Significance
Memory T cells are essential components of immunological memory. In the apparent absence of antigen, numbers of recirculating antigen-specific memory T cells dwindle, provoking the question of whether there is immunological memory without memory T cells. Here we show that human memory T cells can reside in the bone marrow as resting cells in terms of proliferation, transcription, and mobility. The repertoire of bone marrow memory T cells is enriched for systemic pathogens representing persistent, recent, and childhood challenges. In terms of absolute numbers, memory T cells specific for systemic antigens are maintained predominantly in the bone marrow, in particular those representing historic encounters.
Keywords: antigen-specific response, short- and long-term memory, polyfunctional
Abstract
In the bone marrow, a population of memory T cells has been described that promotes efficient secondary immune responses and has been considered to be preactivated, owing to its expression of CD69 and CD25. Here we show that human bone marrow professional memory T cells are not activated but are resting in terms of proliferation, transcription, and mobility. They are in the G0 phase of the cell cycle, and their transcriptome is that of resting T cells. The repertoire of CD4+ bone marrow memory T cells compared with CD4+ memory T cells from the blood is significantly enriched for T cells specific for cytomegalovirus-pp65 (immunodominant protein), tetanus toxoid, measles, mumps, and rubella. It is not enriched for vaccinia virus and Candida albicans-MP65 (immunodominant protein), typical pathogens of skin and/or mucosa. CD4+ memory T cells specific for measles are maintained nearly exclusively in the bone marrow. Thus, CD4+ memory T cells from the bone marrow provide long-term memory for systemic pathogens.
Memory CD4+ and CD8+ T cells are essential components of immunological memory. In humans, analyses of memory T cells have been largely limited to T cells isolated from peripheral blood. Memory T cells of the blood and secondary lymphoid organs (SLOs) are believed to recirculate to find their cognate antigen, presented to them by activated antigen-presenting cells (APCs) in the SLOs. In the apparent absence of antigen their numbers slowly decline (1, 2), suggesting that recirculating memory T cells provide a memory of a limited duration. They have been described as a heterogeneous population with respect to function, based on the expression of either chemokine receptors (3) or cytokines (4). With respect to immunity against infectious pathogens such as HIV, polyfunctional memory Th cells are considered to be the most effective in protection (5).
Recently, memory T cells have been found in many peripheral tissues, such as skin, lung, intestine, and thymus (6–8). These tissue-resident memory T cells (TRM) are thought to provide immediate effector function at the preferred sites of infection with specific pathogens; however, the stability of this memory in humans is not yet clear (9). Memory T cells have also been identified in the bone marrow, which is a privileged tissue in that it is not connected to lymphatic vessels. In humans, memory T cells from the bone marrow express CD69 and thus have been considered to be “preactivated” (10) and, until now, their contribution to immunological memory has remained unclear.
In mice, we have recently identified a population of professional memory CD4+ T cells that is located in the bone marrow in the memory phase of an immune response (1), similar to long-lived plasma cells (11–13). These memory Th cells dock onto IL-7–expressing stroma cells (1), which presumably provide a survival niche for their maintenance (14–16), analogous to the maintenance of long-lived plasma cells by CXCL12-expressing stroma cells (11–13). Murine memory Th cells of the bone marrow provide efficient help to B cells in secondary immune reactions, and their numbers are stably maintained. Furthermore, murine bone marrow memory Th cells express CD69, which is critically required for migration of Th cells into the bone marrow (17). Most importantly, in the absence of bone marrow memory Th cells, secondary immune responses are severely impaired, demonstrating the essential role of murine bone marrow memory Th cells for the maintenance of immunological memory.
Here we analyze and compare memory T lymphocytes from human bone marrow and blood. We show that memory T cells of the bone marrow rest in terms of proliferation, transcription, and mobility, despite expressing CD69. The repertoire of bone marrow CD4+ memory T cells is enriched for T cells recognizing cytomegalovirus (CMV)-pp65, tetanus toxoid (TT), rubella, mumps, and measles virus, all of which are systemic pathogens. CD4+ memory T cells specific for vaccinia virus or Candida (C.) albicans-MP65, representing skin- and/or mucosa-specific pathogens, are not enriched in the bone marrow. In terms of absolute numbers, most memory CD4+ T cells specific for systemic antigens are maintained in the bone marrow, in particular those specific for historic antigens. Memory CD4+ T cells of the bone marrow are polyfunctional, namely they have the capability to express several cytokines simultaneously. Thus, memory T cells of human bone marrow are resting, professional memory cells that provide long-lasting, polyfunctional memory for systemic pathogens.
Results
Human Memory T Cells in Bone Marrow and Peripheral Blood.
