Significance
Although the positive impact of social bonds on individual’s fitness has recently been demonstrated, the mechanisms underlying the motivation to form long-term associations remain largely unknown. Current evidence shows that oxytocin modulates social behavior but evidence of its effects in bond maintenance remains scant, especially in nonreproductive contexts. We provide evidence that in the domestic dog oxytocin enhances social motivation to approach and affiliate with conspecifics and human partners, which constitutes the basis for the formation of any stable social bond. Furthermore, endogenous oxytocin levels increased after dogs engaged in affiliation with their dog partners, indicating a stimulation of the oxytocin system during social interactions. Our findings highlight the important role that oxytocin has in the expression of sociality in mammals.
Keywords: cooperative bonds, cooperative mechanisms
Abstract
Recent evidence suggests that enduring social bonds have fitness benefits. However, very little is known about the neural circuitry and neurochemistry underlying the formation and maintenance of stable social bonds outside reproductive contexts. Oxytocin (OT), a neuropeptide synthetized by the hypothalamus in mammals, regulates many complex forms of social behavior and cognition in both human and nonhuman animals. Animal research, however, has concentrated on monogamous mammals, and it remains unknown whether OT also modulates social bonds in nonreproductive contexts. In this study we provide behavioral evidence that exogenous OT promotes positive social behaviors in the domestic dog toward not only conspecifics but also human partners. Specifically, when sprayed with OT, dogs showed higher social orientation and affiliation toward their owners and higher affiliation and approach behaviors toward dog partners than when sprayed with placebo. Additionally, the exchange of socio-positive behaviors with dog partners triggered the release of endogenous OT, highlighting the involvement of OT in the development of social relationships in the domestic dog. These data provide new insight into the mechanisms that facilitate the maintenance of close social bonds beyond immediate reproductive interest or genetic ties and complement a growing body of evidence that identifies OT as one of the neurochemical foundations of sociality in mammalian species.
According to behavioral ecology theory, sociability evolved either to reduce individuals’ risk from predation or to increase individuals’ ability to find and defend food (1). New evidence from a range of mammalian species, however, shows that competitive success and reproductive performance of social individuals are affected by the nature and quality of the social bonds they form, suggesting that sociability is adaptive in its own right (2, 3). The impact of social bonds on individuals’ fitness extends beyond the benefits derived from associations directly related to mating and parental care (4). For example, in baboons (Papio hamadryas ursinus) (2, 3), house mice (Mus musculus) (5), rats (Rattus norvegicus) (6), horses (Equus equus) (7), and bottlenose dolphins (Tursiops truncatus) (8), individuals that maintain strong, enduring same-sex bonds experience a higher longevity or offspring survival than individuals with weaker bonds. These data parallel evidence from human studies that show that the quality and quantity of individuals’ social relationships is associated not only with better mental health but also with reduced mortality (9).
Beside the above-mentioned fitness benefits of social integration, very little is known about the neural circuitry and neurochemistry underlying the formation and maintenance of social bonds outside reproductive contexts. Although it is likely that brain systems that motivate animals to establish individualized relationships with other group members first evolved to mobilize the maternal care necessary for offspring survival in mammals (10, 11), this possibility remains untested. In this respect, the neuropeptide oxytocin (OT) has recently attracted the attention regarding the neurological basis of prosocial behaviors that facilitate interindividual relationships. This hypothalamic peptide plays an important role in various reproductive effects in mammals, such as parturition and lactation. Extensive animal research has shown that OT is also implicated in the regulation of several behaviors, such as pair-bonding, parental care, sexual behavior, peer recognition, and social memory (for reviews, see refs. 10 and 11). More recent research has revealed that OT influences more complex forms of social behavior and cognition in humans and nonhuman primates. For example, inhaled OT enhances trusting behavior, in-group cooperation, and generosity; it also modulates emotion recognition, social perception, and sensitivity to the experiences of others (for reviews, see refs. 12 and 13).
Previous animal research on OT, however, has concentrated on maternal or pair-bonding between sexual partners, and despite the growing interest in friendships and the fitness benefits of social skills it remains unknown whether OT also modulates other types of social bonds. There is some evidence that variation in levels of OT affects alloparental care (14–16). In addition, correlational studies have shown that positive interaction with close social partners are associated with changes in the levels of peripheral OT in dogs (Canis familiaris) (17) and chimpanzees (Pan troglodytes) (18, 19). Although these results might suggest a role of OT in the maintenance of cooperative associations, the studies failed to prove that OT actually induces changes in behavior that would facilitate those relationships. Furthermore, it is unclear whether the peripheral OT measured in these studies directly feeds back to the brain.
The present study uses a pharmacological intervention to investigate whether in the domestic dog OT modulates the maintenance of enduring, close social bonds in nonreproductive contexts. Understanding the mechanisms that facilitate the formation of social bonds will help us not only to understand the evolution of the biological bases of cooperation, but also the individual variability in social skills observed in many social species (20). Domestic dogs are an excellent model species in which to investigate the physiological underpinnings of social bonds because they are known to be able to form enduring, affective bonds not only with conspecifics but also with humans (21, 22). If brain OT indeed regulates the formation and maintenance of social bonds, we should find that dogs given OT show more positive behaviors toward partners than when given placebo. In particular, we assessed the effects of OT on the behavioral parameters that are normally used to evaluate the strength of social bonds among group mates (i.e., affiliation, approach, and time spent in proximity) (4). Similarly to human studies, we administrated OT to dog subjects using intranasal delivery. We found that OT spray intake increased plasma OT levels in dogs, suggesting transnasal penetration into the brain. After treatment intake, the subjects stayed in an experimental room for 60 min with their owners and a familiar dog partner and their behaviors were analyzed. We also measured urinary OT levels before and after the experimental sessions to establish whether endogenous OT could moderate the effects of exogenous OT administration and to examine if the oxytocinergic system that supports bond formation functions as a bio-behavioral feedback loop.
