Significance
Mutations in the leucine-rich repeat kinase 2 (LRRK2) gene are the most common known genetic cause of late-onset Parkinson disease, but the mechanisms underlying LRRK2 action in neurodegeneration are not clear. We demonstrate that rats deficient in LRRK2 expression are protected from dopaminergic neurodegeneration caused by overexpression of α-synuclein or exposure to LPS. LRRK2 expression is induced in proinflammatory brain myeloid cells under pathological conditions. Our results suggest that LRRK2 inhibition may have important neuroprotective effects by critically modulating neuroinflammatory responses. LRRK2 inhibition may therefore be a potentially efficacious approach to slow or stop the progression of brain disorders where myeloid cell activation drives aspects of dysfunction.
Keywords: PARK8, rat knockout, NACP, SNCA, CD68
Abstract
Missense mutations in the leucine-rich repeat kinase 2 (LRRK2) gene can cause late-onset Parkinson disease. Past studies have provided conflicting evidence for the protective effects of LRRK2 knockdown in models of Parkinson disease as well as other disorders. These discrepancies may be caused by uncertainty in the pathobiological mechanisms of LRRK2 action. Previously, we found that LRRK2 knockdown inhibited proinflammatory responses from cultured microglia cells. Here, we report LRRK2 knockout rats as resistant to dopaminergic neurodegeneration elicited by intracranial administration of LPS. Such resistance to dopaminergic neurodegeneration correlated with reduced proinflammatory myeloid cells recruited in the brain. Additionally, adeno-associated virus-mediated transduction of human α-synuclein also resulted in dopaminergic neurodegeneration in wild-type rats. In contrast, LRRK2 knockout animals had no significant loss of neurons and had reduced numbers of activated myeloid cells in the substantia nigra. Although LRRK2 expression in the wild-type rat midbrain remained undetected under nonpathological conditions, LRRK2 became highly expressed in inducible nitric oxide synthase (iNOS)-positive myeloid cells in the substantia nigra in response to α-synuclein overexpression or LPS exposures. Our data suggest that knocking down LRRK2 may protect from overt cell loss by inhibiting the recruitment of chronically activated proinflammatory myeloid cells. These results may provide value in the translation of LRRK2-targeting therapeutics to conditions where neuroinflammation may underlie aspects of neuronal dysfunction and degeneration.
Missense mutations in the leucine-rich repeat kinase 2 (LRRK2) gene can be found in many families that transmit classical late-onset Parkinson disease (PD) from one generation to the next. Notably, these mutations are prevalent in the Ashkenazi Jewish and North African Arab Berber populations in more than 20% of PD cases (1, 2). Genome-wide association studies further provide evidence that links LRRK2 to PD susceptibility, with several risk alleles being identified in LRRK2 (3, 4). Association studies have also linked LRRK2 to Crohn disease and Hansen disease (5, 6). Although LRRK2 is widely considered an exciting target for therapeutic approaches in PD, the pathobiological role of LRRK2 as a critical modifier of disease susceptibility is not well understood. Additional insights into LRRK2-linked molecular pathways relevant to PD and neurodegeneration may enable more predictive preclinical studies.
PD is pathologically characterized by both α-synuclein inclusions called Lewy bodies and Lewy neurites and the profound loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc). The absence of LRRK2 has been shown to impair α-synuclein inclusion formation, neuron loss, and microglia activation in mice overexpressing mutant α-synuclein (7). However, other studies that have crossed LRRK2 knockout mice with different α-synuclein transgenic mice did not observe robust protection from the formation of α-synuclein pathology or differences in animal survival rates (8, 9). Because these α-synuclein transgenic mice do not have loss of dopaminergic neurons in the SNpc, it is not known whether LRRK2 may modify α-synuclein–mediated dopaminergic cell loss and associated critical phenotypes relevant to PD.
