Significance
Global food security demands the development of new technologies to increase and secure cereal production on finite arable land without increasing water and fertilizer use. Although the use of heterosis through hybrid breeding has produced tremendous economic benefits in worldwide crop production, less than 1% of the global wheat area is planted with hybrids. One of the greatest bottlenecks in breeding hybrid wheat is the lack of an efficient sterility system to block self-pollination. This report describes a recessive system for pollination control in wheat. We demonstrate the implementation and feasibility of the system for generation and maintenance of the male-sterile parent, hybrid seed production and full restoration of fertility in the hybrid wheat seed.
Keywords: hybrid wheat breeding, split-gene approach, site-specific recombination, intein-mediated protein splicing
Abstract
Hybrid wheat plants are superior in yield and growth characteristics compared with their homozygous parents. The commercial production of wheat hybrids is difficult because of the inbreeding nature of wheat and the lack of a practical fertility control that enforces outcrossing. We describe a hybrid wheat system that relies on the expression of a phytotoxic barnase and provides for male sterility. The barnase coding information is divided and distributed at two loci that are located on allelic positions of the host chromosome and are therefore “linked in repulsion.” Functional complementation of the loci is achieved through coexpression of the barnase fragments and intein-mediated ligation of the barnase protein fragments. This system allows for growth and maintenance of male-sterile female crossing partners, whereas the hybrids are fertile. The technology does not require fertility restorers and is based solely on the genetic modification of the female crossing partner.
Hybrids often display increased yield, enhanced yield stability, and improved abiotic and biotic stress resistance that result from heterosis (hybrid vigor) (1). However, hybrid seeds cannot be saved without a significant loss in performance, a fact that protects the intellectual property of seed companies. The benefits of hybrid systems are countered by the increased seed production costs that result from complex breeding schemes. Therefore, there is a strong incentive to develop systems that facilitate hybrid production, particularly in crops where hybrid production has been impossible or difficult to develop.
Hybrid plants are produced by cross-pollination of genetically diverse parental lines. Several prerequisites must be fulfilled for commercial hybrid seed production. First, for self-pollinating (autogamous) species, self-pollination of the “female” must be precluded to ensure that the plant will only be cross-pollinated by the chosen crossing partner (i.e., the “male” line) (2). Second, because male-sterile plants cannot be multiplied by selfing, technical solutions must be available for the multiplication of the sterile crossing partner (3). Third, in crops for which seeds are the harvest product (e.g., all cereals), sterility must be overcome in the hybrid generation to obtain a full yield in the commercialized product (2).
The most efficient way to promote cross-pollination in bisexual plants is male sterility, which can be achieved by various means. In maize, which is the world’s most valuable hybrid, the female crossing partner can be sterilized by manual detasselling (removal of the anthers) (4); however, the detasselling technique cannot be applied to crop plants that do not have a physical separation of the male and female flower parts. The most well-established genetic system is cytoplasmic male sterility (CMS), which is based on mutated mitochondrial DNA (5). The effect of the male sterility-inducing cytoplasm can be neutralized by fertility restorers (i.e., nuclear genes that are specific to each CMS system). CMS is reasonable for hybrid breeding only if mutants for cytoplasmic sterility and fertility restoration are available in a given crop, which renders CMS a relatively inflexible system. The introgression of CMS mutants is complex, because the different genetic components have to be discovered and brought together separately. CMS is functional in important cereals, such as rice and rye; however, in hexaploid wheat, CMS is difficult to develop, complex to maintain, and only marginally reliable. In particular, reliable restorer genes are not yet available, making F1 fertility restoration difficult. The lack of availability of restorer genes has been a major factor in limiting the application of CMS to wheat (6, 7). Moreover, CMS systems are sensitive to environmental factors, such as temperature and photoperiod (8). As a result, no wheat CMS system with more than a regional application is currently used in hybrid seed production (9).
All of the hybrid cultivars that are registered for the European wheat market were produced by sterilizing the pollen recipient through the application of a chemical hybridization agent (CHA) (gametocide) (10). The plant growth regulator Croisor 100 (sintofen; formerly Dupont-Hybrinova, Saaten Union Recherche) is currently the only wheat CHA that is used for commercial European hybrid wheat production (www.hybridwheat.net). Sterile females are grown in alternating stripes in close proximity to untreated plants that display good pollen shedding properties, and the hybrid seed is harvested from the females after cross-pollination (2, 9). However, the commercial deployment of CHAs is hampered by several problems, including compromised seed production in wheat females that are treated with CHAs (11) and narrow application windows attributable to prevailing environmental factors, such as rain, wind, and heat (10, 12). Thus, the use of CHAs bears a substantial financial risk.
