Significance
The recently discovered type VI secretion system (T6SS) is used by Gram-negative bacteria to deliver effector proteins into both eukaryotic and prokaryotic neighboring cells to mediate virulence and competition, respectively. Even though several T6SS effector families have been described, many T6SSs are not associated with known effectors. In this work, we report the discovery of a conserved motif named MIX (marker for type six effectors) that is often located near the T6SS genome neighborhood and is found in numerous proteins from diverse Proteobacteria, among them several T6SS effectors. We show that the MIX motif can be used as a marker to identify new T6SS effectors, thereby significantly enlarging the list of known T6SS effector families.
Keywords: bacterial competition, colicin, vibrio, toxin
Abstract
Bacteria use diverse mechanisms to kill, manipulate, and compete with other cells. The recently discovered type VI secretion system (T6SS) is widespread in bacterial pathogens and used to deliver virulence effector proteins into target cells. Using comparative proteomics, we identified two previously unidentified T6SS effectors that contained a conserved motif. Bioinformatic analyses revealed that this N-terminal motif, named MIX (marker for type six effectors), is found in numerous polymorphic bacterial proteins that are primarily located in the T6SS genome neighborhood. We demonstrate that several MIX-containing proteins are T6SS effectors and that they are not required for T6SS activity. Thus, we propose that MIX-containing proteins are T6SS effectors. Our findings allow for the identification of numerous uncharacterized T6SS effectors that will undoubtedly lead to the discovery of new biological mechanisms.
The type VI secretion system (T6SS), a recently discovered protein secretion machinery (1), is a tool used by Gram-negative bacteria to inject effector proteins into recipient cells (2). During the type VI secretion process, an intracellular tube complex composed of hexameric rings of haemolysin coregulated proteins (Hcp) capped with a trimer of valine-glycine repeat protein G (VgrG) and a proline-alanine-alanine-arginine (PAAR) repeat-containing protein is surrounded by a sheath made of VipA/VipB heterodimers (also known as TssB/TssC). Upon an extracellular signal, the sheath contracts, leading to secretion of the tube complex into an adjacent target cell (2–4). Multiple T6SSs can be encoded within a single bacterial genome (5), and each T6SS can have more than one cognate Hcp, VgrG, or PAAR repeat-containing protein (4).
T6SS effectors are predicted to be loaded onto the tube complex by several distinct mechanisms: as toxin domains fused to VgrG or PAAR repeat-containing proteins, as proteins that bind the inner surface of the Hcp tube, or as proteins that interact with VgrG or PAAR repeat-containing proteins (2). Two T6SS effector families have been characterized: peptidoglycan hydrolases (6) and phospholipases (7). Additional effector activities, such as nucleases (8), actin cross-linking (9), ADP ribosylation (10), and pore-forming (11), have also been described. Notably, T6SS effectors with antibacterial activities are paired with a cognate immunity protein encoded downstream of the effector gene to prevent self-intoxication (6, 12).
We have recently described an antibacterial activity for T6SS1 of the marine bacterium Vibrio parahaemolyticus, a leading cause of gastroenteritis (13), and identified the environmental conditions required for its activation (14). Surprisingly, no known T6SS effectors are found in the genome of the V. parahaemolyticus RIMD 2210633 isolate, suggesting this strain harbors previously unidentified T6SS effectors.
Here, we set out to identify V. parahaemolyticus T6SS1 effectors that mediate its antibacterial activity. Using comparative proteomics, we identified several T6SS effectors and their cognate immunity proteins. Remarkably, we found a motif named MIX (marker for type six effectors) that was shared by two of the newly identified effectors. We hypothesized and subsequently showed that this motif is found in numerous bacterial proteins with diverse predicted or established bacteriocidal and virulence activities, among them several confirmed T6SS effectors. Thus, we propose that proteins containing the MIX motif are polymorphic T6SS effectors.
Results
Identification of T6SS Effectors.
To identify the V. parahaemolyticus T6SS1 effectors that mediate its antibacterial activity, we set out to compare the secretomes of strains with an active or inactive T6SS1 under inducing conditions. To this end, we used a V. parahaemolyticus strain with a deletion in opaR, that encodes the quorum-sensing master regulator, which we have previously shown to derepress T6SS1 (14). We then verified that the ΔopaR strain was able to mediate bacterial killing under T6SS1-inducing conditions, whereas ΔopaRΔhcp1, with an inactive T6SS1, was not (Fig. S1).
Next, we used mass spectrometry (MS) to analyze the secretomes of these two strains under T6SS1-inducing conditions. We identified 13 proteins that were only found in the supernatant fractions of the ∆opaR strain, whereas secretion of other proteins was largely comparable between the two strains (Dataset S1). Of these 13 proteins, seven (VP0035, VP0070, VP0762, VP0793, VP2731, VP2767, and VPA1376) are also encoded by V. parahaemolyticus AQ3810, a strain that does not carry T6SS1 (15), and were thus excluded as potential effectors. The remaining six proteins are VP1388, VP1390, VP1393, VP1394, VP1415, and VPA1263. VP1393, VP1394, and VP1415 are the Hcp1, VgrG1, and PAAR repeat-containing proteins, respectively, that compose the tube complex of T6SS1 (14) and are encoded within the T6SS1 gene cluster on chromosome I. These proteins are known to be secreted by the T6SS, and thus served as positive controls. VP1388 and VP1390 are also encoded within the T6SS1 gene cluster, whereas VPA1263 is encoded on chromosome II. VP1388, VP1390, and VPA1263 are not known structural components of the T6SS1, and thus represented putative effectors. VP1415 and VPA1263 have a predicted AHH nuclease and a PyocinS/colicin DNase domain, respectively, that could mediate antibacterial toxicity. VP1388 has no recognizable conserved domains, and VP1390 contains an OmpA_C peptidoglycan-binding domain.