We determined the frequencies of CD3+CD4+CD45RO+CD45RA− and CD3+CD8+CD45RO+CD45RA− bona fide memory T cells among all CD3+ T lymphocytes from 12 paired bone marrow and blood samples of 50- to 60-y-old individuals by flow cytometry. In blood, 52% of CD3+CD4+ and 36% of CD3+CD8+ T cells were CD45RO+CD45RA− memory cells; in bone marrow, 51% of CD3+CD4+ and 42% of CD3+CD8+ T cells were CD45RO+CD45RA− memory cells (Fig. 1A). Of the CD3+ T cells in blood, 73% were CD4+ and 19% were CD8+, whereas in bone marrow, 54% were CD4+ and 32% were CD8+ (Fig. 1B). For both CD4+ and CD8+ T cells, their distributions in blood and bone marrow were likewise consistent between analyzed donors (Fig. 1 A and B). Based on the reasonable estimate that the number of T cells in bone marrow is 25 × 109 and that of blood is 7 × 109 (18, 19), human bone marrow thus contains three to four times more memory T lymphocytes than blood (Fig. 1C), roughly 10 × 109 cells. In addition to CD45RO+CD45RA− memory T cells, blood and bone marrow also contain CD45RA+CD45RO−CCR7− memory T cells (TEMRA) (5 × 108 and 3 × 109, respectively) (Fig. S1 A and B). Among CD4+ T cells, TEMRA from bone marrow and blood were 25 and 29 times less frequent than CD45RO+CD45RA− memory cells, respectively, whereas among CD8+ T cells, TEMRA were as similarly frequent as CD45RO+CD45RA− cells in blood and bone marrow.
Fig. 1.
Memory T cells in human bone marrow and peripheral blood. (A and B) Frequencies of CD45RO+CD45RA− memory CD4+ and CD8+ T cells (A) and frequencies of CD4+ and CD8+ T cells (B) from paired bone marrow (BM) and peripheral blood (PB) samples. (C) Absolute numbers were calculated according to the frequencies of memory CD4+ and CD8+ T cells (A) and of CD4+ and CD8+ T cells (B) and according to the estimated numbers of T cells within BM and PB of healthy young adults (18, 19). (D) Memory CD4+ and CD8+ T cells of BM express CD69. A representative plot of overlaid histograms of CD69 expression on memory CD4+ or CD8+ T cells of BM and PB, and the average frequencies and absolute numbers of CD69+ memory CD4+ and CD8+ T cells are shown. In A–D, n = 12; P values were obtained as described in SI Materials and Methods. (E) CD137 expression on CD69+ and CD69− memory CD4+ and CD8+ T cells from BM and PB samples. Data shown are representative of four separate experiments. (F) Average frequencies of CD25+ cells among CD69+ or CD69− memory CD4+ T cells of five paired BM and PB samples. Error bars represent SEM. (G) CD127 and FOXP3 expression on CD69+CD25+ and CD69−CD25+ memory CD4+ T cells of BM and on CD25hiCD69− and CD25−CD69− memory CD4+ T cells of PB. Data shown are representative of five separate experiments.
Human Bone Marrow Memory T Cells Express CD69 but Are Not Activated.
CD69 is a calcium-dependent type II transmembrane receptor of the lectin superfamily (20). Expression of CD69 is induced upon activation of T lymphocytes and is therefore sometimes regarded as an activation marker (21). Less than 1% of human CD3+CD45RO+CD45RA− memory T cells from peripheral blood expressed CD69. Strikingly, in bone marrow, 62.8% of CD8+ and 28.6% of CD4+ memory T cells expressed CD69, whereas in terms of absolute numbers, bone marrow contained equal numbers of CD69+ memory CD4+ and CD8+ T cells (Fig. 1D). With the exception of CD69, bone marrow memory T cells did not express other putative activation markers. For example, ex vivo isolated memory T cells from blood or bone marrow did not express CD137 (Fig. 1E). This was not due to anergy or refraction, because memory T cells from blood and bone marrow readily expressed CD137 upon restimulation with anti-CD3/CD28 (Fig. S2). CD25 was expressed by 9% of the (CD69−) CD4+ cells from blood and by 2.1 and 5.5% of the bone marrow CD69+ and CD69− memory CD4+ T cells, respectively (Fig. 1F). However, more than 80% of the cells expressing CD25 did not express the IL-7 receptor (CD127low) but rather expressed FOXP3 (forkhead box P3; scurfin) (Fig. 1G and Fig. S3), highlighting that these cells are bona fide regulatory T cells (22). Therefore, bone marrow also contains a substantial fraction of regulatory memory T cells and, moreover, bona fide memory T cells residing in the bone marrow are not in an activated state, despite their expression of CD69.
Human Bone Marrow Memory T Cells Rest in Terms of Proliferation, Transcription, and Mobility.