Results
Oxytocin and Affiliation.
The effect of OT on dogs’ social behaviors was evaluated via separate linear mixed models (LMM) for each of the four behavioral measures (i.e., affiliation, social proximity, social approach, and social orientation). Treatment (OT or saline), dog’s sex, and their interaction were entered as fixed factors in the models, and the dogs’ identity as a random factor. In the set of analyses involving dog-to-dog interactions, kinship was also included as a fixed factor. Given that individual variation in endogenous OT could crucially moderate the effects of exogenous OT administration, we measured OT concentrations in the urine samples collected immediately before the start of each experimental session. Although basal OT levels can vary, individuals’ urinary OT values of the two experimental sessions were significantly correlated, suggesting stability over time (Spearman correlation: rs = 0.631; n = 16, P = 0.009). Thus, the pretest urinary OT level was also entered in all models as a fixed factor along with its interaction with treatment and sex.
Affiliation with Owners.
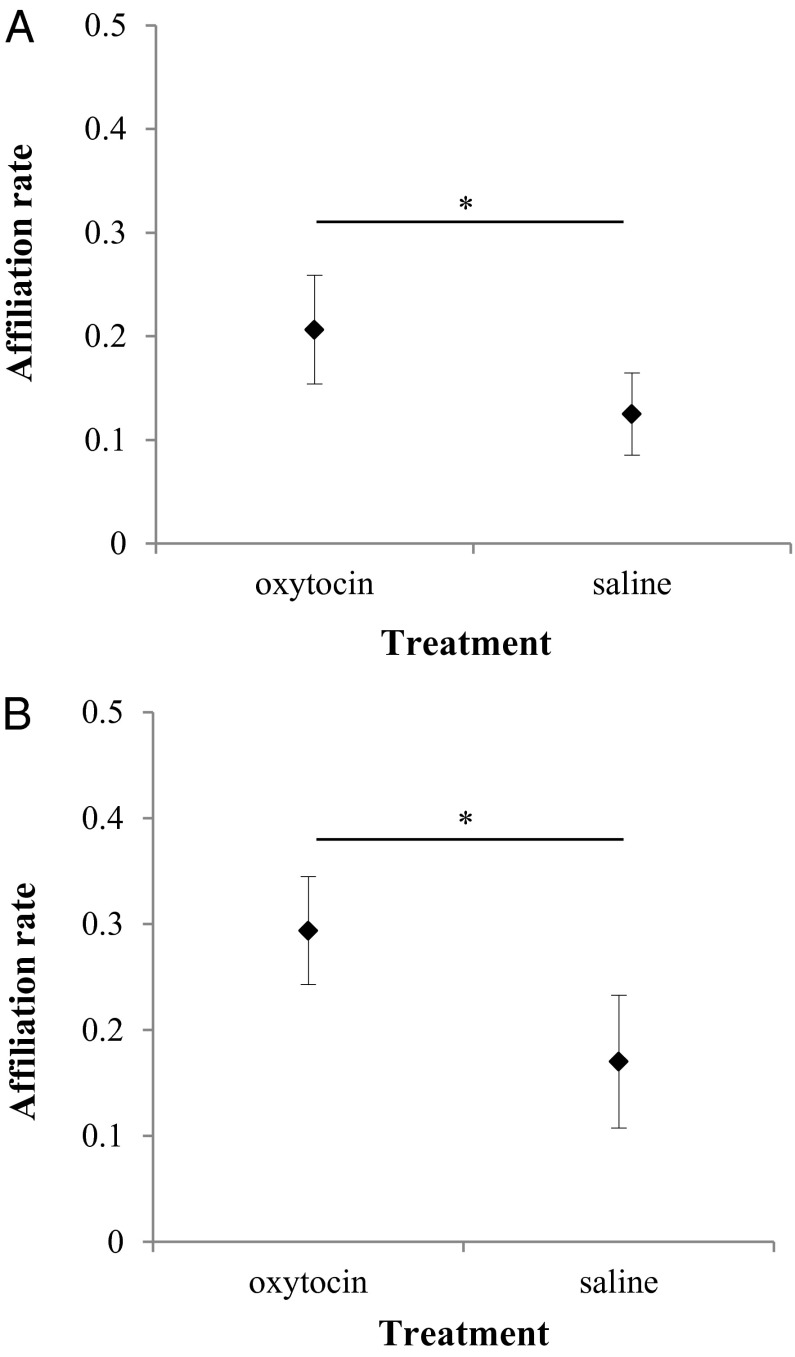
The owners of the dogs were instructed to sit quietly in the experimental room and not to actively interact with their dogs. Thus, any behavior directed from the dogs to their owners was either ignored (e.g., dogs received no response when they sniffed or licked their owners) or briefly reciprocated (e.g., dogs received a gentle brief touch or push back when they tried to lick their owner’s face). Despite this instruction, dogs administered with OT initiated affiliation toward their owners more often than when administered with saline solution (LMM: β = −0.081, SE = 0.019, t = −4.097, P = 0.001) (Fig. 1A). We then examined the total amount of time dogs spent in close social proximity (<1 m) with their owners. The only variable remaining in the best model was pretest OT level, although it did not reach statistical significance (β = 2.761, SE = 1.719, t = 1.606, P = 0.098). On the other hand, the type of treatment dogs were administered with did affect their social orientation (i.e., staring, looking at owner or no clear gaze direction but head frontally oriented to owner). OT treatment was associated with increased social orientation to their owners (LMM: β = −0.109, SE = 0.035, t = −3.100, P = 0.005) (Fig. S1).
Fig. 1.
The effect of OT on dogs’ affiliative behaviors. Mean hourly rate (+SEM) of a combined measure of all affiliative behaviors that dogs directed to their owners (A) or dog partners (B). *P < 0.05.
Affiliation with Dog Partners.