There have been major efforts to deploy transgenic technology for hybrid systems (2); however, Seedlink from Bayer CropScience is the only commercialized transgenic male-sterility system. The Seedlink method relies on the expression of a cytotoxic ribonuclease from Bacillus amyloliquefaciens (barnase) in the male reproductive tissue tapetum, which is essential for pollen development (13). The male-sterile line is maintained by backcrossing with a nontransgenic plant, which results in a mixed population comprising 50% sterile plants. By linking glufosinate resistance (LibertyLink) to the barnase transgene, male-sterile plants can be selected. Fertility restoration in the F1 hybrid progeny results from the expression of the barstar gene, which is delivered by the father. Barstar counteracts barnase by forming a specific and stable one-to-one protein complex (14). Although this method is used successfully for hybrid canola production, the dominant dual-component system requires the expression of transgenes in each crossing partner, which involves additional breeding efforts.
Here, we describe a recessive “split-gene” system that has the potential to overcome several difficulties associated with the systems that are currently used. Our system relies on the expression of a barnase gene that is encoded by two nonoverlapping sequences at complementary genetic loci (15). Following translation, the barnase fragments are assembled into an active phytotoxic enzyme via an autocatalytic process of intein-mediated trans-splicing (16, 17). The tapetum is destroyed after the phytotoxic barnase is formed, which prevents the formation of viable pollen. Both barnase gene fragments reside at identical chromosomal positions on physically distinct homologous chromosomes (“isoloci”). These partial genes functionally mimic a pair of recessive alleles because a plant will produce active barnase and exhibit a male-sterile phenotype only as a “heterozygote” (i.e., when the plant carries both complementary “alleles”). These plants can then be used as the female (male-sterile) crossing partner in hybrid breeding. During meiosis, the members of a pair of barnase loci segregate into separate gametes. Consequently, the hybrid progeny inherit only one of the complementary fragments after pollination, so they remain fertile. This genetic design facilitates a mode of fertility restoration that should be robust. Moreover, because only the female crossing partner is genetically manipulated, this hybridization system is more flexible to use than are systems that also require a genetically modified male parent.
Wheat is one of the most important staple crops. Research has underscored that the implementation of hybrid wheat breeding has the potential to achieve breakthrough increases in yield, yield stability, and sturdiness against biotic and abiotic stresses. Recent large-scale phenotyping involving extensive collections of inbred lines and hybrids revealed that hybrids were superior to the mean of their parents for grain yield, susceptibility to frost, leaf rust, and Septoria tritici blotch (18, 19). A consistently higher grain yield stability for wheat hybrids compared with lines was observed in multilocation field trials (20). The yield benefits and the robustness of hybrids may provide additional benefits related to the future climatic changes that are predicted to impair crop production (21). Therefore, the development of technologies that enable the benefits of heterosis to be captured in a major crop, such as wheat, is of paramount importance.
Results
Description of the Vector Constructs and the Experimental Strategy.
The cloning of the barnase-containing “provectors” used in this study has been described previously (22). The vectors that contain nonoverlapping fragments of a barnase gene from Bacillus amyloliquefaciens are fused to the N- and C-terminal sequences of an intein that is derived from the Synechocystis sp. DnaB helicase gene (Fig. 1A). For tapetum-specific expression, the barnase–intein fusion is controlled by the rice osg6B promoter (23). Upon translation, the inteins covalently fuse the barnase fragments in an autocatalytic reaction to produce an intact cytotoxic barnase protein (Fig. 1D), which leads to male sterility.
Fig. 1.
Vector constructs used in this study. (A and B) The genetic structure of the barnase expression vector (“pro-vector”) (A) and the phiC31-integrase-expression vector (B). The provector harbors attP and attB sequences that serve as targets for the site-specific recombinase phiC31. Note that only the T-DNA part of the vectors is illustrated (not drawn to scale). (C) Phage phiC31 integrase-mediated site-specific recombination results in the derivative loci A1 and A2. Recombination results in the hybrid products attR and attL. (D) A schematic illustration of the assembly of active barnase cytotoxin via the intein-mediated trans-splicing of two precursor molecules. Bar-N and Bar-C, N- and C-terminal gene fragments of the Bacillus amyloliquefaciens barnase gene; HPTII, hygromycin phosphotransferase; IntN and IntC, N- and C-terminal intein sequences from the DnaB gene of Synechocystis sp.; LB and RB, T-DNA left and right borders; ocs, octopine synthase terminator; NLS, SV40 T antigen nuclear localization signal (amino acids PKKKRKV); nos, nopaline synthase terminator; ocs, octopine synthase terminator; phiC31, phage phiC31 recombinase coding sequence; PSK, intron PSK7-i3 from Petunia hybrida; Ptap, tapetum-specific osg6B promoter from rice (23); Pubi, maize ubiquitin 1 promoter; S, (GGGGS)3 flexible peptide linker; UBQ, intron UBQ10-i1 from Arabidopsis thaliana.