To analyze steady-state proliferation of memory T cells in paired bone marrow and blood samples at the single-cell level, cells were stained for expression of Ki67, an antigen that is expressed in all phases of the cell cycle except G0 (23). On average, 4.2% of memory CD4+ and 5.0% of memory CD8+ T cells isolated ex vivo from blood expressed Ki67. In the bone marrow of the same donors, 1.2% of CD4+ and 1.7% of CD8+ memory T cells expressed Ki67. Some of those Ki67+ cells of blood and bone marrow in all likelihood represent recently generated effector cells, because they express only low levels of CD127 (24) (Fig. S4). Thus, more than 98% of the bone marrow memory T cells are resting in the G0 phase of the cell cycle (Fig. 2A). In relation to CD69 expression by memory CD4+ and CD8+ T cells of the bone marrow, Ki67 expression was largely confined to CD69− memory CD4+ and CD8+ T cells (Fig. 2B). To further identify memory T cells in the S and G2/M phases of the cell cycle, DNA content was assessed quantitatively by propidium iodide (PI) staining (Fig. S5). On average, 0.185% of memory CD4+ cells from blood and 0.175% from bone marrow, and 0.067% of memory CD8+ T cells from blood and 0.2% from bone marrow, were in the S and G2/M phases of the cell cycle (Fig. 2C). Importantly, memory T cells from both blood and bone marrow could be readily reactivated with anti-CD3/CD28, resulting in 15–22% of the CD4+ and CD8+ memory T cells entering the S and G2/M phases of the cell cycle (Fig. 2C and Fig. S5). Therefore, steady-state memory T cells of human bone marrow are resting in the G0 phase of the cell cycle.
Fig. 2.

Cell-cycle status of ex vivo human memory CD4+ and CD8+ T cells from BM and PB. (A) Frequencies of Ki67+ memory CD4+ and CD8+ T cells in seven paired BM and PB samples. P values were obtained as described in SI Materials and Methods. (B) Sample flow cytometry expression profiles showing the counterstainings for Ki67 and CD69 expression on memory CD4+ and CD8+ T cells. (C) Frequencies of ex vivo (n = 6) and 72-h anti-CD3/CD28 activated (n = 2) memory CD4+ and CD8+ T cells in the cell-cycle S+G2/M phase. Error bars represent SEM.
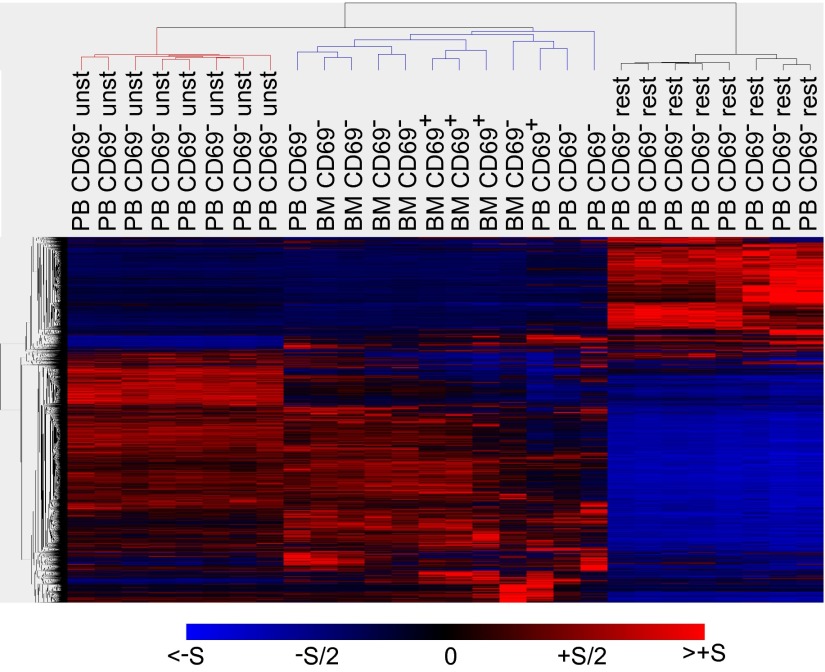
To further determine the global transcriptional activity of bone marrow memory CD4+ T cells, gene expression was analyzed by Affymetrix microarrays. Memory CD4+ T cells were isolated ex vivo from four paired blood and bone marrow samples. Transcriptomes of these cells were compared with those of memory CD4+ T cells from blood of eight unrelated donors, directly after ex vivo isolation or after stimulation for 3 h with phorbol-myristic acid (PMA)/ionomycin. Transcriptomes of memory CD4+ T cells from blood and bone marrow were very similar and clearly distinct from transcriptomes of activated memory CD4+ T cells (Fig. 3). In memory CD4+ T cells from blood and bone marrow, compared with activated T cells, genes indicating activation were down-regulated, as exemplified by IL22, FOXP1, and IFNGR2, whereas genes such as RCOR3 and HDAC1, indicating repression of transcription, were up-regulated (Table S1). Transcriptional inactivity of ex vivo cells was indicated by down-regulation of IRF4, RNPS1, SLAMF1, and NFAT5, and proliferative rest was indicated by down-regulation of MKI67IP, CDK6, and WEE1 and up-regulation of RBL2. In addition, mobility arrest was indicated by up-regulation of ARHGDIB and down-regulation of RHOA and SVIL. As expected, and of particular relevance, is the selective down-regulation of S1PR1, a receptor for S1P, in CD69+ memory CD4+ T cells from bone marrow. This receptor would be required for S1P-mediated egress into the blood (25–27). With respect to genes involved in survival, memory T cells did not express FASL and other apoptotic regulators such as CRADD and TNFAIP8, and thus are not prone to FAS-mediated induction of apoptosis, as expected, whereas activated T cells did express FASL, CRADD, and TNFAIP8. In addition, expression of antiapoptotic regulators XAF1 and CD27 was up-regulated in the resting memory T cells, compared with the activated cells. With respect to functional diversity, expression of the T-cell differentiation lineage-determining transcription factors GATA3, TBX21 (TBET), RORC (nuclear receptor ROR-gamma), EOMES, and BCL6 was detected in memory T cells from both blood and bone marrow, indicating a broad functional repertoire. Otherwise, the global gene expression profiles of memory T cells from blood and bone marrow confirm their resting state in terms of proliferation and mobility, and are clearly distinct from activated cells.
