Significance
We investigate the balance between two mechanisms that remove carbon from the atmosphere and oceans over long timescales—weathering of terrestrial silicates and alteration of the ocean floor. We show that this balance should strongly influence atmospheric oxygen concentration, since it dictates the delivery rate of the ultimate limiting nutrient phosphorus to the ocean. Increasing solar luminosity and declining seafloor spreading rates over Proterozoic time are expected to have shifted the balance of carbon removal toward terrestrial weathering. This leads to the prediction of a gradually increasing oxygen concentration over Proterozoic time, with transient higher oxygen concentration in the late Neoproterozoic.
Keywords: carbon cycle, biogeochemistry, Precambrian
Abstract
A shift toward higher atmospheric oxygen concentration during the late Proterozoic has been inferred from multiple indirect proxies and is seen by many as a prerequisite for the emergence of complex animal life. However, the mechanisms controlling the level of oxygen throughout the Proterozoic and its eventual rise remain uncertain. Here we use a simple biogeochemical model to show that the balance between long-term carbon removal fluxes via terrestrial silicate weathering and ocean crust alteration plays a key role in determining atmospheric oxygen concentration. This balance may be shifted by changes in terrestrial weatherability or in the generation rate of oceanic crust. As a result, the terrestrial chemical weathering flux may be permanently altered—contrasting with the conventional view that the global silicate weathering flux must adjust to equal the volcanic CO2 degassing flux. Changes in chemical weathering flux in turn alter the long-term supply of phosphorus to the ocean, and therefore the flux of organic carbon burial, which is the long-term source of atmospheric oxygen. Hence we propose that increasing solar luminosity and a decrease in seafloor spreading rate over 1,500–500 Ma drove a gradual shift from seafloor weathering to terrestrial weathering, and a corresponding steady rise in atmospheric oxygen. Furthermore, increased terrestrial weatherability during the late Neoproterozoic may explain low temperature, increases in ocean phosphate, ocean sulfate, and atmospheric oxygen concentration at this time.
Increases in ocean oxygen concentration during the Neoproterozoic Era are supported by evidence from iron speciation (1) and enrichments in molybdenum concentration (2). The atmospheric concentration of oxygen is also likely to have increased over this time, resulting in higher ocean sulfate concentration (3) and increased isotopic fractionation of chromium (4) and sedimentary sulphides (5). Oxygenation of the deep oceans at ∼580 Ma is well defined by redox proxies (1, 6); however, the mechanisms controlling this change are uncertain. It is not clear if this required a rise in atmospheric oxygen (7) and, if so, whether this was relatively rapid or the result of a steady rise over the Proterozoic.
The major long-term source of atmospheric oxygen is the burial of organic carbon in sediments, and a drift toward more positive δ13C values over the period 1,500–800 Ma (8) suggests a gradual rise in organic carbon burial and atmospheric O2 (9). Extreme positive fractionation in δ13C observed in parts of the late Neoproterozoic record may indicate periods of elevated organic carbon burial, although the signal is at least partially a consequence of the aftermath of global glaciations occurring at this time (10, 11).
Long-term control of ocean productivity, and ultimately of organic carbon burial rates, is linked to the concentration of the ultimate limiting nutrient phosphorus, which is supplied via chemical weathering on the continents. However, changes in chemical weathering fluxes are constrained in several existing biogeochemical models (12, 13), due to a simplified view of the carbon cycle wherein the rate of CO2 removal via silicate weathering and carbonate deposition (plus any excess of organic carbon burial over oxidative weathering) must equal the rate of volcanic CO2 degassing over long timescales (14). This equality is maintained by negative feedback between CO2 concentration, global temperature, and silicate weathering rates (15).
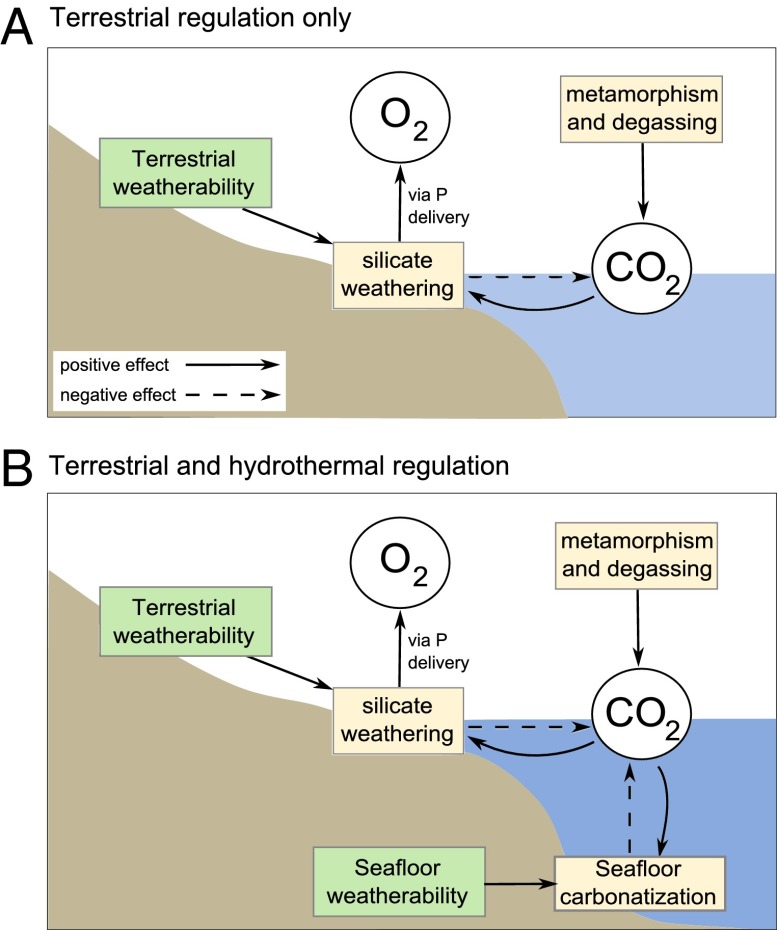
Assuming this model is correct, an increase in terrestrial “weatherability”—a dimensionless parameter describing the ease with which the terrestrial surface is weathered (16)—will cause an initial decrease in CO2 concentration due to an increased silicate weathering flux. Over time, however, lower CO2 and associated lower global temperatures and humidity will act to reduce chemical weathering rates until a balance is struck and the flux of silicate weathering returns to the amount required to balance CO2 input from degassing (Fig. 1A). Thus, at steady state, a change in terrestrial weatherability (which may be caused by a more active land biota or exposure of more easily weathered silicate rocks at the surface) cannot alter the weathering flux but results in lower atmospheric CO2 and a colder, drier global environment. This has important implications for atmospheric oxygen levels: Under the assumption that long-term phosphorus input to the ocean scales with global weathering rates, the marine burial flux of organic matter and therefore the atmospheric oxygen concentration at steady state should not be significantly altered by a change in weatherability (7, 17).
