Abstract
Cancer progression is associated with inflammation, increased metabolic demand, infection, cachexia, and eventually, death. Myeloid-derived suppressor cells (MDSCs) commonly expand during cancer and are associated with adaptive immune suppression and inflammatory metabolite production. We propose that cancer-induced cachexia is driven at least in part by the expansion of MDSCs. MDSC expansion in 4T1 mammary carcinoma-bearing hosts is associated with induction of a hepatic acute phase protein response and altered host energy and fat metabolism, and eventually, reduced survival to polymicrobial sepsis and endotoxemia. Similar results are also seen in mice bearing a Lewis lung carcinoma and a C26 colon adenocarcinoma. However, a similar cachexia response is not seen with equivalent growth of the 66C4 subclone of 4T1, where MDSC expansion does not occur. Importantly, reducing MDSC numbers in 4T1-bearing animals can ameliorate some of these late responses, and reduce susceptibility to inflammation-induced organ injury and death. In addition, administering MDSCs from both tumor and nontumor-bearing mice can produce an acute phase response. Thus, we propose a previously undescribed mechanism for the development of cancer cachexia, whereby progressive MDSC expansion contributes to changes in host protein and energy metabolism, and reduced resistance to infection.
Keywords: Inflammation, acute phase protein, myeloid derived suppressor cells
Introduction
Cancer-associated cachexia is a multi-factorial syndrome that develops in the latter stages of malignancy, and is associated with an acute phase response, loss of body protein and/or fat, and immune dysregulation (1–3). Cachexia is a chronic inflammatory syndrome that manifests predominantly as the progressive wasting of body fat and/or protein, and changes in energy balance that cannot be explained by anorexia alone, and is associated with an ongoing hepatic acute phase response, anemia, hyperlipidemia and presence of sickness syndromes (4). Clinically, cancer patients who develop cachexia are more susceptible to infection and sepsis (5). The systemic release of proinflammatory cytokines, such as TNFα, IL-1β, and IL-6, from tumor-derived tissues and innate immune effector cells are still often promulgated as the primary etiology of cancer cachexia (3, 6), but multiple clinical studies have demonstrated that these inflammatory mediators cannot be wholly responsible for the complexity of this syndrome (7–9). Therefore, the processes which drive development of cancer-associated cachexia remain unanswered.
Several groups have hypothesized that activation of innate immunity and inflammation during active tumor growth may play a role in the development of cachexia (2, 3, 10–12). Tumor-induced amplification of the myeloid compartment leads to the expansion of myeloid-derived suppressor cells (MDSCs) which are immature precursors of the myeloid lineage (13, 14). MDSCs were initially discovered in abnormally high numbers in the setting of advanced malignancy, although they have since been demonstrated in a variety of other inflammatory conditions including autoimmunity and sepsis, with their primary function being to suppress adaptive immune function (14, 15).
We demonstrate here that regardless of the tumor type, increasing tumor burden and MDSC expansion greater than 100-fold over the course of tumor progression is associated with increased energy expenditure, loss of tissue adiposity, a hepatic acute phase protein response, and ultimately reduced resistance to infection. These responses are not seen to the same degree in animals with a histologically-similar, primary tumor burden, but in the absence of MDSC expansion. Additionally, depletion of all phagocytic cells, including MDSCs, in advanced tumor growth improves outcome to a subsequent inflammatory challenge. Administration of putative MDSCs from either a nontumor or tumor-bearing mouse to a healthy animal induces an acute phase response. Although a direct causal relationship between the development of cancer cachexia and increased mortality to sepsis and inflammation with the expansion of MDSCs cannot be conclusively proven, we can conclude that the development of cachexia is at least partly explained by the massive expansion of immature myeloid populations associated with a growing tumor. Therapies already in the clinic targeting MDSC expansion in patients with advanced cancer may have the additional benefit of reducing sickness syndromes and cachexia.
Methods
Mice
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Florida, College of Medicine before their initiation. Mice were maintained on standard food and water ad libitum. Six to eight week-old Balb/c and C57BL/6j female mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and were used between 7 and 9 weeks of age. All mice were maintained in specific pathogen-free conditions at a breeding colony at the University of Florida Health Science Center.
Cell lines
The murine mammary carcinoma, 4T1, was obtained from Drake LaFace, Merck Research Laboratories (Palo Alto, CA) and the 66C4 subclone of 4T1 was obtained from Fred Miller, Karmanos Cancer Institute (Detroit, MI). Lewis lung carcinoma (LLC) and Colon-26 adenocarcinoma (CT26) cells were obtained originally from American Type Cell Culture (Manassas, VA). All cell lines were grown in Dulbecco’s Modified Eagle Medium (DMEM) with fetal bovine serum and penicillin/streptomycin (Cellgro; Manassas, VA). On the day of tumor inoculation, cultured cells were trypsinized, washed in phosphate buffered saline (PBS; Invitrogen GIBCO; Carlsbad, CA), and injected into the right flank of mice. A total of 7 × 103 tumor cells per animal were injected.
Magnetic bead enrichment.of Gr-1+ cells for adoptive transfer or in vitro experiments
A splenocyte cell suspension was made from spleens harvested from day 28 post 4T1 inoculation animals or healthy, nontumor-bearing animals. To remove red blood cells (RBC), splenocyte suspensions were then overlayed on top of Histopaque 1119 and centrifuged (Sigma Aldrich; St. Louis, MO). RBC depleted splenocyte suspensions were then stained with Anti-Gr-1-APC (Miltenyi Biotech; Auburn, CA), anti-APC-microbeads (Miltenyi),and run over a MACS® column twice (Miltenyi). Purity was verified via flow cytometry and yields were consistently >95% pure.
For adoptive transfer experiments, naïve Balb/c animals were injected intravenously with 1 × 107 magnetically-enriched Gr-1+ cells from 4T1-bearing animals at days 1, 3, and 5. Serum was collected at days 2, 4, 6, 10, and 14 and assayed for concentrations of serum amyloid A, haptoglobin, and hemopexin.
Flow cytometry
Spleens were harvested and single cell suspension made by passing cells through 70-µm pore-size cell strainer (Falcon; Heidelberg, Germany). Erythrocytes were lysed with an ammonium chloride lysis solution. Cells were then stained with either anti-Gr-1 allophycocyanin (APC), anti-Ly6G APC, anti-Ly6C peridinin-chlorophyll Cy™5.5 (PerCP Cy™5.5), CD11b R-phycoerythrin-Cy7 (PE-Cy7), CD31 R-phycoerythrin. All antibodies were purchased from BD-Pharmingen (San Jose, CA) unless otherwise noted.
Body Composition
Total body adipose, lean, and fluid measurements were taken at 0, 14, and 28 days post tumor inoculation by time domain-nuclear magnetic resonance (TD-NMR) (Bruker Corporation; Madison, WI). In tumor-bearing animals, the primary tumor was removed before measurement.
Oxygen Consumption
Oxygen consumption was assessed in mice with an oxygen analyzer (model S-3A, AEl Technologies; Naperville, IL). Flow rates were 300 mL/min with a one-minute sampling time at eight minute intervals. The mice were acclimated to the measurement chambers for at least an hour before measurements were taken. Oxygen consumption was then assessed for the next hour. Food was not available during this time period and all measurements were taken during the light portion of the diurnal cycle. Results are expressed as mL/minute/ kg body weight or mL/minute/ per kg tumor-free lean body mass (kg). Lean body weight was assessed using TD-NMR.
Cyto/chemokine detection
Serum was collected via intracardiac puncture from day 28 post 4T1, LLC and CT26 inoculation, and day 35 post 66C4 inoculation and assayed via Luminex multiplex cytokine array (Miltenyi). Serum concentrations of IL-1β, TNFα, IL-6, and IFN-γ were then determined.
