Significance
Few studies have asked at what spatial scale environmental stimuli regulate plant development and when during the patterning process these signals act. We have discovered that plant roots can sense microscale heterogeneity in water availability across their circumference, which causes dramatic differences in the patterning of tissues along this axis. Root branching is a target of such hydropatterning; lateral roots only form on the side of the main root contacting water in soil or agar. We show that hydropatterning is a conserved process in Arabidopsis, maize, and rice and reveal the importance of auxin biosynthesis and transport in regulating this process.
Keywords: moisture regulation, root development, root system architecture, adaptive root response, auxin-regulated root patterning
Abstract
The architecture of the branched root system of plants is a major determinant of vigor. Water availability is known to impact root physiology and growth; however, the spatial scale at which this stimulus influences root architecture is poorly understood. Here we reveal that differences in the availability of water across the circumferential axis of the root create spatial cues that determine the position of lateral root branches. We show that roots of several plant species can distinguish between a wet surface and air environments and that this also impacts the patterning of root hairs, anthocyanins, and aerenchyma in a phenomenon we describe as hydropatterning. This environmental response is distinct from a touch response and requires available water to induce lateral roots along a contacted surface. X-ray microscale computed tomography and 3D reconstruction of soil-grown root systems demonstrate that such responses also occur under physiologically relevant conditions. Using early-stage lateral root markers, we show that hydropatterning acts before the initiation stage and likely determines the circumferential position at which lateral root founder cells are specified. Hydropatterning is independent of endogenous abscisic acid signaling, distinguishing it from a classic water-stress response. Higher water availability induces the biosynthesis and transport of the lateral root-inductive signal auxin through local regulation of TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS 1 and PIN-FORMED 3, both of which are necessary for normal hydropatterning. Our work suggests that water availability is sensed and interpreted at the suborgan level and locally patterns a wide variety of developmental processes in the root.
The root system of plants is a branched network whose architecture is determined by endogenous and environmental cues and serves as a model for pattern formation (1). In Arabidopsis, lateral roots (LRs) are initially specified as founder cells (FCs) within the internal pericycle cell layer of the primary root (2). A temporally oscillating transcriptional network that results in periodic fluctuations in auxin response controls the patterning of FCs along the longitudinal axis of the primary root (3, 4). Moments of peak auxin response are maintained in fixed positions termed prebranch sites (PBS), which mark presumptive FCs and can be visualized using the ProDR5:LUC+ reporter (4). In Arabidopsis, LRs only develop from pericycle cells that overlie one of the two xylem poles (5). Although two such populations of cells exist along the circumferential axis of the root, pericycle cells adjacent to only one xylem pole will be selected. How the xylem pole is chosen and whether environmental stimuli affect this process is currently unclear.
We report that when roots are grown vertically on the surface of an agar medium, LRs predominantly form on the side of the primary root that contacts agar. Through our developmental analysis, we show that the local environment impacts LR patterning by providing spatial cues that select one of the two xylem poles at which LR FCs will be specified. We observe this patterning phenomenon in all flowering plant species examined and also in a realistic soil environment. Because this phenomenon is elicited by exposing roots to an asymmetric distribution of available water, we have termed the process “hydropatterning.”
Results
Patterning of Root Tissues Is Determined by the Local Availability of Water.
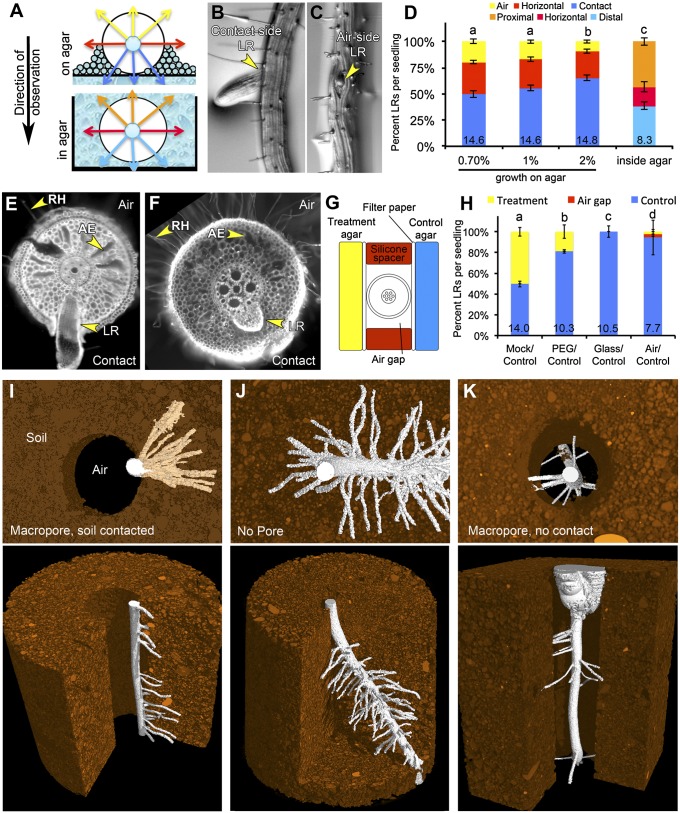
Soil is a heterogeneous environment containing particles and aggregated structures of different sizes with pockets of air and nonuniform distributions of water and nutrients (6). Our understanding of how roots sense and interpret microscale heterogeneity is poor due, in part, to a lack of model experimental systems for studying such phenomena. Interestingly, growing Arabidopsis seedling roots along the surface of an agar medium creates spatial asymmetries in the environment the primary root is exposed to, but the effect of these differences has not been explored before. Under these conditions, one side of the primary root is in contact with the agar medium and the film of water that forms on its surface (contact side), whereas the other side of the primary root is exposed to air in the headspace of the Petri dish (air side) (Fig. 1A). We found that seedlings grown on agar media infrequently developed LRs that grew straight out into the air, suggesting that their patterning might be influenced by this environmental asymmetry (Fig. 1 B, C, and D). We created a phenotyping key to quantify the emergence patterns of LRs across the circumferential axis by categorizing emerged LRs as air side, horizon side, or contact side (SI Appendix, SI Materials and Methods, provides a full description of criteria used). Although we would have a priori expected that the chance of an LR emerging from any particular side of the primary root would be nearly equivalent, we instead observed a strong bias in LR emergence toward the contact side (Fig. 1D). This bias was lost when roots were grown through agar (Fig. 1 A and D), indicating that the observed phenomenon requires an asymmetric environment. Root curvature influences at which side of the root (concave or convex) a LR will initiate (3). We found that inducing a 90-degree bend in the root through gravitropic stimulation had no significant influence on the bias in LR development that occurred along the air–agar axis suggesting that these two processes act independently on the distribution of LRs (SI Appendix, Fig. S1).