Fig. 1.
Simplified long-term CO2 feedbacks. Boxes show processes; ovals show hydrospheric constituents. Solid arrows show positive effect; dashed arrows show negative effect. (A) If terrestrial weathering is the only CO2 regulator, the weathering flux must have the same steady state value regardless of weatherability. Increases in silicate weathering are nullified by pCO2 falling. (B) Adding independent deep sea regulation allows for stable CO2 under an altered silicate weathering flux, with stability maintained by a concurrent change in seafloor carbonatization rate. The balance dictates O2 production, and may be shifted by enhancement of either process.
Here we investigate the effects of accounting for additional climate regulation via seafloor weathering on phosphorus input to the ocean and hence atmospheric oxygen. Carbonate precipitation in low-temperature, off-axis hydrothermal systems accounts for a carbon flux into the ocean crust of ∼0.5–3 × 1012 mol/y (18–20), making it important for the long-term carbon cycle (21, 22). The degree to which the flux is affected by ocean and atmosphere carbon concentration is highly debated, and a direct link via pH has been ruled out (23). However, recent modeling of the differences between Mesozoic and Cenozoic drill cores (24) supports a dependency of the rate of seafloor weathering on crustal K-feldspar uptake, and on bottom water temperature (25).
Both of these mechanisms constitute an indirect negative feedback on CO2 concentration. Indeed, Coogan and Gillis (24) conclude that during the late Mesozoic, the strength of the seafloor weathering–CO2 feedback may have been of a similar magnitude to the feedback between CO2 and terrestrial silicate weathering. In the present-day system, the rate of CO2 removal via seafloor weathering is significantly smaller than the removal flux from terrestrial weathering [around 1/4 of the magnitude (26)]. However, the dependence of seafloor weathering on the mantle heat flux and ridge generation rate suggests a much more significant role in the Precambrian carbon balance (27), such that it was the primary sink for CO2 in the Archean (28).
While all basalts contain phosphorus in fluorapatite, the localized alkalinity generation and carbonate precipitation during seafloor weathering does not generate a phosphorus source to seawater (29). Hydrothermal basalt alteration is a weak sink for phosphate in the present-day system (30) due to combination with ferric iron or carbonates (31). In contrast, phosphorus is released to solution during acidic terrestrial chemical weathering and can pass through the land and ocean ecosystems before reaching the seafloor. As a conservative estimate, and taking into account the weakening of phosphorus sinks in an anoxic and possibly high-alkalinity Precambrian ocean (32), we assume here there is no P flux associated with seafloor weathering.
Under the operation of the two independent, climate-dependent sinks for CO2 (Fig. 1B), the link between degassing rates and silicate weathering rates is weakened substantially. CO2 degassing must now be balanced by a combination of terrestrial silicate weathering and seafloor weathering, plus any imbalance in the organic carbon cycle. In this system, a decrease in CO2 concentration due to increasing terrestrial weatherability would reduce both the terrestrial and deep ocean carbon sinks until the total carbon removal flux again equaled the degassing input. In the new stable state, the seafloor weathering flux is reduced below its initial value, and the terrestrial silicate weathering flux is increased permanently relative to the previous stable state. It is probable therefore that an increase in terrestrial weatherability would lead to a permanent increase in terrestrial chemical weathering fluxes, and therefore phosphorus supply to the ocean, while maintaining stability of the long-term carbon cycle. An increase in the weatherability of the ocean floor will similarly shift the balance in favor of seafloor weathering, reducing the terrestrial silicate weathering flux and phosphorus supply.
To quantitatively test the biogeochemical implications of changes in the CO2 removal balance, a dynamical representation of the seafloor weathering process is added to a simplified version of the carbon oxygen phosphorus sulphur evolution (COPSE) Earth system model (13). COPSE combines the long-term carbon cycle from the GEOCARB (geochemical carbon cycle) model (33) with a model of ocean nutrient and atmospheric oxygen cycling (34, 35), to estimate paleoconcentrations of carbon dioxide, oxygen, and ocean sulfate (see SI Text for full model description). In the model, imbalances in the organic carbon cycle are self-limiting due to negative feedback that maintains stable oxygen levels over long timescales (i.e., dependency of oxidative weathering rates on the crustal inventory of organic carbon, and on atmospheric oxygen concentration). Thus, in our model, the organic carbon cycle may be driven by the inorganic cycle via weathering rates and nutrient input.
Following Sleep and Zahnle (27), seafloor weathering rate is linked to the global spreading rate, and the dependence on relative atmospheric CO2 concentration (RCO2) is denoted by a power law relationship: . The limited laboratory experiments of Brady and Gislason (25) point to a dependency of α = 0.23, which is the only available data on the magnitude of this important parameter and is used as the model baseline. This is a very weak feedback; for example, rewriting the silicate weathering rate approximation used in the GEOCARB models (36) in this form gives a dependency of . Evidence for a strong seafloor weathering feedback in the Mesozoic (24) suggests that α = 0.23 may be a low estimate.