Because antibacterial T6SS effectors should have cognate immunity proteins encoded downstream of the effector gene, we identified small ORFs that possibly encode such immunity proteins downstream of vp1415 (vp1416) and vp1388 (vp1389). However, no gene encoding a colicin-immunity protein (16) was annotated downstream of the putative colicin effector vpa1263. We therefore scanned the genomic region between vpa1263 and vpa1264 and identified a short ORF (V. parahaemolyticus RIMD 2210633 chromosome II position 1344453–1344737) that is predicted to encode a 94-aa long colicin-immunity protein (pfam01320). We named this gene vti2 (Vibrio type VI immunity 2). No putative immunity gene was found downstream of vp1390 because, as we have previously shown, vp1391 encodes a T6SS1 regulator (14). Hence, we hypothesized that VP1388/VP1389, VPA1263/Vti2, and VP1415/VP1416 are T6SS effector/immunity pairs, and we excluded VP1390 from subsequent analyses.
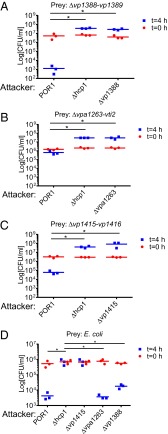
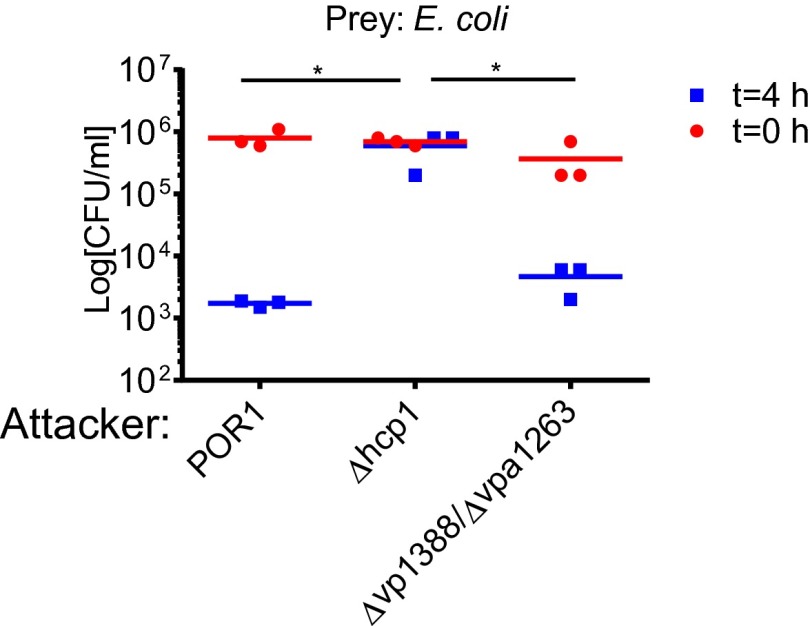
To test our hypothesis, we monitored the ability of a V. parahaemolyticus POR1 strain, which is immune to self-intoxication (14), to kill strains with deletions in the putative effector/immunity gene pairs. Indeed, POR1 was able to kill strains with deletions in vp1415/vp1416, vp1388/vp1389, and vpa1263/vti2 when cocultured under T6SS1-inducing conditions (Fig. 1 A–C). This killing activity was mediated by T6SS1, because a ∆hcp1 strain was not toxic to these strains (Fig. 1 A–C). Moreover, deletion of the putative effectors abolished killing of the strains deleted for the cognate immunity genes, suggesting that these effectors mediated the T6SS1-dependent killing (Fig. 1 A–C). Importantly, strains with deletions in either vp1388 or vpa1263 were able to kill Escherichia coli prey, indicating that these two effectors were not required for T6SS1 activity, whereas a deletion in vp1415 abolished E. coli killing (Fig. 1D) because it encodes the structural PAAR repeat-containing protein that is essential for T6SS activity (4). In addition, ectopic expression of the putative immunity genes reconstituted immunity against self-intoxication (Fig. S2). Taken together, these results demonstrate that VP1388/VP1389, VPA1263/Vti2, and VP1415/VP1416 constitute T6SS effector/immunity pairs.
Fig. 1.
VP1388/VP1389, VPA1263/Vti2, and VP1415/VP1416 are T6SS effector/immunity pairs. Viability counts of prey strains before (0 h) and after (4 h) coculture with the indicated attacker strains are shown. Prey strains used were as follows: POR1Δvp1388-vp1389 (A), POR1Δvpa1263-vti2 (B), POR1Δvp1415-vp1416 (C), and E. coli (D). Asterisks mark statistical significance between sample groups at t = 4 h (P < 0.05).
Widespread Motif Found in Functionally Diverse Bacterial Proteins.