Serum acute phase biomarkers
Blood was harvested via intracardiac puncture from tumor or nontumor-bearing mice at 0, 7, 14, 21, 28, and 35 days post tumor inoculation. Plasma was then assayed via ELISA for serum amyloid A (SAA), haptoglobin, and hemopexin (Life Diagnostics Inc; Westchester, PA).
AST/ALT determination
Serum was collected from day 21 post 4T1 inoculation, day 28 post 66C4 inoculation, and NT bearing animals at 0, 6, 12 hours post LPS administration and assayed for the presence of AST/ALT via a Dimension® Xpand® Plus integrated Chemistry System (Siemens; Deerfield, IL).
Endotoxicosis
Nontumor-bearing mice and animals having similar primary tumor burdens (66C4-bearing animals 38 days post inoculation and 4T1-bearing animals 28 days post inoculation) were injected intraperitoneally (ip) with 50 µg lipopolysaccharide from Escherichia coli O26:B6 (Sigma-Aldrich; St Louis, MO). Mice were followed every six hours for 72 hours for survival.
Cecal ligation and puncture
Polymicrobial sepsis was induced via cecal ligation and puncture as previously described (16, 17). Briefly, laparotomies were performed and the cecum was ligated and punctured through and through with a 25 gauge needle in nontumor-bearing mice and animals having similar primary tumor burdens (66C4-bearing animals 35 days post inoculation and 4T1-bearing animals 28 days post inoculation). Animals were followed every 12 hours for seven days post procedure for survival.
Clodronate liposome depletion
Balb/Cj mice 28 days post 4T1 inoculation were injected with either PBS or clodronate liposomes (Encapsula Nanosciences, Nashville TN) i.p. at 100 uL of liposome suspension/ 10 g of body weight. An additional cohort of non-tumor bearing mice were also injected with PBS liposomes. Animals were then monitored for 36–48 hours. Next, mice were injected with LPS (50 µg) i.p. and followed for 72 hrs for survival. A separate cohort of PBS and clodronate liposome injected mice was euthanized and necropsied. Spleens were harvested and analyzed for the presence absolute numbers of CD11b+ and Gr-1+ cells via flow cytometry.
Lung and liver tissue processing
Lung and liver tissues were harvested at 0, 6, and 12 hours post sublethal endotoxin challenge. Tissues were subsequently fixed in 10% formalin overnight and paraffin embedded. Five micron sections of liver and lung were then stained with hematoxylin and eosin. Paraffin embedded lung tissues were also stained with Ly6G+ via immunohistochemistry. Briefly, five micron sections were deparaffinized and exposed to sodium citrate for antigen retrieval. Sections were then stained with anti-Ly6G (Ebioscience, San Diego, CA), developed using DAB (Vector Laboratories; Burlingame, CA), and counterstained with hematoxylin.
Statistics
Differences among groups were evaluated by Student’s t test, Fisher’s exact test, or one-way ANOVA using SigmaPlot v11 (Systat Software, San Jose, CA). Significance was determined at the 95% confidence level.
Results
Characterization of tumor in vivo kinetics and MDSC expansion
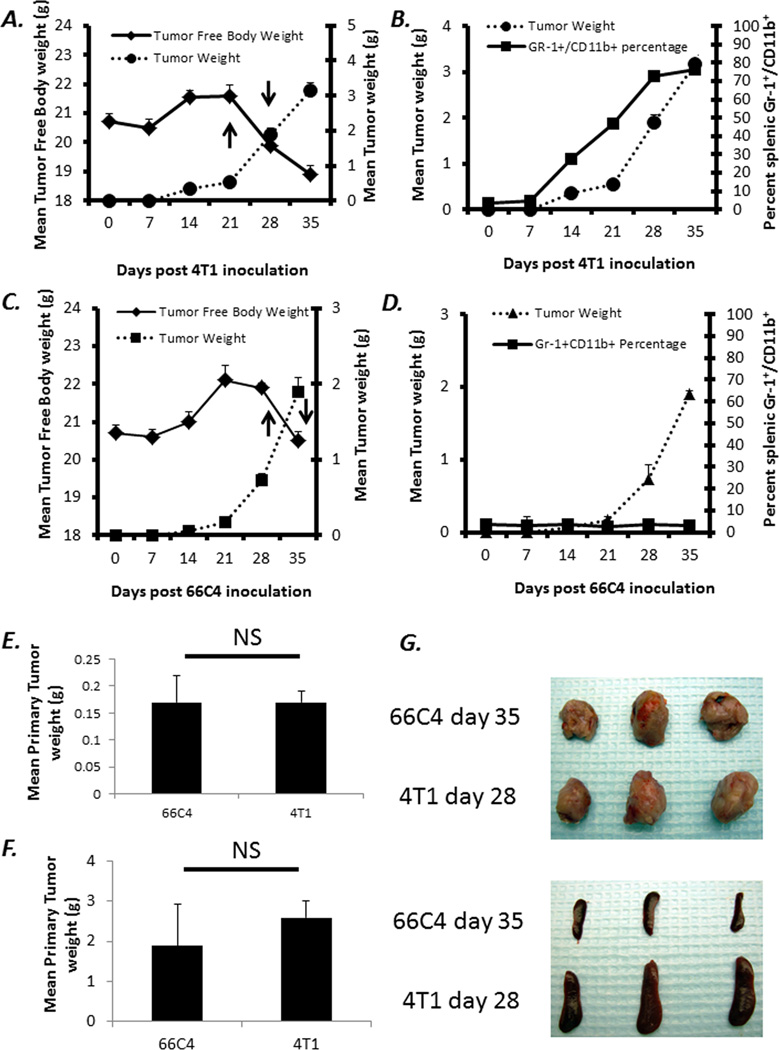
MDSCs have been demonstrated by multiple investigators to not only expand to greater than 100-fold in tumor-bearing animals, but also to produce increased amounts of both ROS and NO (13, 18). Though these properties have been assumed to explain the suppression of antigen-specific T cell responses, they are also potent mediators of anti-microbial pathogen defense (13, 18, 19). As shown in Figure 1, 4T1 tumor growth is associated with a massive increase in the number of Gr-1+CD11b+ splenocytes. By 28 days, the number of splenic Gr-1+CD11b+ is increased to greater than 1 × 108 cells/spleen. We have phenotyped these cells and approximately 5% are CD31+ and greater than 90% are MHC-IIdim/− (data not shown and Supplemental Figure 1). In contrast, the 4T1 subclone, 66C4, does not exhibit any increase in the number of Gr-1+CD11b+ cells (Figure 1). These expanded Gr-1+CD11b+ cells produce increased amounts of ROS and suppress T cell proliferation compared to Gr-1+CD11b+ cells from 66C4 and non-tumor bearing animals (data not shown)(13). Due to the fact that the in vivo growth of 66C4 is slower than 4T1, 66C4-bearing animals were inoculated approximately seven days before 4T1 animals so that experiments were conducted in mice with comparable primary tumor burdens and after the animals had experienced a loss in total tumor-free body weight (Figure 1).
Figure 1. In vivo growth kinetics of 4T1 and 66C4 mammary carcinoma.
4T1 and 66C4 tumor cells were inoculated into Balb/c mice and followed for 35 days. (A and C) Body and Tumor weight measurements were taken at seven day intervals up to day 35. (B and D) Spleens were harvested from animals and assayed via flow cytometry for MDSCs also at seven day intervals (Gr-1+,CD11b+). Values represent percentage of live cells ± SD (n=5). (E) Comparison of primary tumor burden at day 35 post 66C4 inoculation and day 28 post 4T1 inoculation (NS: Not Significant) (F) Comparison of primary tumor burden at day 35 post 66C4 inoculation and day 28 post 4T1 inoculation (NS=Not Significant) (G) Primary tumors and lungs from 4T1 (28 days post inoculation) and 66C4 (35 days post inoculation).
