/eid1206.051194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Shi X, Zhang H, et al. Recurrence of human Streptococcus suis infections in 2007: three cases of meningitis and implications that heterogeneous S. suis 2 circulates in China. Zoonoses Public Health. 2009;56:506–514. doi: 10.1111/j.1863-2378.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Tan Z, Zhu L, et al. Streptococcus suis serotype 2 caused streptococcal toxic shock syndrome (STSS) in a patient. J Nanjing Med Univ. 2008;22:313–316. [Google Scholar]
- Chau PY, Huang CY, Kay R. Streptococcus suis meningitis. An important underdiagnosed disease in Hong Kong. Med J Aust. 1983;1:414–416, 417. [PubMed] [Google Scholar]
- Ho AK, Woo KS, Tse KK, French GL. Infective endocarditis caused by Streptococcus suis serotype 2. J Infect. 1990;21:209–211. doi: 10.1016/0163-4453(90)91883-f. [DOI] [PubMed] [Google Scholar]
- Kay R, Cheng AF, Tse CY. Streptococcus suis infection in Hong Kong. QJM. 1995;88:39–47. [PubMed] [Google Scholar]
- Woo J. Streptococcus suis meningitis in man in Hong Kong. Trans R Soc Trop Med Hyg. 1986;80:848–849. doi: 10.1016/0035-9203(86)90403-7. [DOI] [PubMed] [Google Scholar]
- Woo J, Li EK. Streptococcus suis meningitis requires prolonged treatment with penicillin. Infection. 1987;15:129–130. doi: 10.1007/BF01650216. [DOI] [PubMed] [Google Scholar]
- Ng HW. Overwhelming postsplenectomy sepsis presenting as purpura fulminans due to Streptococcus suis infection. Hong Kong J Emerg Med. 2013;20:155–160. [Google Scholar]
- Ibaraki M, Fujita N, Tada M, Ohtaki O, Nagai H.[A Japanese case of Streptococcus suis meningitis associated with lumbar epidural abscess.] Rinsho Shinkeigaku 200343176–179.Japanese. [PubMed] [Google Scholar]
- Matsuo H, Sakamoto S.[Purulent meningitis caused by Streptococcus suis in a pig breeder.] Kansenshogaku Zasshi 200377340–342.Japanese. [DOI] [PubMed] [Google Scholar]
- Chang B, Wada A, Ikebe T, et al. Characteristics of Streptococcus suis isolated from patients in Japan. Jpn J Infect Dis. 2006;59:397–399. [PubMed] [Google Scholar]
- Ishigaki K, Nakamura A, Iwabuchi S, et al. [A case of Streptococcus suis endocarditis, probably bovine-transmitted, complicated by pulmonary embolism and spondylitis.] Kansenshogaku Zasshi 200983544–548.Japanese. [DOI] [PubMed] [Google Scholar]
- Mori K, Ishii N, Mochizuki H, Taniguchi A, Shiomi K, Nakazato M. Bilateral sensorineural hearing impairment due to Streptococcus suis meningitis 20 days after swine bite. Rinsho Shinkeigaku. 2013;53:732–735. doi: 10.5692/clinicalneurol.53.732. [DOI] [PubMed] [Google Scholar]
- Lee GT, Chiu CY, Haller BL, Denn PM, Hall CS, Gerberding JL. Streptococcus suis meningitis, United States. Emerg Infect Dis. 2008;14:183–185. doi: 10.3201/eid1401.070930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambyah PA, Kumarasinghe G, Chan HL, Lee KO. Streptococcus suis infection complicated by purpura fulminans and rhabdomyolysis: case report and review. Clin Infect Dis. 1997;24:710–712. doi: 10.1093/clind/24.4.710. [DOI] [PubMed] [Google Scholar]
- Chan YC, Wilder-Smith A, Ong BK, Kumarasinghe G, Wilder-Smith E. Adult community acquired bacterial meningitis in a Singaporean teaching hospital. A seven-year overview (1993–2000) Singapore Med J. 2002;43:632–636. [PubMed] [Google Scholar]
- Tan JH, Yeh BI, Seet CS. Deafness due to haemorrhagic labyrinthitis and a review of relapses in Streptococcus suis meningitis. Singapore Med J. 2010;51:e30–33. [PubMed] [Google Scholar]