Results and Discussion
We first investigate the model response to changes in external forcing factors—terrestrial weatherability (W, Fig. 2), seafloor spreading rate (Fig. 3), and the incoming solar flux (Fig. 4). We then use reconstructions of these forcings over 1,500–500 Ma to produce predictions for Proterozoic climate (Fig. 5). Figs. 2–4 show model steady states with other parameters fixed at values for 1,000 Ma and the initial size of the crustal inventories of buried carbon chosen based on an existing model (44).
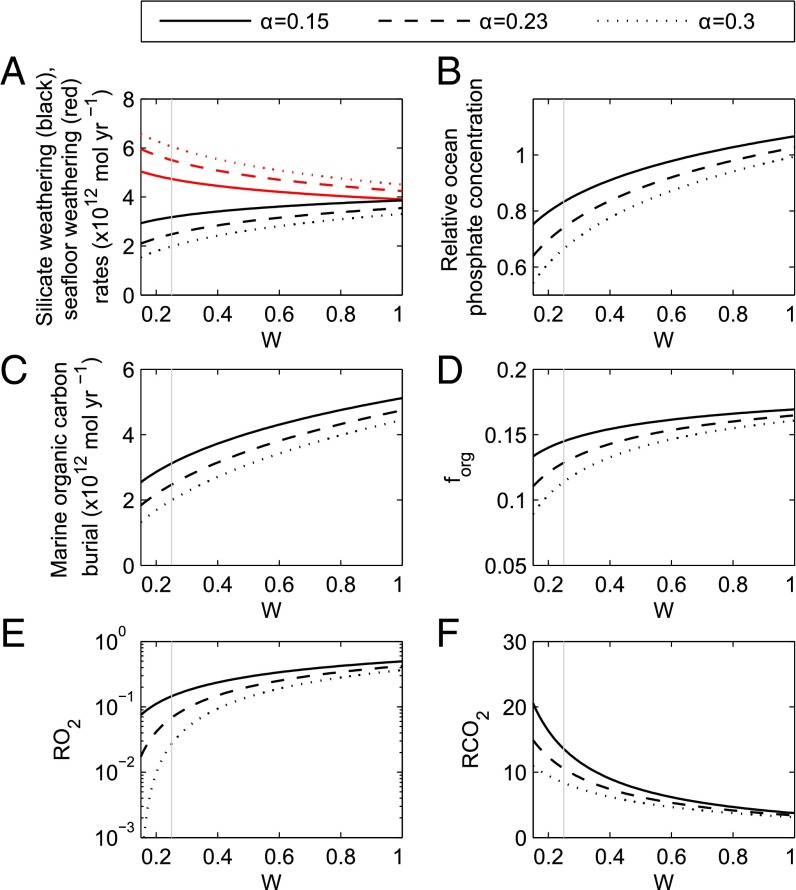
Fig. 2.
Model steady states at 1,000 Ma as a function of terrestrial weatherability, W. Line type shows choices for the dependency of seafloor weathering rate on atmospheric CO2 concentration. Rates of silicate and seafloor weathering (A), relative ocean phosphate concentration (B), organic carbon burial rate (C), the fraction of carbon buried organically (D), relative atmospheric oxygen (E), and CO2 concentrations (F). Weatherability is defined as the global weathering flux that would be expected under present-day conditions, relative (i.e., normalized) to the present-day flux. Gray vertical line denotes best guess for W at 1,000 Ma. We assume a relative spreading rate and solar forcing of 1.83 (37) and 0.923 (38), respectively.
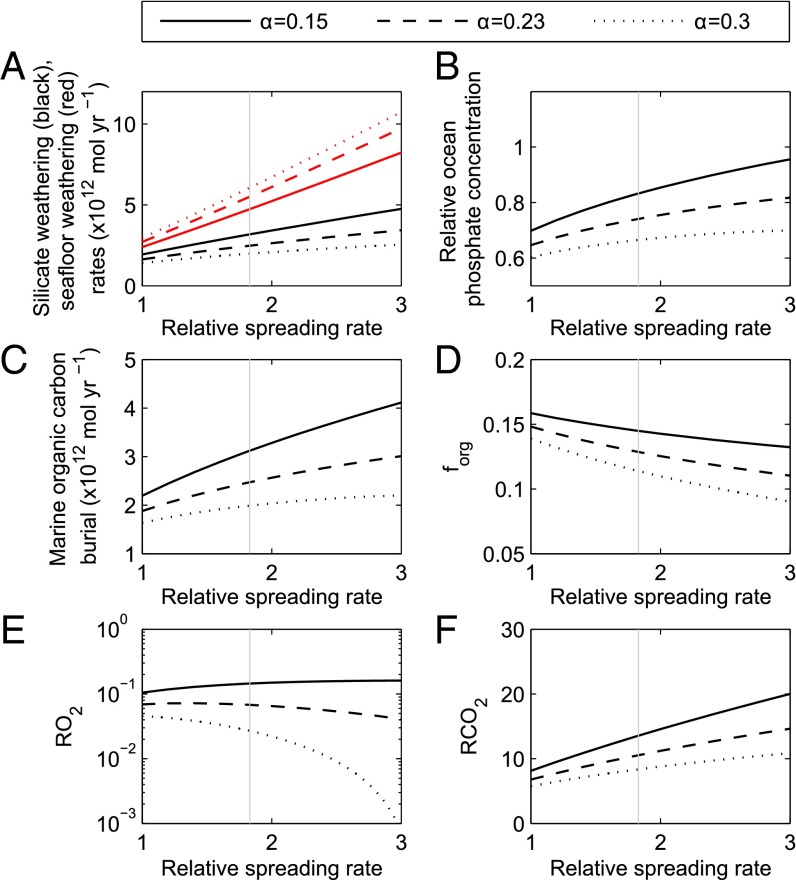
Fig. 3.