Loss of total adipose tissue, increase in oxygen consumption, and increase in acute phase reactants in 4T1 compared to 66C4 bearing animals
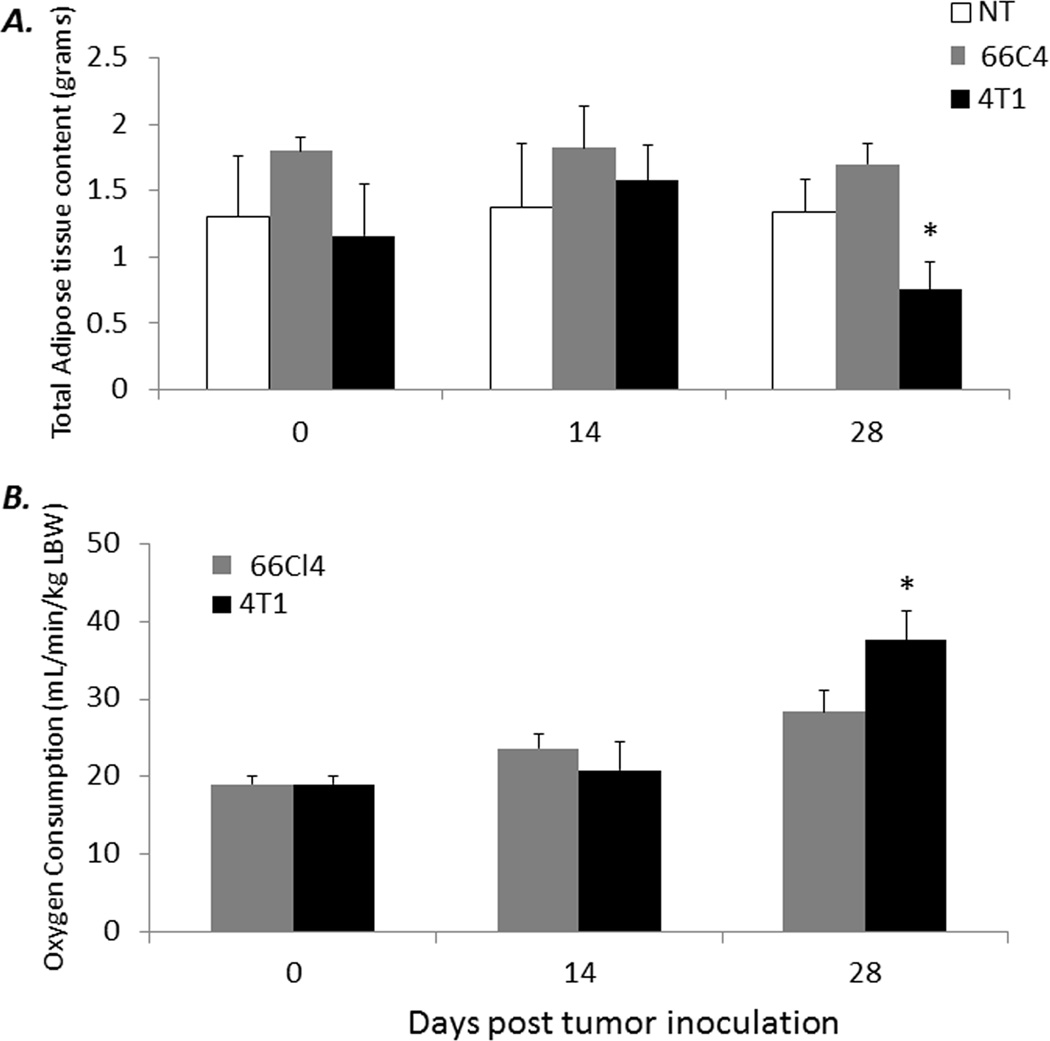
Cancer cachexia is generally associated with advanced tumor growth in most human and murine cancers (1–3). The energy demands incurred by progressive tumor growth and its effect on host tissues leads to changes in body composition (i.e. changes in total lean or adipose tissue) and is one of the primary components responsible for cachexia in cancer-bearing hosts (1, 2). To address the question of whether the expansion of MDSCs can explain the increases in energy expenditure or oxygen consumption (VO2) in tumor-bearing animals, we compared both body composition and energy expenditure via TD-NMR and VO2, respectively, in 4T1, 66C4, and nontumor-bearing mice at 0, 14, and 28 days post tumor inoculation. Although no losses in nontumor lean tissue are observed, a significant decrease in total adiposity is noted in 4T1-bearing mice at day 28, compared to 66C4- (at day 35) or nontumor-bearing animals (p <0.05) (Figure 2A). Similarly, a comparison of VO2 per tumor-free, lean body weight demonstrates a significant increase in oxygen consumption in 4T1-bearing animals compared to 66C4- or nontumor-bearing mice (Figure 2B). Importantly, these observations are not associated with significant differences in systemic cytokine concentrations typically associated with cachexia (Supplemental Figure 2).
Figure 2. Changes in body composition, increased oxygen consumption and increased acute phase responses in 4T1-bearing but not 66C4-bearing animals.
(A) 4T1-, 66C4-, and nontumor- (NT) bearing mice were assayed at 0, 14, and 28 days post 4T1 tumor inoculation for total adipose tissue content via TD-NMR. (n=4–5 per group; * Tukey’s One way-ANOVA p <0.001). (B) Oxygen consumption (VO2) per total lean tumor free body weight in 4T1- and 66C4- was measured at 0, 14, and 28 days post 4T1 tumor inoculation. Values represent mean ± SD (n=4–5 per group; * Student’s t-test p =0.004)
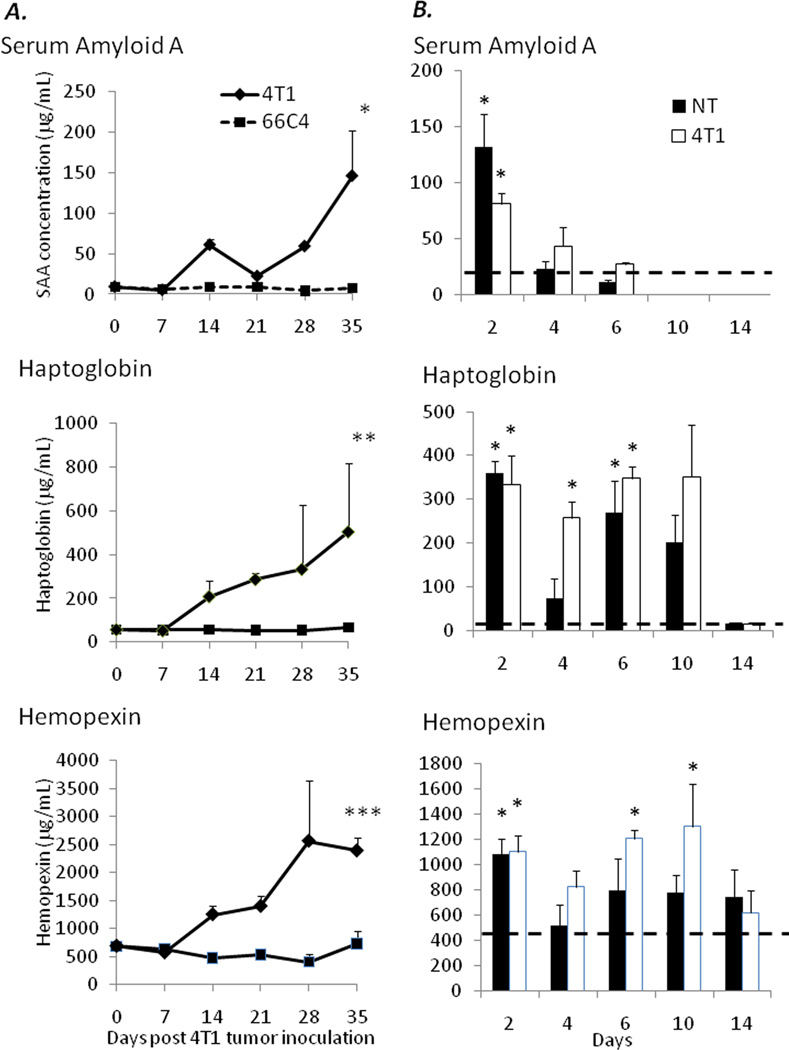
Another facet of tumor-induced cachexia is a chronic inflammatory state due to soluble factors secreted by both the tumor and innate immune effector cells (2, 20, 21). This clinical state is associated with the induction and increase in hepatic acute phase proteins (APP) during advanced stages of disease. As MDSCs have been shown to secrete increased quantities of inflammatory cytokines and chemokines (22, 23), we addressed whether or not the expansion of MDSCs contributes to the initiation or increased APP responses observed in cachexia. To this end, plasma was collected from 4T1- and 66C4-bearing animals at 0, 7, 14, 21, 28, and 35 days post tumor growth and assayed for the presence of three representative murine APPs, serum amyloid A, haptoglobin and hemopexin.