The localization of both barnase gene fragments to the same locus on homologous host chromosomes is achieved by transforming plants with a construct that contains both genetic elements (the provector). Next, the primary prolocus is derivatized by using site-specific deletions. For this purpose, the provectors contain attB and attP recombination sites to serve as targets for phiC31 integrase (a site-specific recombinase) (24, 25). Site-specific recombination is induced by crossing a plant that carries the prolocus with a plant that carries the pICH13130 locus as the integrase source (Fig. 1B) (26). Site-specific deletions may lead to two different loci that contain only the N- or the C-terminal fragment of the barnase (hereafter referred to as derivative loci “A1” and “A2”; Fig. 1C). In the case of only an N-terminal fragment, a hybrid attL sequence is produced via a reaction between attB and attP2; in the case of only a C-terminal fragment, a reaction between attP1 and attB creates a hybrid attR sequence. attL and attR are not substrates for the phiC31 integrase in plants. Consequently, the described phiC31 integrase-mediated recombination reactions are irreversible, and the two reactions are mutually exclusive.
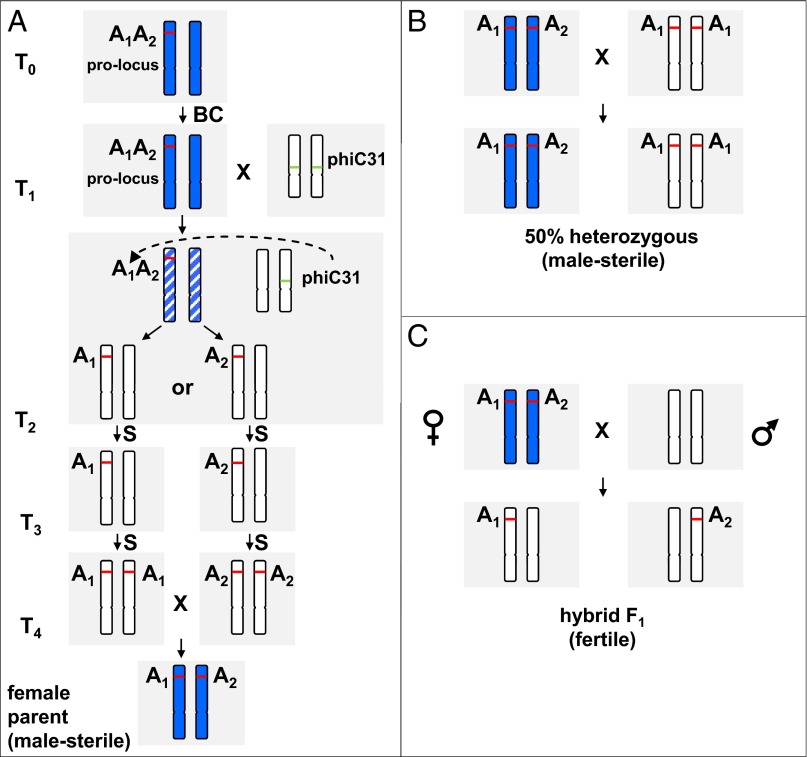
Fig. 2 describes the different experimental steps required to develop the system including the predicted genotypes and phenotypes.
Fig. 2.
Experimental design for the production of hybrid seed. Genotypes that have an expected male-sterile phenotype are symbolized in blue. (A) The generation of the male-sterile parent for hybrid breeding. After transforming the provector, the primary transformants (T0) are expected to be male-sterile as a result of barnase expression. To ensure that the phenotype is stable, and to create more target plants for recombination, the T0 plants are backcrossed. In the event of a single-copy integration of the prolocus, the T1 progeny are expected to segregate so that ∼50% of the plants are male-sterile (transgenic) and 50% are fertile (null-segregants). In the next step, the sterile T1 plants are crossed with plants that express a phiC31 site-specific recombinase (encoded by pICH13130). In the developing T2 plants, the integrase may catalyze the site-specific deletions between the attP and attB sites of the prolocus. Two alternative reactions can lead to the deletion of either the C-terminal or the N-terminal part of the locus, which results in the formation of the derivative loci A1 or A2. The derivative loci will naturally reside at exactly the same genetic position on two homologous chromosomes (because they originate from one prolocus). Depending on the time point at which recombination occurs, the F2 plants may be genetic chimera that contain recombined and nonrecombined sectors. Plants carrying only A1 or A2 should be fertile, because no complete barnase protein can be produced. The A1 and A2 lines are then crossed (either as T3 plants or T4 plants after one round of self-pollination), and a portion of the resulting progeny is expected to carry both the A1 and A2 loci (25% of the progeny if both parents are hemizygous for A1 and A2, and 50% or 100% of the progeny if one or both parents are homozygous for A1 or A2). These heterozygous genetic segregants are expected to be male-sterile because of the coexpression of barnase from the A1 and A2 loci. (B) A strategy for maintaining the male-sterile line. Reproduction of the heterozygous male-sterile line A1A2 can be accomplished by crossing such lines with a homozygous fertile “maintainer” line, A1A1 or A2A2. The progeny of this cross may segregate the seeds so that 50% will have the genotype A1A2 (like the female parent) and, depending on the male parental line that was used, 50% will have the genotype A1A1 or A2A2. (C) Hybrid seed production. Male-sterile plants with the genotype A1A2 are used as the female crossing partners for hybrid seed production. The allelic position of the complementary barnase fragments enforces 100% segregation during meiosis. This results in male fertility and seed set in the hybrid progeny, because all segregants carry only an inactive barnase fragment (either A1 or A2). BC, backcrossing step; S, self-pollination step.