Model steady states at 1,000 Ma as a function of relative seafloor spreading rate. Line type shows choices for the dependency of seafloor weathering rate on atmospheric CO2 concentration. Rates of silicate and seafloor weathering (A), relative ocean phosphate concentration (B), organic carbon burial rate (C), the fraction of carbon buried organically (D), relative atmospheric oxygen (E), and CO2 concentrations (F). Gray vertical line denotes assumed spreading rate at 1,000 Ma (37). We assume a terrestrial weatherability and solar forcing of 1/4 (12) and 0.923 (38), respectively.
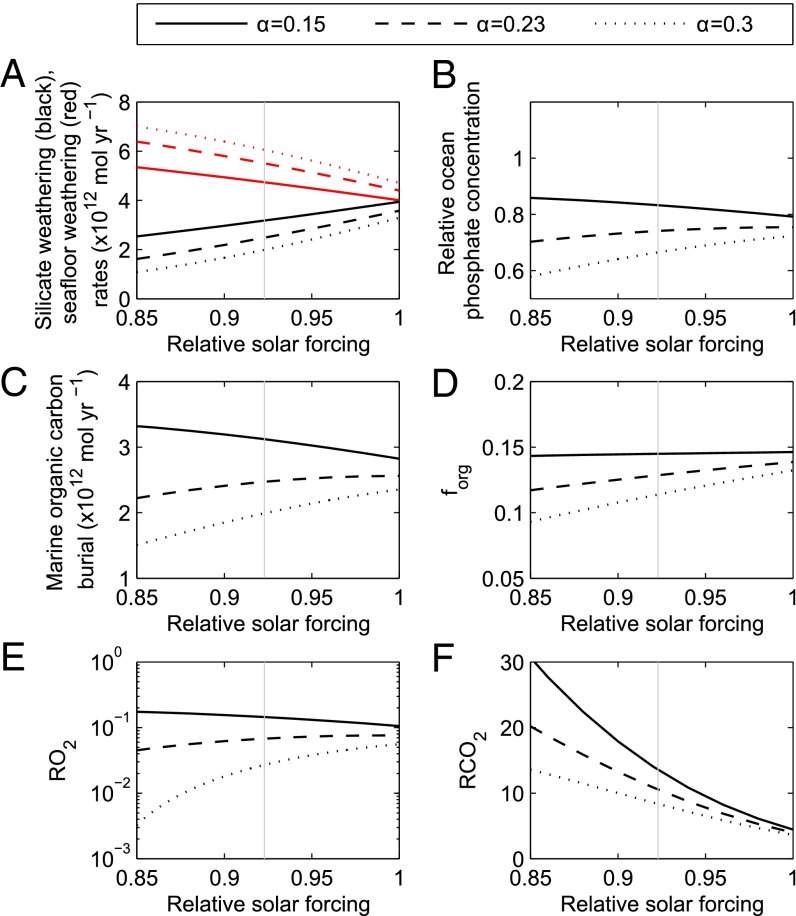
Fig. 4.
Model steady states at 1,000 Ma as a function of relative solar flux. Line type shows choices for the dependency of seafloor weathering rate on atmospheric CO2 concentration. Rates of silicate and seafloor weathering (A), relative ocean phosphate concentration (B), organic carbon burial rate (C), the fraction of carbon buried organically (D), relative atmospheric oxygen (E), and CO2 concentrations (F). Gray vertical line denotes assumed solar flux at 1,000 Ma (38). We assume a relative terrestrial weatherability and spreading rate of 1/4 (12) and 1.83 (37), respectively.
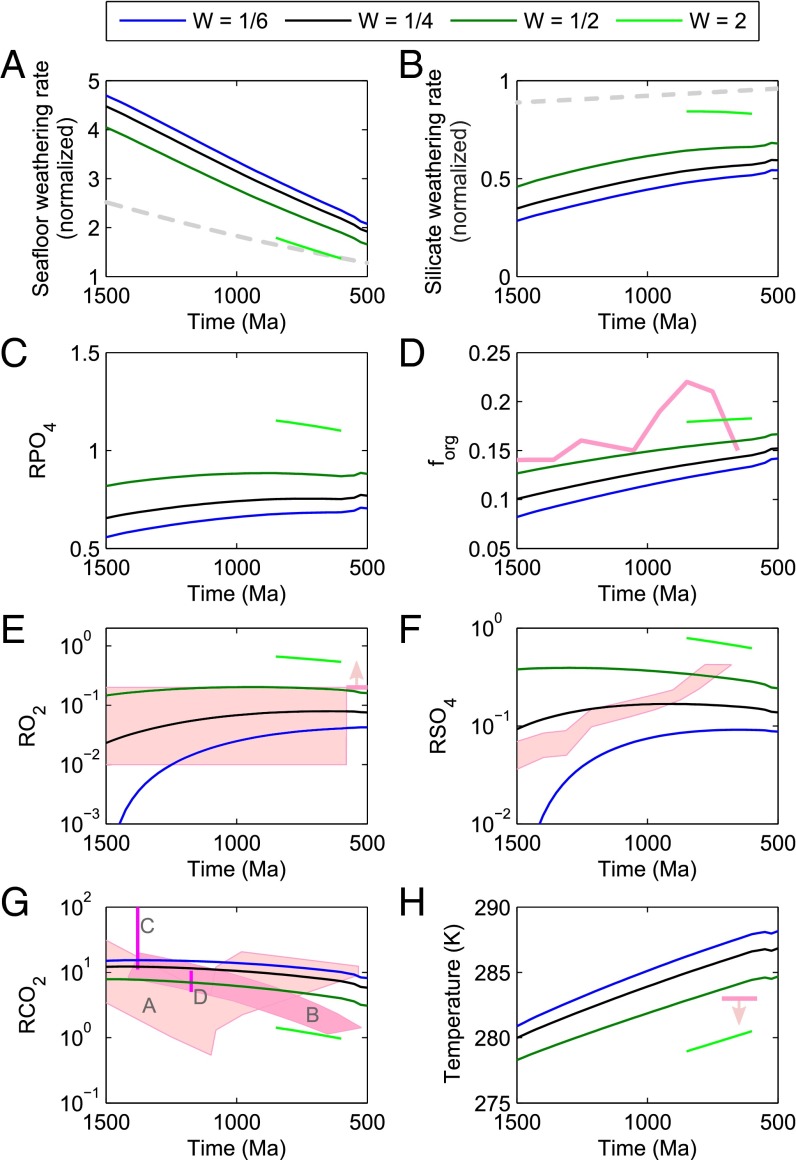
Fig. 5.