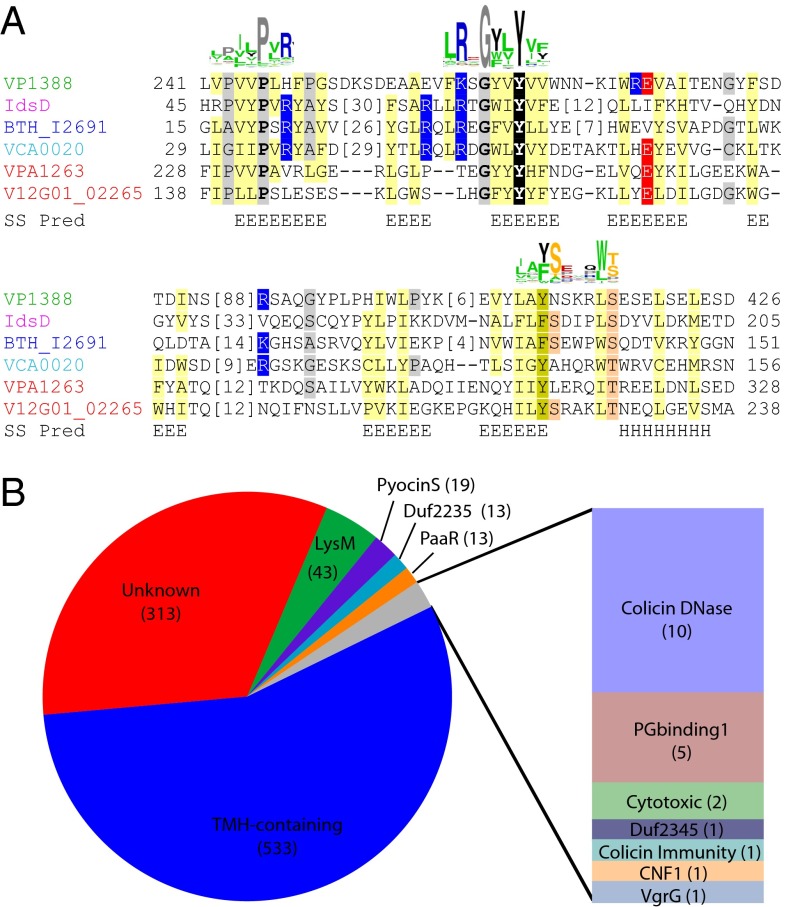
While examining the amino acid sequences of the two T6SS1 effectors, VP1388 and VPA1263, we noticed that they each possess an N-terminal region that is conserved in related sequences. In fact, position-specific iterative (PSI)-BLAST initiated with the N-terminal region of VP1388 (residues 1–300) confidently identified a number of bacterial protein sequences, including the N terminus of VPA1263 (after five iterations, E-value of 3−18). The PSI-BLAST link between VP1388 and VPA1263 spanned ∼225 amino acids (residues 65–284 in VP1388 and residues 37–266 in VPA1263) and shared 18% identity over the aligned region (45 identical residues out of 245 PSI-BLAST aligned positions). Because this region was found in two polymorphic T6SS effectors, we hypothesized that the other sequences identified as having this region might represent new T6SS effectors. To define this region better and identify all protein sequences that possess it, we initiated a transitive series of PSI-BLAST searches using various sequences identified by the VP1388 N terminus (Dataset S2). Remarkably, three of the additional proteins that we identified as having the region were previously shown to be T6SS effectors: the Vibrio cholerae VCA0020 (named VasX) (11, 17), the Burkholderia thailandensis BTH_I2691 (6), and the Proteus mirabilis IdsD (18), supporting our hypothesis (Fig. 2A). Notably, with only a few exceptions, all identified proteins were in Gram-negative T6SS component-encoding Proteobacteria (mainly gamma-Proteobacteria, beta-Proteobacteria, and delta-Proteobacteria, with a few sequences from bacteroidetes and one sequence each from Acidobacteria and Annelida) (Fig. S3). Considering the presence of multiple known T6SS effectors among the identified proteins, as well as their taxonomic distribution, we propose that proteins containing this region function as T6SS effectors, and we named it MIX.
Fig. 2.
MIX motif is found in T6SS effectors and is fused to various toxic domains. (A) Multiple sequence alignment of MIX from known T6SS effectors with start and end positions indicated. Consensus secondary structure predictions are indicated below [E (strand) and H (helix)], and conservation logos of the motifs generated with the full list of MIX sequences are indicated above. Residues are highlighted according to group-wise conservations: mainly hydrophobic (yellow), mainly small (gray), mainly positive (blue), mainly negative (red), and motif Y (black). (B) Pie chart represents domains found fused to MIX. PG, peptidoglycan; TMH, transmembrane helix.
MIX sequences exhibit considerable diversity, which complicated defining its boundaries and aligning the entire dataset. To overcome this problem, we first grouped representative sequences using all against all pairwise similarities. The resulting clusters split the MIX sequences into five clans, which we named MIX I–MIX V (Fig. S4). Analysis of the amino acid sequences revealed that MIX is composed of a widely conserved central motif, hRxGhhYhh (where h represents hydrophobic residues), as well as two somewhat less conserved motifs at the N terminus (shhPhR) and the C terminus (hhF/YSxxxWS/T) (Fig. 2A).