Fig. 1.
Hydropatterning of root development in Arabidopsis, maize and rice. (A) Diagram showing asymmetries in the local environment generated when seedlings grow on the surface of an agar-based media or the symmetric environment generated when roots are grown through agar. (B and C) LR primordia emerging from the contact (B) or air side (C) of the primary root. (D) Quantification of LR emergence patterns from the primary root under different conditions (n > 10). Various phenotypic categories are indicated with different colors and are marked in A. (E) Cross-section of a rice primary root grown on agar, stained with calcofluor. Image shows the development of aerenchyma (AE) and root hairs (RH) on the air side and an LR emerging from the contact side. (F) Cross-section of a maize root grown on agar and stained with propidium iodide. (G) Diagram showing the construction of “agar sandwiches” used to test the effects of local differences in media composition on LR development in maize. (H) LR outgrowth is induced on two sides by contact with agar (Mock/Control); this effect is diminished on the “Treatment” side when the water potential of the media is reduced using PEG infusion (PEG/Control). Contact of the root with a glass surface does not induce LR outgrowth (Glass/Control). Growth of roots along a single agar surface results in the suppression of LR development on the air side (Air/Control) (n > 10). (I–K) MicroCT-generated images of maize seedlings grown through a macropore of air (I and K) or a continuous volume of soil (J). The root in K is growing in air, whereas in I the root is contacting the soil surface. Root tissue is false-colored in white, and soil is false-colored in brown. Average number of LRs per seedling (D) and per centimeter of primary root (H) is shown at base of columns in bar charts. Error bars indicate SEM. Significant differences based on Fisher’s exact test (P < 0.05) with similar groups are indicated using the same letter.
Changing agar concentration in the growth media from 1 to 2% decreased water potential (Ψw) and the amount of expressed water on the surface of the media, which reduced the circumferential area of the root contacting liquid water (SI Appendix, Fig. S1) (7). This change in media composition also significantly affected the bias in the distribution of LRs (Fig. 1D). Use of different growth substrates indicated that no specific component of the media besides water was necessary to elicit biased LR development, although these media differed significantly in water potential (range, −0.22 MPa) (SI Appendix, Fig. S2). These data suggest that contact with water, in or on the surface of the media, had a greater influence on the local induction of LR development than small differences in water potential.
Growth of Oryza sativa (rice) and Zea mays (maize) seedlings on agar also resulted in the development of LRs predominantly on the contact side of the root (Fig. 1 E, F, and H and SI Appendix, Fig. S3). In maize, LR development was also locally induced when seedlings were grown on wet germination paper, again indicating that no specific component of the medium besides water was necessary to elicit biased LR development (SI Appendix, Fig. S3). Using a similar experimental system as Karahara et al. (8) (Fig. 1G), we tested the effects of placing the maize primary root between two slabs of control media. Interestingly, LRs developed along both sides contacting the media, demonstrating that multiple distinct domains along the circumferential axis of the root can simultaneously form LRs with intervening areas lacking LR development (Fig. 1H).
X-ray microscale computed tomography (microCT) visualization of maize roots growing through a macropore (large air space) in the soil matrix revealed a similar positioning of LRs biased toward the root face in direct contact with the soil (Fig. 1I, Movie S1, and SI Appendix, Table S1). When roots were grown in pots without a macropore, LRs developed around the entire circumference of the primary root (Fig. 1J and Movie S2). Interestingly, when roots did not contact the soil surface in the macropore (Fig. 1K and Movie S3), LRs emerged sporadically in all directions, suggesting that a nonuniform environment is required for the bias in LR development but that contact is not required for LR development per se in this condition. These data support the physiological relevance of the patterning phenomenon observed in vitro.
In addition to LR emergence, rice and maize seedlings showed preferential accumulation of aerenchyma (air pockets forming in the cortex cell layers that may aid in gas exchange) on the air side of the root (Fig. 1 E and F and SI Appendix, Fig. S3). In maize, the pigment anthocyanin accumulated on the air side of the root whereas it was depleted from the contact side, especially in those regions where preemergent LRs were developing (SI Appendix, Fig. S3). This provided a useful visual marker to distinguish contact and air sides in root cross-sections. Anthocyanin biosynthesis is light-dependent; however, hydropatterning of LRs was not disrupted by growth of plants in the dark (SI Appendix, Fig. S3).