Fig. 3.
Global resting gene expression profiles of ex vivo memory CD4+ T cells from BM and PB. Transcriptomes of memory CD4+ T-cell subsets from four paired BM and PB samples were compared with transcriptomes of CD4+ memory T cells from PB of eight unrelated donors, directly after isolation (ex vivo) or after stimulation for 3 h with PMA/ionomycin. Differentially expressed genes (7,383) were hierarchically clustered and displayed. The normalized induction (red) or repression (blue) is shown for each gene. Transcriptomes of the following samples are shown: from paired ex vivo BM and PB samples, BM CD69+, BM CD69−, and PB CD69−; from PB samples of unrelated donors, PB CD69− unstimulated (unst) or restimulated with PMA/ionomycin (rest).
The Repertoire of Bone Marrow Memory CD4+ T Cells.
To assess the repertoire of bone marrow memory CD4+ T cells, we determined the frequencies of memory CD4+ T cells specific for CMV-pp65, TT, measles, rubella, mumps, vaccinia virus, and C. albicans-MP65 in paired bone marrow and blood samples of individual donors aged 40–70 y. Bone marrow and blood mononuclear cells were restimulated with antigen and CD28 antibodies for 12 or 16 h (the latter for vaccinia virus). CD4+ T cells reacting to the antigen were identified according to the expression of CD154 (28, 29) and one or more of the cytokines IL-2, TNF-α, and IFN-γ, as assessed by intracellular immunofluorescence (Fig. S6A). Frequencies of cells reacting to stimulation with “anti-CD28 only” were subtracted from the frequencies of cells reacting to “anti-CD28 plus antigen.” The superantigen SEB was included as a high control for stimulations (Fig. S6B). Furthermore, to exclude that any potential differences in frequencies of reactive cells were due to inadequate antigen presentation in either blood or bone marrow, for example different types or numbers of APCs, we performed control experiments mixing bone marrow and blood cells at 1:1 ratios, with one or the other fraction being carboxyfluorescein succinimidyl ester (CFSE)-labeled, and stimulating them with CMV-pp65. The frequencies of reactive cells from bone marrow and blood were the same in the mixed culture as in the separate pure blood and bone marrow control cultures, demonstrating that APCs from both bone marrow and blood are equally efficient at presenting antigen (Fig. S6C).