As demonstrated in Figure 3, as early as 14 days post tumor growth, 4T1-, but not 66C4-bearing animals have significantly increased plasma concentrations of all three APPs. As the increased production of APPs is one of the hallmarks distinguishing inflammation-associated cachexia from simple inanition (1), these data further strengthen the hypothesis that MDSCs contribute to the chronic inflammatory milieu present during advanced tumor growth that may accelerate the development of cachexia in tumor-bearing hosts.
Figure 3.
(A) Plasma was harvested from 4T1- and 66C4-bearing mice at 0, 7, 14, 21, 28, and 35 days post tumor inoculation and assayed for serum amyloid A and haptoglobin via ELISA. Values represent mean concentration in ng/mL and are ± SD (n=4–5/group) (* Mann- Whitney Rank Sum Test p=0.01) (** Mann Whitney Rank Sum Test p=0.002) (*** Student’s t-test p <0.001). (B) Naïve Balb/c animals were injected intravenously with 1 × 107 magnetically enriched Gr-1+ cells from healthy, nontumor and 4T1-bearing animals at days 1, 3, and 5. Serum was collected at days 2, 4, 6, 10, and 14 and assayed for concentrations of serum amyloid A, haptoglobin, and hemopexin. Hashed lines represent values obtained from sham injections (* p<0.05).
We also intravenously administered 1×107 GR-1+ splenocytes obtained from both nontumor-bearing animals and 28 day 4T1 tumor-bearing mice into naïve nontumor-bearing animals on days 1, 3, and 5. Serum was collected at days 2, 4, 6, 10, and 14 and assayed for concentrations of serum amyloid A, haptoglobin, and hemopexin. As shown in Figure 3, although the acute phase protein response was only modestly lower in animals receiving GR-1+ cells from a healthy than a tumor-bearing animal, both administrations produced a dramatic increase in the acute phase response. This suggests that simply expanding the numbers of these cells are required to elicit an acute phase response.
Expansion of MDSCs in classic tumor models of cachexia are associated with an increase in the loss of adipose tissue, increase in VO2, and increase in the production acute phase reactants
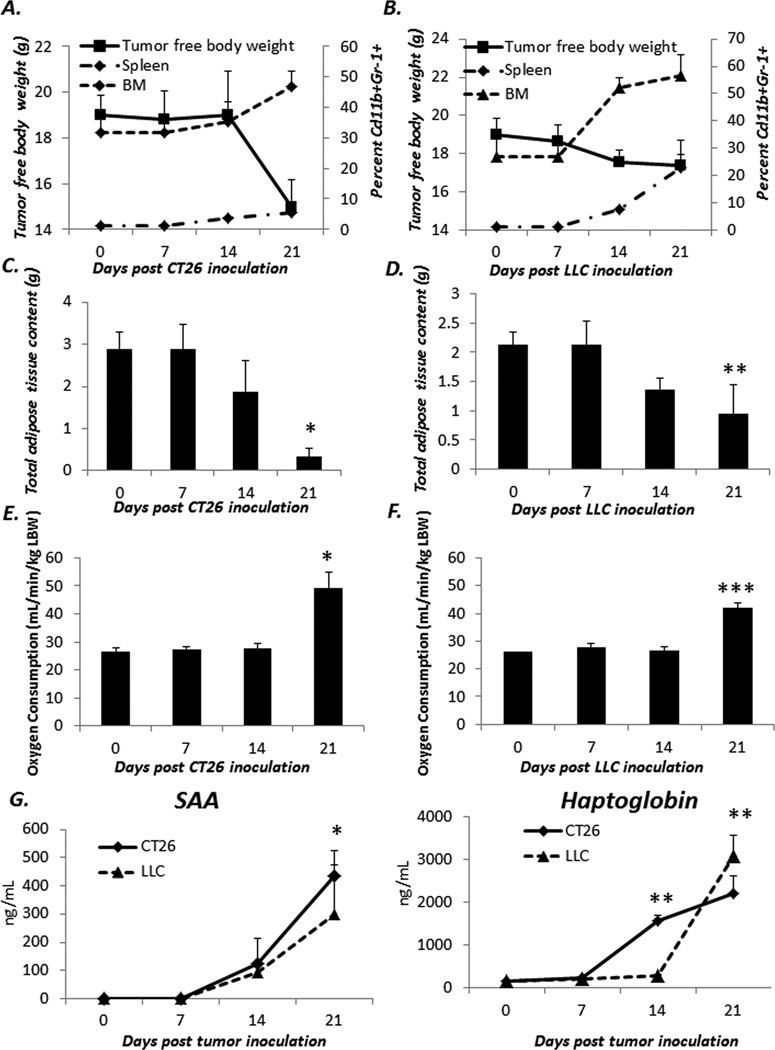
While these metabolic and immunologic changes are evident in the 4T1 tumor model in which MDSCs do expand compared to the 66C4 model where MDSCs do not, it is possible that these observations are only true for 4T1. Therefore, we examined if these changes in MDSCs also occurred in other classic models of murine cancer cachexia, namely Lewis lung carcinoma (LLC) and colon carcinoma 26 (CT26). As shown in Figure 4A and B, murine hosts inoculated with either LLC or CT26 undergo a significant CD11b+Gr-1+ cell expansion in both the spleen and bone marrow. In addition, similar to 4T1, a smaller more immature CD31+ subset also expands proportionally with tumor growth. To determine if these changes in myeloid cells also correlated with changes in body composition, LLC or CT26 bearing animals were analyzed via TD-NMR. As demonstrated in 4T1 bearing animals, both LLC and CT26 bearing animals had significant loss of adiposity over the course of tumor growth (Figure 4C and D). Similarly, both LLC and CT26 animals had significant increases in oxygen consumption (Figure 4E and F). Finally, we also measured the serum concentrations of serum amyloid-A and haptoglobin (Figure 4G) and show that LLC and CT26 bearing hosts have significant increases in these acute phase reactants as these MDSCs expand. Together these data support the findings from the 4T1 model that the expansion of MDSCs during tumor growth promote and/or exacerbate cachexia in tumor bearing hosts.