Rice, maize, and Arabidopsis also showed a clear bias in root hair development on the air side (Fig. 1 E and F and SI Appendix, Fig. S4). In Arabidopsis, suppression of root hair development often occurred before the initiation stage but was not associated with obvious changes in the expression of genes involved in root hair patterning (SI Appendix, Fig. S4). The presence of root hairs was used as a visual marker to distinguish air and contact sides of roots removed from the media for imaging. Root hair initiation on the contact side could be rescued by treatment with abscisic acid (ABA) or the ethylene precursor 1–aminocyclopropane-1–carboxylic acid (ACC), suggesting that the lack of root hair development was not simply a consequence of physical impedance of the growth medium (SI Appendix, Fig. S4). Together these data demonstrated that plant roots are adept at sensing and developmentally responding to local differences in the environment in ways that we hypothesize take advantage of microscopic variations in the distribution of liquid water and air in soil.
The rate at which water is absorbed by the root (Jv) is the product of the driving force for water flow (ΔΨw, the difference in water potential between the root and the growth medium) and the resistance to water flow (inversely proportional to the hydraulic conductivities of the medium and the root, Lp) (9). In our in vitro growth systems, the air is likely at water-potential equilibrium with the culture medium; thus water potential does not distinguish these environments. Hydraulic conductivity, however, differs dramatically; the conductivity of agar (1 × 10−5 m2 s−1 MPa−1) is orders of magnitude higher than that of air (4.18 × 10−12 m2 s−1 MPa−1) (9, 10).
To specifically test the effects that media water potential and hydraulic conductivity have on the local regulation of LR development, we again used the “agar sandwich” approach to vary the media contacting the maize root (treatment agar slab) while a second agar slab contacting the root served as a control. In rice, Karahara et al. (8) previously showed that growth of roots between two slabs of agar results in asymmetries in aerenchyma development if one of the slabs contains mannitol, which reduces water potential of the medium. We performed similar experiments using polyethylene glycol (PEG; infused agar Ψw was −0.63 ± 0.02 MPa, and control agar was −0.10 ± 0.01 MPa) and observed a significant reduction in LR emergence on the treatment side, which partially mimicked the effect of air (Fig. 1H) (11). We severely reduced hydraulic conductivity by placing various non-water-conducting materials between the root and the treatment agar slab. This eliminated the inductive effect of this media on LR development, indicating that hydraulic conductivity of the contacted surface, rather than contact alone, was important for hydropatterning (SI Appendix, Fig. S3). Similar results were obtained when a sheet of glass or silicone rubber was used to contact the root, suggesting that the pliancy of the material was inconsequential (Fig. 1H and SI Appendix, Fig. S3). Together these data suggest that the rate with which water is absorbed by a root from the media determines whether a contacted surface will induce LR development.
To describe the environmental response phenomena shown here, we have designated the term hydropatterning: a nonuniform distribution of available water causes asymmetries in root development. This term is primarily used to simplify discussion of the process, and we do not intend to imply any specific physiological or molecular mechanisms used by the plant to detect differences in water availability.
Hydropatterning Affects Lateral Root Development During FC Specification.
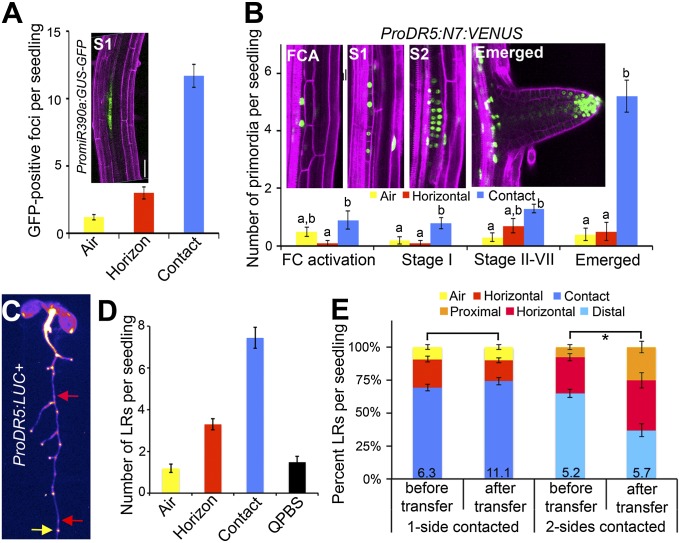
In Arabidopsis, the developmental steps involved in LR patterning have been well defined (12). Previous studies have clearly shown that environmental stimuli can affect the initiation and emergence of LRs (1); however, evidence is lacking regarding an earlier role. We predicted that if hydropatterning acts after LR initiation we should observe an accumulation of preemerged LR primordia on the air side, which would account for the lower relative number of emerged LRs on this side.
The ProMiR390a:GFP-GUS reporter is expressed in the xylem pole and associated pericycle cells and marks stage-I LR primordia and later stages (13) (Fig. 2A). Confocal imaging of contact and air sides of seedling primary roots showed a clear bias in the number of GFP-positive foci observed between these sides (Fig. 2A). The ProDR5:VENUS-N7 reporter is initially expressed in adjacent xylem pole pericycle cells during FC activation and can be used to visualize the migration of nuclei from two neighboring pericycle cells to the common anticlinal cell plate before cell division and stage-I LR initiation (Fig. 2B) (14, 15). Interestingly, several stages of LR development showed a bias between the contact and air sides of the root, and no obvious accumulation of paused or quiescent LR primordia was observed on the air side that could account for the difference in emerged primordia between these sides (Fig. 2B). Quantification of LR primordia in seedlings after tissue clearing revealed similar results (SI Appendix, Fig. S5). These data indicate that hydropatterning acts at or before the earliest stages of LR initiation.
Fig. 2.