- Kim H, Lee SH, Moon HW, et al. Streptococcus suis causes septic arthritis and bacteremia: phenotypic characterization and molecular confirmation. Korean J Lab Med. 2011;31:115–117. doi: 10.3343/kjlm.2011.31.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh HJ, Park KJ, Jang JH, et al. Streptococcus suis meningitis with bilateral sensorineural hearing loss. Korean J Lab Med. 2011;31:205–211. doi: 10.3343/kjlm.2011.31.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SM, Cho BH, Choi KH, et al. Meningitis caused by Streptococcus suis: case report and review of the literature. J Clin Neurol. 2012;8:79–82. doi: 10.3988/jcn.2012.8.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh YJ, Song SH. A case of Streptococcus suis infection causing pneumonia with empyema in Korea. Tuberc Respir Dis. 2012;73:178–181. doi: 10.4046/trd.2012.73.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HY, Liao CH, Liu CY, Huang YT, Teng LJ, Hsueh PR. Streptococcus suis infection in Taiwan, 2000–2011. Diagn Microbiol Infect Dis. 2012;74:75–77. doi: 10.1016/j.diagmicrobio.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Takamatsu D, Wongsawan K, Osaki M, et al. Streptococcus suis in humans, Thailand. Emerg Infect Dis. 2008;14:181–183. doi: 10.3201/eid1401.070568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdsin A, Dejsirilert S, Puangpatra P, et al. Genotypic profile of Streptococcus suis serotype 2 and clinical features of infection in humans, Thailand. Emerg Infect Dis. 2011;17:835–842. doi: 10.3201/eid1705.100754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi D, Kerdsin A, Pienpringam A, et al. Population-based study of Streptococcus suis infection in humans in Phayao Province in northern Thailand. PLoS One. 2012;7:e31265. doi: 10.1371/journal.pone.0031265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanterdsith B, Tharavichitkul P, Mahanupab P, Raksamat W. Postmortem diagnosis of sudden unexpected death from Streptococcus suis type 2 infection: a case report. J Forensic Leg Med. 2013;20:347–349. doi: 10.1016/j.jflm.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Kerdsin A, Oishi K, Sripakdee S, et al. Clonal dissemination of human isolates of Streptococcus suis serotype 14 in Thailand. J Med Microbiol. 2009;58:1508–1513. doi: 10.1099/jmm.0.013656-0. [DOI] [PubMed] [Google Scholar]
- Kerdsin A, Dejsirilert S, Sawanpanyalert P, et al. Sepsis and spontaneous bacterial peritonitis in Thailand. Lancet. 2011;378:960. doi: 10.1016/S0140-6736(11)60923-9. [DOI] [PubMed] [Google Scholar]
- Leelarasamee A, Nilakul C, Tien-Grim S, Srifuengfung S, Susaengrat W. Streptococcus suis toxic-shock syndrome and meningitis. J Med Assoc Thai. 1997;80:63–68. [PubMed] [Google Scholar]
- Chotmongkol V, Janma J, Kawamatawong T. Streptococcus suis meningitis: report of a case. J Med Assoc Thai. 1999;82:922–924. [PubMed] [Google Scholar]
- Teekakirikul P, Wiwanitkit V. Streptococcus suis infection: overview of case reports in Thailand. Southeast Asian J Trop Med Public Health. 2003;34 (Suppl 2:178–183. [PubMed] [Google Scholar]
- Wangkaew S, Chaiwarith R, Tharavichitkul P, Supparatpinyo K. Streptococcus suis infection: a series of 41 cases from Chiang Mai University Hospital. J Infect. 2006;52:455–460. doi: 10.1016/j.jinf.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Wangsomboonsiri W, Luksananun T, Saksornchai S, Ketwong K, Sungkanuparph S. Streptococcus suis infection and risk factors for mortality. J Infect. 2008;57:392–396. doi: 10.1016/j.jinf.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Rusmeechan S, Sribusara P. Streptococcus suis meningitis: the newest serious infectious disease. J Med Assoc Thai. 2008;91:654–658. [PubMed] [Google Scholar]
- Navacharoen N, Chantharochavong V, Hanprasertpong C, Kangsanarak J, Lekagul S. Hearing and vestibular loss in Streptococcus suis infection from swine and traditional raw pork exposure in northern Thailand. J Laryngol Otol. 2009;123:857–862. doi: 10.1017/S0022215109004939. [DOI] [PubMed] [Google Scholar]
- Fongcom A, Pruksakorn S, Netsirisawan P, Pongprasert R, Onsibud P. Streptococcus suis infection: a prospective study in northern Thailand. Southeast Asian J Trop Med Public Health. 2009;40:511–517. [PubMed] [Google Scholar]