Generation of the A1 and A2 Loci.
A total of 180 sterile primary transformants (T0) carrying the prolocus were produced by genetic transformation and were backcrossed to wild-type plants (Fig. 2A). More than 95% of the backcrosses led to vital seeds, which demonstrates that sterility was restricted to the male gametes and did not affect fertility in general. For further analysis, we preferentially selected lines that carried a single barnase locus (indicated by a 1:1 segregation of transgenic sterile vs. nontransgenic fertile T1 plants; assayed using fertility assays and Southern blot analyses).
To induce deletions at the prolocus, 96 male-sterile T1 plants (derived from 46 independent T0 plants) were crossed with doubled-haploid (DH) plants that expressed an active phiC31 integrase recombinase (Fig. 2A). The genotypes of 862 fertile T2 plants were screened for the occurrence of the A1 and A2 loci by performing PCRs that amplified the barnase-intein regions of the A1 and A2 loci (Table S1 and Fig. S1). As expected, all plants carried the integrase transgene. A total of 56% (482 plants) harbored the barnase locus; however, the loss of the barnase locus through segregation was expected for a proportion of the T2 plants because the T1 generation is hemizygous for the target loci. To confirm the site-specific recombination, PCRs were conducted to amplify the “excision empty donor site” of the derivative A1 and A2 loci, including the corresponding hybrid sequences attL or attR (Fig. S1). The presence of attL and attR was confirmed by sequencing the PCR products. A total of 97% (467 plants) of the cotransgenic T2 plants harbored recombinant loci. Of these plants, 78% (365 plants) carried both A1 and A2 loci. We identified 102 T2 plants (derived from 24 independent lines) that carried only an A1 or A2 locus. All of these hemizygous segregants displayed a fertile phenotype.
Thus, we conclude that the switch from a sterile phenotype in the T0 and T1 plants into a fertile phenotype in the T2 plants invariably correlates with the loss of an N- or C-terminal barnase fragment.
Four of the lines (3493, 7031, 7335, and 9855) segregated both A1 and A2 progeny plants (Table S1). We did not observe any sectors of sterility and fertility or recombinant and nonrecombinant sectors in the T2 plants. This result indicates that the recombination events occurred early in the developing embryos after the T1 plants were hybridized with the integrase line, which is consistent with previous results (26).
Generation and Propagation of the Male-Sterile Female Line 7031-A1A2.
Fertile T3 plants that carried isolocus A1 were crossed with fertile T3 plants carrying isolocus A2. The resulting T4 progeny were analyzed for the presence of the isoloci and for fertility. Whereas heterozygous segregants carrying both A1 and A2 from lines 3493, 7335, and 9855 displayed male fertility or partial male sterility (Fig. S2 G–I), crosses of 7031-A1 with 7031-A2 led to progeny populations that displayed the expected phenotype without exception. Specifically, all heterozygous plants were male-sterile (Fig. S2 D–F), whereas 7031-A1, 7031-A2, and the null-segregants were male-fertile. Thus, line 7031 was chosen to develop the system. We selected the parental 7031-A1 and 7031-A2 T3 plants from the successful crosses and propagated them by selfing (resulting in T4 progeny; Fig. 2A). The resulting lines (hemizygous and homozygous) were crossed: all 190 of the resulting male-sterile progeny plants from the 7031-A1 × 7031-A2 crosses contained both A1 and A2 loci, and the male-fertile plants lacked either one or both loci (Table 1 and Fig. 3A). These data demonstrate the functional complementation of the split-barnase loci A1 and A2 in heterozygous wheat plants.
Table 1.
Production of the male-sterile line for hybrid seed production
| 7031-A1 × 7031-A2 from T4 | A1 only | A2 only | Neither | Both |
| No. of progeny | 123 | 95 | 85 | 190 |
| Fertile | 123 | 95 | 85 | 0 |
| Male-sterile | 0 | 0 | 0 | 190 |
Genotypes and phenotypes of genetic segregants resulting from a cross between lines carrying 7031-A1 and 7031-A2. Note that homozygous and hemizygous parental lines were used.
Fig. 3.
Phenotype of the female crossing partner and the hybrid F1. (A) The morphology of the ears from the female parent that carries both isoloci 7031-A1 and 7031-A2. The ears display the typical “open floret” phenotype and contain no seeds. Other ears of these plants were used as pollen acceptors for the production of hybrid F1 plants (B). All hybrid progeny plants displayed full fertility and carry either A1 or A2.
To maintain heterozygous male-sterile plants, crosses were performed between 10 male-sterile heterozygous A1A2 segregants and plants that carried only the A1 locus (according to Fig. 2B). Among the 79 genetic segregants that were produced, 41 were heterozygous for the A1 and A2 loci and showed male sterility, and 38 carried only the A1 locus and were fertile. Control crosses with T2 plants carrying only the N-terminal loci (A1 × A1) or the C-terminal loci (A2 × A2) resulted only in fertile progeny. The aforementioned crosses were performed reciprocally, and no differences in the phenotypes of the progeny were detected.