Hydropatterning acts during FC specification to affect LR patterning. (A) The PromiR390a:GUS-GFP reporter is expressed at stage 1 of LR initiation and later. Confocal imaging of contact and air sides of the primary root showed a strong bias in the number of GFP-positive foci (n ≥ 10). (B) The ProDR5:N7:VENUS reporter marks pericycle cell nuclei at the stage of FC activation (FCA), Stage 1 (S1), Stage 2 (S2), and later stages. Most stages showed greater numbers of primordia on the contact side than on the air side. (C) Seedling expressing the ProDR5:LUC+ reporter. LR emergence patterns were quantified in the region of the primary root containing all emerged LRs (in this example, the region above the yellow arrow). Luciferase activity was then visualized, and foci of reporter activity, included outgrown LRs, were counted (n = 27). Quiescent PBSs (red arrows) are sites of reporter expression that showed no signs of LR emergence. (D) Chart showing the number of emerged LRs and quiescent PBSs (QPBS). (E) Seedlings were grown for 5 d on 1% agar, then a second agar slab was applied to the former air side of the root. Seedlings were grown for five additional days, and the position of emerged LRs was quantified in the region of the primary root that formed before and after treatment. Control seedlings were grown similarly; however, a second agar slab was not applied. The proximal domain is defined as the region of the primary root in contact with the second applied agar slab, whereas the distal domain is toward the original agar slab on which seedlings were germinated. In B, significant differences were analyzed on a per-stage basis using Student’s t test (P < 0.05); statistically similar groups are indicated using the same letter. For E, asterisk indicates a significant difference based on Fisher’s exact test (P < 0.05). Average number of LRs per centimeter of primary root shown at base of columns in bar charts. Error bars indicate SEM. (Scale bar, 50 μm.)
The orientation of the xylem pole determines the angle with which LRs emerge (5). Using the ProS32:erGFP reporter to mark the orientation of the vascular pole we found no significant bias with respect to the air–agar axis, eliminating this as a possible contributor to hydropatterning (SI Appendix, Fig. S5).
The earliest visual marker for the position of future LR primordia is the ProDR5:LUC+ reporter, which marks PBSs. Moreno-Risueño et al. (4) previously showed that growth of roots on the surface or through agar had no significant influence on the number of PBSs specified, indicating that hydropatterning acts subsequent to PBS specification. Between the specification of PBSs along the longitudinal axis and the activation of asymmetric divisions in FCs, an additional decision must be made that has received less attention. In Arabidopsis, LRs will only develop from pericycle cells that overlie one of the two xylem poles (5). Although two such populations of cells exist along the circumferential axis of the root, pericycle cells adjacent to only one xylem pole are chosen. We postulated two models for how hydropatterning affects LR patterning during this developmental interval (SI Appendix, Fig. S6). In the first model, xylem pole selection and the subsequent specification of FCs at this pole are independent of the local environment, with the later stage of LR initiation being the target of hydropatterning. This model predicts that a similar number of FCs should be specified on the air and contact sides, with most FCs on the air side remaining quiescent. The second model postulates that local environmental differences across the circumferential axis bias xylem pole selection and FC specification toward the contact side. Based on this second model, we would expect few quiescent PBSs as most would be specified toward the permissive environment of the contacted surface to begin with.
Under our growth conditions, seedlings expressing the ProDR5:LUC+ reporter developed on average 11.9 total emerged LRs after 10 d of growth with 7.4 LRs emerged toward the agar and 1.2 toward the air (Fig. 2 C and D). Thus, the difference in emerged LRs between air and contact sides is ∼6.3. To determine if there existed a quantity of quiescent PBSs that would account for this difference, we visualized the ProDR5:LUC+ reporter and found 13.4 LUC-marked sites. We verified that reporter expression in PBSs was maintained throughout the time frame of the experiment (SI Appendix, Fig. S7). Based on the difference between the number of LUC-expressing foci and emerged LRs, we calculated that 1.5 PBSs were quiescent per seedling. This number is significantly lower than the number expected based on the first model (6.3 quiescent PBSs). Thus, our data are consistent with the second model and suggest that hydropatterning likely acts during FC specification.
If hydropatterning acts at FC specification, then mature regions of the primary root where FCs have already been specified should not be responsive to future changes in the distribution of water in the environment. By this reasoning, we predicted that regions of the root previously exposed to air would not develop new LRs if they subsequently came into contact with a wet surface. We directly tested this prediction by applying a sheet of agar to the air side of the root of an Arabidopsis seedling 5 d postgermination. The orientation of LR emergence was quantified in regions of the primary root that had formed before and after the application of the agar slab. In regions of the primary root previously exposed to air, the spatial distribution of LRs was similar in the treated roots to the untreated control, whereas in regions of the primary root that formed after application of the agar slab, LRs developed in all directions (Fig. 2E). These results are consistent with our model that hydropatterning acts at the time of FC specification and suggest that the orientation of LRs is determined through sensing of the local environment near the root tip and subsequently becomes fixed.
Auxin Biosynthesis Is Moisture-Induced and Necessary for Hydropatterning.
We next asked which signaling pathways act downstream of moisture during hydropatterning. Previous work has shown that under water-limiting conditions, LR development is strongly suppressed (16). Severe water limitation induces ABA signaling, which is known to inhibit the development of LRs (17). We found that several mutants that disrupt ABA signaling did not have a significant effect on hydropatterning (SI Appendix, Fig. S8). Of particular importance, the pyr/pyl 112458 mutant, which is highly resistant to ABA treatment (18), showed normal hydropatterning (SI Appendix, Fig. S8). These data differentiate hydropatterning from a classic water-stress response and indicate that signaling pathways other than ABA are involved.