Besides neuronal LRRK2 expression, high levels of LRRK2 have been recently detected in activated myeloid lineage cells that include macrophages and microglia (10, 11). In cultured rat microglia cells, we found that LRRK2 expression is induced upon activation. RNA-interference knockdown of LRRK2 reduces immune responses induced by the toll-like receptor 4 (TLR4) agonist lipopolysaccharide (LPS) (10). Both LPS exposure and overexpression of human α-synuclein via viral vectors produce selective dopaminergic neuron death in rodents (12, 13), but LRRK2 function has not been previously evaluated in either model system. The LPS receptor TLR4 is poorly or not expressed in neurons and requires the function of innate immune cells including microglia and macrophages to elicit neurodegeneration. α-synuclein–related toxicities in the midbrain also appear to require innate immune cell action, as MHC-II knockout mice are protected from dopaminergic neurodegeneration (14). We therefore hypothesized that LRRK2 pathobiological function may not be fully appreciated without significant neuroinflammatory mechanisms driving aspects of neuronal dysfunction and degeneration in model systems.
Here, we sought to clarify the role of LRRK2 in neurodegeneration using several paradigms of dopaminergic neuronal loss that are known to depend, in part, on the effects of neuroinflammation. We observed profound neuroprotection from LPS exposures as well as inflammation and dopaminergic degeneration caused by the overexpression of human α-synuclein in LRRK2 knockout rats. These studies should provide a rationale, as well as possible preclinical platforms, for the evaluation of future therapeutics (e.g., clinical-candidate LRRK2 kinase inhibitors) that reduce LRRK2 activity.
Results
Recently, LRRK2 KO rats in the outbred Long–Evans background (Charles Rivers) became commercially available. Zinc-finger nucleases were used to genetically modify the LRRK2 gene with an in-del in exon 30 that results in a premature stop codon in the same exon (Fig. S1A). We verified complete knockdown of protein using both N-terminal and C-terminal targeting LRRK2 monoclonal antibodies (Fig. S1B). We performed stereological analysis in a cohort of rats to estimate the normal content of tyrosine hydroxylase (TH)-positive dopaminergic neurons in the SNpc. We observed no differences, indicating that LRRK2 is not required for dopaminergic neuron development in rats (Fig. S1C).
In vitro evidence suggests that LRRK2 knockdown attenuates proinflammatory responses induced by LPS exposure (10). We first evaluated several endotoxin dosages of ultrapurified LPS (Invivogen, Inc.) in rodents and found that 50 kE.U. (endotoxin units) produced a significant lesion 2 wk after introduction to the SNpc. Because outbred strains like Long–Evans are known for heterogeneity in phenotype and often demand larger groups for appropriately powered analyses, we assembled a cohort of 38 LRRK2 WT and KO rats (n = 19 per genotype) to evaluate the effects of LRRK2 knockdown on dopaminergic neuron survival after LPS exposure. Intracranial injection of LPS into the SNpc of WT rats resulted in an average loss of ∼40% of the population of TH-positive neurons in comparison with the loss of ∼20% observed in an equally sized cohort of LRRK2 knockout rats (Fig. 1 A and B and Fig. S1D). In contrast, introduction of the vehicle control, PBS, into the SNpc failed to cause any significant loss of TH-positive neurons in either LRRK2 WT or KO rats (Fig. S1E).
Fig. 1.