Figure 4. Changes in adiposity, oxygen consumption, and acute phase reactants also occur in other tumor models of cachexia following expansion of Gr-1+CD11b+ cells in the spleen and bone marrow.
CT26 and LLC was inoculated into naïve mice and followed for 21 days. Spleen and bone marrow (A and B) were harvested from tumor bearing animals at 7 day intervals and plotted against tumor free body weight (n=5/group). Total host adipose tissue content (C and D) and oxygen consumption (E and F) were measured at 7 day intervals as well. Values represent mean ± SD (n=5/group; * One way ANOVA on ranks p<0.001; ** Kruskall-Wallis One way ANOVA on ranks p=0.002; *** ANOVA on ranks p=0.009). Finally, serum from tumor bearing host was assayed via ELISA for the presence of serum amyloid A (SAA) and Haptoglobin (G) Values represent mean concentration in ng/mL and are ± SD (n=5/group; * One way ANOVA on ranks p<0.05; **Kruskall-Wallis One way ANOVA on ranks p<0.001).
Advanced stage 4T1-bearing, but not 66C4-bearing mice, are more susceptible to endotoxemia and polymicrobial sepsis
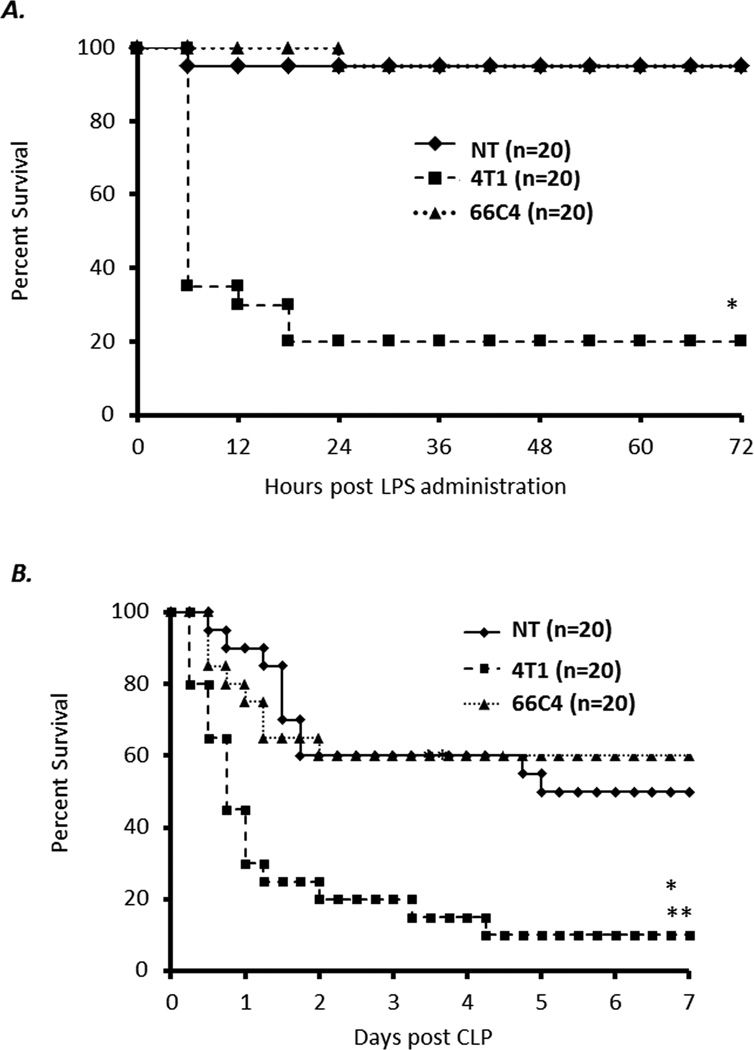
Patients with advanced cancer are known to be at increased risk for severe infection and sepsis (24, 25). To examine whether or not the continued expansion of MDSCs observed in 4T1-bearing animals increases the susceptibility of these mice to infection, 4T1-, 66C4- and nontumor-bearing animals with advanced tumor growth (day 28 post 4T1 inoculation) were exposed to a sublethal dose of endotoxin or polymicrobial sepsis. Again due to the fact that the in vivo growth of 66C4 is slower than 4T1, 66C4 bearing animals were inoculated approximately 7 days before 4T1 animals so that experiments were conducted in mice with comparable primary tumor burdens (day 28 for 4T1 and day 35 for 66C4). As shown in Figure 5A and B, advanced 4T1-bearing mice are more sensitive and have significantly higher mortality to sublethal endotoxin administration and polymicrobial sepsis compared to advanced 66C4- or nontumor-bearing animals.
Figure 5. Late stage 4T1-bearing animals have an increased susceptibility to endotoxemia and polymicrobial sepsis compared to 66C4- and nontumor-bearing animals.
(A) 4T1-, 66C4- and nontumor- (NT) bearing mice at 28–30 days post tumor inoculation were exposed to sublethal dose of endotoxin (n=20 per group; Fisher’s exact test * p < 0.001) (B) 4T1-, 66C4- and nontumor- (NT) bearing mice at 28–30 days post tumor inoculation were challenged via cecal ligation and puncture (CLP) (n=20 per group; Fisher’s Exact Test * NT vs 4T1 p = 0.014 ** 66C4 vs 4T1 p = 0.031).
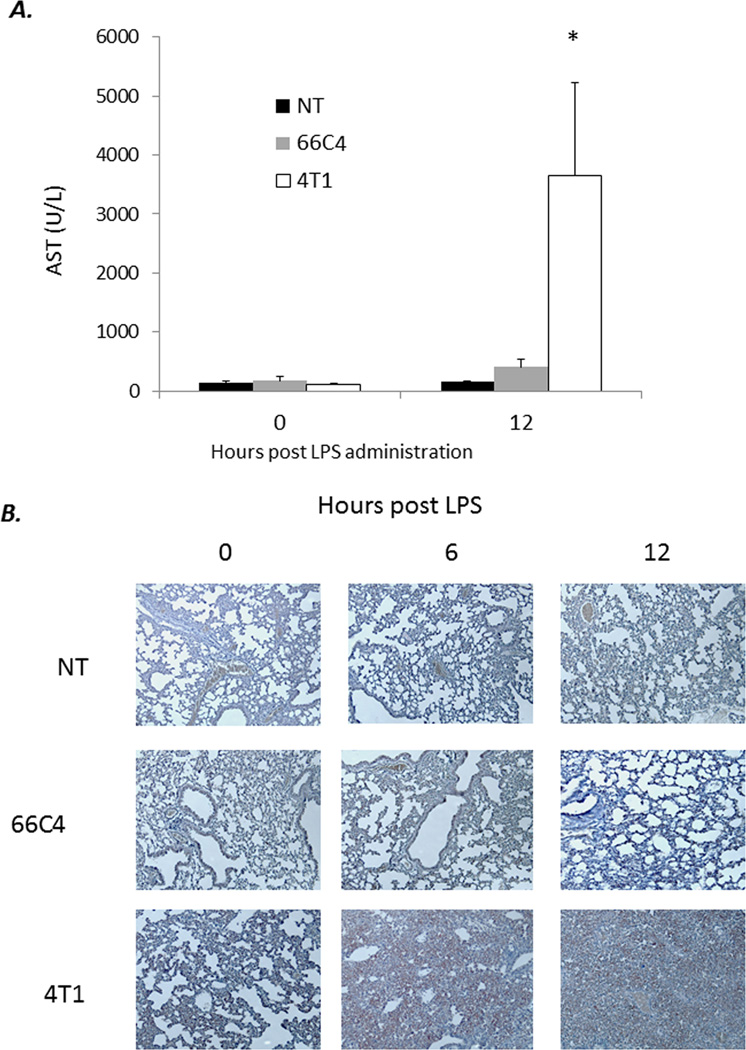
To understand the mechanism of mortality in 4T1-bearing mice in response to endotoxicosis, we harvested lungs and liver tissue from 4T1, 66C4, and nontumor-bearing animals at 0, 6, and 12 hours post endotoxin administration. In contrast to 66C4 and nontumor-bearing animals, 4T1-bearing mice have significantly increased pulmonary infiltrate and liver necrosis/damage (Figure 6). To further investigate if this susceptibility to endotoxemia was dependent on the expansion and overwhelming numbers of MDSCs present in 4T1-bearing animals, 4T1- and nontumor-bearing mice were administered clodronate-encapsulated or PBS-encapsulated liposomes at 28 days post 4T1 inoculation, and then subjected to sublethal endoxtoxemia.