Production of Hybrid Seed and the Restoration of Fertility.
Male-sterile heterozygous plants from line 7031 were crossed with wild-type plants (Fig. 2C). This step corresponds to commercial hybrid seed production. A total of 849 progeny plants were obtained (hereafter designated as the “hybrid F1” generation). Among these plants, 98.5% displayed the expected hybrid genotype. Of these, 411 plants (49%) carried the A1 locus exclusively, and 425 plants (51%) carried the A2 locus exclusively. As demonstrated by pollen analyses and visual inspections, these plants were completely fertile without exception (Fig. 3B). Both the A1 and A2 loci were detected in 13 F1 plants (1.5%), whereas no plant lacked both the A1 and A2 loci.
Discussion
In this study, we established a transgenic pollination control system for the generation of hybrid wheat seed. This proof-of-principle study was performed under greenhouse conditions for a noncommercial, prototype version of the split-gene system. Nevertheless, to our knowledge, this report is the only one to detail the successful application of transgenic technology for hybrid seed production in wheat.
Our data suggest that the complementary barnase fragments are suitable for producing male sterility in wheat plants, regardless of whether they are located on one transfer (T)-DNA (prolocus, or “cis”-configuration) or whether they are in an allelic relationship on two homologous chromosomes (“trans”-configuration). After crossing 7031-A1 lines with 7031-A2 lines, all 190 of the resulting heterozygous A1A2 plants displayed male sterility, whereas the remaining 303 progeny plants (carrying only one or no barnase locus) were fertile. This result represents the first intein-mediated “trans-activation” of an agriculturally important trait in plants that were derived from the sexual hybridization of parental plants that did not express the trait. The potential practical applications of “recessive” systems that use two components “linked in repulsion” are not limited to hybrid seed systems. The principle may lead to numerous technical solutions for efficient germplasm or trait control including the control of transgene flow, thereby enabling the generation of more biologically contained transgenic plants. The linkage of a second split gene providing a trait of interest to a split pollen or seed-specific expressed barnase gene prevents the sexual transmission of the trait of interest (27, 28).
Fertility restoration in the hybrid progeny is robust, because all 836 F1 plants that carried either the A1 locus (411 plants, 49%) or the A2 locus (425 plants, 51%) were fully fertile. As is expected in the event of isoallelic loci, we did not detect any F1 plants that lacked both A1 and A2 loci. However, 13 of the 849 F1 plants (1.5%) were identified as carrying the genotype A1A2. These individuals occurred sporadically (the 13 nonhybrid progeny plants were derived from 12 independent female plants). It cannot be excluded that a proportion of these plants resulted from unintended cross-pollination or contamination during handling in the greenhouse. However, the more likely explanation is that in some cases, florets of the female plants may produce an amount of viable pollen that is sufficient for self-pollination. Consistent with this hypothesis, in occasional cases, 10–20% fertile pollen grains were detected (Fig. S2F).
The split-gene system provides several benefits that primarily originate from the isoallelic (allelic) position of the complementary barnase fragments. It relies on the genetic modification of just one of the two parents. In contrast, genetically manipulated pollinators are required in the case of dual-component dominant systems, such as Seedlink or CMS, which limits the flexibility of dual-component dominant systems in practical applications. This property is of particular importance for the cleistogamous crop wheat because most wheat varieties lack the particular floral traits that enable a high and consistent level of pollen shed (29). Thus, the breeding of plants that may act as pollinators for hybrid wheat breeding is complex and should not be constrained by the requirement for transgenes in the male crossing partner (7). Moreover, the split-gene system does not require restorer genes for robust fertility restoration. Finally, the split-gene concept is expected to be broadly applicable to many wheat varieties and other plant species because the integral genetic elements (integrase, barnase, and inteins) are generic rather than plant-specific, and these elements have been shown to function in several monocotyledonous and dicotyledonous plant species (2, 30, 31).
A penalty that is associated with the development of the split-gene system is the complexity of the establishment of loci A1 and A2. Transformation of the primary constructs was performed using particle bombardment. In the majority (78%) of T2 plants that displayed recombination, the loci A1 and A2 were not separated. Therefore, we conclude that most of the primary transformants carried rather complex DNA integrations that may have been resolved into unpredictable products by single or multiple consecutive phiC31 recombination events. In the future, Agrobacterium-mediated transformation may be applied to produce less complex events that segregate more A1 and A2 isoloci following recombinase-mediated separation, thus making the system development more efficient. An alternative approach may be to transform constructs carrying only A1 and A2 independently, followed by screening for closely integrated events that display an acceptable rate of recombination between the two loci. This approach has the advantage of saving the integrase-mediated recombination step; however, it is compromised by the requirement of screening a large number of integration events and by the incomplete repulsion of the events.
Importantly, to apply the split-gene system to other wheat varieties, the A1 and A2 loci are not required to be developed de novo. Rather, using standard marker-assisted breeding, line conversion may be accomplished by repetitive backcrossing of the A1 and A2 loci independently.
