Autoreactive antibodies can be pathogenic in a myriad of diseases. Consequently, the adaptive immune system actively removes or inactivates self-reactive B cells, while promoting the survival of B cells that recognize exogenous antigens, including microbial pathogens (1, 2). These physiological mechanisms of immune tolerance are often defective in autoimmune diseases (3, 4), and much effort is currently being expended learning how to mitigate autoreactive B-cell responses in the setting of autoimmune diseases. An unwelcome consequence of tolerizing selection is reduction in the potential diversity of the primary B-cell repertoire that limits the availability of B cells capable of generating protective responses against pathogens (5). An example of immunological censoring by tolerance is apparent in the protective antibodies elicited by HIV-1 infection, called broadly reactive neutralizing antibodies (bnAbs) (6). The majority of bnAbs are either polyreactive (reactive with many unrelated molecules), autoreactive (reactive with one specific self-antigen), or both (7), and in some cases, reactivity with host antigens is tightly linked to HIV-1–neutralizing activity (8, 9). Thus, in contrast to autoimmunity where the goal is to reduce autoreactive B-cell responses, vaccinologists are trying to activate and expand disfavored bnAb B-cell clonal lineages subject to control by immune tolerance (6–13).
In PNAS, Sabouri et al. (14) outline a surprising pathway for the utilization or “redemption” of autoreactive anergic B cells. The authors demonstrate that B cells that recognize both foreign and self-antigens can be activated by immunization and recruited into germinal centers (GC) where hypermutation of the B-cell antigen receptor (BCR) can reduce self-reactivity while maintaining the capacity of the redeemed B cells to recognize an exogenous antigen (14).
This is a surprising finding, not only because anergic B cells are refractory to most activating stimuli (1, 15, 16), but also because this finding emphasizes the poorly understood capacity of GC to select BCR mutants with lowered affinity for abundant, soluble antigen (17). Anergic B cells, it now seems, are capable of making substantial contributions to humoral immunity. An important and exciting corollary of this work is the potential elucidation of novel cellular pathways for vaccines to target anergic B cells for the induction of antibody to microbial epitopes that mimic host antigens (e.g., HIV-1 bnAbs).
B cells develop from progenitors that generate functional BCR by genomic rearrangements of V (variable), D (diversity), and J (joining) gene segments (18). This process results in a highly diverse set of BCR capable of reacting with virtually any antigen but also produces autoreactive B cells (19, 20). Indeed, some 70% of newly generated (“late pre-B”) human BCR are autoreactive (19, 21); the majority of these self-reactive BCR are eliminated or inactivated by immune tolerance mechanisms (19), including the induction of anergy or B-cell unresponsiveness (16, 22–24).
In GC, antigen-specific B cells recruited to follicular dendritic cells respond to follicular dendritic cell-associated antigen by proliferation and the accumulation of V(D)J mutations introduced by activation-induced cytidine deaminase. GC mutant B cells are selected for increasing BCR affinity to antigen in a Darwinian process mediated by competition for T-follicular helper cell (TFH) survival/proliferation signals (2). Dominance and persistence of GC B-cell clonal lineages is determined by mutant BCR avidity, but these mutations also generate BCR with affinity for self-antigens (25, 26). For this reason, it has long been thought that the GC should be capable of tolerizing autoreactive mutants that arise during affinity maturation (17, 27).