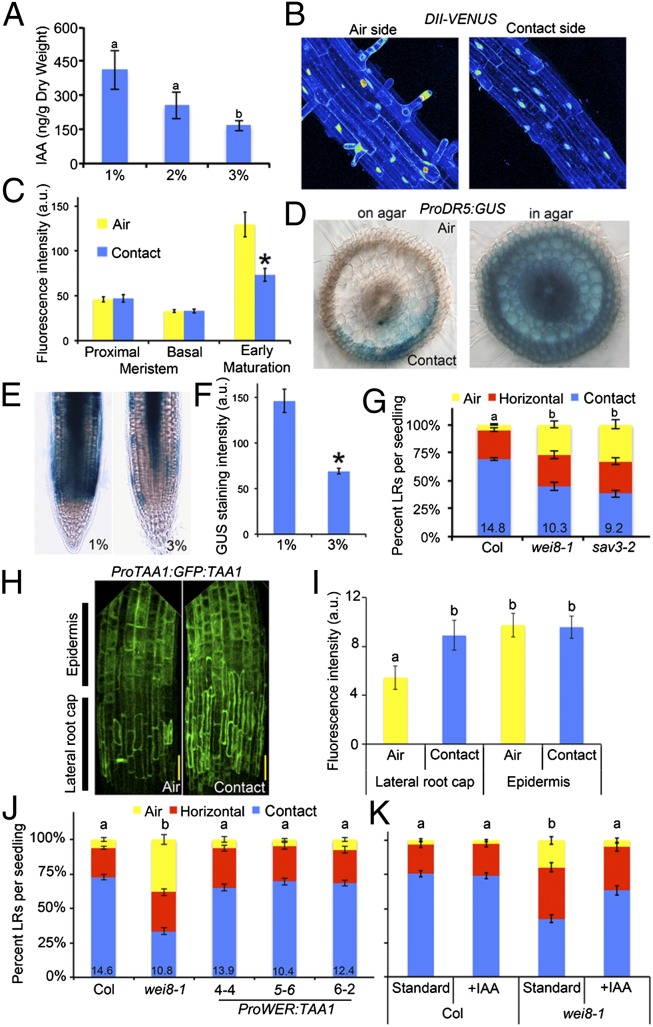
Auxin is an important signaling molecule contributing to all stages of LR development (19). Quantification of indole-3–acetic acid (IAA) concentration in whole roots of Arabidopsis showed a significant increase when seedlings were grown on media with a lower concentration of agar (Fig. 3A). Measurements of endogenous auxin signaling using the DII-VENUS sensor, which is degraded in an auxin concentration-dependent manner (20), showed similar results (SI Appendix, Fig. S9). Furthermore, a transcriptional reporter of auxin response, ProDR5:erGFP, showed an increase in fluorescence in the outer tissue layers of the root at 1% agar compared with 3% agar conditions (SI Appendix, Fig. S9). These results suggest that water availability promotes auxin accumulation, signaling, and response.
Fig. 3.
Moisture activates auxin biosynthesis and response. (A) IAA levels quantified by liquid chromatography tandem mass spectrometry in whole roots grown on media containing different concentrations of agar (n = 3). (B) Maximum projects of confocal image stacks show DII-VENUS reporter expression is higher on the air side of the root relative to the contact side. Fluorescence intensity is shown using a 16-color look-up table. (C) Quantitation of DII-VENUS average nuclear fluorescence intensity in the epidermis shown for different regions of the root. (D) Cross-sections of rice roots expressing the ProDR5:GUS reporter showing local induction of the reporter on the contact side of the root (on agar) and uniform activation of the reporter when roots are grown in agar. (E and F) The ProTIR2:TIR2:GUS reporter shows stronger expression in the outer tissue layers of seedlings grown on 1% compared with 3% agar, quantified in F. (G) Two mutant alleles of tryptophan aminotransferase of Arabidopsis (TAA1) show a strong suppression of hydropatterning (n ≥ 20). (H) Maximum projections of confocal image stacks show ProTAA1:GFP:TAA1 reporter expression in the LRC and epidermis of the transition and elongation zones. (I) GFP fluorescence quantified for cell types on the air and contact sides (n ≥ 8). (J) The ProWER:TAA1 transgene was able to rescue the wei8-1 hydropatterning defect in multiple independent transgenic lines as was growth of seedlings on media supplemented with IAA (K). Average number of LRs per seedling shown at base of columns in bar charts. Error bars indicate SEM. Significant differences based on Fisher’s exact test (P < 0.05) (G, J, and K) or Student’s t test (P < 0.05) (A, C, F, and I) with similar groups indicated using same letter. (Scale bars, 50 μm.)
We asked whether the auxin pathway was locally regulated by contact with a wet surface. Expression of the DII-VENUS sensor was lower on the contact side of the early maturation zone, relative to the air side, although no significant differences were observed elsewhere (Fig. 3 B and C). These data suggest that higher levels of auxin may be present on the contact side of the primary root. Determining differences in auxin signaling at the elongation zone and pericycle cell layers was not possible using the DII-VENUS sensor, as fluorescence intensity was very low in these regions of the root. In rice, where radial cross-sections are more easily examined, the auxin transcriptional response reporter, ProDR5:GUS, exhibited higher staining on the contact side of the early maturation zone compared with the air side, whereas uniform activation of the reporter was observed in roots grown through agar (Fig. 3D). Together these data suggest that water availability can locally promote auxin accumulation and its response.
TAA1 (also known as WEI8, SAV3 and TIR2) encodes an l-tryptophan pyruvate aminotransferase that converts tryptophan to indole-pyruvic acid, a direct biosynthetic precursor of the auxin, IAA (21–26). The ProTIR2:TIR2:GUS reporter showed an increase in expression in the outer tissue layers at 1% agar compared with 3% agar media, suggesting TAA1 may control water-dependent changes in auxin biosynthesis (Fig. 3 E and F). Indeed the ProTAA1:TAA1:GFP reporter line showed significant differences in expression level between the contact and air sides of the root (Fig. 3 H and I) for the lateral root cap (LRC); however, in the epidermis no differences were observed.