- Laohapensang K, Rutherford RB, Arworn S. Mycotic abdominal aortic aneurysm due to Streptococcus suis: a case report. Surg Infect. 2010;11:179–181. doi: 10.1089/sur.2008.111. [DOI] [PubMed] [Google Scholar]
- Pachirat O, Taksinachanekit S, Mootsikapun P, Kerdsin A. Human Streptococcus suis endocarditis: echocardiographic features and clinical outcome. Clin Med Insights Cardiol. 2012;6:119–123. doi: 10.4137/CMC.S9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson DJ, Trott DJ, Clarke IL, Mwaniki CG, Robertson ID. Population structure of Australian isolates of Streptococcus suis. J Clin Microbiol. 1993;31:2895–2900. doi: 10.1128/jcm.31.11.2895-2900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nga TV, Nghia HD, Tu le TP, et al. Real-time PCR for detection of Streptococcus suis serotype 2 in cerebrospinal fluid of human patients with meningitis. Diagn Microbiol Infect Dis. 2011;70:461–467. doi: 10.1016/j.diagmicrobio.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghia HD, Hoa NT, Linh le D, et al. Human case of Streptococcus suis serotype 16 infection. Emerg Infect Dis. 2008;14:155–157. doi: 10.3201/eid1401.070534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheim HF, Nguyen HN, Taylor W, et al. Streptococcus suis, an important cause of adult bacterial meningitis in northern Vietnam. PLoS One. 2009;4:e5973. doi: 10.1371/journal.pone.0005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Nguyen K, Nguyen D, et al. The spectrum of central nervous system infections in an adult referral hospital in Hanoi, Vietnam. PLoS One. 2012;7:e42099. doi: 10.1371/journal.pone.0042099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Dang Trung N, Le Thi Phuong T, Wolbers M, et al. Aetiologies of central nervous system infection in Viet Nam: a prospective provincial hospital-based descriptive surveillance study. PLoS One. 2012;7:e37825. doi: 10.1371/journal.pone.0037825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiss HK, Kofler M, Hausdorfer H, Pfausler B, Schmutzhard E.[Streptococcus suis meningitis and neurophysiology of the acoustic system. First case report from Austria.] Nervenarzt 199970738–741.German. [DOI] [PubMed] [Google Scholar]
- Colaert J, Allewaert M, Magerman H, Vandeven J, Vandepitte J. Streptococcus suis meningitis in man. First reported observation in Belgium. Acta Clin Belg. 1985;40:314–317. doi: 10.1080/22953337.1985.11719099. [DOI] [PubMed] [Google Scholar]
- Cammaert T, Verstraete W, Baeck E.[Deafness–blindness caused by Streptococcus suis meningitis—epidemiology and rehabilitation.] Acta Otorhinolaryngol Belg 19904437–41.Dutch. [PubMed] [Google Scholar]
- Hantson P, Vekemans MC, Gautier P, et al. Fatal Streptococcus suis meningitis in man. Acta Neurol Belg. 1991;91:165–168. [PubMed] [Google Scholar]
- Coolen L, Dens J, Baeck E, et al. Streptococcus suis meningitis, permanent perceptive deafness and endophthalmitis. Intensive Care Med. 1989;15:545. [PubMed] [Google Scholar]
- Perch B, Kjems E. Group R Streptococcus infections in man. JAMA. 1971;216:1484. doi: 10.1001/jama.1971.03180350060026. [DOI] [PubMed] [Google Scholar]
- Koldkjaer O, Nielsen G. Haemolytic Streptococcus group R infection. Br Med J. 1972;3:765. doi: 10.1136/bmj.3.5829.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjems E, Perch B. [Serious infections in man caused by group R streptococci.] Danish; Ugeskr Laeger. 1975;137:682–683. [PubMed] [Google Scholar]
- Poggenborg R, Gaini S, Kjaeldgaard P, Christensen JJ. Streptococcus suis: meningitis, spondylodiscitis and bacteraemia with a serotype 14 strain. Scand J Infect Dis. 2008;40:346–349. doi: 10.1080/00365540701716825. [DOI] [PubMed] [Google Scholar]
- Hedegaard SS, Zaccarin M, Lindberg J.[Septic arthritis caused by Streptococcus suis.] Ugeskr Laeger 20131751574–1575.Danish. [PubMed] [Google Scholar]