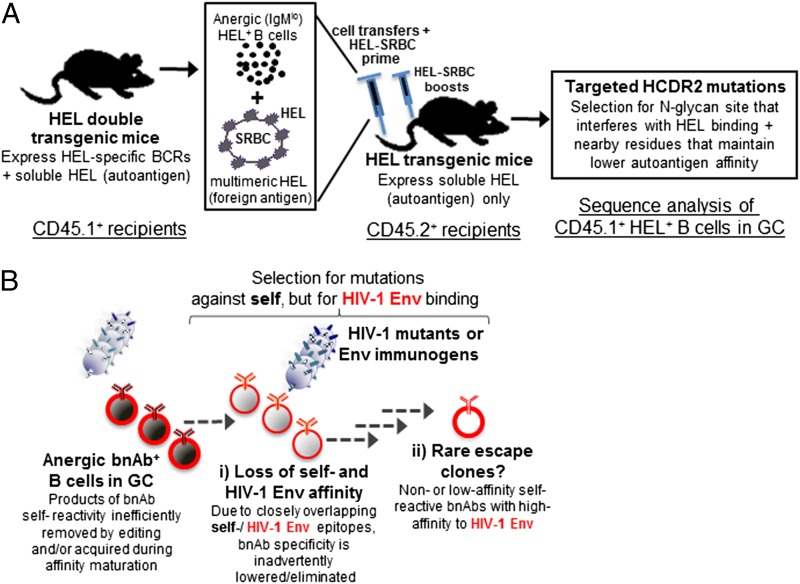
Although the mechanisms that ensure self-tolerance in GC are not well understood, the work of Sabouri et al. (14) calls attention to the potential of V(D)J hypermutation not only to generate autoreactivity but also to silence it, and to redeem autoreactive B cells. Sabouri et al. infer clonal redemption by the analysis of BCR mutations in human B cells that abrogate the intrinsic autoreactivity of VDJ rearrangements containing the IGHV4-34*01 gene segment (28), and by study of transgenic mice that constitutively express soluble hen egg lysozyme (HEL) and the high affinity, HEL-specific Hy10 BCR. In the latter model, mutations in the VH gene segment were found to suppress binding to an HEL self-epitope and, in contrast to experiments demonstrating apoptosis by Hy10 GC B-cell exposed to soluble HEL (27), Sabouri et al. (14) demonstrate that a subset of HEL-reactive Hy10 GC B cells do not die, but proliferate and diversify following immunization with HEL. This expansion of Hy10 B cells depends on mutations in the complementarity determining regions of the Hy10 heavy chain (HCDR2) that lower affinity for HEL (14).
These HCDR2 mutations relax the autoreactivity of Hy10 B cells but not the ability to respond to exogenous antigen ligands, leading to their “redemption” (14). Paradoxically, anergic B cells are able to generate more progeny in GC than do nonanergic, naïve B cells (14), an observation seemingly in tension with previous studies (27, 29) but consistent with reports indicating that GC B cells loaded with antigen expand extensively in the GC dark zone (23, 30). These findings are of interest for those trying to develop vaccines against pathogens bearing neutralizing epitopes that cross-react with self, with HIV-1 as a prominent example (6, 10, 12). Only a subset of HIV-1–infected individuals (∼20%) make high levels of HIV-1 bnAbs, and Env vaccination does not induce bnAbs. Knock-in mice that express bnAb BCR exhibit blockade of B-cell development at the first tolerance checkpoint with deletion of >90% of B cells; however, deletion is not complete, with a minority of anergic bnAb B cells surviving (11, 12, 31). These anergic bnAb B cells can be triggered by vaccine antigens to generate high titers of bnAb and, similar to the findings of Sabouri et al. (14), a fraction of bnAb B cells acquired T-cell–dependent BCR mutations that decreased both
The work of Sabouri et al. calls attention to the potential of V(D)J hypermutation not only to generate autoreactivity but also to silence it.
autoreactivity and binding to the HIV-1 Env, a process termed affinity reversion (Fig. 1) (12, 32). The similarities and differences between autoantibody redemption and affinity reversion merit additional investigation.
Fig. 1.