Model steady states at discrete time points for 1,500–500 Ma for α = 0.23. Line color denotes relative weatherability of the continents, W. Relative spreading rate (37) shown as dashed gray line in A; relative solar forcing (38) shown as dashed gray line in B. Geological constraints are shown in pink. Plotted are normalized rates of seafloor (A) and silicate (B) weathering; relative ocean phosphate concentration (C); the fraction of carbon buried organically (D); relative atmospheric oxygen concentration (E) against the constraints from refs. 6 and 39; relative ocean sulfate concentration (F) against ref. 3; relative CO2 concentration (G) against data from ref. 9 (A), ref. 40, (B), ref. 41 (C), and ref. 42 (D); and global average surface temperature (H) shown with expected low temperature during the Neoproterozoic glacial period (43).
The results confirm that a change in terrestrial weatherability (Fig. 2) can translate to a permanent change in the terrestrial chemical weathering fluxes by altering the balance of CO2 sinks, and that oxygen concentration at steady state is extremely sensitive to this balance. This holds for all values of the seafloor weathering feedback strength, providing some feedback exists (α > 0). Allowing changes in terrestrial weatherability (W) to affect oxidative weathering of organic matter (an O2 sink) in addition to silicate and carbonate weathering weakens its effect on atmospheric O2, but does not change its sign unless α is very small (see Fig. S1).
Changes in seafloor spreading rate (Fig. 3) have a more complex effect on oxygen concentration. Increasing the spreading rate shifts the carbon removal balance toward seafloor weathering as would be expected; however, it also increases the rate of CO2 release from subduction zones, which results in higher rates of CO2 removal via both pathways (due to a larger CO2 source). So, while an increase in spreading rate always drives a decrease in the fraction of carbon that is buried as organic matter (forg), it only results in a significant oxygen decrease if the feedback strength is at least the value predicted by Brady and Gislason (25) (α ≥ 0.23). In COPSE, the rate of CO2 degassing also depends on the carbon content of the crust, which has increased over time (44). Thus, considering steady states at 500 Ma shows a weaker link between spreading rate and oxygen concentration (Fig. S2); however, oxygen still decreases under an increase in spreading rate for α ≥ 0.23.
Altering the solar flux (S, Fig. 4) has a similar effect to changing the terrestrial weatherability, because terrestrial weathering rates are highly dependent on surface temperature. The difference is that while the weatherability parameter, W, is assumed to enhance silicate and carbonate weathering equally, the solar flux has a stronger effect on silicate weathering due to its assumed stronger temperature dependence (36). Carbonate weathering and deposition has no net effect on the carbon cycle but does release phosphate. This means that for low values of α, increasing the solar flux may result in a decreased rate of carbonate weathering at steady state (due to large decreases in CO2 concentration), and a lower phosphate and hence O2 concentration. For α ≥ 0.23, an increase in the solar flux produces a significant increase in steady state oxygen concentration. Some direct effect of surface temperature change on seafloor weathering rates is also expected, via deep water temperature, which is not considered in COPSE. However, the temperature dependence of terrestrial weathering fluxes is much stronger (25).
Model burial fluxes for phosphate have been modified to take into account the high concentration of dissolved silica in the Precambrian, before the evolution of radiolarians and diatoms (45). In the baseline model used, the flux of iron-sorbed phosphorus burial is removed from COPSE following the assumption of extreme limitation under high silica (32). The model has also been run (see Figs. S3−S6) with further limitation of calcium-associated phosphorus burial, which may have occurred due to high alkalinity during the Precambrian (32). This results in higher concentrations of phosphate and oxygen for all scenarios. However, with a feedback strength of α ≥ 0.23, oxygen still decreases under an enhanced spreading rate and increases under an enhanced solar flux.
Because of the simplicity of our model, the results should be viewed as “pseudo”-steady states. The model does not include transfer between the mantle and the surface Earth system, or escape of hydrogen to space, and therefore does not represent a complete model of the oxygen cycle. However, the processes that we are interested in are not affected by these simplifications. Values for α outside of the range we have considered affect the model in a predictable way, shifting O2 predictions further from the baseline (α = 0.23) results.
Fig. 5 shows model steady states at discrete time points between 1,500 Ma and 500 Ma for the case α = 0.23. Results are plotted, where possible, against geological data. The choices for weatherability, W, reflect the uncertainty in the value of this parameter before the evolution of vascular plants in the Phanerozoic (46); the best guess of W = 1/4 (12) is shown in black. Declining spreading rate over the Proterozoic [taken from the model of Lowell and Keller (37) and shown as a gray dashed line in Fig. 5A] alongside an increasing solar flux [following Caldeira and Kasting (38) and shown as a gray dashed line in Fig. 5B] leads to a gradual weakening of the seafloor weathering carbon sink and a shift in the carbon removal balance toward terrestrial weathering. The result is a gradual increase over time in phosphorus delivery to the ocean and organic carbon burial, causing an increase in atmospheric oxygen over 1,500–500 Ma.