For pp65 and TT, CD154+cytokine+ cells were readily detectable both in blood and bone marrow, but frequencies (Fig. 4A) were higher in bone marrow than in blood, except for one donor showing a higher frequency of TT-specific cells in blood than in bone marrow. Moreover, absolute numbers (Fig. 4B) of pp65-reactive cells were significantly higher in bone marrow than in blood. Similarly, TT-reactive cells in bone marrow outnumbered those in blood by five times (Fig. 4B). Interestingly, CD154+cytokine+ memory T cells reacting to measles were below the level of reliable detection in blood in five out of six donors, that is, at frequencies below 10−4 of memory CD4+ T cells and less than 3 × 105 in the entire organ (Fig. 4 A and B and Fig. S7). However, in bone marrow, measles-specific CD154+cytokine+ cells were readily detectable in all donors, at frequencies of 10−4 to 10−3 of memory CD4+ T cells (Fig. 4A and Fig. S7), corresponding to absolute numbers of 8 × 105 to 1 × 107 cells (Fig. 4B). For rubella, CD154+cytokine+ cells were detectable in bone marrow in similar frequencies as TT-reactive cells, whereas they were not detectable in blood in two out of four donors (Fig. 4A and Fig. S7). However, in the two donors with detectable responding cells in blood, the frequencies as well as absolute numbers of rubella-specific cells were higher in bone marrow than in blood (Fig. 4 A and B and Fig. S7). Also similar to TT, mumps-specific CD154+cytokine+ cells were present in higher frequencies in bone marrow in three out of four donors (Fig. 4 A and B and Fig. S7). The higher frequencies of CD4+ memory T cells in bone marrow recognizing CMV, TT, measles, rubella, and mumps, compared with blood, reflect a preferential recruitment of memory T cells specific for systemic antigens to the bone marrow. The exclusive presence of memory Th cells specific for the measles virus antigen in bone marrow in five out of six donors as well as for the rubella antigen in two out of four donors shows that the bone marrow is able to maintain long-term memory, even in the apparent absence of circulating memory cells. For C. albicans-MP65 or vaccinia virus, CD154+cytokine+ memory Th cells were detectable both in bone marrow and blood in three or four out of five donors, respectively, without significant differences in absolute numbers between these two tissue compartments (Fig. 4 A and B and Fig. S7). The differential distribution of long-term T-cell memory for systemic pathogens (measles, rubella, and mumps) versus that for skin/mucosal pathogens (C. albican-MP65 and vaccinia) may reflect the different immunization routes and/or different immune responses as, at least in mice, a prominent skin-resident T-cell memory for vaccinia is known to develop (30).
Fig. 4.

Comparison of antigen specificity and diversity of memory CD4+ T cells from paired BM and PB samples. Mononuclear cells isolated from four to seven paired BM and PB samples were stimulated with the indicated antigens, and the induced cytokine production (IFN-γ, IL-2, or TNF-α) in memory CD4+ T cells was examined according to CD154 expression. For each subpopulation, the background (as detected in the anti-CD28 stimulated but otherwise equally treated control samples) was subtracted. (A) Frequencies and (B) estimated absolute numbers of antigen-specific CD154+cytokine+ (total cytokine-producing) cells are shown. Absolute numbers of antigen-specific memory CD4+ T cells were calculated using the frequency of these cells and the estimated numbers of T cells within BM and PB of healthy young adults (18, 19). Symbols in gray indicate frequencies under reliable detection limit (10−4 of memory CD4+ T cells) or estimated cell numbers calculated according to such frequencies. P values were obtained as described in SI Materials and Methods.
Memory CD4+ T cells that reacted with the reexpression of two or more of the cytokines TNF-α, IL-2, and IFN-γ are polyfunctional and considered to be more protective in secondary immune reactions to infectious pathogens than those expressing only one of the three cytokines (5, 31). Both in terms of frequencies and absolute numbers, polyfunctional memory Th cells specific for pp65 or TT predominantly resided in bone marrow (Figs. S7 and S8). In absolute numbers, and with considerable individual variation, 5–50 times more pp65- or TT-specific polyfunctional memory Th cells resided in the bone marrow than in the blood (Fig. S8). Polyfunctional memory Th cells specific for vaccinia, at frequencies of 0.017–0.189%, corresponding to absolute numbers of 4 × 105 to 1.8 × 107, were found both in the bone marrow and in blood (Figs. S7 and S8). Polyfunctional memory Th cells specific for measles were only found in the bone marrow in five out of six donors, at an average frequency of 0.016% of memory CD4+ T cells (Figs. S7 and S8).
Discussion
In the present study, we have compared phenotype, gene expression, repertoire of antigen specificity, and persistence of memory T cells from bone marrow and blood of individual human donors. Both CD4+ and CD8+ memory T cells from bone marrow are quiescent in their activation status and rest in the G0 phase of the cell cycle, and their gene expression profile suggests that they are immobile and protected from apoptosis.
It has been known for some time that bone marrow contains a prominent population of CD4+ and CD8+ cells with a memory/effector phenotype, both in humans (32–34) and in mice (1, 35–37). We have shown previously that in murine immune responses to defined antigens, that are, those resembling vaccines, antigen-specific memory CD4+ T cells translocate to the bone marrow within 30–60 d, and thereafter are maintained almost exclusively in the bone marrow whereas they slowly decline in blood and SLOs (1). Similarly, the slow contraction of memory T cells in blood has also been described for humans (2), provoking the question of whether there is immunological memory without memory T cells (38). Despite this, the analysis of memory T cells from blood has until now continued to be the major basis for the assessment of T-cell memory in humans. However, in light of the apparent contraction of memory T cells in the periphery, memory T cells specific for some pathogens at best represent “short-term” memory. The analysis of murine memory Th cells from bone marrow has put bone marrow memory T cells center-stage, constituting a population of persisting professional memory cells resulting in stable systemic Th memory (1, 16). Moreover, although recently TRM cells have been described in humans (39) and mice (6–8) and are discussed as a major effector of local long-term memory, it remains unclear as to how they are stably maintained over time (9).