Figure 6. Overwhelming MDSC infiltration into lung and liver parenchyma following sublethal endotoxemia.
(A) Lungs were harvested from 4T1-, 66C4-, and nontumor- (NT) bearing mice at 0, 6, and 12 hours following sublethal endotoxin adminsitration, fixed in formalin, and stained with hematoxylin and eosin. Images taken at 10×. (B) Lung tissue was harvested from 4T1, 66C4, and NT bearing animals at 0 and 12 hours post LPS challenge and placed in formalin. Tissues were then sectioned and stained with hematoxylin and eosin. Arrows denote zone of necrosis. Images wre taken at 10×. (C) AST serum concentrations from 4T1-, 66C4-, and nontumor- (NT) bearing mice at 0 and 12 hours (*n=5 per group; Tukey’s One way ANOVA p<0.05).
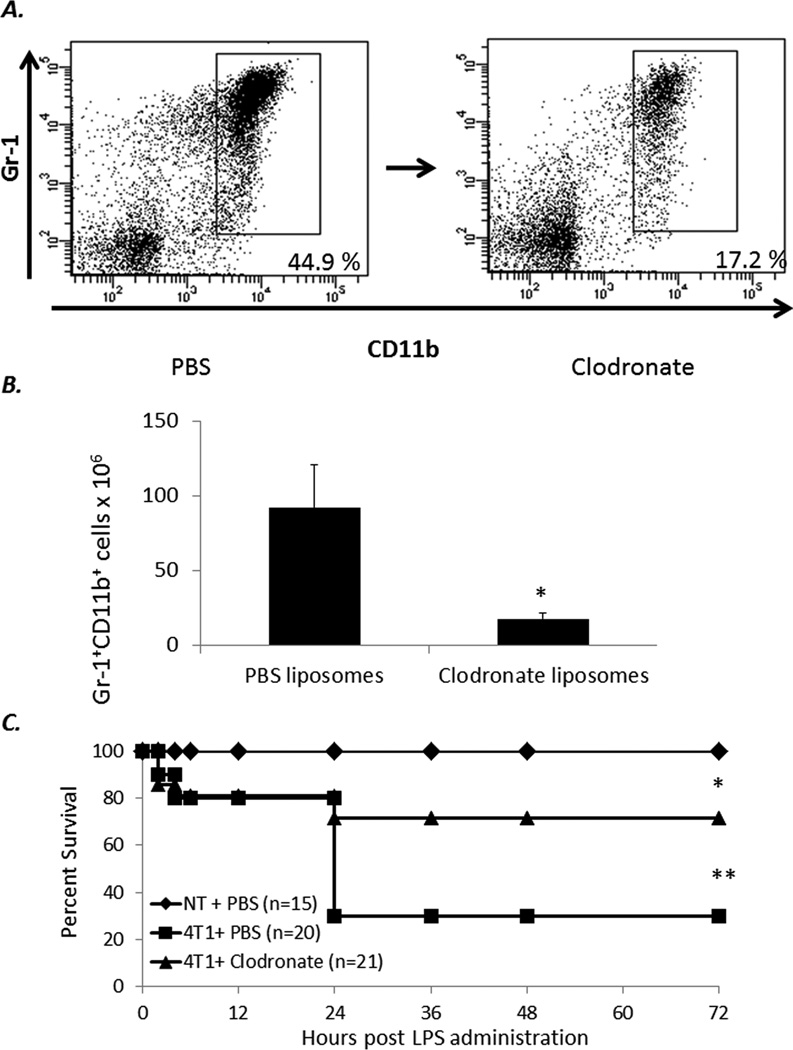
As shown in Figure 7, depleting phagocytic cells with clodronate-encapsulated liposomes reduces the numbers of MDSCs and reverses the susceptibility of these animals to endotoxemia. These data support the findings from the previous experiments and show that the overexpansion of phagocytic cells, including MDSCs in tumor-bearing hosts is deleterious during advanced disease.
Figure 7. Depletion of MDSCs improves survival to endotoxemia in 4T1 bearing animals.
(A) Balb/C J 28 days post 4T1 inoculation were injected with either PBS or clodronate liposomes (Encapsula nanosciences, Nashville TN) IV at 100 uL of Liposome suspension/ 10 g of body weight. Animals were then euthanized after 48 hours. (B) Splenocytes from either clodronate (n=5) or PBS (n=3) injected animals were stained for CD11b+Gr-1+ cells and absolute numbers of MDSCs were then calculated (* Student t-test p<0.001). (C) 4T1 and NT-bearing mice at 28–30 days post tumor inoculation were administered either Clodronate or PBS liposomes IV. After 36–48 hours mice were then injected with LPS (50 µg) ip and followed for 72 hours (* Fisher’s exact test NT vs 4T1 +PBS p<0.001, ** 4T1 + Clodronate vs 4T1 +LPS p =0.013). Panel represents the summary of two experiments.
Discussion
The role of tumor-induced myelopoiesis during the progression of cancer, leading to the expansion of MDSCs, is likely to have multiple effects on the host. Not only have MDSCs been found to contribute to the evasion of immune surveillance through the impairment of CD4+, CD8+ and regulatory T cell responses (19), they also have been shown to increase the growth and metastatic potential of cancer directly and indirectly through promotion of angiogenesis (13, 26). However, some of the soluble factors (NO, ROS etc) that have been shown to cause indirect adaptive immune suppression also promote enhanced innate immunity, and therefore increased resistance to microbial invasion. In addition, MDSCs produce multiple inflammatory factors that not only promote their immunosuppressive activity, but also predispose them to anaphylactoid responses (22). Therefore, we propose that the initial expansion of MDSCs and other myeloid-derived cells early during tumor growth may actually serve to increase innate immunity and pathogen surveillance during cancer, and may represent a conserved host response to systemic stress and inflammation (27). Like any acute phase response, this enhanced immune surveillance has metabolic costs to the tumor-bearing host, including increased energy expenditure, a hepatic acute phase response, and expansion of the myeloid compartment which in the end, may also contribute to the development of cachexia (22, 23, 28). In this manner, the proposed model and mechanisms behind cancer cachexia are novel and may be expanded to include other inflammatory states associated with wasting and MDSC expansion, such as sepsis and active autoimmune diseases (29). MDSC expansion may have short term benefits to the host, but when prolonged as occurs in advanced tumor growth, the early benefits of an initial MDSC expansion are outweighed by the adverse effects associated with cancer cachexia.
We have used two independent approaches to support these conclusions, although both approaches have significant limitations. First, we have used the 4T1 mammary cancer tumor model because it is a well-established model of MDSC expansion (22, 30, 31), and has the added advantage that a well-described subclone of this tumor cell line, 66C4, does not exhibit MDSC expansion (22). 4T1 behaves very similar to the Lewis Lung carcinoma (LLC) as a murine cachexia model. Like the 4T1 tumor-bearing mice used in this study, we have shown LLC tumor-bearing mice lose non-tumor body weight that is predominantly fat, have a hepatic acute phase response, and exhibit anemia and hyperlipidemia (32). Some investigators have also seen body protein losses in LLC-bearing mice with very advanced tumor growth (33).