TAA1 loss-of-function alleles wei8-1 and sav3-1 both showed a significant reduction in hydropatterning (Fig. 3G), indicating that TAA1-mediated auxin biosynthesis is necessary for the response. To test if the spatial pattern of TAA1 expression is important for hydropatterning, we attempted to rescue wei8-1 defects by introducing transgenes that drove constitutive expression in the epidermis (ProWEREWOLF:TAA1) or throughout the root (ProUBIQUTIN10:TAA1). Both constructs were able to rescue hydropatterning in wei8-1 and did not cause obvious gain-of-function defects (Fig. 3J and SI Appendix, Fig. S9). Furthermore, exogenous IAA added to the media could similarly rescue wei8-1 defects (Fig. 3K). These data suggest that TAA1-mediated auxin biosynthesis is necessary for hydropatterning but that localized expression of TAA1 and local biosynthesis of IAA are not required to communicate positional information generated by the local environment.
Auxin Efflux and Response Pathways Are Necessary for Hydropatterning.
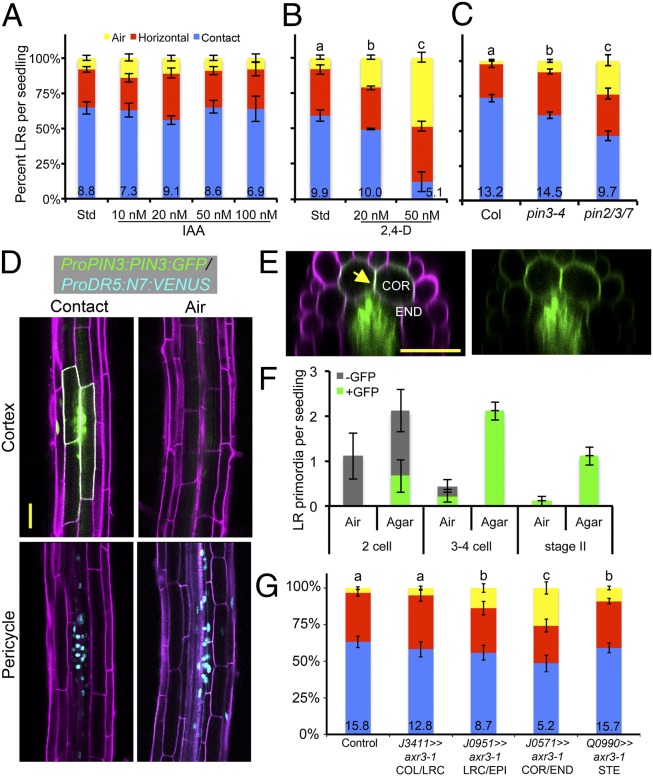
Polar auxin transport is required to generate localized gradients of auxin important for patterning the site of future lateral organs (27). We treated seedlings with various concentrations of a transportable form of auxin, IAA, or 2,4-dichlorophenoxyacetic acid (2,4-D), which is not efficiently effluxed from cells (Fig. 4 A and B) (28). Although IAA did not have a significant effect, 2,4-D could strongly disrupt hydropatterning even at low concentrations, suggesting that the ability of the root to transport auxin may be important for hydropatterning. We surveyed various genetic backgrounds affected in PIN-mediated auxin efflux and found that the Pro35S:PIN1 line strongly disrupted the process (SI Appendix, Fig. S10). Loss of pin-formed 3 (PIN3) function had a modest effect, which was enhanced in the pin2/3/7 mutant background (Fig. 4C) (29). The pin7 and the pin3/7 double mutant also showed significant defects, whereas other pin alleles did not affect hydropatterning (SI Appendix, Fig. S10). These data indicate that a normal auxin transport pathway is required for hydropatterning, and this can be disrupted through misexpression of, or loss-of-function mutations in, certain transport pathway members.
Fig. 4.
Auxin efflux transport pathways are necessary for hydropatterning. Effect of IAA (A) or 2,4-D (B) treatment on hydropatterning (n ≥ 20). (C) pin3-4 and pin2/3/7 mutants showed defects in hydropatterning (n ≥ 20). (D) Roots expressing the ProPIN3:PIN3:GFP and ProDR5:N7:VENUS reporters. Optical cross-sections at the cortex cell layer (Upper) or at the pericycle (Lower). Propidium iodide counter stain (magenta), PIN3:GFP (green, plasma membrane localized), and N7:VENUS (cyan, nuclear). (E) A radial cross-section reveals strong localization to the lateral cross-walls between cortex cells (yellow arrow). (F) Frequency with which early stages of LR development are observed on the air and contact sides of the primary root and whether these primordia are associated with PIN3:GFP expression in ground tissue (n = 9). (G) Transactivation of axr3-1 expression in the COR/END had the strongest effect in suppressing hydropatterning (n ≥ 20). Average number of LRs per seedling shown at base of columns in bar charts. Error bars indicate SEM. Significant differences based on Fisher’s exact test (P < 0.05) with similar groups indicated using same letter. (Scale bars, 50 μm.)