Absence of LRRK2 protects from LPS-induced dopaminergic neurodegeneration. LRRK2 WT or KO rats, aged 10–12 wk, were unilaterally injected with either 50 kE.U. (from 10 μg of LPS) or vehicle (PBS) control, and killed 2 wk postinjection. (A) Representative coronal sections of TH-stained SNpc and (B) unbiased stereological counts of TH-positive neurons in the SNpc, with injected side (ipsilateral) counts and noninjected side (contralateral) counts given. Counts expressed as a percentage TH-positive cell loss are shown in Fig. S1 D and E. (C) Stereological counts of CD68-positive myeloid cells in the ipsilateral midbrain sections. Contralateral midbrain sections have negligible CD68 immunoreactivity (<100 cells) and counts are not shown. (D) Representative coronal sections showing CD68 immunoreactivity in the SNpc injected with LPS. (E) Correlation between recruitment of CD68-positive myeloid cells and percentage of surviving TH cells in the SNpc. (F) Perimeter analysis of cells labeled with Iba1 through the midbrain (n = 3 animals per group, >1,000 Iba1 cells analyzed per group). (G) Representative confocal images that span the ipsilateral (LPS-exposed) SNpc. [Scale bar, 0.5 mm for A and D and 100 μm (5 μm for high-magnification insets) for G.] P values are calculated by one-way ANOVA with Tukey’s post hoc test (B, C, D, and F), and linear regression analysis for E (Pearson’s r = −0.75). Error bars represent SEM.
LRRK2 expression has been detected in activated myeloid cells in the periphery and brain (10, 11). We used CD68, a glycoprotein expressed in cells of the myeloid lineage, as a marker for activated microglia, macrophages, and monocytes. Although few or no CD68-positive cells could be detected in the noninjected SNpc in rats (always <100 cells across the whole SNpc in the cohort; Fig. 1D), LPS exposure induced a significant recruitment of CD68-positive cells in the injected SNpc (Fig. 1 C and D). Vehicle control injection alone resulted in only modest CD68-positive cell induction (Fig. 1C). In rats lacking LRRK2, stereological counts demonstrated a significant reduction in CD68-positive cell recruitment compared with WT rats (Fig. 1C). The number of CD68-positive cells recruited to the midbrain correlated with dopaminergic cell death in the SNpc (Fig. 1E). A shift from ramified to amoeboid morphologies of ionized calcium-binding adapter molecule 1 (Iba1)/CD68-positive cells is indicative of myeloid cell activation (15). In LPS-exposed tissue, all CD68-positive cells were also Iba1-positive, and analysis of cell contours illuminated by Iba1 staining in the midbrain demonstrated a shift from ramified to amoeboid cells in LPS- versus PBS-exposed SNpc tissue in WT rats. LRRK2 KO rats failed to develop comparable amoeboid cell morphologies despite robust CD68 expression (Fig. 1F). Inducible nitric oxide synthase (iNOS) is a classical proinflammatory marker and could be detected in all CD68-positive cells in both LRRK2 WT and KO myeloid cells after LPS exposure. However, levels of iNOS were much more prominent in CD68-positive cells in the WT rats compared with the LRRK2 KO rats (Fig. 1G). These results demonstrate that ablation of LRRK2 expression significantly reduced both dopaminergic neurodegeneration induced by LPS exposure as well as CD68-positive myeloid cell recruitment.
LPS has been applied to both in vitro and in vivo paradigms to investigate myeloid cell-derived neuroinflammatory responses. Direct α-synuclein exposures to microglia cells have been described to activate many of the same responses (16). α-synuclein overexpression in the SNpc using recombinant adeno-associated virus (rAAV) has been previously found to produce a selective and relatively robust lesion of dopaminergic neurons, comparable to the level of neurodegeneration induced by LPS exposure (12, 13). We titrated the rAAV virus expressing human WT α-synuclein to determine the minimum amount required to reliably transduce >90% of dopaminergic neurons in the SNpc of Long–Evans LRRK2 WT and KO rats, as well as to ensure that LRRK2 WT and KO rats were similarly susceptible to viral transduction. We selected the amount of 6 × 109 viral particles delivered to the SNpc, and no differences were noted in the efficiency of transduction in LRRK2 WT or KO rats (Fig. S2 B and C). Next, we measured the expression of exogenous and endogenous α-synuclein in both LRRK2 WT and KO rat midbrains 2 wk after intracranial injections. We found comparable expression levels of total α-synuclein levels as well as comparable soluble and insoluble α-synuclein levels (Fig. S3 A–C).