- Avril MF, Ghanassia JP, Leger JM, Bure A, Modai J.[Meningitis by group R Streptococcus. About two observations.] Med Maladies Infect 19777531–534.French. [Google Scholar]
- Paul G, Ancelle JP, Lionsquy G, Brochard C, Brassac R, Nevot P.[Human meningitis by group R Streptococcus: an unknown anthropozoonosis?] Med Maladies Infect 19777525–529.French. [Google Scholar]
- Faucqueur B, Proust J.[Streptococcus suis meningitis. An occupational disease.] Presse Med 1983121821French. [PubMed] [Google Scholar]
- Bonmarchand G, Massari P, Humbert G, et al. Group R streptococci: wild boars as a second reservoir. Scand J Infect Dis. 1985;17:121–122. doi: 10.3109/00365548509070431. [DOI] [PubMed] [Google Scholar]
- Desjars P, Reynaud AE, Tasseau F, Courtieu AL.[Streptococcus suis infections: about a meningitis in a slaughterhouse worker.] Med Maladies Infect 198717469–471.French. [Google Scholar]
- Dupas D, Vignon M, Geraut C. Streptococcus suis meningitis. A severe noncompensated occupational disease. J Occup Med. 1992;34:1102–1105. [PubMed] [Google Scholar]
- Bezian MC, Diallo BS, Chachia A, Gabinski C, Beyloi J.[Purpura fulminans during Streptococcus suis meningitis and septicemia.] Med Maladies Infect 199626349–351.French. [Google Scholar]
- Tayoro J, Besnier JM, Laudat P, Cattier B, Choutet P. Infective endocarditis due to Streptococcus suis serotype 2. Eur J Clin Microbiol Infect Dis. 1996;15:765–766. doi: 10.1007/BF01691970. [DOI] [PubMed] [Google Scholar]
- Caumont H, Gerard N, Depernet B, Brasme L, Eschard JP, Etienne JC.[Streptococcus suis L3–L4 spondylodiscitis in a butcher.] Presse Med 199625French. [PubMed] [Google Scholar]
- Bouchaud L, Bourgain C, Aouar M, Grise G, Morcamp D, Deséchalliers JP.[Streptococcus suis type II meningitis. About a case.] Med Maladies Infect 199727317–318.French. [Google Scholar]
- François B, Gissot V, Ploy MC, Vignon P. Recurrent septic shock due to Streptococcus suis. J Clin Microbiol. 1998;36:2395. doi: 10.1128/jcm.36.8.2395-2395.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand F, Périno CL, Recule C, et al. Bacteriological diagnosis of Streptococcus suis meningitis. Eur J Clin Microbiol Infect Dis. 2001;20:519–521. doi: 10.1007/pl00011299. [DOI] [PubMed] [Google Scholar]
- Bensaid T, Bonnefoi-Kyriacou B, Dupel-Pottier C, Bellon O, Lagier E, Chardon H.[Streptococcus suis meningitis following wild boar hunting.] Presse Med 2003321077–1078.French. [PubMed] [Google Scholar]
- Pedroli S, Kobisch M, Beauchet O, Chaussinand JP, Lucht F.[Streptococcus suis bacteremia.] Presse Med 200332599–601.French. [PubMed] [Google Scholar]
- Fauveau L, Mourtada Y, Hazouard E.[Meningoencephalitis related-to Streptococcus suis in a butcher: relevance of occupational questionnaire at emergency room.] Ann Fr Anesth Reanim 200726814–815.French. [DOI] [PubMed] [Google Scholar]
- Bahloul H, Mofredj A, Mrabet A, Gineyt G, Rousselier P.[Streptococcus suis meningitis after oral contamination?] Med Maladies Infect 200838281–282.French. [DOI] [PubMed] [Google Scholar]
- Rosenstingl S, Rouede Y, Galanti MJ, Gatfosse M.[Streptococcus suis septicaemia in a hunter's wife.] Med Maladies Infect 200838S177French. [Google Scholar]
- Piech C, Guettrot-Imbert G, Delevaux I, André M, Lhoste A, Aumaître O.[Streptococcus suis spondylodiscitis and oligoarthritis in a hunter.] Rev Med Intern 200930S142French. [Google Scholar]
- Kaufhold A, Lutticken R, Litterscheid S.[Systemic infection caused by Streptococcus suis.] Dtsch Med Wochenschr 19881131642–1643.German. [DOI] [PubMed] [Google Scholar]
- Bungener W, Bialek R. Fatal Streptococcus suis septicemia in an abattoir worker. Eur J Clin Microbiol Infect Dis. 1989;8:306–308. doi: 10.1007/BF01963457. [DOI] [PubMed] [Google Scholar]
- Grebe T, Bergenthal D, Fahr AM, Scheja HW.[Meningitis caused by Streptococcus suis type 2 in an adult.] Dtsch Med Wochenschr 19971221244–1247.German. [DOI] [PubMed] [Google Scholar]