Experimental demonstration of autoantibody redemption in the HEL transgenic mouse system. (A) The immunization schema that allowed detection of rescued anergic autoreactive B cells following HEL-RBC immunization. HEL-specific B cells from double-transgenic mice, rendered anergic because of their prior encounter with soluble-expressed HEL, were injected into HEL transgenic mice, together with a HEL-SRBC immunogen. Immunized mice driven to generate anergic HEL-specific GC B cells were induced to acquire mutations in the HEL antibody HCDR2 region that resulted in decreased affinity for HEL and increased B-cell survival. (B) An illustration of how such a mechanism may contribute to bnAb responses during HIV-1 infection, and how B-cell populations generated by this process could be harnessed in the setting of HIV-1 vaccination. Vaccination with HIV-1 Env may result in either a population of anergic GC B cells, which have accumulated somatic mutations that generate bnAb specifcity (red) and self-reactivity (black), but may also generate rare B cells with BCR that harbor decreased self-reactivity, while retaining bnAb HIV-1 Env affinity. The hypothesis is that Env vaccination targeted at disfavored bnAb B-cell clonal lineages could drive otherwise unfavored and rare HIV-1–specific B-cell clones in GC to survive and proliferate.
Finally, the important findings of Sabouri et al. (14) raise significant questions. Is the phenomenon of autoantibody redemption generalizable to naturally found human autoantigens to impact microbe vaccine development? To what degree is autoantibody redemption able to patch “holes” in the primary BCR repertoire created by immune tolerance? Can vaccination strategies be devised to harness autoantibody redemption to drive the expansion and persistence of normally disfavored, autoreactive B cells with BCR that recognize microbes? Answers to these questions will be crucial to understanding how microbial pathogens avoid robust immunity and to defining the role of BCR mutation in controlling pathogenic B cells in autoimmune diseases.
Supplementary Material
Acknowledgments
This work was supported by the Division of AIDS, the National Institute of Allergy and Infectious Diseases (NIAID), the National Institutes of Health (NIH); Grant UM1-AI100645 for the Center for HIV/AIDS Vaccine Immunology-Immunogen Discovery; NIH, NIAID Grant R01 AI87202; and a Collaboration for AIDS Vaccine Discovery grant from the Bill & Melinda Gates Foundation.
Footnotes
The authors declare no conflict of interest.
See companion article on page E2567.
References
- 1.Goodnow CC, Sprent J, Fazekas de St Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435(7042):590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 2.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 3.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol. 2008;20(6):632–638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Menard L, Samuels J, Ng YS, Meffre E. Inflammation-independent defective early B cell tolerance checkpoints in rheumatoid arthritis. Arthritis Rheum. 2011;63(5):1237–1245. doi: 10.1002/art.30164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowes T, et al. Tolerance to self gangliosides is the major factor restricting the antibody response to lipopolysaccharide core oligosaccharides in Campylobacter jejuni strains associated with Guillain-Barré syndrome. Infect Immun. 2002;70(9):5008–5018. doi: 10.1128/IAI.70.9.5008-5018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haynes BF, Verkoczy L. Host controls of HIV neutralizing antibodies. Science. 2014;344(6184):588–589. doi: 10.1126/science.1254990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang G, et al. Identification of autoantigens recognized by the 2F5 and 4E10 broadly neutralizing HIV-1 antibodies. J Exp Med. 2013;210(2):241–256. doi: 10.1084/jem.20121977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alam SM, et al. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc Natl Acad Sci USA. 2009;106(48):20234–20239. doi: 10.1073/pnas.0908713106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mouquet H, Warncke M, Scheid JF, Seaman MS, Nussenzweig MC. Enhanced HIV-1 neutralization by antibody heteroligation. Proc Natl Acad Sci USA. 2012;109(3):875–880. doi: 10.1073/pnas.1120059109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haynes BF, Kelsoe G, Harrison SC, Kepler TB. B-cell–lineage immunogen design in vaccine development with HIV-1 as a case study. Nat Biotechnol. 