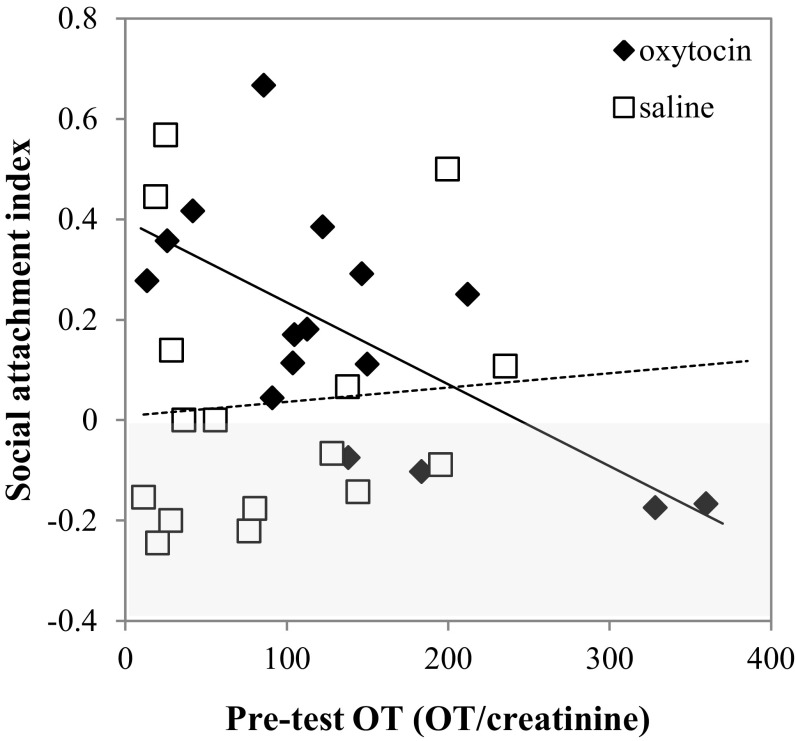
When analyzing the affiliative behaviors directed to dog partners, we found that affiliation was affected by the type of treatment administered but not by sex, pretest OT levels, or kinship. After OT intake, dogs affiliated with their partners significantly more often than after saline treatment (LMM: β = −0.113, SE = 0.048, t = −2.339, P = 0.026) (Fig. 1B). We did not find, however, any effect of the studied variables on the amount of time the subjects spent in close proximity with their partners (LMM full model main effects; treatment: β = −0.192, SE = 0.135, t = –1.426, P = 0.146; sex: β = −0.051, SE = 0.179, t = −0.283, P = 0.748; pre-OT: β = 0.001, SE = 0.001, t = −1.319, P = 0.158; kinship: β = 0.267, SE = 206, t = 1.297, P = 0.154). Given that the time two individuals spend in close proximity could be because of the approaches or departures of either of them, we examined the subject’s relative role in maintaining close proximity to his/her partner by calculating the proportion of approaches minus the proportion of departures effected by the subject. In this case, treatment (LMM: β = −0.163, SE = 0.075, t = −2.158, P = 0.037) and its interaction with pretest OT levels (β = 0.002, SE = 0.001, t = 2.633, P = 0.007) significantly affected the contribution of the subjects to the maintenance of social proximity, which was higher when they received the OT treatment than when they received the placebo. Furthermore, this effect was significantly higher for dogs with low levels of pretest OT than for dogs with high levels of endogenous OT at the start of the experimental session (Fig. 2). Finally, with regard to social orientation, we did not find any significant effect of treatment, subject’s sex, pre-OT levels, or kinship (LMM full model main effects; treatment: β = −0.067, SE = 0.060, t = −1.112, P = 0.231; sex: β = 0.039, SE = 0.060, t = 0.649, P = 0.482; pretest OT: β = −0.0002, SE = 0.0003, t = −0.773, P = 0.414; kinship: β = −0.031, SE = 0.069, t = −0.442, P = 0.631).
Fig. 2.
OT effects on dogs’ tendency to approach their partners. Relation between the social approach index for OT (black) and saline (white) treatments and subject endogenous OT levels before experimental sessions. Lines represent the trend lines for OT (solid) and saline (dashed) datasets.
Affiliation and Endogenous OT.
Several correlational studies have suggested an association between endogenous OT and positive interactions (17, 19, 23). To explore this possibility, urine samples were collected at the end of each experimental session to assess posttest OT levels. Because we were interested in the effect of affiliation on endogenous OT, we limited the analyses to the saline condition to remove the confounding effects of exogenous OT. The increase OT ratio (ΔOT = posttest OT levels/pretest OT levels) did not show a significant association with the time subjects spent in close proximity to their owners or dog partners, nor with the affiliation the subjects provided to or interchanged with them (i.e., affiliation given and received) (Table S1). We did find, however, a positive association between ΔOT and the proportion of affiliation that was reciprocated from their dog partners (Spearman correlation: rs = 0.471; n = 16, P = 0.032), suggesting that the immediate reciprocation of affiliation triggered the release of endogenous OT.
Intranasal Administration of OT.