- Strangmann E, Froleke H, Kohse KP. Septic shock caused by Streptococcus suis: case report and investigation of a risk group. Int J Hyg Environ Health. 2002;205:385–392. doi: 10.1078/1438-4639-00165. [DOI] [PubMed] [Google Scholar]
- Rosenkranz M, Elsner HA, Sturenburg HJ, Weiller C, Rother J, Sobottka I. Streptococcus suis meningitis and septicemia contracted from a wild boar in Germany. J Neurol. 2003;250:869–870. doi: 10.1007/s00415-003-1103-3. [DOI] [PubMed] [Google Scholar]
- Heidt MC, Mohamed W, Hain T, Vogt PR, Chakraborty T, Domann E. Human infective endocarditis caused by Streptococcus suis serotype 2. J Clin Microbiol. 2005;43:4898–4901. doi: 10.1128/JCM.43.9.4898-4901.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S, Jechart G, Emmerling U, Ehret W.[Meningitis caused by Streptococcus suis.] Dtsch Med Wochenschr 20071321098–1100.German. [DOI] [PubMed] [Google Scholar]
- Mazokopakis EE, Kofteridis DP, Papadakis JA, Gikas AH, Samonis GJ. First case report of Streptococcus suis septicaemia and meningitis from Greece. Eur J Neurol. 2005;12:487–489. doi: 10.1111/j.1468-1331.2005.00998.x. [DOI] [PubMed] [Google Scholar]
- Voutsadakis IA. Streptococcus suis endocarditis and colon carcinoma: a case report. Clin Colorectal Cancer. 2006;6:226–228. doi: 10.3816/CCC.2006.n.041. [DOI] [PubMed] [Google Scholar]
- Baddeley PG. Streptococcus suis infection. Occup Med. 1995;45:222. doi: 10.1093/occmed/45.4.222-a. [DOI] [PubMed] [Google Scholar]
- Manzin A, Palmieri C, Serra C, et al. Streptococcus suis meningitis without history of animal contact, Italy. Emerg Infect Dis. 2008;14:1946–1948. doi: 10.3201/eid1412.080679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camporese A, Tizianel G, Bruschetta G, Cruciatti B, Pomes A. Human meningitis caused by Streptococcus suis: the first case report from north-eastern Italy. Infez Med. 2007;15:111–114. [PubMed] [Google Scholar]
- Perseghin P, Bezzi G, Troupioti P, Gallina M. Streptococcus suis meningitis in an Italian blood donor. Lancet. 1995;346:1305–1306. doi: 10.1016/s0140-6736(95)91912-0. [DOI] [PubMed] [Google Scholar]
- Kloppenburg M, Mulder NH, Houwerzijl J. Septicaemia due to porcine streptococci. Lancet. 1975;306:1218. doi: 10.1016/s0140-6736(75)92719-1. [DOI] [PubMed] [Google Scholar]
- Zanen HC, Engel HW. Porcine streptococci causing meningitis and septicaemia in man. Lancet. 1975;305:1286–1288. [PubMed] [Google Scholar]
- van Jaarsveld BC, van Kregten E, van Kesteren RG, Rozenberg-Arska M, Bartelink AK.[Fulminant sepsis caused by Streptococcus suis.] Ned Tijdschr Geneeskd 19901341462–1464.Dutch. [PubMed] [Google Scholar]
- Bartelink AK, van Kregten E. Streptococcus suis as threat to pig-farmers and abattoir workers. Lancet. 1995;346:1707. doi: 10.1016/s0140-6736(95)92874-x. [DOI] [PubMed] [Google Scholar]
- Halaby T, Hoitsma E, Hupperts R, Spanjaard L, Luirink M, Jacobs J. Streptococcus suis meningitis, a Poacher's risk. Eur J Clin Microbiol Infect Dis. 2000;19:943–945. doi: 10.1007/pl00011230. [DOI] [PubMed] [Google Scholar]
- Peetermans WE, Moffie BG, Thompson J. Bacterial endocarditis caused by Streptococcus suis type 2. J Infect Dis. 1989;159:595–596. doi: 10.1093/infdis/159.3.595. [DOI] [PubMed] [Google Scholar]
- Arend SM, van Buchem MA, van Ogtrop ML, Thompson J. Septicaemia, meningitis and spondylodiscitis caused by Streptococcus suis type 2. Infection. 1995;23:128. doi: 10.1007/BF01833885. [DOI] [PubMed] [Google Scholar]
- van de Beek D, Spanjaard L, de Gans J. Streptococcus suis meningitis in the Netherlands. J Infect. 2008;57:158–161. doi: 10.1016/j.jinf.2008.04.009. [DOI] [PubMed] [Google Scholar]
- de Ceuster LM, van Dillen JJ, Wever PC, Rozemeijer W, Louwerse ES.[Streptococcus suis meningitis in a meat factory employee.] Ned Tijdschr Geneeskd 2012156A5080Dutch. [PubMed] [Google Scholar]