We examined the spatial localization pattern of the PIN3 protein using a GFP reporter (ProPIN3:PIN3:GFP) and found specific enrichment in cortex and endodermal cells (ground tissue) overlying early-stage LR primordia on the contact side (Fig. 4 D and E). PIN3:GFP was localized to all surfaces of these cells and was particularly enriched at the cross-wall between neighboring cortex cells overlying LR primordia (Fig. 4E). Few early-stage primordia developed on the air side, and these were generally associated with very weak or absent PIN3:GFP expression. Quantification of these results revealed that early stage-I LR primordia were preferentially associated with these patches of PIN3:GFP expression on the contact side, and these primordia were able to progress through to later developmental stages (Fig. 4F). We did not observe ground tissue-associated PIN3 expression in the absence of a primordium, suggesting that PIN3 likely acts after FC specification and may promote initiation of LR development on the contact side. This role for PIN3 is consistent with a previous study showing that PIN3 acts in the endodermis to promote LR initiation (30). Analysis of the PIN2 reporter did not reveal obvious differences in expression pattern between contact and air sides of the root (SI Appendix, Fig. S10). These data suggest that PIN transporters could play a role in maintaining local differences in auxin concentration between the air and contact sides but likely do not generate such gradients through differential expression or localization between these sides.
To probe where auxin transcriptional regulation is important for hydropatterning, we used the GAL4/UAS transactivation system to misexpress axr3-1 in different cell layers (31). The axr3-1 mutant allele encodes a dominant suppressor of auxin transcriptional responses (32). We observed significant reductions in hydropatterning in the J0571>>axr3-1 line [cortex (COR) and endodermis (END)], and less in the J0951>>axr3-1 [epidermis (EPI), weak cortex expression] and Q0990>>axr3-1 [stele (STE), no pericycle (PER) expression] lines (Fig. 4G). Thus, ground tissue layers may be important conduits and response centers for auxin during hydropatterning. These data suggest that the perception of local moisture may occur through a cascade of signaling events initiated in the outer tissue layers that are transmitted ultimately to the pericycle.
Discussion
Through our studies of hydropatterning, we have revealed that the local distribution of water around the root tip plays an important role in the establishment of root system architecture by biasing the position selected for future branch sites around the circumferential axis. Conservation of this process in eudicot and monocot species indicates that root systems of flowering plants have developed mechanisms to interpret and respond to microscale differences in the environment, which are relevant in natural soils. Hydropatterning also results in the biased development of other tissues such as aerenchyma and root hairs. Understanding the spatial scale at which stimuli are perceived and responses elicited is essential for predicting the impact that changes in the natural environment will have on plant physiology. Our studies of hydropatterning reveal that the regulation of LRs, hairs, and aerenchyma can result in distinct developmental zones over very small distances. Thus, perception, signaling, and response likely occur at the suborgan level. Signaling events controlling LR and root hair patterning occur at the root tip and become developmentally fixed. This has important implications for our understanding of root development under field conditions as we predict that periodic episodes of water deficit may have a long-term impact on root system architecture.
Our findings suggest that the availability of water from the environment, a product of hydraulic conductivity and water potential, is the critical environmental variable regulating LR development during hydropatterning. In the in vitro growth systems used here, differences in hydraulic conductivity are likely to be the major contributor to hydropatterning, as agar and air differ in this property by several orders of magnitude. We have also observed that hydropatterning is influenced by water potential; however, the effects are significant only with relatively large changes. If the water potential of the media is the same or lower than the water potential of the root itself, this would eliminate or reverse the flow of water from the media to the root and likely eliminate the LR-inducing effects of the media. How cells can actually sense differences in the availability of water from contacting surfaces will be an important and challenging question for future research.
Auxin biosynthesis, transport, and signaling are essential for hydropatterning. We have shown that differences in the availability of water affect the level of auxin that accumulates in the root and the expression level of TAA1 in the outer tissue layers. Our data suggest that auxin levels may differ across the air-contact axis in Arabidopsis and rice roots and that specific PIN-type auxin efflux transporters may be important for maintaining gradients of auxin across the circumferential axis. Based on the spatial expression pattern of PIN transporters and mathematical-modeling approaches, it has been proposed that auxin is transported to the root tip through the central stele and then redirected away from the tip through the outer tissue layers (33, 34). We hypothesize that higher levels of auxin on the contact side are transported shootward through the outer tissues and delivered to the elongation and early maturation zones where they promote FC specification in pericycle cells at the proximal xylem pole. In the absence of TAA1 or a properly functioning auxin transport pathway, auxin signaling may not reliably exceed the required threshold on the contact side to influence the spatial distribution of FCs. Root curvature is proposed to act after FC specification (4), thus explaining why we do not observe an interaction with hydropatterning.
Materials and Methods
Plant materials and methods for genetic analysis, plant growth conditions, transgene construction, phenotypic analysis, and X-ray microscale computed tomography (microCT) are described in SI Appendix, SI Materials and Methods. Arabidopsis thaliana ecotypes Columbia, Col-0, and Landsberg erecta [La(er)] were used. Maize inbred line B73 and rice cultivar Kitaake and Taichung 65 were used.
Supplementary Material
Acknowledgments
We thank José Alonso, Fred Berger, Eva Benkova, Matt Evans, Annemarie Meijer, Ben Scheres, and Jian Xu for providing materials, and the J.R.D. laboratory for comments on the manuscript. Funding was provided by a Carnegie Institution for Science endowment, the National Research Foundation of Singapore, a National Science Foundation Graduate Research Fellowship under Grant DGE-1147470 (to N.E.R.), European Research Council FUTUREROOTS (C.J.S., M.C.T., S.J.M., and M.J.B.), and Biotechnology and Biological Sciences Research Council (C.J.S., S.J.M., and M.J.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400966111/-/DCSupplemental.