In the present analysis, we also have compared the repertoire of memory Th cells from human blood and bone marrow (of the same donors) for specificities representing presumably persistent antigens, namely pp65 of CMV and MP65 of C. albicans; antigens encountered within the last 10 y, namely TT; and antigens presumably encountered only in childhood (40–70 y ago in the case of our study), namely measles, rubella, mumps, and vaccinia virus or vaccine. In the bone marrow of the donors analyzed, significant populations of memory Th cells specific for any of these antigens are present, ranging in numbers from 106 to 108.
Memory CD4+ T cells specific for vaccinia were found both in blood and bone marrow in four out of five analyzed donors, without significant differences in absolute numbers between these two tissue compartments. This could be due to a preferential location of vaccinia-specific memory T cells in the skin, as has been recently reported in mice (30), and/or a preferential generation of circulating memory cells, compared with bone marrow memory cells. It has been shown previously that the numbers of vaccinia-specific CD4+ memory T cells in human blood decline over time, with a half-life of 8–15 y (2). Also for Candida, CD4+ memory T cells were present in blood at higher frequencies than in bone marrow, in three out of four donors, suggesting that the repertoire of memory T cells specific for pathogens addressing skin and mucosa are excluded from the bone marrow and may be maintained in the tissues affected as tissue-resident memory cells (30, 40).
CD4+ memory Th cells specific for measles were exclusively detectable in bone marrow in five out of six donors, in numbers comparable to TT-specific cells. Also, in two out of four donors, CD4+ memory T cells specific for rubella were only detectable in bone marrow, and not in blood. Memory Th cells specific for CMV, TT, mumps, and rubella, the latter in two out of four donors, were also found in the blood, but at frequencies among CD4+ memory T cells of at least twofold less than in bone marrow. Thus, the repertoire of human bone marrow CD4+ T-cell memory is significantly enriched for T cells specific for systemic pathogens and vaccines. In terms of absolute cell numbers, CD4+ T cells specific for these systemic antigens were even more prominent, 5–20 times, in the bone marrow than in the blood. This observation is in line with a stable maintenance of memory Th cells in the bone marrow, whereas their numbers would dwindle in the blood (1). Moreover, this observation shows that memory Th cells in the bone marrow can be maintained in the apparent absence of memory Th cells in blood, in other words, that the bone marrow cells are not circulating but remain residents of the bone marrow.
This notion of bone marrow residency is supported by differences in the phenotypes of memory T cells from blood and bone marrow. For example, we found that 30% of CD4+ and 60% of CD8+ memory T cells from bone marrow expressed CD69, commonly regarded as an activation marker (21). Accordingly, memory T cells from bone marrow have been considered to be in a “heightened” activation state (10, 41). However, here we show that despite expression of CD69, these cells are quiescent. They do not express other activation markers, such as CD137, they show a gene transcription profile of resting cells, very similar to that of memory T cells from blood and clearly distinct from that of activated T cells, and they are in the G0 phase of the cell cycle, as determined by Ki67 staining. This resembles the situation in the mouse, where 30% of the memory Th cells of bone marrow express CD69 yet are resting in terms of proliferation and overall transcriptional activity (1). In mice, expression of CD69 on activated Th cells is critical for their migration into the bone marrow and the establishment of Th cell memory as such (17). Furthermore, expression of CD69 may also be critical to retain the cells in the bone marrow, because expression of a receptor for S1P, which mediates egress into the blood (25–27), is down-regulated in CD69+ bone marrow memory Th cells.
Bone marrow CD4+ memory T cells are imprinted for efficient reexpression of effector cytokines. Memory cells specific for pp65 of CMV, TT, measles, rubella, mumps, MP65 of C. albicans, or vaccinia, when restimulated, readily expressed two or three of the cytokines TNF-α, IL-2, and IFN-γ, marking them as “polyfunctional” memory T cells. In immune responses to HIV, it had been shown that this polyfunctionality is essential for efficient immune responses to the pathogen (5, 31). For systemic pathogens (CMV-pp65, TT, measles, rubella, and mumps), specific polyfunctional memory T cells preferentially reside in bone marrow. Even if one considers that not all cycling memory T cells are in the blood but more are in the SLOs (42) and tissues such as the skin (43), CMV-, TT-, and measles-specific polyfunctional memory T cells are largely restricted to the bone marrow. Interestingly, polyfunctional CD4+ memory T cells specific for C. albicans-MP65- and vaccinia-specific ones are not, again indicating that CD4 T-cell memory for these antigens may be maintained in the tissues affected, namely skin and mucosa.