Although both 4T1 and 66C4 are highly metastatic, not surprisingly, the 4T1 tumor cell line grows more rapidly than 66C4 as a solid flank tumor, and is presumed to become metastatic earlier. Whether this more rapid tumor growth and metastasis are secondary to the MDSC expansion is open to conjecture. We attempted to address these issues, however, by injecting 66C4 seven days before inoculation with 4T1, thus giving these animals an additional seven days for the size of the primary tumor (Figure 1 and Supplementary Figure 1) and presumably the metastatic potential to be roughly similar.
Secondly, we recognize that the use of clodronate-liposomes to deplete MDSCs in the cachectic 4T1 animals does not only deplete MDSCs, but also most other phagocytic cells, such as Kupffer cells, macrophages, etc. Therefore, it is difficult to distinguish the depletion of MDSCs from other phagocytic myeloid populations and their contribution to the improvement in survival observed in the clodronate-treated animals in response to endotoxin. However, the fact that depletion of phagocytic cells, including MDSCs, improved outcome to an inflammatory challenge (Figure 7), coupled with the observation that 4T1-, but not 66C4-, bearing mice with massive expansion of MDSCs, were susceptible to the same inflammatory challenge (Figure 6) suggests that overexpansion of MDSCs, are contributory.
Here we show that in late stage 4T1-, LLC- or CT26-bearing mice, a tumor setting in which MDSCs expand dramatically, animals display increased loss of adiposity, increased oxygen consumption, a hepatic acute phase response, and increased susceptibility to infection. These data suggest that the presence of large numbers of myeloid cells, including MDSCs, in the 4T1 tumor-bearing host is sufficient to initiate chronic inflammation, and demonstrate that these cells may play a novel and previously unrecognized role in the tumor-induced cachexia syndrome.
One obvious question is through what mechanism(s) are these cachectic animals susceptible to inflammatory challenges, including endotoxemia and polymicrobial sepsis. Although these studies cannot resolve this question completely, the data leads to a strong suggestion. We looked at plasma cytokine concentrations in these animals, presuming that animals with expansion of their MDSCs and other phagocytic populations would respond to endotoxin and polymicrobial sepsis with an exaggerated inflammatory response. This was not seen. Changes in both plasma pro- and anti-inflammatory cytokines after endotoxin administration or polymicrobial sepsis were no different in 4T1-bearing mice than 66C4- or nontumor-bearing animals (data not shown). Secondly, we thought that perhaps the animals would be immune suppressed, and the cachectic 4T1-bearing mice would have rapid dissemination of bacteria from the peritoneal cavity after polymicrobial sepsis. This was also not seen. Blood bacteremia levels did not differ among the groups (data not shown) after induction of sepsis.
In addition, the mortality in our model was not associated with either anapyhlactoid responses, anemia, or thrombocytopenia (data not shown). Rather, the findings were more consistent with infiltration of large numbers of myeloid cells (see Figure 6) into organs of the reticuloendothelial system, such as lung and liver, leading to organ injury. Although MDSCs from tumor-bearing animals are known to produce more inflammatory mediators (such as NO, and cytokines)(19), administering Gr-1+ cells from a healthy animal produced a similar acute phase response as did GR-1+ cells from a tumor-bearing animal. Such findings suggest that the sheer numbers of these cells, rather than their phenotype may be more important for at least some of the acute phase responses. It is important to note that we could not fully demonstrate that it was indeed the supraphysiologic numbers of these myeloid cells that are the mechanistic culprit for the increase mortality in the 4T1 animals. In fact, many investigators have used adoptive transfer techniques (up to 5 × 106 cells) to demonstrate gain/loss of function with MDSCs in either tumor or transplantation studies (19, 34–37). However, the adoptive transfer 100s of millions of Gr-1+ cells into either naïve or 66Cl4 animals to recapitulate the massive infiltration of MDSCs into the lung following sepsis seen in the 4T1 animals is many fold higher than what has been used by previous investigators, and is not possible in these animals. Administration of this number of GR-1+ cells would likely lead to pulmonary embolus and death, as the first pass of any intravenous injection is through the lung.
Such findings have direct clinical relevance since some current anti-neoplastic therapies are aimed at blocking the expansion of MDSCs in advanced cancer (38, 39). Although such approaches are primarily targeting the immunosuppressive properties of MDSCs, an unexpected beneficial result of these studies may be a reduction in sickness syndromes and cachexia. Despite these limitations, the data within the manuscript suggest a previously unrecognized and novel association between the expansion of MDSCs and cancer-associated cachexia. As MDSCs have been recognized in multiple disease settings beyond cancer, their biological role is more than likely not limited to simply T cell suppression, though this is clearly an important obstacle to overcome in the application of tumor vaccines and other immune therapeutics (13, 40, 41). Therefore, based on these findings and other published data (13, 23, 27, 42, 43), the expansion of MDSCs during disease is likely a conserved host response to a systemic inflammatory insult and when unchecked, may lead to cachexia. Clearly, additional studies are required to verify and expand on these findings, but the current results provide strong evidence that one of the many results of MDSC expansion in advanced cancer is its contribution to the inflammatory and cachexia-producing responses.
Supplementary Material
Acknowledgments
Supported in part by GM-40586 and GM-81923, awarded by the National Institute of General Medical Sciences (NIGMS), P.H.S. AGC, MJD, KMK-S were supported by the Ruth Kirschstein NRSA training grant (T32 GM-08721) in burns and trauma, awarded by NIGMS. AGC was also supported by an individual NRSA training grant (F32 GM-093665), awarded by NIGMS.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare
References
- 1.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, Macdonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. The lancet oncology. 2011 doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 2.Winfield RD, Delano MJ, Pande K, Scumpia PO, Laface D, Moldawer LL. Myeloid-derived suppressor cells in cancer cachexia syndrome: a new explanation for an old problem. JPEN J Parenter Enteral Nutr. 2008;32:651–655. doi: 10.1177/0148607108325075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 4.Moldawer LL, Copeland EM., 3rd Proinflammatory cytokines, nutritional support, and the cachexia syndrome: interactions and therapeutic options. Cancer. 1997;79:1828–1839. [PubMed] [Google Scholar]
- 5.Ambrus JL, Ambrus CM, Mink IB, Pickren JW. Causes of death in cancer patients. J Med. 1975;6:61–64. [PubMed] [Google Scholar]
- 6.Penna F, Minero VG, Costamagna D, Bonelli G, Baccino FM, Costelli P. Anti-cytokine strategies for the treatment of cancer-related anorexia and cachexia. Expert opinion on biological therapy. 2010;10:1241–1250. doi: 10.1517/14712598.2010.503773. [DOI] [PubMed] [Google Scholar]
- 7.Robinson DW, Jr, Eisenberg DF, Cella D, Zhao N, de Boer C, DeWitte M. The prognostic significance of patient-reported outcomes in pancreatic cancer cachexia. The journal of supportive oncology. 2008;6:283–290. [PubMed] [Google Scholar]