The majority of MIX-containing sequences include long extended C-termini lacking identifiable relationships with known domains (Fig. 2B). MIX sequences that possess known domains include two different peptidoglycan-binding domains (LysM and peptidoglycan-binding1), PyocinS and colicin DNase bacteriocidal domains, a bacterial ribosomal inactivating RNase domain (cytotoxic), and a Rho-activating domain of cytotoxic necrotizing factor (CNF1). MIX also exists in proteins with domains of unknown function, Duf2235 and Duf2345, which are mostly found fused to VgrG and PAAR repeat-containing proteins. Interestingly, MIX motifs are found in several VgrG and PAAR repeat-containing proteins, further supporting a link between MIX and T6SS (Fig. 2B). Remarkably, many MIX proteins contain predicted transmembrane helices (TMHs) in the C terminus (Fig. 2B and Fig. S5), suggesting they could function as pore-forming toxins, as was shown recently for VCA0020 (11). Indeed, the C terminus of several of the predicted TMH-containing proteins confidently identifies colicin pore-forming structures using HHpred (19): Vibrio coralliilyticus VCR_19700 (GI|518106880, range 427–711) identifies the pore-forming domain of the Colicin B structure (1rh1: range 279–509, probability 93.7%), and Pseudoalteromonas haloplanktis PHAL_03780 (GI|515077299, range 749–901) identifies the pore-forming domain of the Colicin A structure (1col: 34–194, probability 90.6%).
Genome Neighborhoods Link MIX to the T6SS.
Large-scale genome analysis on T6SS components has revealed their phylogenetic distribution, gene content, organization, and evolution (5, 20). Importantly, T6SS genes tend to occur closely within genomic neighborhoods (5). The conserved genomic organization among these T6SS loci has been used to justify functional associations of various components (4, 21). Given this precedence for conserved genomic context, we sought to strengthen our proposal that MIX-containing proteins function as T6SS effectors by comparing the gene neighborhood association network of the core T6SS component groups with those of the five MIX clans. Given the observation that MIX can occur as fusions within T6SS components, we considered both gene neighborhood and gene fusion data in our analysis.
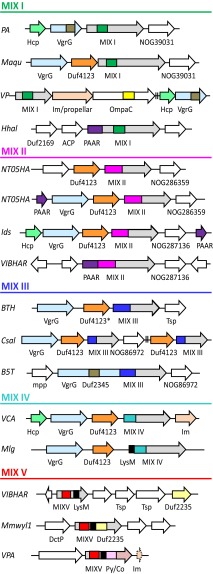
Similar to previous observations (5, 20), the core T6SS components form a highly connected network of confident genome neighborhood linkages (scores >0.7; Fig. S6). Compared with core T6SS components, MIX-containing groups include substantially fewer representatives with more limited taxonomic distribution (Table 1). The MIX-encoding genes display variable domain organizations and rapidly diverging sequences that further complicate groupings. Accordingly, links between conserved core components are more confident than links of these peripheral components to the conserved core T6SS. Strikingly, many of the MIX-encoding genes occur together with VgrG in genome neighborhoods (Fig. 3 and Fig. S5) and are likely within the same operons, further supporting a link between MIX and T6SSs.
Table 1.
MIX functional associations
| MIX | Connected node | ||||
| Clan* | NOG† | Rep. gene‡ | COG/NOG† | Name | STRING score§ |
| I | NOG135787 12/9 | VP1388 | NOG39031 | Unknown TMH | 0.825 |
| NOG09810 | VC_A0118 family | 0.663 | |||
| COG3518 | ImpF | 0.485 | |||
| II | NOG272743 6/4 | VIBHAR_03070 | NOG283659 | Unknown TMH | 0.825 |
| NOG132149 | Duf4123 | 0.762 | |||
| COG4104 | PAAR | 0.308 | |||
| III | NOG126038 54/29 | BTH_I2691 | NOG282135 | Unknown TMH | 0.885 |
| NOG86170 | Duf4123 | 0.881 | |||
| NOG296811 | Unknown TMH | 0.849 | |||
| NOG132306 | Duf4123 | 0.849 | |||
| NOG86972 | Unknown TMH | 0.843 | |||
| NOG73587 | TPR repeat | 0.762 | |||
| COG4253 | VgrG/Duf2345 | 0.734 | |||
| NOG70522 | Transposase IS3 | 0.643 | |||
| COG3521 | VasD | 0.605 | |||
| IV | NOG40297 21/16 | VCA0020 | NOG43078 | Duf4123 | 0.875 |
| NOG235932 | Unknown TMH | 0.825 | |||
| COG4104 | PAAR | 0.673 | |||
| COG3157 | Hcp | 0.419 | |||
| V | NOG45572 43/32 | Mmwyl1_ 0527 | NOG12776 | Duf2235 | 0.836 |
| COG3501 | VgrG | 0.771 | |||
| COG5529 | Pyocin_large | 0.737 | |||
| NOG75169 | Duf4123 | 0.709 | |||
| NOG219546 | Duf2931 | 0.709 | |||
| COG3157 | Hcp | 0.690 | |||
| COG4253 | VgrG/Duf2345 | 0.663 | |||
ImpF, impaired in nodulation F; VasD, virulence-associated secretion D.
STRING database group most representative of all MIX-containing sequences from the indicated clan: nonorthologous group (NOG) or clusters of orthologous groups (COGs). Confidently networked T6SS component groups from Fig. S6 are bolded.
Representative (Rep.) gene name from STRING group. Known T6SS components are bolded.