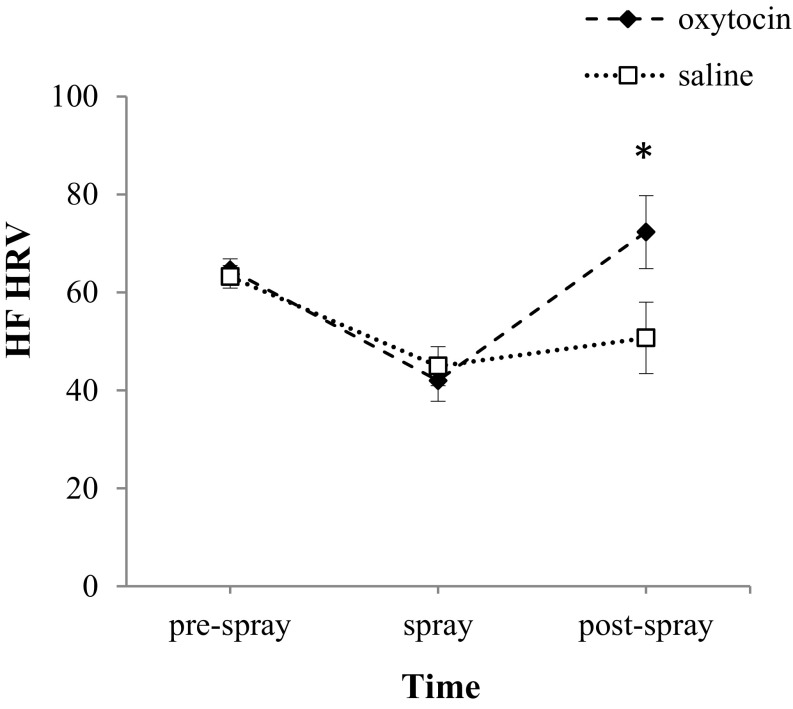
Similarly to human studies, we administrated OT to dog subjects using intranasal delivery. To evaluate the effectiveness of this method in dogs, we combined heart rate, blood, and urine sampling from dogs receiving OT or saline treatment. During the experimental sessions dogs’ heart rate variability (HRV) was telemetrically measured and the high frequency (HF), a well-validated index of parasympathetic control (24), calculated (SI Text). To maximize observable effects, data on HF HRV were calculated for 5-min intervals occurring before and after spray intake. The obtained values were subjected to repeated-measures ANOVA with two factors: treatment (OT, saline) and time (prespray, spray, postspray) (Fig. 3). We found a significant effect of time [ANOVA: F(2, 22) = 14.21, P < 0.001] as well as a significant time by treatment interaction [ANOVA: F(2, 22) = 4.55, P = 0.022]. Independent of the treatment received, right after spray administration, subjects displayed a significant decrease of HF HRV relative to prespray values (Bonferroni post hoc multiple comparisons: prespray vs. spray time, OT: P = 0.007; saline: P = 0.027) (Fig. 3), suggesting that the administration of the spray could have caused some transitory discomfort in the dogs. When subjects were treated with saline solution, their HF HRV values did not significantly vary in the subsequent 5 min. However, when they were treated with OT, subjects displayed a significant increase in HF HRV relative to both OT spray time (P < 0.001) and saline postspray (P = 0.015), suggesting OT transnasal penetration into the central nervous system.
Fig. 3.
The effects of intranasal OT on parasympathetic cardiac control. “Prespray” corresponds to the 5-min interval before spray intake, “spray” to the 5-min interval right after spray intake, and “postspray” to the subsequent 5-min interval (i.e., 5–10 min). *P < 0.05 versus basal. Markers represent the mean HF HRV values (+SEM) for the OT (black) and saline (white) treatments.
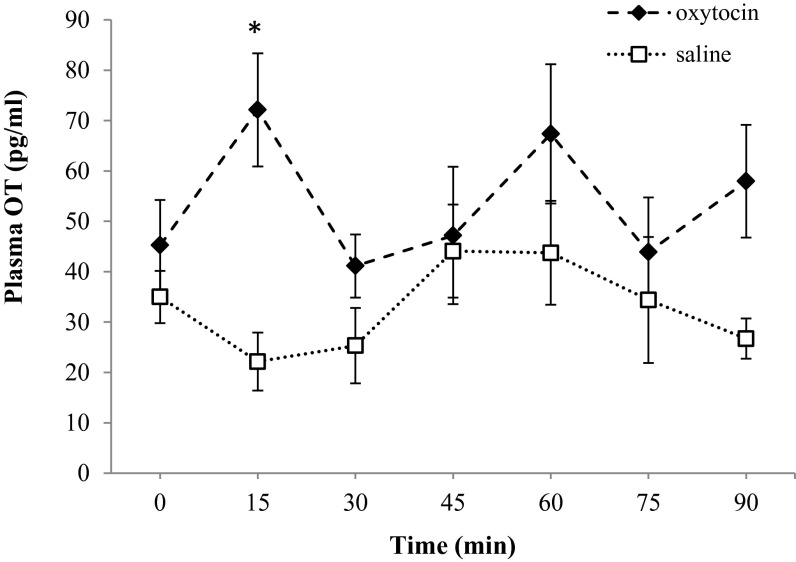
Because blood collection during the experimental sessions would have disturbed the subjects, blood samples were collected from an additional group of five dogs receiving the treatment while at rest. Blood samples were drawn before spray administration (baseline) and every 15 min after intranasal treatment over a time window of 90 min (i.e., six samples; see SI Text for details). There were no significant differences in plasma OT levels between the saline and the OT baseline data (Wilcoxon signed rank test, z = −0.674, n = 5, P = 0.500). However, when dogs were treated with OT, subjects displayed a significant increase in plasma OT relative to saline postspray (LMM, β = −0.297, SE = 0.139, t = −2.125, P = 0.033) (Fig. 4), which might indicate an activation of central OT neurons. Plasma OT levels after OT administration were significantly elevated at 15 min relative to both baseline (z = −2.023, n = 5, P = 0.043) and saline condition (z = −2.023, n = 5, P = 0.043). OT concentrations at the end of the experimental session (time 90 min) were also higher after OT administration than after saline spray, although the difference did not reach statistical significance (z = −1.753, n = 5, P = 0.080). On the other hand, after saline administration, dogs displayed equivalent basal levels of plasma OT throughout the experimental session.
Fig. 4.
Plasma kinetics of OT. Plasma OT concentrations (mean + SEM) at baseline (0 min) and every 15 min after intranasal delivery of OT (black) or saline (white) treatment. *P < 0.05 versus basal, Wilcoxon signed rank test.