2012;30(5):423–433. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verkoczy L, et al. Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance. Proc Natl Acad Sci USA. 2010;107(1):181–186. doi: 10.1073/pnas.0912914107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verkoczy L, et al. Induction of HIV-1 broad neutralizing antibodies in 2F5 knock-in mice: Selection against membrane proximal external region-associated autoreactivity limits T-dependent responses. J Immunol. 2013;191(5):2538–2550. doi: 10.4049/jimmunol.1300971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haynes BF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308(5730):1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 14.Sabouri Z, et al. Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proc Natl Acad Sci USA. 2014;111:E2567–E2575. doi: 10.1073/pnas.1406974111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodnow CC, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334(6184):676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 16.Goodnow CC. Transgenic mice and analysis of B-cell tolerance. Annu Rev Immunol. 1992;10:489–518. doi: 10.1146/annurev.iy.10.040192.002421. [DOI] [PubMed] [Google Scholar]
- 17.Han S, Zheng B, Dal Porto J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. IV. Affinity-dependent, antigen-driven B cell apoptosis in germinal centers as a mechanism for maintaining self-tolerance. J Exp Med. 1995;182(6):1635–1644. doi: 10.1084/jem.182.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fugmann SD, Lee AI, Shockett PE, Villey IJ, Schatz DG. The RAG proteins and V(D)J recombination: Complexes, ends, and transposition. Annu Rev Immunol. 2000;18:495–527. doi: 10.1146/annurev.immunol.18.1.495. [DOI] [PubMed] [Google Scholar]
- 19.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 20.Jankovic M, Casellas R, Yannoutsos N, Wardemann H, Nussenzweig MC. RAGs and regulation of autoantibodies. Annu Rev Immunol. 2004;22:485–501. doi: 10.1146/annurev.immunol.22.012703.104707. [DOI] [PubMed] [Google Scholar]
- 21.Wardemann H, Nussenzweig MC. B-cell self-tolerance in humans. Adv Immunol. 2007;95:83–110. doi: 10.1016/S0065-2776(07)95003-8. [DOI] [PubMed] [Google Scholar]
- 22.Adams E, Basten A, Goodnow CC. Intrinsic B-cell hyporesponsiveness accounts for self-tolerance in lysozyme/anti-lysozyme double-transgenic mice. Proc Natl Acad Sci USA. 1990;87(15):5687–5691. doi: 10.1073/pnas.87.15.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodnow CC, Adelstein S, Basten A. The need for central and peripheral tolerance in the B cell repertoire. Science. 1990;248(4961):1373–1379. doi: 10.1126/science.2356469. [DOI] [PubMed] [Google Scholar]
- 24.Merrell KT, et al. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25(6):953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Tiller T, et al. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26(2):205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mietzner B, et al. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci USA. 2008;105(28):9727–9732. doi: 10.1073/pnas.0803644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shokat KM, Goodnow CC. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995;375(6529):334–338. doi: 10.1038/375334a0. [DOI] [PubMed] [Google Scholar]
- 28.Richardson C, et al. Molecular basis of 9G4 B cell autoreactivity in human systemic lupus erythematosus. J Immunol. 2013;191(10):4926–4939. doi: 10.4049/jimmunol.1202263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan TD, et al. Elimination of germinal-center-derived self-reactive B cells is governed by the location and concentration of self-antigen. Immunity. 2012;37(5):893–904. doi: 10.1016/j.immuni.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 30.Schwickert TA, et al. A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J Exp Med. 2011;208(6):1243–1252. doi: 10.1084/jem.20102477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, et al. Common tolerance mechanisms, but distinct cross-reactivities associated with gp41 and lipids, limit production of HIV-1 broad neutralizing antibodies 2F5 and 4E10. J Immunol. 2013;191(3):1260–1275. doi: 10.4049/jimmunol.1300770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verkoczy L, Diaz M. Autoreactivity in HIV-1 broadly neutralizing antibodies: Implications for their function and induction by vaccination. Curr Opin HIV AIDS. 2014;9(3):224–234. doi: 10.1097/COH.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]