Combined STRING genome neighborhood and gene fusion score (high confidence, >0.7; medium confidence, >0.4).
Fig. 3.
MIX genome neighborhoods link to T6SS components. Representative MIX domain genes from five clans (colored according to clan as in Fig. S4 and labeled MIX I–MIX V) are represented by arrows indicating the direction of translation. MIX-containing and neighboring genes are labeled with gene names below. Gene abbreviations are shown to the left and correspond to the following species (with the MIX domain gene name in parentheses): PA, Pseudomonas aeruginosa PAO1 (PA5265); Maqu, Marinobacter aquaeolei VT8 (Maqu_3717); VP, V. parahaemolyticus RIMD 2210633 (VP1388); Hhal, Halorhodospira halophila SL1 (Hhal_0928); NT05HA, Aggregatibacter aphrophilus NJ8700 (NT05HA_0925 and NT05HA_2120); Ids, P. mirabilis BB2000 (IdsD); VIBHAR, Vibrio campbellii ATCC BAA-1116 (VIBHAR_03070 and VIBHAR_06795); BTH, B. thailandensis E264 (BTH_I2691); Csal, Chromohalobacter salexigens DSM 3043 (Csal_2252); B5T, Alcanivorax dieselolei B5 (B5T_00667); VCA, V. cholerae O1 biovar El Tor str. N16961 (VCA0020); Mlg, Alkalilimnicola ehrlichii MLHE-1 (Mlg_0648); Mmwyl1, Marinomonas sp. MWYL1 (Mmwyl1_0527); and VPA, V. parahaemolyticus RIMD 2210633 (VPA1263). Functionally linked neighboring genes are colored according to domain, including the T6SS components Hcp (light green), VgrG (light blue), and PAAR (purple), as well as several additional domains of unknown function, including Duf4123 (orange), Duf2235 (light yellow), and Duf2345 (dark gray). One gene contains a divergent Duf4123* domain that can be identified with less stringent cutoffs. Known immunity genes are colored light orange, and potential immunity genes with functionally linked domains are labeled: NOG39031, NOG286359, and NOG86972. Additional genes with domains of known function are colored: peptidoglycan binding domains OmpA_C (yellow) and LysM (black), as well as bacterial toxins PyocinS (pink) and colicin DNase (salmon). ACP, malonate decarboxylase delta subunit; DctP, TRAP dicarboxylate transporter subunit; Im, immunity; mpp, enterobacter aerogenes GpdQ and related proteins, metallophosphatase domain; OmpaC, outer membrane protein A C-terminal domain; Py/CO, PyocinS/colicin; Tsp, transposase and inactivated derivatives.
Genes classified as MIX I, III, and IV establish numerous genome neighborhood links of medium or high confidence to the core T6SS network (Table 1). A few examples of proteins from MIX I, II, and III clans exist where MIX is fused to a T6SS core component (Fig. 3), providing gene fusion linkage to the T6SS. MIX II links to the T6SS are of low confidence (to PAAR repeat-containing proteins: 0.308), potentially due to an observed mobility of MIX II duplications within genomes. Whereas one copy remains associated with the T6SS, the duplicated genes tend to migrate to distant sites (Fig. 3, NT05HA). MIX duplications in the genome are also evident for MIX III and IV.
MIX V maps to three different orthologous sets that are defined by their more well-defined C-terminal domains: Duf2235, PyocinS/colicin nuclease (not in Table 1), and LysM (not in Table 1). These C-terminal domains can be found alone, fused to MIX V, or fused to other T6SS components. Thus, neighborhood analysis for Duf2235 paradoxically provides the strongest evidence for association of MIX V with the T6SS, where Duf2235 genes from the orthologous set that do not necessarily contain MIX V provide highly confident (0.771) neighborhood and gene fusion links to VgrG. In fact, Duf2235 domain architectures include fusions to VgrG, Hcp, and PAAR repeat-containing proteins. Such a mobile distribution of the C-terminal domains fused to MIX V highlights its ability to acquire potential new T6SS effector functions through C-terminal fusions.
Validating a T6SS MIX Effector.