The variation in plasma OT after spray intake was also reflected in the OT levels of the urine samples collected before and after the experimental sessions. We found a significant positive correlation between the accumulate value of plasma OT and the increase in urinary OT levels (Spearman correlation: r = 0.639,P = 0.047) (Fig. S2). Furthermore, the LMM analysis showed that dogs’ urinary OT levels significantly differed between the sampling time points (pretest, posttest) and the treatment received (OT, saline) (sampling time × treatment: β = −0.642, SE = 0.328, t = −1.954, P = 0.039) (Fig. S3). Specifically, posttest urinary OT concentrations were significantly elevated following intranasal OT administration compared with pretest OT levels of the same experimental session (P = 0.012) and with posttest OT levels after saline spray (P = 0.047).
Discussion
In this study we investigated whether OT could modulate social bonding in the domestic dog. OT caused dogs to engage in higher levels of affiliation, social orientation, and social approach with their owners and dog partners. In humans and some nonhuman animals, individuals establish highly differentiated associations with other group members (25). These strong social bonds are usually defined in terms of high rates of cooperative behaviors exchanged over time (4). The present study demonstrates that OT enhances social motivation to approach and affiliate with close social partners, which constitutes the basis for the formation of any stable social bond and facilitates its maintenance over time. Because enduring social relationships have adaptive value (2, 5–8), it is likely that natural selection has favored neurological mechanisms that promote their maintenance (10, 11). That exogenous OT promotes a suite of behaviors directly related with social bonding in dogs supports the idea that the same hormonal and neuroendocrine factors that promote parental behavior and pair-bonding in mammals (i.e., the oxytocinergic system) also contribute to the formation of other types of social relationships. These findings are in line with studies in humans reporting the effects of OT on behaviors that facilitate interpersonal relations, from perception bias to social motivation to trusting behavior (12, 13).
Our study shows previously undescribed effects of OT on interspecific relationships. Although some animals may form permanent cross-species associations (26), marked preferences for particular individuals of another species are not common (27). Through cohabitation with humans, dogs are able to establish close bonding and attachment with people (21, 28), which in turn may modulate their behavioral and emotional responses (28, 29). Here we show that the same neurological mechanisms implicated in the regulation of intraspecific bonds in mammal also modulate cooperative associations between individuals from different species, suggesting that this hormonal mechanism might underlie the formation of stable cooperative associations between genetically unrelated individuals.
Similar to human (30) and recent nonhuman primate studies (31), the OT treatment was administered nasally, a minimally stressful method. One of the main technical issues of this method is whether the exogenous OT is able to cross the blood–brain barrier and exert direct effect on the brain. We found that in contrast with saline treatment, intranasal administration of OT significantly increased plasma OT concentrations. Given the short peripheral half-life values of OT [<5 min (32)], together with the magnitude of the observed increase in plasma OT levels, our results can hardly be explained by a continuous uptake from the nasal membranes; they rather suggest a peripheral release by activation of hypothalamic OT neurons. Furthermore, that intranasal administration of OT increased HF HRV is consistent with the idea that exogenous OT produced an increase of the parasympathetic autonomic cardiac control. The present study, though, cannot rule out the possibility that the observed changes in HRV are a result of the influence of peripheral OT receptors (33). However, the findings that OT treatment is also associated with behavioral changes, together with previous studies in human and nonhuman animals reporting a rapid accumulation of peptides in brain tissue and cerebrospinal fluid after their intranasal administration (34–36), suggest a more central mechanism. Our results parallel neuropharmacological research showing elevated OT levels in the extracellular fluid within the septum and dorsal hippocampus of rodents after intranasal administration, which was accompanied by a sharp rise in plasma OT (37). Activation of OT neurons, which project simultaneously to the posterior pituitary and forebrain regions (38, 39), can induce peripheral and central OT release and exert direct effects both at the cellular and behavioral level (40). Taken all together, the results of the present study suggest that OT can penetrate into the central nervous system in dogs when administered intranasally.
Although our study does not explicitly address the mechanisms through which OT may affect social behavior, our data better support the idea that OT directly promotes motivation to affiliate rather than reducing social anxiety, or altering the perceptual salience of social cues (12). Because in this experiment subjects stayed in a room with familiar associates and were not asked to perform any task, it is unlikely that variation in anxiety could explain the observed results. On the other hand, although our data on social orientation partially support the idea that OT may increase the salience of social cues (41), we used a general measure of social attention that does not distinguish between looking toward faces, perhaps the most important communicative part, or to other parts of the body. Thus, it is possible that OT increased a general tendency for social orientation in dogs rather than enhancing the salience of social cues. Our findings, though, provide stronger evidence for the notion that OT motivates social engagement. When dogs received the OT treatment they not only affiliated more often with their owners and dog partners, but also, their contribution to the maintenance of social proximity was higher, and this result held after having controlled for partners’ actions. These findings parallel evidence in humans and rodents showing that OT is implicated in attraction and bonding behavior (10, 42, 43). That OT promotes social approach and affiliation in relationships other than mother–infant or –mates should not be interpreted as an indiscriminate effect of OT on motivation to seek social contact. If anything, the accumulative evidence on OT shows that its effects on social behavior and cognition are context-dependent. In human and nonhuman animals OT can both enhance affiliation and cooperation and increase territoriality and out-group bias, depending on behavioral context (44–47). This finding may also hold for dogs because they form strong bonds with in-group members [including humans (21, 28)] and could show territorial aggression toward strangers. This hypothesis could be tested by evaluating the effects of OT on dogs’ behavior in the presence of strangers.
Our analysis also revealed that the observed effect of exogenous OT on dogs’ tendency to approach their partners was modulated by individual differences in endogenous OT, with dogs with high levels of endogenous OT being less responsive to the effects of administered OT. There is considerable literature proving that there exists individual—and population—variation in the endogenous OT system and that such variation is behaviorally relevant (reviewed in refs. 10 and 12). There is also the suspicion that this natural variation could affect the outcomes of OT administration. Despite these findings and theories, few studies have measured endogenous OT levels in conjunction with OT administration (12). Our results support the idea that basal levels of OT may affect individuals’ responses to exogenous OT, and further underline the importance of considering the interaction of applied doses and endogenous OT levels in studies using pharmacological intervention.