To determine whether LRRK2 modifies α-synuclein–induced dopaminergic neurodegeneration, α-synuclein or eGFP viruses were unilaterally injected in 10–12-wk-old LRRK2 WT and KO rats (n = 100). Animals were killed at 4 wk, 8 wk, and 12 wk postinjection. Four animals, three KO and one WT, were excluded from this study due to an incomplete viral transduction of the SNpc and/or unsuccessful tissue processing. α-synuclein expression was evaluated in tissue sections using a human-isoform–specific monoclonal antibody (Syn208) or an antibody selective for α-synuclein pathologic inclusions (Syn514). In SNpc neurons in WT or KO rats labeled by either antibody, intracellular distribution of α-synuclein showed comparable punctate cytoplasmic staining (Fig. 2 B and C). Surprisingly, LRRK2 KO animals showed more robust abnormal α-synuclein reactivity across the SNpc (Fig. 2A).
Fig. 2.
Absence of LRRK2 protects from α-synuclein–induced dopaminergic neurodegeneration. Analysis of 10–12-wk-old LRRK2 WT and KO rats unilaterally injected with 6 × 109 rAAV2 α-synuclein viral particles. (A) Representative DAB images with Nissl counterstain showing human α-synuclein immunoreactivity in neurons of the ipsilateral SNpc 4 wk after viral injection. (B) Representative high-magnification SNpc neurons showing human α-synuclein immunoreactivity and (C) reactivity with a α-synuclein antibody that selective recognizes pathologic inclusions (23). SNpc sections were analyzed for α-synuclein aggregates in Nissl-positive cells at high magnification. (D) Representative coronal sections showing TH immunoreactivity in the SNpc, 4 wk after viral injection. (E) Unbiased stereological analysis of TH-positive neurons in the SNpc with respect to ipsilateral (ipsi, injected) and contralateral (contra, uninjected) sides. Stereological counts of Nissl-positive cells and dopaminergic neurodegeneration, calculated as percentage of remaining neurons, are provided in Fig. S4. (F) Linear regression analysis for SNpc neurodegeneration, determined by stereological counts of TH cells in the SNpc, and the percentage of cells that remain with α-synuclein aggregates. Additional representative images are provided in Fig. S5. (G) Representative coronal sections showing CD68 immunoreactivity in the SNpc and (H) unbiased stereological analysis of CD68 cells, 4 wk postinjection of rAAV2 α-synuclein viral particles. (I) Perimeter analysis of cells labeled with Iba1 through the midbrain (n = 3 animals per group, >1,000 Iba1 cells analyzed per group). (J) Representative confocal sections that span the ipsilateral (α-synuclein transduced) SNpc. CD68 immunoreactivity was never detected on the contralateral side. [Scale bar, 0.5 mm for A, D, and G and 50 μm (and 5 μm for the high-magnification inset) for J.] P values were calculated by one-way ANOVA with Tukey’s post hoc test, and error bars represent SEM.
Gross losses of SNpc neurons were apparent in WT but not LRRK2 KO rats (Fig. 2D). An overall average loss of ∼30% of TH-positive dopaminergic neurons in WT rats was seen across the 4, 8, and 12 wk post–α-synuclein virus injection (Fig. 2E and Fig. S4 A and B). The extent of neurodegeneration did not vary between the three time points (one-way ANOVA, P > 0.1), indicating a nonprogressive loss of cells. At each of these time points, no significant loss of TH-positive neurons could be detected in LRRK2 KO rats. To verify that the reduction of TH-positive neurons in α-synuclein–transduced WT rats occurred due to cell loss instead of loss of TH expression, Nissl-positive cells in the SNpc were also counted, and similar differences were observed in WT but not KO animals (Fig. S4A). As with the LPS-injected rat cohorts, no significant sex effects were observed in any group. The effects of matched titers of EGFP control virus did not vary between the LRRK2 WT and KO animals (Fig. S4C). Prominent α-synuclein pathology could be detected in SNpc in both WT and LRRK2 KO rats. We observed a significant (P < 0.003) inverse correlation between α-synuclein pathology and the extent of neurodegeneration (Fig. 2F). In rats with severe loss of TH-positive SNpc cells (e.g., 40%), fewer neurons were detected with abnormal α-synuclein expression (Fig. S5). Because viral transduction and α-synuclein expression is equivalent at earlier time points before cell loss (Figs. S2 and S3), transduced SNpc neurons with abnormal α-synuclein were lost in WT but not LRRK2 KO rats.