The genetic design of the male-sterile female line provides the opportunity for maintaining the sterile lines, which is a prerequisite for a future commercial application of this technique. Crossing the heterozygous female line (A1A2) with a homozygous fertile “maintainer” line (e.g., A1A1 or A2A2) results in ∼50% of the male-sterile progeny having the female genotype. In future approaches, linking the isolocus that is not present in the maintainer line to a transgene conferring herbicide resistance may enable the selection of the female genotype A1A2. However, this in-field production of the male-sterile lines would require overplanting and eliminating half of the sown plants to attain a pure stand of male-sterile female plants on the stripe-breeding production field. Alternatively, the female A1A2 line may be produced by crossing homozygous A1A1 and A2A2 lines. This cross would result in 100% male-sterile plants (A1A2). For this purpose, CHAs may be used to sterilize either the A1A1 line or the A2A2 line in small-scale applications. The resulting pure, sterile populations would enable the mixed planting of male and female parental lines in the production field, a strategy that would improve the economics of hybrid wheat seed production because almost the entire area could be used for hybrid seed harvesting (32). The use of CHAs on a small scale is, however, technically difficult.
Recently, several other transgenic systems were suggested in which male sterility was induced by external stimulation of an otherwise fertile plant (“conditional male-sterility systems”). Syngenta generated tobacco plants that expressed a modified form of d-amino acid oxidase (DAAO) in the tapetal cells (33). When d-glufosinate, the nonphytotoxic enantiomer of the commercial herbicide glufosinate, is sprayed as a “protoxin,” DAAO oxidizes d-glufosinate to 2-oxo-4-(methylphosphinyl)-butanoic acid; phytotoxic l-glufosinate is subsequently formed via intermediates. This compound causes tapetal cell death, which in turn induces male sterility. In a system that has been used by Monsanto, the expression of a 5-enolpyruvylshikimate 3-phosphate synthase (EPSPS), which confers herbicide tolerance, is specifically inhibited in tapetal cells by either miRNA-mediated translational inhibition or transcriptional repression (34, 35). Thus, cells that are necessary for pollen development are specifically unprotected against the phytotoxic effects of glyphosate and are killed during herbicide treatment. However, the benefits of conditional male sterility are compromised by the requirement for the large-scale spraying of chemicals onto production fields, resulting in increased costs and short biological application windows similar to classical CHAs.
Compared with line varieties, hybrid wheat currently occupies only a small niche in the market. Less than 1% of the global wheat area is planted with hybrids (9). Apart from a cost-effective hybridization system, there are several biological bottlenecks that hamper the implementation of a cost-efficient hybrid seed production system. Because the floral biology of most wheat varieties is ill-adapted to cross-fertilization (29), a “redesigning” of the cleistogamic wheat flower has been suggested (7). Ideally, both male and female parental plants would possess open flowering spikelets. For optimal hybrid seed production, the sterile females must open their flowers simultaneously with those of the male crossing partners, which must release much long-lived viable pollen with good aerodynamics outside the floret. However, wheat produces only a comparably low level of pollen that settles quickly because it is relatively heavy and is viable only for a relatively short period (36).
Finally, because the heterosis of a hybrid may increase with genetic divergence between the parents, it will be of importance to group wheat lines into genetically divergent heterotic pools that combine the maximum use of yield heterosis with a high end-user quality (9). Currently, no divergent “heterotic pools” exist for wheat.
This report describes the successful implementation of a transgenic approach for hybrid wheat production. However, whether the prospects of the split-gene system will be translated into commercial hybrid wheat breeding and products in the future remains open. Ultimately, the successful realization of any genetically modified organism-based technology for agriculture will depend on industrial and consumer acceptance and on international trade regulations (37). Despite these issues, we hope that the split-gene system described here will be a valuable future contribution to unblocking bottlenecks associated with hybrid wheat breeding.
Materials and Methods
Transgenic Wheat Plants.
Spring wheat (Triticum aestivum L. cultivar Bobwhite) was used throughout this study. The plants were grown under controlled greenhouse conditions with 16 h of light at 20 °C and 8 h of darkness at 16 °C. The provectors were transformed using biolistic particle bombardment using a modified protocol that has been described previously (38).
Transgenic calli were selected on a medium containing 100 mg/L hygromycin B (Roche). Transgenic plantlets were selected in a medium containing 50 mg/L hygromycin B. For maintenance and propagation, male-sterile plants were pollinated by placing pollen-shedding anthers of untransformed plants into the closed flower. Plants were propagated by embryo rescue on half strength Murashige and Skoog medium, (M0222, Duchefa) supplemented with 15 g/L sucrose, 0.1 g/L casein hydrolysate, 0.2 mg/L copper(II) sulfate pentahydrate, 0.5 mg/L thiamine, and 50 mg/L myo-inositol.
Molecular Analyses of the Transgenic Plants.
The experimental strategies used for polymerase chain reactions and DNA gel blot analyses are detailed in Table S2 and Fig. S1.
Fertility Assays.