The influence of hormones in behavior is not simply unidirectional (48). Although OT acts as a modulator of neural pathways of social behavior, socio-positive behaviors can also trigger an OT response, functioning as a bio-behavioral feedback loop (49). For example, in rats maternal–infant touch and contact increase the expression of OT (50). Interestingly, in the present study we found that an increase in dogs’ OT levels was associated with affiliation being reciprocated, rather than with the sole act of providing it or receiving it. Our finding thus supports recent studies in human (51) and nonhuman animals (18), showing that social contact alone is not sufficient to raise OT levels, and suggests that psychological, physical or social factors may modulate the activation of the oxytocinergic system.
The present research demonstrates not only that OT motivates social bonding beyond genetic ties or reproductive interest, or even between individuals from phylogenetically very distant species, but also that the exchange of social-positive behaviors associated with bond maintenance triggers the release of OT. Although in humans and nonhuman animals stable collaborative associations with nonkin have fitness benefits (7), the proximate motivation to develop a long-term relationship is most likely not modeled on ultimate functions (i.e., the subsequent benefits), but on previous rewards individuals received (52, 53). Because OT enhances reward via dopamine-dependent mesolimbic reward pathways (54), it is likely that positive feedback, through behaviorally induced OT secretion, facilitates repeated interactions with individuals with whom positive interactions have already occurred. In our study, the administration of OT increased dogs’ affiliation toward conspecifics and humans, and the engagement in affiliation with their social partners was associated with an increase of endogenous OT, supporting the idea of an OT-mediated positive feedback loop in dog’s social bonding. As such, our findings suggest that OT might be an important mechanism that allowed the evolution of enduring cooperative bonds between related and unrelated individuals without requiring any cognitive understanding or estimate of the probability of future benefits.
Materials and Methods
Ethical Statement.
The present study was approved by the Ethics Committee of Azabu University (Japan) (No. 130304–2). Dogs were recruited through owners’ responses to flyer postings at veterinary hospitals and training centers. Written informed consent for participation in this study was obtained from the owners.
Subjects.
Sixteen dogs older than 12 mo of age served as subjects of this study [females, n = 8; males, n = 8; mean age 6.1 y (SE = 0.7)]. The group consisted of nine Standard Poodles, four Labrador retrievers, one German shepherd, one Shetland sheep dog, and one Border Collie. All dogs were companion dogs that lived in human households. Dogs living in the same house (n = 12) or dogs having daily contact with the subjects (n = 4) acted as partners during the experimental sessions. Partners were usually subjects themselves in other tests. Six subjects were genetically related with their partners (i.e., siblings) and all subjects had a long-term association with their owners and dog partners (i.e., more than 1 y).
Experimental Procedures.
The testing was conducted at the Companion Animal Research center of Azabu University (Japan). The only objects in the experimental room (11.5 × 6.5 m) were a chair where the owner sat and a blanket for the dog placed in front of the chair. The testing consisted of two phases. In the preadministration phase (30 min) the heart-rate device (see below) was activated and the dog and his/her owner entered the room. The owner was instructed to sit quietly and not to actively interact with the subject; the dog could move freely in the room. To promote the dogs’ activity, every 10 min the owner moved the chair and the blanket to a different predesignated position.
After 30 min had elapsed, 40 IU of OT or saline in 100-μL solution, depending on the testing condition, were nasally administered to the dog using a hand compressing air spray bottle (cat#1–9560-01, Asone). We administered fairly large doses of OT (i.e., 40 UI) to ensure that part of the solution was absorbed. All subjects received both conditions. The order of the treatments was counterbalanced across dogs and each condition carried out on different days. Owners were unaware of which treatment their dogs received. Immediately after spray intake, the dog acting as partner entered the room and a second blanket was placed in front of the chair. The postadministration phase lasted 60 min. As in the preadministration phase, every 10 min the owner changed the position of the chair and the blankets to the next designated point. All sessions were video-recorded for the subsequent analysis of dogs’ behaviors using four video cameras (VC-C50iR, Canon).
Blood and Urine Sample Collection.
In a separate experiment, blood samples were collected from five additional dogs (one male, four females, mean age = 6 y) at baseline (i.e., 3 min before spray administration) and every 15 min after intranasal treatment over a time window of 90 min (i.e., time 15, 30, 45, 60, 75, 90 min) while the dogs remained calm and were accompanied by their owners (SI Text). In both experiments, immediately before and after each experimental session (i.e., separated 90-min apart), urine samples were collected from all subjects. After their collection, the samples were centrifuged at 4 °C in a refrigerated centrifuge and frozen at −80 °C until assay.
OT Measurement.
To measure urinary and plasma OT concentrations we followed the radioimmunoassay procedure developed by Higuchi et al. (55) and later validated in dogs (56). Solid-phase extraction of samples was performed to eliminate interference with known peptides and proteins and to concentrate the levels of OT, and OT validation of accuracy was conducted satisfactorily (SI Text and Fig. S4). To compensate for variation in urine concentration, creatinine levels were measured and OT values were expressed as OT/creatinine ratio.
Heart Rate Measures.
Dogs’ HRV was telemetrically measured using a Polar RS800CX digital system device attached to dogs’ chests (57). For the HRV analyses the frequency domain indices were calculated using Kubios Heart Rate Variability Analysis Software 2.0 for Windows (57). Results from three subjects (one male and two females) were excluded from HRV analysis because of the poor quality of their data. For further details, see SI Text.
Behavioral Measures.