- Zalas-Wiecek P, Michalska A, Grabczewska E, Olczak A, Pawlowska M, Gospodarek E. Human meningitis caused by Streptococcus suis. J Med Microbiol. 2013;62:483–485. doi: 10.1099/jmm.0.046599-0. [DOI] [PubMed] [Google Scholar]
- Taipa R, Lopes V, Magalhaes M. Streptococcus suis meningitis: first case report from Portugal. J Infect. 2008;56:482–483. doi: 10.1016/j.jinf.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Dragojlovic J, Milosevic B, Sasic N, Pelemis M, Sasic M.[Streptococcus suis infection–clinical manifestations.] Med Pregl 200558236–239.Serbian. [DOI] [PubMed] [Google Scholar]
- Tarradas C, Luque I, de Andres D, et al. Epidemiological relationship of human and swine Streptococcus suis isolates. J Vet Med B Infect Dis Vet Public Health. 2001;48:347–355. doi: 10.1046/j.1439-0450.2001.00460.x. [DOI] [PubMed] [Google Scholar]
- Vela AI, Aspiroz C, Fortuno B, et al. Meningitis caused by an unusual genotype (ST3) of Streptococcus suis. Infection. 2013;41:701–703. doi: 10.1007/s15010-012-0382-y. [DOI] [PubMed] [Google Scholar]
- Martinez Aviles P, Jusdado Ruiz-Capillas JJ, Gomez Rodrigo J, Solis Villa J.[Sacroiliitis caused by Streptococcus suis type II.] An Med Interna 199411309Spanish. [PubMed] [Google Scholar]
- Juncal AR, Pardo F, Rodriguez I, Perez del Molino ML.[Meningitis by Streptococcus suis.] Enferm Infecc Microbiol Clin 199715120–121.Spanish. [PubMed] [Google Scholar]
- de la Hoz Adame ME, de la Rubia Martin F, Dominguez Fuentes B, Garcia Gil D.[Acute meningitis caused by Streptococcus suis: review of a case in a splenectomized patient.] An Med Interna 200522507Spanish. [PubMed] [Google Scholar]
- Alonso-Socas Mdel M, Aleman-Valls R, Roldan-Delgado H, Gomez-Sirvent JL.[Endocarditis and spondylodiscitis caused by Streptococcus suis.] Enferm Infecc Microbiol Clin 200624354–355.Spanish. [DOI] [PubMed] [Google Scholar]
- Luengo-Alvarez J, Martin-Ruiz C, Sanchez Munoz-Torrero JF, Iniguez-Ovando R.[Meningitis due to Streptococcus suis: a case report.] Enferm Infecc Microbiol Clin 200624352–354.Spanish. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Ropero F, Palacios R, Garcia V, Santos J. Meningitis due to Streptococcus suis with no contact with pigs or porcine products. J Infect. 2007;55:478. doi: 10.1016/j.jinf.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Riquelme E, Escribano E, Blanch JJ, Crespo MD.[Acute Streptococcus suis meningitis in a woman working in a meat market.] Enferm Infecc Microbiol Clin 200826256–257.Spanish. [DOI] [PubMed] [Google Scholar]
- Aspiroz C, Vela AI, Pascual MS, Aldea MJ.[Acute infective endocarditis due to Streptococcus suis serotype 2 in Spain.] Enferm Infecc Microbiol Clin 200927370–371.Spanish. [DOI] [PubMed] [Google Scholar]
- Galbarro J, Franco-Alvarez de Luna F, Cano R, Angel Castano M.[Acute meningitis and spondylodiscitis due to Streptococcus suis in a patient who had no contact with pigs or porcine products.] Enferm Infecc Microbiol Clin 200927425–427.Spanish. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ferro J, Lopez-Gonzalez FJ, Pardo F, Pias-Peleteiro JM.[Acute Streptococcus suis meningitis in a pig breeder.] Enferm Infecc Microbiol Clin 201129396–397.Spanish. [DOI] [PubMed] [Google Scholar]
- Christensen P, Kronvall G.[A case of Streptococcus suis meningitis—a new occupational disease in Sweden?] Lakartidningen 198582119Swedish. [PubMed] [Google Scholar]
- Hickling P, Cormack FC. Meningitis caused by group R haemolytic streptococci. Br Med J. 1976;2:1299–1300. doi: 10.1136/bmj.2.6047.1299-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agass MJ, Willoughby CP, Bron AJ, Mitchell CJ, Mayon-White RT. Meningitis and endophthalmitis caused by Streptococcus suis type II (group R) Br Med J. 1977;2:167–168. doi: 10.1136/bmj.2.6080.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLendon BF, Bron AJ, Mitchell CJ. Streptococcus suis type II (group R) as a cause of endophthalmitis. Br J Ophthalmol. 1978;62:729–731. doi: 10.1136/bjo.62.10.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joynson DH. Infections of man and Group R Streptococci. Br J Clin Pract. 1980;34:147–149, 154. [PubMed] [Google Scholar]