From the transcriptomes of bone marrow memory cells, it is obvious that all of the lineage-determining transcription factors of memory T-cell differentiation are expressed, including TBX21, GATA3, RORC, EOMES, and BCL6. FOXP3 is also expressed, suggesting that a fraction of the memory cells in bone marrow are regulatory memory T cells. Indeed, 2.1% of the CD69+ and 5.5% of the CD69− Th memory cells of bone marrow have the phenotype CD25+CD127low and FOXP3+, qualifying them as bona fide Treg cells.
As we show here, memory T cells of the bone marrow are resting and maintain antigen-specific memory over long periods of time, presumably decades, even when memory T cells of the same specificity are no longer detectable in the blood. This is a drastic reversal of the current concept that memory T cells recirculate throughout the body to find their cognate antigen, presented to them by APCs in SLOs. If memory T cells reside and rest in the bone marrow, antigen-sampling and/or -presenting cells would have to come to the bone marrow to find and mobilize the memory T cells specific for their antigen. At present it remains enigmatic how this works. However, it has been shown already for murine memory Th cells from bone marrow that they are superior helper cells in secondary immune responses, compared with memory T cells from spleen, efficiently promoting antibody class switching and affinity maturation of activated B cells (1, 17).
Materials and Methods
Sample Collection and Preparation.
Unpaired blood samples were collected from anonymous healthy adult donors (mean age ± SEM: 47.5 ± 3.6; n = 8) and paired bone marrow and peripheral blood samples from anonymous systemically healthy adults (63.8 ± 1.2; n = 61) undergoing hip replacement operations. All samples were obtained with local ethical committee (Ethikkommission der Charité-Univerisitätsmedizin Berlin) approvals and informed consent in accordance with the Declaration of Helsinki. All freshly obtained samples were subjected to immediate preparation. Mononuclear cells of blood and bone marrow were isolated by density gradient sedimentation using Ficoll-Hypaque (Sigma-Aldrich).
Flow Cytometry Analysis.
Eight- to 12-color flow cytometry was performed for the analysis of cell phenotype, cytokine profile, and cell sorting using a BD FACSAria cell sorter, LSRFortessa flow cytometer (BD Biosciences), or MACSQuant (Miltenyi Biotec). The following fluorochrome-conjugated mouse anti-human antibodies were used to stain cells: CD14 (Beckman Coulter), CD19 and CD4 (house conjugate), Ki67 (eBioscience), CD154 (Miltenyi Biotec), CD3, CD45RA, and CD45RO (BD Biosciences), CD8, CD69, CD25, CD127, CCR7, CD137, TNF-α, IFN-γ, and IL-2 (BioLegend). DAPI or PI was used as a dead cell exclusion marker. Stained cells were acquired using FACSDiva (BD Biosciences) or MACSQuantify software and data were analyzed with FlowJo (Tree Star).
FOXP3 Staining.
Cells isolated from paired bone marrow and blood samples were first stained for surface markers followed by fixation/permeabilization and intracellular staining using the Foxp3 Staining Buffer Set (eBioscience) according to the manufacturer’s recommendations.
Cell-Cycle Analysis.
Cell-cycle status was assessed by staining cells with Ki67 or PI. Briefly, live cells were stained with surface markers followed by fixation/permeabilization using FACS Lysing Solution/FACS Permeabilizing Solution 2 (BD Biosciences) for Ki67 or Foxp3 Fix/Perm buffers (eBioscience) for PI. In the case of PI staining, cells were treated with RNaseA (200 µg/mL) and stained for PI (20 µg/mL) in PBS at 37 °C for 15 min. Samples were acquired via MACSQuant (Miltenyi Biotec).
Additional methods are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We are thankful to Antje Blankenstein and the Core Unit Cell Harvesting at the Berlin-Brandenburg Center for Regenerative Therapies for providing paired peripheral blood and bone marrow samples. We acknowledge the assistance of the Flow Cytometry Core Facility at Deutsches Rheuma-Forschungszentrum Berlin. R.K. and C.S. were in part supported by The José Carreras Leukaemia Foundation. This work was supported by a European Research Council (ERC) Advanced Grant [ERC-2010-AdG_20100317 Grant 268987 (to A.R.)] and Priority Programme 1468 Immunobone of the German Research Council, and in part by the International Max Planck Research School for Infectious Diseases and Immunology Generation 2011.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data discussed in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE50677).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318731111/-/DCSupplemental.