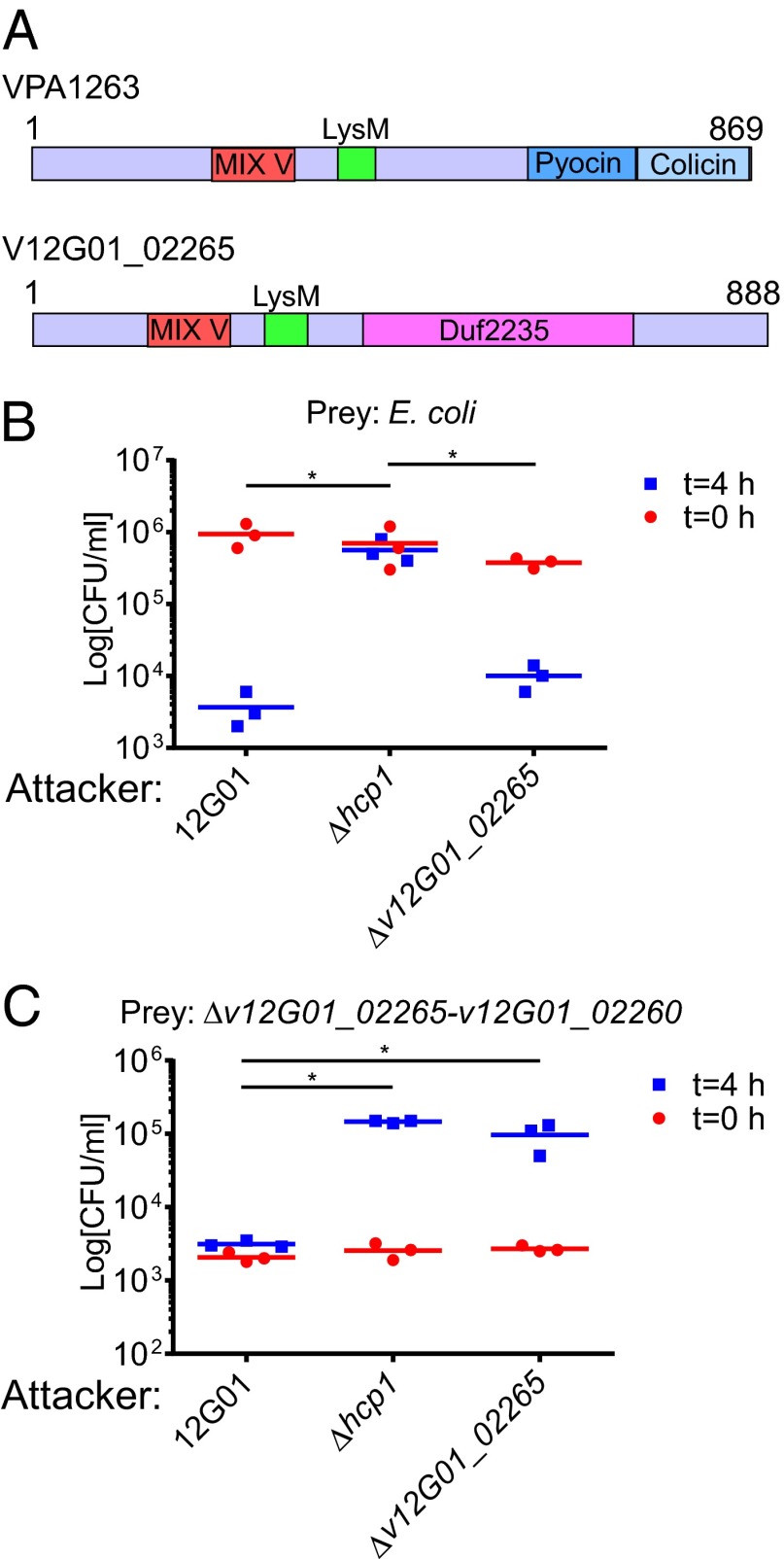
To test our hypothesis directly that MIX-containing proteins are T6SS effectors, we set out to characterize the Vibrio alginolyticus v12G01_02265 that encodes a member of the MIX V clan with a C-terminal Duf2235 (Figs. 2A and 4A). Notably, v12G01_02265 does not neighbor any known T6SS component. First, we demonstrated that V. alginolyticus can use its T6SS1 (22) for interbacterial competition by showing that it is able to kill E. coli in a T6SS1-dependent manner (Fig. 4B). Next, we constructed a V. alginolyticus strain deleted for v12G01_02265-v12G01_02260, which encodes the putative T6SS effector/immunity pair, and showed that it lost its immunity against self-intoxication when cocultured with the WT 12G01 strain (Fig. 4C). Remarkably, strains deleted for hcp1 or v12G01_02265 were no longer toxic toward the Δv12G01_02265-v12G01_02260 strain, indicating that self-intoxication was mediated by T6SS1, and specifically by v12G01_02265 (Fig. 4C). Importantly, the Δv12G01_02265 strain was still toxic toward E. coli, indicating that V12G01_02265 is not required for T6SS1 activity (Fig. 4B). Taken together, these results support our hypothesis and demonstrate that V12G01_02265 and V12G01_02260 are a T6SS effector/immunity pair.
Fig. 4.
V12G01_02265 and V12G01_02260 are a T6SS effector/immunity pair. (A) Schematic representation compares the MIX V-containing T6SS effectors VPA1263 and V12G01_02265. Viability counts of E. coli (B) and V. alginolyticus Δv12G01_02265-v12G01_02260 (C) prey before (0 h) and after (4 h) coculturing with V. alginolyticus 12G01 and its indicated derivative attacker strains. Asterisks mark statistical significance between sample groups at t = 4 h (P < 0.05).
MIX-Containing Effectors Are Not Required for T6SS Activity.
To determine whether MIX plays an essential structural role in the T6SS machinery, we constructed a V. parahaemolyticus strain with deletions in vp1388 and vpa1263, the only two genes encoding a MIX-containing protein in V. parahaemolyticus RIMD 2210633. This ∆vp1388∆vpa1263 strain was still toxic toward E. coli (Fig. 5), indicating that MIX is not a component required for T6SS activity and that additional effectors, such as VP1415, are sufficient to exert toxicity.
Fig. 5.
MIX is not required for T6SS activity. Viability counts of E. coli prey before (0 h) and after (4 h) coculturing with V. parahaemolyticus POR1, POR1Δhcp1, or POR1Δvp1388Δvpa1263 attacker strains. Asterisks mark statistical significance between sample groups at t = 4 h (P < 0.05).