It is not absolutely certain that the global spreading rate has declined over time. One recent model (47) predicts a gradually increasing mantle heat flow and spreading rate over the Proterozoic. When incorporated into our model (Fig. S7), this significantly reduces the rate of oxygen increase. However, it is insufficient to reverse the O2 rise, which is caused in this case by the increasing solar forcing.
High positive carbonate δ13C observed across the Neoproterozoic and inferred high ocean phosphate, as well as low temperature associated with global glaciation, may be linked to a period of tectonically enhanced weatherability due to supercontinent breakup, unusually equatorial continental configuration, and the outpouring of large igneous provinces (48, 49). In our model, this would translate into a large increase in weatherability, perhaps to levels higher than the present day. The light green line in Fig. 5 shows W = 2 for the period 850–600 Ma. Note that O2 and phosphorus increases in the model significantly predate current estimates for deep ocean oxygenation occurring around 635–580 Ma (50), although there is some evidence for rising O2 throughout the breakup of Rodinia (1, 4).
Geologically forced increases in weatherability in the Neoproterozic were likely transient, as the supercontinent Pangaea began to reform early in the Phanerozoic. This would be expected to cause oxygen concentration to drop in the Paleozoic after the Neoproterozoic rise. Alternatively, if an expansion of land-based photosynthetic organisms occurred over the Proterozoic (51, 52), their enhancement of the terrestrial weathering process would be expected to cause a permanent shift toward higher weatherability and atmospheric O2.
Our model results for forg (the fraction of carbon buried as organic matter) are consistently lower than the isotope-derived model it is compared with (8) (Fig. 5D). However, our model predictions for this fraction are largely dependent on the assumed present-day inorganic carbon fluxes in COPSE and the reduction in organic carbon burial due to the absence of land plants, which are both uncertain. Assuming extreme limitations on phosphate burial (32) (Fig. S6), our predicted forg fits the isotope model (8) more closely. However, the present work is primarily concerned with the trends in this parameter rather than the absolute value. An alternative isotope model incorporates seafloor weathering as a carbon removal pathway (28) and produces similar forg predictions to the current work. This requires that seafloor weathering imparts a negative carbon isotope fractionation, which has been challenged by recent data (53).
Modeled temperature for >1,000 Ma is much lower than the absence of glaciation in the geologic record would suggest. Our model is, however, consistent with the evidence for modest CO2 concentrations at this time (9, 42). A possible solution is that methane—a greenhouse gas not included in our model—was at a higher concentration under low Proterozoic oxygen concentrations (54), and would have boosted global temperature. We would expect higher surface temperature to shift the carbon removal balance toward silicate weathering, thus increasing oxygen concentration. However, high methane concentration would also increase the oxygen sink due to photochemical reaction between O2 and CH4 (55).
The results here show that enhancements of the continental weathering process may result in permanent changes in phosphorus supply to the ocean, which in turn influences organic carbon burial and ultimately atmospheric oxygen concentration. Hence we propose that planetary oxygenation during the Proterozoic may have depended on speeding up the terrestrial side of the carbon cycle, shifting the balance away from deep sea carbon removal.
Our model prediction of rising O2 over the Proterozoic requires the dependence of seafloor weathering rate on CO2 concentration to be at least as strong as the results of Brady and Gislason (25) suggest (i.e., α ≥ 0.23). The conclusions of Coogan and Gillis (24) point toward a stronger relationship, but, assuming a weak relationship of α ≤ 0.15, the O2 response to changes in spreading rate and solar forcing is reversed (Figs. 3 and 4) and our model would predict a gradual fall in O2 over the Proterozoic. The argument presented here is largely theoretical: Further observational work to establish the changing rate of carbon uptake by the oceanic crust, and its power as a feedback mechanism, will clearly be important in determining the history of atmospheric oxygen.
Methods
Transfer of carbon from the hydrosphere to the crust via seafloor weathering follows Sleep and Zahnle (27) and the formulation of other fluxes in COPSE:
| [1] |
where is the relative spreading rate, RCO2 denotes the relative concentration of CO2 in the atmosphere, and is the assumed present-day rate, taken between current estimates (18–20). We follow ref. 27 in letting α expresses the dependency of hydrothermal carbonatization on ocean CO2. The seafloor weathering flux is added to COPSE as a transfer of carbon from the combined ocean/atmosphere reservoir to the buried carbonate reservoir. Other minor alterations are made to the COPSE model to make it more applicable to the Precambrian and to improve robustness to temperature and oxygen concentration outside the Phanerozoic window. See SI Text for list of modifications and full model equations.
Supplementary Material
Acknowledgments
We thank the anonymous reviewers of this work for their constructive input. B.M. thanks the University of East Anglia for a Dean’s studentship. A.J.W.’s contribution was supported by a Royal Society Research Professorship and T.M.L.’s contribution by a Royal Society Wolfson Research Merit Award. B.M. is supported by the UK Natural Environment Research Council (NERC) project (NE/G018332/2) “Ocean circulation, nutrient cycling and atmospheric carbon dioxide.” A.J.W. and T.M.L. are supported by the NERC project (NE/I005978/1) “Reinventing the planet: The Neoproterozoic revolution in oxygenation, biogeochemistry and biological complexity.” T.M.L. is also supported by the Leverhulme Trust (RPG-2013-106).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321679111/-/DCSupplemental.