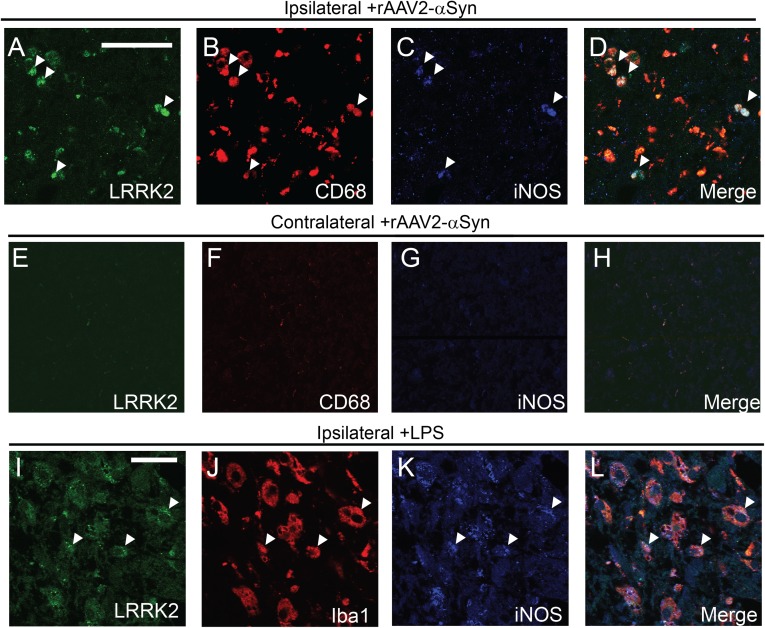
Microglial activation has been previously described in rAAV2–α-synuclein–transduced rodents (12, 14, 17). In WT rats transduced with α-synuclein, we observed a significant recruitment of CD68-positive cells within the SNpc (Fig. 2 G–J). Consistent with observations made in rats with LPS exposures (Fig. 1), the number of CD68-positive cells recruited in response to α-synuclein expression were reduced in LRRK2 KO rats compared with WT rats (Fig. 2H). In addition, there was a significant shift from ramified to amoeboid Iba1-positive cells in WT rats (Fig. 2 I and J). These amoeboid, CD68/Iba1-positive cells that accumulated in the WT, but not LRRK2 KO, rats also demonstrated higher iNOS expression than ramified cells (Fig. 2J). To probe LRRK2 expression within the myeloid cell fields induced by α-synuclein expression, we costained sections using LRRK2 monoclonal antibodies with optimized immunohistochemical protocols (18) in both ipsilateral and contralateral SNpc (Fig. 3). LRRK2 signal overlapped well with CD68-positive cells. Colabeling experiments demonstrated that the highest LRRK2-expressing myeloid cells were also the strongest iNOS-expressing cells (Fig. 3 A–D). In contrast, LRRK2 was not or weakly expressed in noninjected SNpc cells (Fig. 3E). Similarly, LPS-induced Iba1/iNOS-positive cells also expressed LRRK2 protein (Fig. 3 I–L). Thus, in WT rats, proinflammatory myeloid cells were the only LRRK2-positive cells that were detected in the midbrain. In LRRK2 KO rats, these proinflammatory myeloid cells were reduced in number and had reduced iNOS expression.
Fig. 3.