Affiliative behaviors, including sniffing, licking, gentle touching with the nose or paw, play bouts, and body contact (i.e., the subject sits, rests, or stands up in physical contact, excluding tails, with his/her owner or partner), were recorded using an all occurrence sampling. Rates per hour were calculated for a combined measure of all affiliative behaviors. A behavior was considered to be reciprocated if the subject received an affiliative behavior from the partner in return within the next 5 s. The proportion of affiliation reciprocated was calculated as the total number of behaviors reciprocated divided by the total number of behaviors directed to the partner.
Additionally, we calculated the time (in seconds) spent in close proximity (i.e., within 1 m) to the owner or the partner, along with a proximity index as a measure of the subject’s responsibility for proximity maintenance. The index equals the proportion of times the pair was united (<1 m) by subject’s movements minus the proportion of time the pair was separated (>1 m) by subject’s movements. The index ranges from 1 to −1, with positive values indicating a greater responsibility to maintain proximity to subject’s partners than vice versa.
Furthermore, the social orientation of the subject toward the owner and the partner was assessed, recording the greatest orientation observed every 30 s, ranging from socially oriented (i.e., staring, looking at owner/partner or no clear gaze direction but head frontally oriented to owner/partner for at least 5 s), laterally oriented (i.e., head laterally oriented without clearly looking at owner/partner), and turned away (i.e., partially or completely turned away from owner/partner). The level of social orientation was then calculated as the proportion of 30-s blocks in which the subject was socially oriented toward his/her owner or partner.
To examine whether the occurrence of the different behavioral measures was affected by the treatment, LMM were used [lmer function, R package lme4 (58)]. Analyses were conducted via separate LMM for each variable, entering treatment (OT/saline), subject’s sex, pretest OT level, and their interactions as fixed factors, and the identity of the subject as a random factor. Analyses for behaviors directed to humans were run independently from those directed to dog partners. In this later case, the kinship relationship between the subject and the dog partner was also included as a fixed factor. LMM we also used to examined whether changes in plasma and urinary OT concentrations were affected by the intranasal administration of the treatment. For these analyses, OT concentrations were log10-transformed to fit a normal distribution. Dogs’ identity was entered as a random factor, and treatment, sampling point time (0, 15, 30, 45, 60, 75, 90 min for blood samples, and pretest, posttest for urine samples) and their interaction as fixed factors. The best model was selected by using Akaike’s information criteria.
Supplementary Material
Acknowledgments
We thank the dogs and their owners for their participation in the study; Maki Katayama for her help in the collection of the blood samples; and Leanne Proops and three anonymous referees for helpful discussion and comments on an earlier version of this manuscript. This research was partially supported by the Japan Society for the Promotion of Science Research Fellowships for Foreign Researchers (P10311) (to T.R.); the Ministry of Education, Culture, Sports, Science and Technology with a Grant-in-aid for Challenging Exploratory Research (23650132) (to T.H.); and Grant-in-Aid for Scientific Research on Innovative Areas 4501 (to T.K. and T.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.S.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322868111/-/DCSupplemental.
References
- 1.Alexander RD. The evolution of social behavior. Annu Rev Ecol Syst. 1974;5:325–383. [Google Scholar]
- 2.Silk JB, Alberts SC, Altmann J. Social bonds of female baboons enhance infant survival. Science. 2003;302(5648):1231–1234. doi: 10.1126/science.1088580. [DOI] [PubMed] [Google Scholar]
- 3.Silk JB, et al. The benefits of social capital: Close social bonds among female baboons enhance offspring survival. Proc Biol Sci. 2009;276(1670):3099–3104. doi: 10.1098/rspb.2009.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seyfarth RM, Cheney DL. The evolutionary origins of friendship. Annu Rev Psychol. 2012;63:153–177. doi: 10.1146/annurev-psych-120710-100337. [DOI] [PubMed] [Google Scholar]
- 5.Weidt A, Hofmann SE, Konig B. Not only mate choice matters: Fitness consequences of social partner choice in female house mice. Anim Behav. 2008;75(3):801–808. [Google Scholar]
- 6.Yee JR, Cavigelli SA, Delgado B, McClintock MK. Reciprocal affiliation among adolescent rats during a mild group stressor predicts mammary tumors and lifespan. Psychosom Med. 2008;70(9):1050–1059. doi: 10.1097/PSY.0b013e31818425fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron EZ, Setsaas TH, Linklater WL. Social bonds between unrelated females increase reproductive success in feral horses. Proc Natl Acad Sci USA. 2009;106(33):13850–13853. doi: 10.1073/pnas.0900639106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frère CH, et al. Social and genetic interactions drive fitness variation in a free-living dolphin population. Proc Natl Acad Sci USA. 2010;107(46):19949–19954. doi: 10.1073/pnas.1007997107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Med. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30(4):534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Insel TR. The challenge of translation in social neuroscience: A review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65(6):768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: Context and person matter. Trends Cogn Sci. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30(4):548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Kendrick KM, Da Costa AP, Hinton MR, Keverne EB. A simple method for fostering lambs using aneustrous ewes with artificially induced lactation and maternal-behaviour. Appl Anim Behav Sci. 1992;34(4):345–357. [Google Scholar]
- 15.Bales KL, Pfeifer LA, Carter CS. Sex differences and developmental effects of manipulations of oxytocin on alloparenting and anxiety in prairie voles. Dev Psychobiol. 2004;44(2):123–131. doi: 10.1002/dev.10165. [DOI] [PubMed] [Google Scholar]
- 16.Madden JR, Clutton-Brock TH. Experimental peripheral administration of oxytocin elevates a suite of cooperative behaviours in a wild social mammal. Proc Biol Sci. 2011;278(1709):1189–1194. doi: 10.1098/rspb.2010.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odendaal JSJ, Meintjes RA. Neurophysiological correlates of affiliative behaviour between humans and dogs. Vet J. 2003;165(3):296–301. doi: 10.1016/s1090-0233(02)00237-x. [DOI] [PubMed] [Google Scholar]