Fertility was evaluated by determining the occurrence of seeds on mature spikes. Alternatively, to determine fertility before manual pollination, the amount and vitality of the pollen was assayed. For this purpose, pollen from two flowers of two to seven ears from each plant was stained with Alexander stain (39) and examined under a light microscope. The assays were performed 2–3 d before anthesis.
Supplementary Material
Acknowledgments
We thank Manja Franke, Corinna Schollmeier, Silvana Fischer, Susanne Knüpffer, Robert Jerchel, and Kerstin Denzin for technical assistance and greenhouse management. We thank Dr. Ingo Schubert, Dr. Florian Mette, and Dr. Heike Gnad for their comments on the manuscript and Dr. Renate Schmidt for fruitful discussions. We thank the Bundesministerium für Bildung und Forschung for funding the project KMU-Innovativ (Grant FZK0315889B) as part of a joint project between the Leibniz Institute of Plant Genetics and Crop Plant Research and Nordsaat GmbH.
Footnotes
Conflict of interest statement: The cooperation partner Nordsaat Saatzucht GmbH holds a patent that protects key elements of the split-gene system (Eur Patent Publ EP2294204).
This article is a PNAS Direct Submission.
See Commentary on page 9024.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1402836111/-/DCSupplemental.
References
- 1.Schnable PS, Springer NM. Progress toward understanding heterosis in crop plants. Annu Rev Plant Biol. 2013;64:71–88. doi: 10.1146/annurev-arplant-042110-103827. [DOI] [PubMed] [Google Scholar]
- 2.Kempe K, Gils M. Pollination control technologies for hybrid breeding. Plant Breed. 2011;27(4):417–437. [Google Scholar]
- 3.Perez-Prat E, van Lookeren Campagne MM. Hybrid seed production and the challenge of propagating male-sterile plants. Trends Plant Sci. 2002;7(5):199–203. doi: 10.1016/s1360-1385(02)02252-5. [DOI] [PubMed] [Google Scholar]
- 4.Smith CW, Betrán J, Runge E. Corn: Origin, History, Technology, and Production. Hoboken, NJ: Wiley; 2004. [Google Scholar]
- 5.Chen L, Liu Y-G. Male sterility and fertility restoration in crops. Annu Rev Plant Biol. 2014;65:5.1–5.28. doi: 10.1146/annurev-arplant-050213-040119. [DOI] [PubMed] [Google Scholar]
- 6.Angus WJ. United Kingdom wheat pool. In: Bonjean AP, Angus WJ, editors. The World Wheat Book. Paris: Lavoisier; 2001. pp. 103–125. [Google Scholar]
- 7.Whitford R, et al. Hybrid breeding in wheat: Technologies to improve hybrid wheat seed production. J Exp Bot. 2013;64(18):5411–5428. doi: 10.1093/jxb/ert333. [DOI] [PubMed] [Google Scholar]
- 8.Kaul MLH. Male Sterility in Higher Plants. Berlin, Heidelberg: Springer; 1988. [Google Scholar]
- 9.Longin CF, et al. Hybrid breeding in autogamous cereals. Theor Appl Genet. 2012;125(6):1087–1096. doi: 10.1007/s00122-012-1967-7. [DOI] [PubMed] [Google Scholar]
- 10.Cisar G, Cooper D. Hybrid wheat. In: Curtis BC, Rajaram S, Gomez Macpherson H, editors. Bread Wheat: Improvement and Production. Rome: Food and Agriculture Organization; 2002. pp. 157–174. [Google Scholar]
- 11.Adugna A, Nanda GS, Singh K, Bains NS. A comparison of cytoplasmic and chemically-induced male sterility systems for hybrid seed production in wheat (Triticum aestivum L.) Euphytica. 2004;135(3):297–304. [Google Scholar]
- 12.Pickett AA. Advances in Plant Breeding. Vol 15. Berlin: Paul Parey Scientific; 1993. Hybrid wheat—results and problems. [Google Scholar]
- 13.Mariani C, De Beuckeleer M, Truettner J, Leemans J, Goldberg RB. Induction of male sterility in plants by a chimaeric ribonuclease gene. Nature. 1990;374(6295):737–741. [Google Scholar]
- 14.Mariani CV, et al. A chimaeric ribonuclease-inhibitor gene restores fertility to male sterile plants. Nature. 1992;357(6377):384–387. [Google Scholar]
- 15.Gils M, et al. A novel hybrid seed system for plants. Plant Biotechnol J. 2008;6(3):226–235. doi: 10.1111/j.1467-7652.2007.00318.x. [DOI] [PubMed] [Google Scholar]
- 16.Saleh L, Perler FB. Protein splicing in cis and in trans. Chem Rec. 2006;6(4):183–193. doi: 10.1002/tcr.20082. [DOI] [PubMed] [Google Scholar]
- 17.Perler FB. Protein splicing of inteins and hedgehog autoproteolysis: Structure, function, and evolution. Cell. 1998;92(1):1–4. doi: 10.1016/s0092-8674(00)80892-2. [DOI] [PubMed] [Google Scholar]
- 18.Gowda M, Longin CFH, Lein V, Reif JC. Relevance of specific versus general combining ability in winter wheat. Crop Sci. 2012;52(6):2494–2500. [Google Scholar]