- Shneerson JM, Chattopadhyay B, Murphy MF, Fawcett IW. Permanent perceptive deafness due to Streptococcus suis type II infection. J Laryngol Otol. 1980;94:425–427. doi: 10.1017/s0022215100089040. [DOI] [PubMed] [Google Scholar]
- Twort CH. Group R streptococcal meningitis (Streptococcus suis type II): a new industrial disease. Br Med J (Clin Res Ed) 1981;282:523–524. doi: 10.1136/bmj.282.6263.523-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements MR, Hamilton DV, Clifton-Hadley FA, O'Reilly JF. Streptococcus suis type II infection. A new industrial disease. Practitioner. 1982;226:323–325. [PubMed] [Google Scholar]
- McNeil NI, Gordon T. Meningitis caused by Streptococcus suis type II. Postgrad Med J. 1986;62:743–744. doi: 10.1136/pgmj.62.730.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay PE, Cunniffe JG, Kramer G, France AJ, Gray JA, Watt B. Two cases of Streptococcus suis meningitis. Br J Ind Med. 1989;46:352–353. doi: 10.1136/oem.46.5.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meecham JS, Worth RC. Persistent diplopia following Streptococcus suis type 2 meningitis. J R Soc Med. 1992;85:579–580. doi: 10.1177/014107689208500927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins EJ, Brooksby P, Schweiger MS, Enright SM. Septicaemia in a pig-farm worker. Lancet. 2001;357:38. doi: 10.1016/s0140-6736(00)03570-4. [DOI] [PubMed] [Google Scholar]
- Doube A, Calin A. Bacterial endocarditis presenting as acute monoarthritis. Ann Rheum Dis. 1988;47:598–599. doi: 10.1136/ard.47.7.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher D. Streptococcus suis septicaemia presenting as severe acute gastro-enteritis. J Infect. 1991;22:303–304. doi: 10.1016/s0163-4453(05)80022-2. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Morland AB, Ruddock KH, Gresty MA. Recovery from bilateral vestibular failure: implications for visual and cervico-ocular function. Acta Otolaryngol Suppl. 1995;520 (Pt 2:405–407. doi: 10.3109/00016489509125283. [DOI] [PubMed] [Google Scholar]
- Rao SS, Mariathas A, Teare L. Meningitis in a butcher. Emerg Med J. 2008;25:607–608. doi: 10.1136/emj.2007.052233. [DOI] [PubMed] [Google Scholar]
- Kennedy KJ, Jadeer AA, Ong CW, Senanayake SN, Collignon PJ. Two cases of Streptococcus suis endocarditis in Australian piggery workers. Med J Aust. 2008;189:413. doi: 10.5694/j.1326-5377.2008.tb02100.x. [DOI] [PubMed] [Google Scholar]
- Tramontana AR, Graham M, Sinickas V, Bak N. An Australian case of Streptococcus suis toxic shock syndrome associated with occupational exposure to animal carcasses. Med J Aust. 2008;188:538–539. doi: 10.5694/j.1326-5377.2008.tb01771.x. [DOI] [PubMed] [Google Scholar]
- Rojas MT, Gottschalk M, Velazquez Ordonez V.[Virulence evaluation of serotypes of Streptococcus suis isolated in slaughterhouse workers in the valley of Toluca, State of Mexico, Mexico.] Vet Mex 200132201–205.Spanish. [Google Scholar]
- Quessy S, Dubreuil JD, Caya M, Higgins R. Discrimination of virulent and avirulent Streptococcus suis capsular type 2 isolates from different geographical origins. Infect Immun. 1995;63:1975–1979. doi: 10.1128/iai.63.5.1975-1979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson ID, Blackmore DK. Occupational exposure to Streptococcus suis type 2. Epidemiol Infect. 1989;103:157–164. doi: 10.1017/s0950268800030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbers AR, Vecht U, Osterhaus AD, et al. Low prevalence of antibodies against the zoonotic agents Brucella abortus, Leptospira spp., Streptococcus suis serotype II, hantavirus, and lymphocytic choriomeningitis virus among veterinarians and pig farmers in the southern part of The Netherlands. Vet Q. 1999;21:50–54. doi: 10.1080/01652176.1999.9694991. [DOI] [PubMed] [Google Scholar]