LRRK2 immunoreactivity in CD68/iNOS-positive cells recruited in response to α-synuclein and LPS-transduced SNpc. (A–D) Representative confocal sections of the unilateral SNpc transduced with rAAV2/1–α-synuclein, into 10–12-wk-old LRRK2 WT rats, killed 4 wk postinjection. LRRK2 (green)/CD68 (red)/iNOS (blue)-positive small amoeboid (spherical) cells (e.g., ∼5 μm) are highlighted with arrowheads. (E–H) In the contralateral side (noninjected), no immunoreactivity could be detected for LRRK2, CD68, and iNOS. (I–L) LRRK2 WT rats, aged 10–12 wk, were unilaterally injected with 50 kE.U. (from 10 μg of LPS) and killed 2 wk postinjection. Confocal analysis of a representative area of the ipsilateral SNpc revealed cells positive for LRRK2, Iba1, CD68, and iNOS. Arrowheads indicate points of reference. (Scale bar, 50 μm for A–H and 20 μm for I–L.)
Discussion
Here, we find that LRRK2 KO rats are robustly protected from dopaminergic neurodegeneration elicited by α-synuclein overexpression or LPS exposure. The neuroprotection in the LRRK2 KO rats coincides with reductions in proinflammatory CD68-positive myeloid cells. Activated CD68 myeloid cells also express LRRK2, consistent with previous reports of robust LRRK2 expression in activated cells of myeloid lineage derived from the periphery (11). Overall, these data imply that inhibition of LRRK2 will protect against neuroinflammation and cell loss in PD.
One of the first reports using LRRK2 knockout mice showed a profound neuroprotection from α-synuclein overexpression in the forebrain (7). This neuroprotection was coupled with reductions in associated microglial activation. We extend observations made in the mouse models in four ways: (i) The neuroprotective effects seen in LRRK2 KO mice in the forebrain are also observed in rat midbrain dopaminergic neurons; (ii) LRRK2 expression in myeloid cells is correlated to proinflammatory responses induced by α-synuclein overexpression in the SNpc; (iii) LRRK2 KO rats are protected from dopaminergic neurodegeneration induced by a potent myeloid cell agonist, LPS; and (iv) neuroprotection in the LRRK2 KO was found in the rat midbrain where LRRK2 expression is normally low or nonexistent in both neurons and microglia.
The role of LRRK2 in proinflammatory myeloid cells such as monocytes, macrophages, and microglia is not clear, but it is known that LRRK2 expression is strongly induced upon their differentiation into a proinflammatory state (10, 11). We confirm these results in vivo where we find LRRK2 expression overlaps well with iNOS expression. Moreover, the iNOS/CD68/LRRK2-positive cells described here are reminiscent of differentiated myeloid cells recruited both from resident surveying microglia and invading cells from the periphery. Additional studies are required to resolve the exact origins of LRRK2-positive myeloid cells. Ultimately, we envisage that LRRK2 action in myeloid cells may synergistically act together with other LRRK2-positive cells in the brain to produce susceptibility for PD. We further hypothesize that LRRK2 inhibition might benefit multiple neurodegenerative disorders where proinflammatory processes are associated with disease.
We cannot rule out important effects of LRRK2 function in neurons that are susceptible to degeneration in PD. For example, LRRK2 is likely expressed in dopaminergic neurons in rats but at very low levels that are difficult to detect (19) but nevertheless could drive critical aspects of neurodegeneration in the rats. In addition, LRRK2 expression in neurons outside of the midbrain may exert critical function on dopaminergic neurons in the SNpc in response to α-synuclein toxicities. Susceptible neurons overexpressing α-synuclein in WT rats were lost, whereas neurons in LRRK2 KO rats with comparable α-synuclein expression survived. We also evaluated later time points (i.e., 12-wk postviral injections) to determine if the loss of LRRK2 delayed but did not prevent SNpc cell loss, and still no significant neurodegeneration could be detected at these late time points. However, it is important to distinguish virally delivered human α-synuclein expression from emerging models of PD that harness transmissible α-synuclein fibrils (20). Future studies are required to determine whether LRRK2 has a role in mediating other types of α-synuclein–dependent neurotoxicities.