References
- 1.Malamy JE. Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 2005;28(1):67–77. doi: 10.1111/j.1365-3040.2005.01306.x. [DOI] [PubMed] [Google Scholar]
- 2.Overvoorde P, Fukaki H, Beeckman T. Auxin control of root development. Cold Spring Harb Perspect Biol. 2010;2(6):a001537. doi: 10.1101/cshperspect.a001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Smet I, et al. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development. 2007;134(4):681–690. doi: 10.1242/dev.02753. [DOI] [PubMed] [Google Scholar]
- 4.Moreno-Risueño MA, et al. Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science. 2010;329(5997):1306–1311. doi: 10.1126/science.1191937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parizot B, et al. Diarch symmetry of the vascular bundle in Arabidopsis root encompasses the pericycle and is reflected in distich lateral root initiation. Plant Physiol. 2008;146(1):140–148. doi: 10.1104/pp.107.107870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brady NC, Weil RR. The Nature and Properties of Soils. Revised 14th Ed. Upper Saddle River, NJ: Pearson–Prentice Hall; 2008. [Google Scholar]
- 7.Owens L, Wozniak C. Measurement and effects of gel matric potential and expressibility on production of morphogenic callus by cultured sugarbeet leaf discs. Plant Cell Tissue Organ Cult. 1991;26(2):127–133. [Google Scholar]
- 8.Karahara I, et al. Demonstration of osmotically dependent promotion of aerenchyma formation at different levels in the primary roots of rice using a ‘sandwich’ method and X-ray computed tomography. Ann Bot (Lond) 2012;110(2):503–509. doi: 10.1093/aob/mcs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nobel PS, Cui M. Shrinkage of attached roots of Opuntia ficus-indica in response to lowered water potentials—Predicted consequences for water uptake or loss to soil. Ann Bot (Lond) 1992;70(6):485–491. [Google Scholar]
- 10.Ishida T, Anno T, Matsukawa S, Nagano T. Hydraulic conductivity and diffusion coefficient in gels for plant tissue culture. Environ Control Biol. 2000;38(3):165–171. [Google Scholar]
- 11.Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006;45(4):523–539. doi: 10.1111/j.1365-313X.2005.02593.x. [DOI] [PubMed] [Google Scholar]
- 12.Van Norman JM, Xuan W, Beeckman T, Benfey PN. To branch or not to branch: The role of pre-patterning in lateral root formation. Development. 2013;140(21):4301–4310. doi: 10.1242/dev.090548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marin E, et al. miR390, Arabidopsis TAS3 tasiRNAs, and their AUXIN RESPONSE FACTOR targets define an autoregulatory network quantitatively regulating lateral root growth. Plant Cell. 2010;22(4):1104–1117. doi: 10.1105/tpc.109.072553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laskowski M, et al. Root system architecture from coupling cell shape to auxin transport. PLoS Biol. 2008;6(12):e307. doi: 10.1371/journal.pbio.0060307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Rybel B, et al. A novel aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr Biol. 2010;20(19):1697–1706. doi: 10.1016/j.cub.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Babé A, et al. Repression of early lateral root initiation events by transient water deficit in barley and maize. Philos Trans R Soc Lond B Biol Sci. 2012;367(1595):1534–1541. doi: 10.1098/rstb.2011.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Smet I, et al. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J. 2003;33(3):543–555. doi: 10.1046/j.1365-313x.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Guzman M, et al. Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell. 2012;24(6):2483–2496. doi: 10.1105/tpc.112.098574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavenus J, et al. Lateral root development in Arabidopsis: Fifty shades of auxin. Trends Plant Sci. 2013;18(8):450–458. doi: 10.1016/j.tplants.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Brunoud G, et al. A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature. 2012;482(7383):103–106. doi: 10.1038/nature10791. [DOI] [PubMed] [Google Scholar]
- 21.Stepanova AN, et al. The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. Plant Cell. 2011;23(11):3961–3973. doi: 10.1105/tpc.111.088047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stepanova AN, et al. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell. 2008;133(1):177–191. doi: 10.1016/j.cell.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 23.Yamada M, Greenham K, Prigge MJ, Jensen PJ, Estelle M. The TRANSPORT INHIBITOR RESPONSE2 gene is required for auxin synthesis and diverse aspects of plant development. Plant Physiol. 2009;151(1):168–179. doi: 10.1104/pp.109.138859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao Y, et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell. 2008;133(1):164–176. doi: 10.1016/j.cell.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Won C, et al. Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc Natl Acad Sci USA. 2011;108(45):18518–18523. doi: 10.1073/pnas.1108436108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mashiguchi K, et al. The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci USA. 2011;108(45):18512–18517. doi: 10.1073/pnas.1108434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benková E, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115(5):591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 28.Delbarre A, Muller P, Imhoff V, Guern J. Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta. 1996;198(4):532–541. doi: 10.1007/BF00262639. [DOI] [PubMed] [Google Scholar]
- 29.Blilou I, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433(7021):39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 30.Marhavý P, et al. Auxin reflux between the endodermis and pericycle promotes lateral root initiation. EMBO J. 2013;32(1):149–158. doi: 10.1038/emboj.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swarup R, et al. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat Cell Biol. 2005;7(11):1057–1065. doi: 10.1038/ncb1316. [DOI] [PubMed] [Google Scholar]
- 32.Rouse D, Mackay P, Stirnberg P, Estelle M, Leyser O. Changes in auxin response from mutations in an AUX/IAA gene. Science. 1998;279(5355):1371–1373. doi: 10.1126/science.279.5355.1371. [DOI] [PubMed] [Google Scholar]
- 33.Grieneisen VA, Xu J, Marée AF, Hogeweg P, Scheres B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature. 2007;449(7165):1008–1013. doi: 10.1038/nature06215. [DOI] [PubMed] [Google Scholar]
- 34.Band LR, et al. Systems analysis of auxin transport in the Arabidopsis root apex. Plant Cell. 2014;26(3):862–875. doi: 10.1105/tpc.113.119495. [DOI] [PMC free article] [PubMed] [Google Scholar]