- 8.Wiedenmann B, Malfertheiner P, Friess H, Ritch P, Arseneau J, Mantovani G, Caprioni F, Van Cutsem E, Richel D, DeWitte M, Qi M, Robinson D, Jr, Zhong B, De Boer C, Lu JD, Prabhakar U, Corringham R, Von Hoff D. A multicenter, phase II study of infliximab plus gemcitabine in pancreatic cancer cachexia. The journal of supportive oncology. 2008;6:18–25. [PubMed] [Google Scholar]
- 9.van Zaanen HC, Lokhorst HM, Aarden LA, Rensink HJ, Warnaar SO, van der Lelie J, van Oers MH. Chimaeric anti-interleukin 6 monoclonal antibodies in the treatment of advanced multiple myeloma: a phase I dose-escalating study. British journal of haematology. 1998;102:783–790. doi: 10.1046/j.1365-2141.1998.00835.x. [DOI] [PubMed] [Google Scholar]
- 10.Allavena P, Germano G, Marchesi F, Mantovani A. Chemokines in cancer related inflammation. Experimental cell research. 2011;317:664–673. doi: 10.1016/j.yexcr.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Mantovani A. Molecular pathways linking inflammation and cancer. Current molecular medicine. 2010;10:369–373. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 12.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. Journal of leukocyte biology. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 13.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagaraj S, Collazo M, Corzo CA, Youn JI, Ortiz M, Quiceno D, Gabrilovich DI. Regulatory myeloid suppressor cells in health and disease. Cancer Res. 2009;69:7503–7506. doi: 10.1158/0008-5472.CAN-09-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cripps JG, Gorham JD. MDSC in autoimmunity. International immunopharmacology. 2011 doi: 10.1016/j.intimp.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oberholzer A, Oberholzer C, Bahjat KS, Ungaro R, Tannahill CL, Murday M, Bahjat FR, Abouhamze Z, Tsai V, LaFace D, Hutchins B, Moldawer LL, Clare-Salzler MJ. Increased survival in sepsis by in vivo adenovirus-induced expression of IL-10 in dendritic cells. J Immunol. 2002;168:3412–3418. doi: 10.4049/jimmunol.168.7.3412. [DOI] [PubMed] [Google Scholar]
- 17.Scumpia PO, McAuliffe PF, O'Malley KA, Ungaro R, Uchida T, Matsumoto T, Remick DG, Clare-Salzler MJ, Moldawer LL, Efron PA. CD11c+ dendritic cells are required for survival in murine polymicrobial sepsis. J Immunol. 2005;175:3282–3286. doi: 10.4049/jimmunol.175.5.3282. [DOI] [PubMed] [Google Scholar]
- 18.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nature medicine. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oldenburg HS, Rogy MA, Lazarus DD, Van Zee KJ, Keeler BP, Chizzonite RA, Lowry SF, Moldawer LL. Cachexia and the acute-phase protein response in inflammation are regulated by interleukin-6. European journal of immunology. 1993;23:1889–1894. doi: 10.1002/eji.1830230824. [DOI] [PubMed] [Google Scholar]
- 21.Delano MJ, Moldawer LL. The origins of cachexia in acute and chronic inflammatory diseases. Nutr Clin Pract. 2006;21:68–81. doi: 10.1177/011542650602100168. [DOI] [PubMed] [Google Scholar]
- 22.Pande K, Ueda R, Machemer T, Sathe M, Tsai V, Brin E, Delano MJ, Van Rooijen N, McClanahan TK, Talmadge JE, Moldawer LL, Phillips JH, LaFace DM. Cancer-induced expansion and activation of CD11b+ Gr-1+ cells predispose mice to adenoviral-triggered anaphylactoid-type reactions. Mol Ther. 2009;17:508–515. doi: 10.1038/mt.2008.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. The Journal of experimental medicine. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danai PA, Moss M, Mannino DM, Martin GS. The epidemiology of sepsis in patients with malignancy. Chest. 2006;129:1432–1440. doi: 10.1378/chest.129.6.1432. [DOI] [PubMed] [Google Scholar]
- 25.Safdar A, Armstrong D. Infectious morbidity in critically ill patients with cancer. Crit Care Clin. 2001;17:531–570. vii–viii. doi: 10.1016/s0749-0704(05)70198-6. [DOI] [PubMed] [Google Scholar]
- 26.Mauti LA, Le Bitoux MA, Baumer K, Stehle JC, Golshayan D, Provero P, Stamenkovic I. Myeloid-derived suppressor cells are implicated in regulating permissiveness for tumor metastasis during mouse gestation. The Journal of clinical investigation. 2011 doi: 10.1172/JCI41936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, Heyworth PG, Efron PA, Moldawer LL. A Paradoxical Role for Myeloid-Derived Suppressor Cells in Sepsis and Trauma. Mol Med. 2010;17:281–292. doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K, Ouyang GF, Okada M, Balazs M, Adany R, Shibata T, Takami T. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. 2008;83:1136–1144. doi: 10.1189/jlb.0907611. [DOI] [PubMed] [Google Scholar]
- 29.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, Heyworth PG, Efron PA, Moldawer LL. A Paradoxical Role for Myeloid Derived Suppressor Cells in Sepsis and Trauma. Mol Med. 2010 doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, Rini B, Finke JH, Cohen PA. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer research. 2010;70:3526–3536. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 32.Sherry BA, Gelin J, Fong Y, Marano M, Wei H, Cerami A, Lowry SF, Lundholm KG, Moldawer LL. Anticachectin/tumor necrosis factor-alpha antibodies attenuate development of cachexia in tumor models. FASEB J. 1989;3:1956–1962. doi: 10.1096/fasebj.3.8.2721856. [DOI] [PubMed] [Google Scholar]
- 33.Bennani-Baiti N, Walsh D. Animal models of the cancer anorexia-cachexia syndrome. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2011;19:1451–1463. doi: 10.1007/s00520-010-0972-0. [DOI] [PubMed] [Google Scholar]
- 34.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunological reviews. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhao X, Rong L, Zhao X, Li X, Liu X, Deng J, Wu H, Xu X, Erben U, Wu P, Syrbe U, Sieper J, Qin Z. TNF signaling drives myeloid-derived suppressor cell accumulation. The Journal of clinical investigation. 2012;122:4094–4104. doi: 10.1172/JCI64115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dolcetti L, Peranzoni E, Bronte V. Measurement of myeloid cell immune suppressive activity. Curr Protoc Immunol. 2010;Chapter 14(Unit 14):17. doi: 10.1002/0471142735.im1417s91. [DOI] [PubMed] [Google Scholar]
- 37.Capietto AH, Kim S, Sanford DE, Linehan DC, Hikida M, Kumosaki T, Novack DV, Faccio R. Down-regulation of PLCgamma2-beta-catenin pathway promotes activation and expansion of myeloid-derived suppressor cells in cancer. The Journal of experimental medicine. 2013;210:2257–2271. doi: 10.1084/jem.20130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer immunology, immunotherapy : CII. 2013;62:909–918. doi: 10.1007/s00262-013-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanford DE, Porembka MR, Panni RZ, Mitchem JB, Belt BA, Plambeck-Suess SM, Lin G, Denardo DG, Fields RC, Hawkins WG, Strasberg SM, Lockhart AC, Wang-Gillam A, Goedegebuure SP, Linehan DC. A Study of Zoledronic Acid as Neo-Adjuvant, Perioperative Therapy in Patients with Resectable Pancreatic Ductal Adenocarcinoma. Journal of cancer therapy. 2013;4:797–803. doi: 10.4236/jct.2013.43096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002;51:293–298. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kubler H, Yancey D, Dahm P, Vieweg J. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14:8270–8278. doi: 10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- 42.Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, Khoury SJ. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
- 43.Scumpia PO, Kelly-Scumpia KM, Delano MJ, Weinstein JS, Cuenca AG, Al-Quran S, Bovio I, Akira S, Kumagai Y, Moldawer LL. Cutting edge: bacterial infection induces hematopoietic stem and progenitor cell expansion in the absence of TLR signaling. J Immunol. 2010;184:2247–2251. doi: 10.4049/jimmunol.0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.