Overall, our findings indicate LRRK2 as an exciting potential therapeutic target for neuroprotection strategies in PD. The discovery of potent and specific small molecule inhibitors that are near ready for implementation in vivo should allow for future translation to clinical applications (21). PD cases with LRRK2 mutations that show typical late-onset PD may benefit from LRRK2 inhibition. Should LRRK2 function in a critical and ubiquitous aspect of pathobiology in PD, LRRK2 inhibition may provide benefit to PD cases without LRRK2 mutations. Furthermore, inhibition of LRRK2 may provide protection in other diseases in which neuroinflammation contributes to neurodegeneration.
Materials and Methods
Detailed materials and methods are available in SI Materials and Methods.
Animals.
All experiments were approved by the local Institutional Animal Care and Use Committee. Long–Evans outbred rats in this study originated from the Charles Rivers collection and were obtained directly from SAGE Laboratories. All surgeries were performed in 10–12-wk-old animals.
Virus Production and Intracranial Surgery.
rAAV2/1–α-synuclein and rAAV2/1–EGFP viruses were obtained from the Virus Core of the University of North Carolina. Ultrapurified LPS was obtained from Invivogen. Solutions were injected into the right SNpc at the following empirically derived coordinates: 4.65 mm posterior and 2.25 mm lateral to bregma, and 7.45 mm ventral relative to skull, with the needle bevel facing laterally, and a flow rate of 0.3 μL/min, maximum volume of 4 μL.
Immunohistochemistry and Immunofluorescence.
Rats were PBS/paraformaldehyde perfused and brain sections obtained with a freezing sliding microtome (Leica). Free-floating sections were quenched with hydrogen peroxide followed by a sodium citrate/pH 6.0 antigen retrieval step. Primary antibodies were incubated for 24–48 h followed by secondary antibodies overnight. The following primary antibodies were used: human-specific α-synuclein syn208 and nitrosylated α-synuclein Syn514 (both courtesy of the Virginia Lee laboratory and previously characterized) (19, 22, 23), TH (Sigma and Santa Cruz), CD68 (AdSerotec), EGFP (Santa Cruz), GFAP (Sigma), LRRK2 (NeuroMAB N241), and Iba1 (Wako). For immunohistochemistry, the ABC (Avidin-Biotin Complex reagent, Vector Labs) kit was used with ImmPACT-DAB (3, 3′-diaminobenzidine) substrate (Vector Labs). Most sections were counterstained using a standard Nissl staining protocol. Cell morphological analyses were performed with an Axiovision v4.8 (Carl Zeiss) automatic measurement program.
Stereology and Statistical Analysis.
Unbiased stereological estimation of the total number of TH+/Nissl+/CD68+ cells in the SNpc was performed using an optical fractionator (Microbrightfield). Statistical analysis and graphs were generated with Graphpad software.
Supplementary Material
Acknowledgments
The authors acknowledge the expert technical assistance of Xianzhen Hu and Rita Cowell. Support was provided by the Michael J. Fox Foundation for Parkinson’s Disease Research, National Institutes of Health/National Institute of Neurological Disorders and Stroke R01NS064934, and the American Parkinson’s Disease Association.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1403215111/-/DCSupplemental.