- Smith TC, Capuano AW, Boese B, Myers KP, Gray GC. Exposure to Streptococcus suis among US swine workers. Emerg Infect Dis. 2008;14:1925–1927. doi: 10.3201/eid1412.080162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala V, Colombo A, Gerola L.[Infection Risks of Streptococcus suis Type 2 Localizations in Slaughtered Swines.] Arch Vet It 198940180–184.Italian. [Google Scholar]
- Walsh B, Williams AE, Satsangi J. Streptococcus suis type 2: pathogenesis and clinical disease. Rev Med Microbiol. 1992;3:65–71. [Google Scholar]
- de Greeff A, Wisselink HJ, de Bree FM, et al. Genetic diversity of Streptococcus suis isolates as determined by comparative genome hybridization. BMC Microbiol. 2011;11:161. doi: 10.1186/1471-2180-11-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye C, Bai X, Zhang J, et al. Spread of Streptococcus suis sequence type 7, China. Emerg Infect Dis. 2008;14:787–791. doi: 10.3201/eid1405.070437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Ye C, Jing H, et al. Streptococcus suis outbreak investigation using multiple-locus variable tandem repeat number analysis. Microbiol Immunol. 2010;54:380–388. doi: 10.1111/j.1348-0421.2010.00228.x. [DOI] [PubMed] [Google Scholar]
- Rehm T, Baums CG, Strommenger B, Beyerbach M, Valentin-Weigand P, Goethe R. Amplified fragment length polymorphism of Streptococcus suis strains correlates with their profile of virulence-associated genes and clinical background. J Med Microbiol. 2007;56:102–109. doi: 10.1099/jmm.0.46616-0. [DOI] [PubMed] [Google Scholar]
- Blume V, Luque I, Vela AI, et al. Genetic and virulence-phenotype characterization of serotypes 2 and 9 of Streptococcus suis swine isolates. Int Microbiol. 2009;12:161–166. [PubMed] [Google Scholar]
- Tang Y, Zhao H, Wu W, Wu D, Li X, Fang W. Genetic and virulence characterization of Streptococcus suis type 2 isolates from swine in the provinces of Zhejiang and Henan, China. Folia Microbiol (Praha) 2011;56:541–548. doi: 10.1007/s12223-011-0077-2. [DOI] [PubMed] [Google Scholar]
- Zhu W, Wu C, Sun X, et al. Characterization of Streptococcus suis serotype 2 isolates from China. Vet Microbiol. 2013;166:527–534. doi: 10.1016/j.vetmic.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Ye C, Zhu X, Jing H, et al. Streptococcus suis sequence type 7 outbreak, Sichuan, China. Emerg Infect Dis. 2006;12:1203–1208. doi: 10.3201/eid1208.060232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi H, Sugawara M, Okura M, Osaki M, Takamatsu D. Prevalence of Streptococcus suis genotypes in isolates from porcine endocarditis in East Japan. J Vet Med Sci. 2012;74:1681–1684. doi: 10.1292/jvms.12-0301. [DOI] [PubMed] [Google Scholar]
- Luey CK, Chu YW, Cheung TK, et al. Rapid pulsed-field gel electrophoresis protocol for subtyping of Streptococcus suis serotype 2. J Microbiol Methods. 2007;68:648–650. doi: 10.1016/j.mimet.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Chatellier S, Gottschalk M, Higgins R, Brousseau R, Harel J. Relatedness of Streptococcus suis serotype 2 isolates from different geographic origins as evaluated by molecular fingerprinting and phenotyping. J Clin Microbiol. 1999;37:362–366. doi: 10.1128/jcm.37.2.362-366.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Tang J, Dong W, et al. A glimpse of streptococcal toxic shock syndrome from comparative genomics of S. suis 2 Chinese isolates. PLoS One. 2007;2:e315. doi: 10.1371/journal.pone.0000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecht U, Wisselink HJ, Jellema ML, Smith HE. Identification of two proteins associated with virulence of Streptococcus suis type 2. Infect Immun. 1991;59:3156–3162. doi: 10.1128/iai.59.9.3156-3162.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]