- 19.Longin CFH, et al. Hybrid wheat: Quantitative genetic parameters and consequences for the design of breeding programs. Theor Appl Genet. 2013;126(11):2791–2801. doi: 10.1007/s00122-013-2172-z. [DOI] [PubMed] [Google Scholar]
- 20.Mühleisen J, Piepho HP, Maurer HP, Longin CFH, Reif JC. Yield stability of hybrids versus lines in wheat, barley, and triticale. Theor Appl Genet. 2014;127(2):309–316. doi: 10.1007/s00122-013-2219-1. [DOI] [PubMed] [Google Scholar]
- 21.de Vries GE. Climate changes leads to unstable agriculture. Trends Plant Sci. 2000;5(9):367. [Google Scholar]
- 22.Kempe K, Rubtsova M, Riewe D, Gils M. The production of male-sterile wheat plants through split barnase expression is promoted by the insertion of introns and flexible peptide linkers. Transgenic Res. 2013;22(6):1089–1105. doi: 10.1007/s11248-013-9714-7. [DOI] [PubMed] [Google Scholar]
- 23.Tsuchiya T, Toriyama K, Yoshikawa M, Ejiri S, Hinata K. Tapetum-specific expression of the gene for an endo-beta-1,3-glucanase causes male sterility in transgenic tobacco. Plant Cell Physiol. 1995;36(3):487–494. doi: 10.1093/oxfordjournals.pcp.a078784. [DOI] [PubMed] [Google Scholar]
- 24.Smith MC, Thorpe HM. Diversity in the serine recombinases. Mol Microbiol. 2002;44(2):299–307. doi: 10.1046/j.1365-2958.2002.02891.x. [DOI] [PubMed] [Google Scholar]
- 25.Thorpe HM, Smith MC. In vitro site-specific integration of bacteriophage DNA catalyzed by a recombinase of the resolvase/invertase family. Proc Natl Acad Sci USA. 1998;95(10):5505–5510. doi: 10.1073/pnas.95.10.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kempe K, et al. Transgene excision from wheat chromosomes by phage phiC31 integrase. Plant Mol Biol. 2010;72(6):673–687. doi: 10.1007/s11103-010-9606-7. [DOI] [PubMed] [Google Scholar]
- 27.Gleba Y, Marillonnet S, Klimyuk V. Design of safe and biologically contained transgenic plants: Tools and technologies for controlled transgene flow and expression. Biotechnol Genet Eng Rev. 2004;21:325–367. doi: 10.1080/02648725.2004.10648061. [DOI] [PubMed] [Google Scholar]
- 28.Kempe K, Rubtsova M, Gils M. Intein-mediated protein assembly in transgenic wheat: Production of active barnase and acetolactate synthase from split genes. Plant Biotechnol J. 2009;7(3):283–297. doi: 10.1111/j.1467-7652.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- 29.de Vries AP. Flowering biology of wheat, particularly on view of hybrid seed production-a review. Euphytica. 1971;20(2):152–170. [Google Scholar]
- 30.Evans TC, Jr, Xu MQ, Pradhan S. Protein splicing elements and plants: From transgene containment to protein purification. Annu Rev Plant Biol. 2005;56:375–392. doi: 10.1146/annurev.arplant.56.032604.144242. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Yau YY, Perkins-Balding D, Thomson JG. Recombinase technology: Applications and possibilities. Plant Cell Rep. 2011;30(3):267–285. doi: 10.1007/s00299-010-0938-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maruyama K, Kato H, Araki H. Mechanized production of F1 seeds in rice [Oryza sativa] by mixed planting. Jpn Agric Res Q. 1991;24(4):243–252. [Google Scholar]
- 33.Hawkes T, et al. D-glufosinate as a male sterility agent for hybrid seed production. Plant Biotechnol J. 2011;9(3):301–314. doi: 10.1111/j.1467-7652.2010.00549.x. [DOI] [PubMed] [Google Scholar]
- 34.Conner TW, Fabbri BJ, Huang J. 2002. Methods for translational repression of gene expression in plants. US Patent 20,020,062,499.
- 35.Allen E, et al. 2007. Methods for producing hybrid seed. US patent publication WO 2007047016 (Monsanto Company, Creve Coeur, MO)
- 36.Fritz SE, Lukaszewski AJ. Pollen longevity in wheat, rye and triticale. Plant Breed. 1989;102(1):31–34. [Google Scholar]
- 37.Dunwell JM. Transgenic cereals: Current status and future prospects. J Cereal Sci. 2013;59(3):419–434. [Google Scholar]
- 38.Rubtsova M, et al. Expression of active Streptomyces phage phiC31 integrase in transgenic wheat plants. Plant Cell Rep. 2008;27(12):1821–1831. doi: 10.1007/s00299-008-0604-z. [DOI] [PubMed] [Google Scholar]
- 39.Alexander MP. Differential staining of aborted and nonaborted pollen. Stain Technol. 1969;44(3):117–122. doi: 10.3109/10520296909063335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.