References
- 1.Hulihan MM, et al. LRRK2 Gly2019Ser penetrance in Arab-Berber patients from Tunisia: A case-control genetic study. Lancet Neurol. 2008;7(7):591–594. doi: 10.1016/S1474-4422(08)70116-9. [DOI] [PubMed] [Google Scholar]
- 2.Ozelius LJ, et al. LRRK2 G2019S as a cause of Parkinson’s disease in Ashkenazi Jews. N Engl J Med. 2006;354(4):424–425. doi: 10.1056/NEJMc055509. [DOI] [PubMed] [Google Scholar]
- 3.International Parkinson’s Disease Genomics Consortium (IPDGC) Wellcome Trust Case Control Consortium 2 (WTCCC2) A two-stage meta-analysis identifies several new loci for Parkinson’s disease. PLoS Genet. 2011;7(6):e1002142. doi: 10.1371/journal.pgen.1002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nalls MA, et al. International Parkinson Disease Genomics Consortium Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet. 2011;377(9766):641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danoy P, et al. Australo-Anglo-American Spondyloarthritis Consortium Spondyloarthritis Research Consortium of Canada Association of variants at 1q32 and STAT3 with ankylosing spondylitis suggests genetic overlap with Crohn’s disease. PLoS Genet. 2010;6(12):e1001195. doi: 10.1371/journal.pgen.1001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang FR, et al. Genomewide association study of leprosy. N Engl J Med. 2009;361(27):2609–2618. doi: 10.1056/NEJMoa0903753. [DOI] [PubMed] [Google Scholar]
- 7.Lin X, et al. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson’s-disease-related mutant alpha-synuclein. Neuron. 2009;64(6):807–827. doi: 10.1016/j.neuron.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daher JP, et al. Neurodegenerative phenotypes in an A53T α-synuclein transgenic mouse model are independent of LRRK2. Hum Mol Genet. 2012;21(11):2420–2431. doi: 10.1093/hmg/dds057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herzig MC, et al. High LRRK2 levels fail to induce or exacerbate neuronal alpha-synucleinopathy in mouse brain. PLoS ONE. 2012;7(5):e36581. doi: 10.1371/journal.pone.0036581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moehle MS, et al. LRRK2 inhibition attenuates microglial inflammatory responses. J Neurosci. 2012;32(5):1602–1611. doi: 10.1523/JNEUROSCI.5601-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thévenet J, Pescini Gobert R, Hooft van Huijsduijnen R, Wiessner C, Sagot YJ. Regulation of LRRK2 expression points to a functional role in human monocyte maturation. PLoS ONE. 2011;6(6):e21519. doi: 10.1371/journal.pone.0021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirik D, et al. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci. 2002;22(7):2780–2791. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castaño A, Herrera AJ, Cano J, Machado A. Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J Neurochem. 1998;70(4):1584–1592. doi: 10.1046/j.1471-4159.1998.70041584.x. [DOI] [PubMed] [Google Scholar]
- 14.Harms AS, et al. MHCII is required for α-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci. 2013;33(23):9592–9600. doi: 10.1523/JNEUROSCI.5610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468(7321):253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 16.Fellner L, et al. Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia. 2013;61(3):349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS ONE. 2010;5(1):e8784. doi: 10.1371/journal.pone.0008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies P, et al. Comprehensive characterization and optimization of anti-LRRK2 (leucine-rich repeat kinase 2) monoclonal antibodies. Biochem J. 2013;453(1):101–113. doi: 10.1042/BJ20121742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West AB, et al. Differential LRRK2 expression in the cortex, striatum, and substantia nigra in transgenic and nontransgenic rodents. J Comp Neurol. 2014 doi: 10.1002/cne.23583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luk KC, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cookson MR. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat Rev Neurosci. 2010;11(12):791–797. doi: 10.1038/nrn2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giasson BI, et al. A panel of epitope-specific antibodies detects protein domains distributed throughout human alpha-synuclein in Lewy bodies of Parkinson’s disease. J Neurosci Res. 2000;59(4):528–533. doi: 10.1002/(SICI)1097-4547(20000215)59:4<528::AID-JNR8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Waxman EA, Duda JE, Giasson BI. Characterization of antibodies that selectively detect alpha-synuclein in pathological inclusions. Acta Neuropathol. 2008;116(1):37–46. doi: 10.1007/s00401-008-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.