Discussion
In this work, we used proteomic, bioinformatic, and genetic analyses to reveal numerous functionally diverse T6SS effectors that contain a conserved motif, named MIX. Several findings support our proposal that MIX-containing proteins are T6SS effectors: (i) MIX is genetically linked to the T6SS, (ii) many MIX-containing proteins are found in T6SS gene clusters or fused to T6SS tube complex components, (iii) MIX proteins are not a required structural component of the T6SS machinery, (iv) MIX is found in proteins with predicted cytotoxic domains, and (v) at least six MIX-containing proteins are confirmed T6SS effectors. Remarkably, each of these T6SS effectors (VP1388, VPA1263, VCA0020, BTH_I2691, IdsD, and V12G01_02265) belongs to a different MIX clan, strengthening our hypothesis that members of all MIX clans represent T6SS effectors.
Similar to VgrG and PAAR repeat-containing proteins, MIX proteins contain cytotoxic effector domains (4, 9). However, MIX effectors do not appear to be an essential structural component of the T6SS machinery, because they were not required for the antibacterial activity of the V. parahaemolyticus T6SS1. This finding is supported by previous observations that VCA0020 (VasX), the only MIX-containing protein in V. cholerae, was also not required for T6SS activity (17, 23). We note that although unlikely, it is possible that additional MIX-containing proteins eluding our detection are found in these strains and may mask a role for MIX in T6SS activity.
MIX appears to be located predominantly at the N-terminal end of proteins, with predicted effector activity domains at the C terminus. Compared with clustering of the entire MIX-containing sequence (Fig. S4), clustering of the C-terminal ends presumed to encompass effector activities produced numerous disconnected groups, as well as a few sparsely linked groups. This discrepancy supports a modular structure for MIX-containing T6SS effectors, in which a similar MIX-containing N terminus is fused to different C-terminal effector domains. Such modularity possibly provides bacteria with a diverse pool of toxins that can be deployed via the T6SS, as previously described for contact-dependent toxin delivery systems (24, 25). The modular nature of the MIX-containing N terminus is further highlighted by its tendency to duplicate within genomes while, in some cases, fused to different C-terminal activity domains. In other cases, MIX duplications appear to retain the same C-terminal activity domain. Interestingly, the MIX-adjacent genes presumed to encode immunity proteins have also duplicated in many cases, suggesting that the MIX effectors have retained similar activities despite divergence of their C-terminal sequence. The presence of a transposase IS3 among the top-scoring neighborhood links of MIX III (Table 1) may suggest a mechanism for the observed mobility of MIX-containing sequences.
Notably, our results suggest that MIX-containing effectors are not only antibacterial toxins but may also be eukaryotic virulence factors. For example, V. cholerae was previously shown to require the MIX-containing T6SS effector VCA0020 (VasX) to kill the social amoeba Dictyostelium discoideum (17), and the MIX-containing protein encoded by VPR01S_11_01570 from Vibrio proteolyticus is fused to a CNF1 Rho-activating domain that usually functions as a virulence factor against eukaryotes (26).
In conclusion, we describe a new motif found in numerous bacterial proteins that are predicted to be T6SS effectors with diverse activities. Identification of these T6SS effector families using this motif will undoubtedly lead to a better understanding of intercellular bacterial interactions.
Materials and Methods
Cloning and genetic manipulations were done using standard methods. For bacterial killing assays, cells were cocultured on LB broth agar plates containing 1% or 3% (vol/vol) sodium chloride for 4 h at 30 °C. Proteomics secretome data were analyzed by tandem MS using a QExactive mass spectrometer (Thermo Electron) coupled to an Ultimate 3000 RSLC-Nano liquid chromatography system (Dionex). Genomic neighborhoods encompassing the T6SS machinery were explored using the Microbial Genome Database for Comparative Analysis and the Search Tool for the Retrieval of Interacting Genes (STRING). The STRING database v9.1 describing protein association networks was used to generate genome neighborhood information for core T6SS components and MIX-containing groups.
Detailed information on methods and associated references are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
H.M., D.C.T., and X.G. are supported by Cancer Prevention and Research Institute of Texas Grants R1121 and RP120613 (to H.M.). N.V.G. is funded, in part, by National Institutes of Health (NIH) Grant GM094575 and Welch Foundation Grant I-1505. K.O., J.A.K, and D.S. are supported by NIH Grant R01-AI056404 and Grant I-1561 from the Welch Research Foundation. D.S. is a Chilton Foundation Fellow. K.O. is a Burroughs Welcome Investigator in Pathogenesis of Infectious Disease, Beckman Young Investigator, and W. W. Caruth, Jr., Biomedical Scholar, and has an Earl A. Forsythe Chair in Biomedical Science.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The dataset of tandem MS results reported in this paper has been uploaded onto the PeptideAtlas repository, www.peptideatlas.org/PASS/PASS00442.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406110111/-/DCSupplemental.