References
- 1.Canfield DE, et al. Ferruginous conditions dominated later neoproterozoic deep-water chemistry. Science. 2008;321(5891):949–952. doi: 10.1126/science.1154499. [DOI] [PubMed] [Google Scholar]
- 2.Scott C, et al. Tracing the stepwise oxygenation of the Proterozoic ocean. Nature. 2008;452(7186):456–459. doi: 10.1038/nature06811. [DOI] [PubMed] [Google Scholar]
- 3.Kah LC, Lyons TW, Frank TD. Low marine sulphate and protracted oxygenation of the Proterozoic biosphere. Nature. 2004;431(7010):834–838. doi: 10.1038/nature02974. [DOI] [PubMed] [Google Scholar]
- 4.Frei R, Gaucher C, Poulton SW, Canfield DE. Fluctuations in Precambrian atmospheric oxygenation recorded by chromium isotopes. Nature. 2009;461(7261):250–253. doi: 10.1038/nature08266. [DOI] [PubMed] [Google Scholar]
- 5.Canfield DE, Teske A. Late Proterozoic rise in atmospheric oxygen concentration inferred from phylogenetic and sulphur-isotope studies. Nature. 1996;382(6587):127–132. doi: 10.1038/382127a0. [DOI] [PubMed] [Google Scholar]
- 6.Canfield DE, Poulton SW, Narbonne GM. Late-Neoproterozoic deep-ocean oxygenation and the rise of animal life. Science. 2007;315(5808):92–95. doi: 10.1126/science.1135013. [DOI] [PubMed] [Google Scholar]
- 7.Lenton TM, Boyle RA, Poulton SW, Shields-Zhou GA, Butterfield NJ. Co-evolution of eukaryotes and ocean oxygenation in the Neoproterozoic era. Nat Geosci. 2014;7:257–265. [Google Scholar]
- 8.Des Marais DJ, Strauss H, Summons RE, Hayes JM. Carbon isotope evidence for the stepwise oxidation of the Proterozoic environment. Nature. 1992;359(6396):605–609. doi: 10.1038/359605a0. [DOI] [PubMed] [Google Scholar]
- 9.Sheldon ND. Causes and consequences of low atmospheric pCO2 in the Late Mesoproterozoic. Chem Geol. 2013;362:224–231. [Google Scholar]
- 10.Hoffman PF, Kaufman AJ, Halverson GP, Schrag DP. A neoproterozoic snowball Earth. Science. 1998;281(5381):1342–1346. doi: 10.1126/science.281.5381.1342. [DOI] [PubMed] [Google Scholar]
- 11.Mills B, Watson AJ, Goldblatt C, Boyle R, Lenton TM. Timing of Neoproterozoic glaciations linked to transport-limited global weathering. Nat Geosci. 2011;4:861–864. [Google Scholar]
- 12.Berner RA. GEOCARBSULF: A combined model for Phanerozoic atmospheric O2 and CO2. Geochim Cosmochim Acta. 2006;70:5653–5664. [Google Scholar]
- 13.Bergman NM, Lenton TM, Watson AJ. COPSE: A new model of biogeochemical cycling over Phanerozoic time. Am J Sci. 2004;304(5):397–437. [Google Scholar]
- 14.Berner RA, Lasaga AC, Garrels RM. The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years. Am J Sci. 1983;283(7):641–683. doi: 10.2475/ajs.284.10.1175. [DOI] [PubMed] [Google Scholar]
- 15.Walker JCG, Hays PB, Kasting JF. A negative feedback mechanism for the long-term stabilization of Earth's surface temperature. J Geophys Res. 1981;86(C10):9776–9782. [Google Scholar]
- 16.Kump LR, Arthur MA. In: Tectonic Uplift and Climate Change. Ruddiman WF, editor. New York: Springer; 1997. pp. 399–426. [Google Scholar]
- 17.Schrag DP, Berner RA, Hoffman PF, Halverson GP. On the initiation of a snowball Earth. Geochem Geophys Geosyst. 2002;3(6) doi: 10.1029/2001GC000219. [DOI] [Google Scholar]
- 18.Gillis KM, Coogan LA. Secular variation in carbon uptake into the ocean crust. Earth Planet Sci Lett. 2011;302(3-4):385–392. [Google Scholar]
- 19.Alt JC, Teagle DAH. The uptake of carbon during alteration of oceanic crust. Geochim Cosmochim Acta. 1999;63:1527–1535. [Google Scholar]
- 20.Staudigel H, Hart SR, Schmincke H-U, Smith BM. Cretaceous ocean crust at DSDP sites 417 and 418: Carbon uptake from weathering verses loss by magmatic outgassing. Geochim Cosmochim Acta. 1989;53:3091–3094. [Google Scholar]
- 21.François LM, Walker JCG. Modelling the Phanerozoic carbon cycle and climate: constraints from the 87Sr/86Sr isotopic ratio of seawater. Am J Sci. 1992;292(2):81–135. doi: 10.2475/ajs.292.2.81. [DOI] [PubMed] [Google Scholar]
- 22.Walker JCG. Precambrian evolution of the climate system. Global Planet Change. 1990;82(3-4):261–289. [PubMed] [Google Scholar]
- 23.Caldeira K. Long-term control of atmospheric carbon dioxide: Low temperature seafloor alteration or terrestrial silicate rock weathering. Am J Sci. 1995;295(9):1077–1114. [Google Scholar]
- 24.Coogan LA, Gillis KM. Evidence that low-temperature oceanic hydrothermal systems play an important role in the silicate-carbonate weathering cycle and long-term climate regulation. Geochem Geophys Geosyst. 2013;14(6):1771–1786. [Google Scholar]
- 25.Brady PV, Gislason SR. Seafloor weathering controls on atmospheric CO2 and global climate. Geochim Cosmochim Acta. 1997;61(5):965–973. [Google Scholar]
- 26.Schultz A, Elderfield H. Controls on the physics and chemistry of seafloor hydrothermal circulation. Philos Trans R Soc Lond A Math Phys Eng Sci. 1997;355(1723):387–425. [Google Scholar]
- 27.Sleep NH, Zahnle K. Carbon dioxide cycling and implications for climate on ancient Earth. J Geophys Res. 2001;106(E1):1373–1399. [Google Scholar]