- 18.Crockford C, et al. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc Biol Sci. 2013;280(1755):20122765. doi: 10.1098/rspb.2012.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wittig RM, et al. Food sharing is linked to urinary oxytocin levels and bonding in related and unrelated wild chimpanzees. Proc R Soc B. 2014;281(1778):20133096. doi: 10.1098/rspb.2013.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sih A, Bell A, Johnson JC. Behavioral syndromes: An ecological and evolutionary overview. Trends Ecol Evol. 2004;19(7):372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Topál J, Miklósi A, Csányi V, Dóka A. Attachment behavior in dogs (Canis familiaris): A new application of Ainsworth’s (1969) Strange Situation Test. J Comp Psychol. 1998;112(3):219–229. doi: 10.1037/0735-7036.112.3.219. [DOI] [PubMed] [Google Scholar]
- 22.Prato-Previde E, Custance DM, Spiezio C, Sabatini F. Is the dog-human relationship an attachment bond? An observational study using Ainsworth’s strange situation. Behaviour. 2003;140(2):225–254. [Google Scholar]
- 23.Gordon I, et al. Oxytocin and cortisol in romantically unattached young adults: Associations with bonding and psychological distress. Psychophysiology. 2008;45(3):349–352. doi: 10.1111/j.1469-8986.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 24.von Borell E, et al. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals—A review. Physiol Behav. 2007;92(3):293–316. doi: 10.1016/j.physbeh.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Silk JB, et al. Female chacma baboons form strong, equitable, and enduring social bonds. Behav Ecol Sociobiol. 2010;64(11):1733–1747. doi: 10.1007/s00265-010-0986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anne O, Rasa E. Dwarf mongoose and hornbill mutualism in the Taru desert, Kenya. Behav Ecol Sociobiol. 1983;12(3):181–190. [Google Scholar]
- 27.Proops L, McComb K. Cross-modal individual recognition in domestic horses (Equus caballus) extends to familiar humans. Proc R Soc B. 2012;279(1741):3131–3138. doi: 10.1098/rspb.2012.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagasawa M, Mogi K, Kikusui T. Attachment between humans and dogs. Jpn Psychol Res. 2009;51(3):209–221. [Google Scholar]
- 29.Romero T, Konno A, Hasegawa T. Familiarity bias and physiological responses in contagious yawning by dogs support link to empathy. PLoS ONE. 2013;8(8):e71365. doi: 10.1371/journal.pone.0071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veening JG, Olivier B. Intranasal administration of oxytocin: Behavioral and clinical effects, a review. Neurosci Biobehav Rev. 2013;37(8):1445–1465. doi: 10.1016/j.neubiorev.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang SWC, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proc Natl Acad Sci USA. 2012;109(3):959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uvnäs-Moberg K, et al. Plasma levels of oxytocin increase in response to suckling and feeding in dogs and sows. Acta Physiol Scand. 1985;124(3):391–398. doi: 10.1111/j.1748-1716.1985.tb07674.x. [DOI] [PubMed] [Google Scholar]
- 33.Jankowski M, et al. Oxytocin in cardiac ontogeny. Proc Natl Acad Sci USA. 2004;101(35):13074–13079. doi: 10.1073/pnas.0405324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11(1):1–18. doi: 10.1016/s0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 35.Born J, et al. Sniffing neuropeptides: A transnasal approach to the human brain. Nat Neurosci. 2002;5(6):514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- 36.Striepens N, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep. 2013;3:3440. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38(10):1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Knobloch HS, Grinevich V. Evolution of oxytocin pathways in the brain of vertebrates. Front Behav Neurosci. 2014;8:31. doi: 10.3389/fnbeh.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross HE, et al. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162(4):892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knobloch HS, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73(3):553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 41.Andari E, et al. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci USA. 2010;107(9):4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 43.McCall C, Singer T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat Neurosci. 2012;15(5):681–688. doi: 10.1038/nn.3084. [DOI] [PubMed] [Google Scholar]
- 44.Campbell A. Attachment, aggression and affiliation: The role of oxytocin in female social behavior. Biol Psychol. 2008;77(1):1–10. doi: 10.1016/j.biopsycho.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20(6):858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 46.Pedersen CA. Biological aspects of social bonding and the roots of human violence. Ann N Y Acad Sci. 2004;1036:106–127. doi: 10.1196/annals.1330.006. [DOI] [PubMed] [Google Scholar]
- 47.De Dreu CKW, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJJ. Oxytocin promotes human ethnocentrism. Proc Natl Acad Sci USA. 2011;108(4):1262–1266. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soares MC, et al. Hormonal mechanisms of cooperative behaviour. Philos Trans R Soc Lond B Biol Sci. 2010;365(1553):2737–2750. doi: 10.1098/rstb.2010.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feldman R. Oxytocin and social affiliation in humans. Horm Behav. 2012;61(3):380–391. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 50.Uvnäs-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23(8):819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- 51.Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology. 2010;35(8):1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 52.Schino G, Aureli F. Reciprocal altruism in primates: Partner choice, cognition, and emotions. Adv Study Behav. 2009;39:45–69. [Google Scholar]
- 53.de Waal FBM. Putting the altruism back into altruism: The evolution of empathy. Annu Rev Psychol. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- 54.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322(5903):900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 55.Higuchi T, Honda K, Fukuoka T, Negoro H, Wakabayashi K. Release of oxytocin during suckling and parturition in the rat. J Endocrinol. 1985;105(3):339–346. doi: 10.1677/joe.0.1050339. [DOI] [PubMed] [Google Scholar]
- 56.Mitsui S, et al. Urinary oxytocin as a noninvasive biomarker of positive emotion in dogs. Horm Behav. 2011;60(3):239–243. doi: 10.1016/j.yhbeh.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Jonckheer-Sheehy SM, Vinke CM, Ortolani O. Validation of a Polar human heart rate monitor for measuring heart rate and heart rate variability in adult dogs under stationary conditions. J Vet Behav. 2012;7(4):205–212. [Google Scholar]
- 58.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.