References
- 1.Mougous JD, et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science. 2006;312(5779):1526–1530. doi: 10.1126/science.1128393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho BT, Dong TG, Mekalanos JJ. A view to a kill: The bacterial type VI secretion system. Cell Host Microbe. 2014;15(1):9–21. doi: 10.1016/j.chom.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basler M, Pilhofer M, Henderson GP, Jensen GJ, Mekalanos JJ. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature. 2012;483(7388):182–186. doi: 10.1038/nature10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shneider MM, et al. PAAR-repeat proteins sharpen and diversify the type VI secretion system spike. Nature. 2013;500(7462):350–353. doi: 10.1038/nature12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer F, Fichant G, Berthod J, Vandenbrouck Y, Attree I. Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: What can be learned from available microbial genomic resources? BMC Genomics. 2009;10:104. doi: 10.1186/1471-2164-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell AB, et al. A widespread bacterial type VI secretion effector superfamily identified using a heuristic approach. Cell Host Microbe. 2012;11(5):538–549. doi: 10.1016/j.chom.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell AB, et al. Diverse type VI secretion phospholipases are functionally plastic antibacterial effectors. Nature. 2013;496(7446):508–512. doi: 10.1038/nature12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koskiniemi S, et al. Rhs proteins from diverse bacteria mediate intercellular competition. Proc Natl Acad Sci USA. 2013;110(17):7032–7037. doi: 10.1073/pnas.1300627110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pukatzki S, Ma AT, Revel AT, Sturtevant D, Mekalanos JJ. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc Natl Acad Sci USA. 2007;104(39):15508–15513. doi: 10.1073/pnas.0706532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suarez G, et al. A type VI secretion system effector protein, VgrG1, from Aeromonas hydrophila that induces host cell toxicity by ADP ribosylation of actin. J Bacteriol. 2010;192(1):155–168. doi: 10.1128/JB.01260-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyata ST, Unterweger D, Rudko SP, Pukatzki S. Dual expression profile of type VI secretion system immunity genes protects pandemic Vibrio cholerae. PLoS Pathog. 2013;9(12):e1003752. doi: 10.1371/journal.ppat.1003752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell AB, et al. Type VI secretion delivers bacteriolytic effectors to target cells. Nature. 2011;475(7356):343–347. doi: 10.1038/nature10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newton A, Kendall M, Vugia DJ, Henao OL, Mahon BE. Increasing rates of vibriosis in the United States, 1996-2010: Review of surveillance data from 2 systems. Clin Infect Dis. 2012;54(Suppl 5):S391–S395. doi: 10.1093/cid/cis243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salomon D, Gonzalez H, Updegraff BL, Orth K. Vibrio parahaemolyticus type VI secretion system 1 is activated in marine conditions to target bacteria, and is differentially regulated from system 2. PLoS ONE. 2013;8(4):e61086. doi: 10.1371/journal.pone.0061086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyd EF, et al. Molecular analysis of the emergence of pandemic Vibrio parahaemolyticus. BMC Microbiol. 2008;8:110. doi: 10.1186/1471-2180-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cascales E, et al. Colicin biology. Microbiol Mol Biol Rev. 2007;71(1):158–229. doi: 10.1128/MMBR.00036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyata ST, Kitaoka M, Brooks TM, McAuley SB, Pukatzki S. Vibrio cholerae requires the type VI secretion system virulence factor VasX to kill Dictyostelium discoideum. Infect Immun. 2011;79(7):2941–2949. doi: 10.1128/IAI.01266-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wenren LM, Sullivan NL, Cardarelli L, Septer AN, Gibbs KA. 2013 doi: 10.1128/mBio.00374-13. Two independent pathways for self-recognition in Proteus mirabilis are linked by type VI-dependent export. MBio 4(4). pii: e00374-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hildebrand A, Remmert M, Biegert A, Söding J. Fast and accurate automatic structure prediction with HHpred. Proteins. 2009;77(Suppl 9):128–132. doi: 10.1002/prot.22499. [DOI] [PubMed] [Google Scholar]
- 20.Shrivastava S, Mande SS. Identification and functional characterization of gene components of Type VI Secretion system in bacterial genomes. PLoS ONE. 2008;3(8):e2955. doi: 10.1371/journal.pone.0002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leiman PG, et al. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc Natl Acad Sci USA. 2009;106(11):4154–4159. doi: 10.1073/pnas.0813360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng L, Gu D, Wang Q, Liu Q, Zhang Y. Quorum sensing and alternative sigma factor RpoN regulate type VI secretion system I (T6SSVA1) in fish pathogen Vibrio alginolyticus. Arch Microbiol. 2012;194(5):379–390. doi: 10.1007/s00203-011-0780-z. [DOI] [PubMed] [Google Scholar]
- 23.Dong TG, Ho BT, Yoder-Himes DR, Mekalanos JJ. Identification of T6SS-dependent effector and immunity proteins by Tn-seq in Vibrio cholerae. Proc Natl Acad Sci USA. 2013;110(7):2623–2628. doi: 10.1073/pnas.1222783110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aoki SK, et al. A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature. 2010;468(7322):439–442. doi: 10.1038/nature09490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang D, de Souza RF, Anantharaman V, Iyer LM, Aravind L. Polymorphic toxin systems: Comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biol Direct. 2012;7:18. doi: 10.1186/1745-6150-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boquet P. The cytotoxic necrotizing factor 1 (CNF1) from Escherichia coli. Toxicon. 2001;39(11):1673–1680. doi: 10.1016/s0041-0101(01)00154-4. [DOI] [PubMed] [Google Scholar]
- 27.Frickey T, Lupas A. CLANS: A Java application for visualizing protein families based on pairwise similarity. Bioinformatics. 2004;20(18):3702–3704. doi: 10.1093/bioinformatics/bth444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.