- 28.Bjerrum CJ, Canfield DE. New insights into the burial history of organic carbon on the early Earth. Geochem Geophys Geosyst. 2004;5(8):Q08001. doi: 10.1029/2004GC000713. [DOI] [Google Scholar]
- 29.Baturin GN. Phosphorus cycle in the ocean. Lithol Miner Resour. 2003;38(2):101–119. [Google Scholar]
- 30.Paytan A, McLaughlin K. The oceanic phosphorus cycle. Chem Rev. 2007;107(2):563–576. doi: 10.1021/cr0503613. [DOI] [PubMed] [Google Scholar]
- 31.Wheat CG, Feely RA, Mottl MJ. Phosphate removal by oceanic hydrothermal processes: An update of the phosphorus budget in the oceans. Geochim Cosmochim Acta. 1996;60(19):3593–3608. [Google Scholar]
- 32.Planavsky NJ, et al. The evolution of the marine phosphate reservoir. Nature. 2010;467(7319):1088–1090. doi: 10.1038/nature09485. [DOI] [PubMed] [Google Scholar]
- 33.Berner RA. A model for atmospheric CO2 over Phanerozoic time. Am J Sci. 1991;291(4):339–376. doi: 10.2475/ajs.289.4.333. [DOI] [PubMed] [Google Scholar]
- 34.Lenton TM, Watson AJ. Redfield revisited: II. What regulates the oxygen content of the atmosphere? Global Biogeochem Cycles. 2000;14(1):249–268. [Google Scholar]
- 35.Van Cappellen P, Ingall ED. Redox stabilization of the atmosphere and oceans by phosphorus-limited marine productivity. Science. 1996;271(5248):493–496. doi: 10.1126/science.271.5248.493. [DOI] [PubMed] [Google Scholar]
- 36.Berner RA. GEOCARB II: A revised model of atmospheric CO2 over Phanerozoic time. Am J Sci. 1994;294(1):56–91. [Google Scholar]
- 37.Lowell RP, Keller SM. High-temperature seafloor hydrothermal circulation over geologic time and Archean banded iron formations. Geophys Res Lett. 2003;30(7):1391. [Google Scholar]
- 38.Caldeira K, Kasting JF. The life span of the biosphere revisited. Nature. 1992;360(6406):721–723. doi: 10.1038/360721a0. [DOI] [PubMed] [Google Scholar]
- 39.Kump LR. The rise of atmospheric oxygen. Nature. 2008;451(7176):277–278. doi: 10.1038/nature06587. [DOI] [PubMed] [Google Scholar]
- 40.Kah LC, Bartley JK. Effect of marine carbon reservoir size on the duration of carbon isotope excursions: Interpreting the Mesoproterozoic carbon isotope record. Geol Soc Am Abstr Programs. 2004;36(5):78. [Google Scholar]
- 41.Kaufman AJ, Xiao S. High CO2 levels in the Proterozoic atmosphere estimated from analyses of individual microfossils. Nature. 2003;425(6955):279–282. doi: 10.1038/nature01902. [DOI] [PubMed] [Google Scholar]
- 42.Kah LC, Riding R. Mesoproterozoic carbon dioxide levels inferred from calcified cyanobacteria. Geology. 2007;35(9):799–802. [Google Scholar]
- 43.Hoffman PF, Schrag DP. The snowball Earth hypothesis: Testing the limits of global change. Terra Nova. 2002;14(3):129–155. [Google Scholar]
- 44.Hayes JM, Waldbauer JR. The carbon cycle and associated redox processes through time. Philos Trans R Soc Lond B Biol Sci. 2006;361(1470):931–950. doi: 10.1098/rstb.2006.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Racki G, Cordey F. Radiolarian palaeoecology and radiolarites: Is the present the key to the past? Earth Sci Rev. 2000;52(1-3):83–120. [Google Scholar]
- 46.Berner RA. The rise of plants and their effect on weathering and atmospheric CO2. Science. 1997;276(5312):544–546. [Google Scholar]
- 47.Korenaga J. Plate tectonics, flood basalts and the evolution of Earth’s oceans. Terra Nova. 2008;20(6):419–439. [Google Scholar]
- 48.Li ZX, et al. Assembly, configuration, and break-up history of Rodinia: A synthesis. Precambrian Res. 2008;160(1-2):179–210. [Google Scholar]
- 49.Donnadieu Y, Goddéris Y, Ramstein G, Nédélec A, Meert J. A ‘snowball Earth’ climate triggered by continental break-up through changes in runoff. Nature. 2004;428(6980):303–306. doi: 10.1038/nature02408. [DOI] [PubMed] [Google Scholar]
- 50.Sahoo SK, et al. Ocean oxygenation in the wake of the Marinoan glaciation. Nature. 2012;489(7417):546–549. doi: 10.1038/nature11445. [DOI] [PubMed] [Google Scholar]
- 51.Lenton TM, Watson AJ. Biotic enhancement of weathering, atmospheric oxygen and carbon dioxide in the Neoproterozoic. Geophys Res Lett. 2004;31(5):L05202. [Google Scholar]
- 52.Kennedy M, Droser M, Mayer LM, Pevear D, Mrofka D. Late Precambrian oxygenation; inception of the clay mineral factory. Science. 2006;311(5766):1446–1449. doi: 10.1126/science.1118929. [DOI] [PubMed] [Google Scholar]
- 53.Shilobreeva S, Martinez I, Busigny V, Agrinier P, Laverne C. Insights into C and H storage in the altered oceanic crust: Results from ODP/IODP Hole 1256D. Geochim Cosmochim Acta. 2011;75(9):2237–2255. [Google Scholar]
- 54.Pavlov AA, Hurtgen MT, Kasting JF, Arthur MA. Methane-rich Proterozoic atmosphere? Geology. 2003;31(1):87–90. [Google Scholar]
- 55.Claire MW, Catling DC, Zahnle KJ. Biogeochemical modelling of the rise in atmospheric oxygen. Geobiology. 2006;4(4):239–269. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.