References
- 1.Tokoyoda K, et al. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009;30(5):721–730. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Hammarlund E, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9(9):1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 4.Löhning M, Richter A, Radbruch A. Cytokine memory of T helper lymphocytes. Adv Immunol. 2002;80:115–181. doi: 10.1016/s0065-2776(02)80014-1. [DOI] [PubMed] [Google Scholar]
- 5.Betts MR, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291(5512):2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 7.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10(5):524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann M, Oschowitzer A, Kurzhals SR, Krüger CC, Pircher H. Thymus-resident memory CD8+ T cells mediate local immunity. Eur J Immunol. 2013;43(9):2295–2304. doi: 10.1002/eji.201343519. [DOI] [PubMed] [Google Scholar]
- 9.Bevan MJ. Memory T cells as an occupying force. Eur J Immunol. 2011;41(5):1192–1195. doi: 10.1002/eji.201041377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herndler-Brandstetter D, et al. Human bone marrow hosts polyfunctional memory CD4+ and CD8+ T cells with close contact to IL-15-producing cells. J Immunol. 2011;186(12):6965–6971. doi: 10.4049/jimmunol.1100243. [DOI] [PubMed] [Google Scholar]
- 11.Slifka MK, Matloubian M, Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J Virol. 1995;69(3):1895–1902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388(6638):133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 13.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20(6):707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Kondrack RM, et al. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198(12):1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198(12):1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tokoyoda K, Hauser AE, Nakayama T, Radbruch A. Organization of immunological memory by bone marrow stroma. Nat Rev Immunol. 2010;10(3):193–200. doi: 10.1038/nri2727. [DOI] [PubMed] [Google Scholar]
- 17.Shinoda K, et al. Type II membrane protein CD69 regulates the formation of resting T-helper memory. Proc Natl Acad Sci USA. 2012;109(19):7409–7414. doi: 10.1073/pnas.1118539109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trepel F. Number and distribution of lymphocytes in man. A critical analysis. Klin Wochenschr. 1974;52(11):511–515. doi: 10.1007/BF01468720. [DOI] [PubMed] [Google Scholar]
- 19.Di Rosa F, Pabst R. The bone marrow: A nest for migratory memory T cells. Trends Immunol. 2005;26(7):360–366. doi: 10.1016/j.it.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Ziegler SF, et al. Molecular characterization of the early activation antigen CD69: A type II membrane glycoprotein related to a family of natural killer cell activation antigens. Eur J Immunol. 1993;23(7):1643–1648. doi: 10.1002/eji.1830230737. [DOI] [PubMed] [Google Scholar]
- 21.Testi R, Phillips JH, Lanier LL. T cell activation via Leu-23 (CD69) J Immunol. 1989;143(4):1123–1128. [PubMed] [Google Scholar]
- 22.Miyara M, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 24.Paiardini M, et al. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J Immunol. 2005;174(5):2900–2909. doi: 10.4049/jimmunol.174.5.2900. [DOI] [PubMed] [Google Scholar]
- 25.Shiow LR, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440(7083):540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 26.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 27.Feng C, et al. A potential role for CD69 in thymocyte emigration. Int Immunol. 2002;14(6):535–544. doi: 10.1093/intimm/dxf020. [DOI] [PubMed] [Google Scholar]
- 28.Frentsch M, et al. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11(10):1118–1124. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 29.Chattopadhyay PK, Yu J, Roederer M. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat Med. 2005;11(10):1113–1117. doi: 10.1038/nm1293. [DOI] [PubMed] [Google Scholar]
- 30.Jiang X, et al. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483(7388):227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: Implications for vaccine design. Nat Rev Immunol. 2008;8(4):247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 32.Feuerer M, et al. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med. 2001;7(4):452–458. doi: 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 33.Palendira U, et al. Selective accumulation of virus-specific CD8+ T cells with unique homing phenotype within the human bone marrow. Blood. 2008;112(8):3293–3302. doi: 10.1182/blood-2008-02-138040. [DOI] [PubMed] [Google Scholar]
- 34.Guerreiro M, et al. Human peripheral blood and bone marrow Epstein-Barr virus-specific T-cell repertoire in latent infection reveals distinct memory T-cell subsets. Eur J Immunol. 2010;40(6):1566–1576. doi: 10.1002/eji.200940000. [DOI] [PubMed] [Google Scholar]
- 35.Becker TC, Coley SM, Wherry EJ, Ahmed R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J Immunol. 2005;174(3):1269–1273. doi: 10.4049/jimmunol.174.3.1269. [DOI] [PubMed] [Google Scholar]
- 36.Mazo IB, et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 2005;22(2):259–270. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Quinci AC, et al. IL-15 inhibits IL-7Rα expression by memory-phenotype CD8+ T cells in the bone marrow. Eur J Immunol. 2012;42(5):1129–1139. doi: 10.1002/eji.201142019. [DOI] [PubMed] [Google Scholar]
- 38.Bell EB, Westermann J. CD4 memory T cells on trial: Immunological memory without a memory T cell. Trends Immunol. 2008;29(9):405–411. doi: 10.1016/j.it.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Sathaliyawala T, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38(1):187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207(3):553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Price PW, Cerny J. Characterization of CD4+ T cells in mouse bone marrow. I. Increased activated/memory phenotype and altered TCR Vbeta repertoire. Eur J Immunol. 1999;29(3):1051–1056. doi: 10.1002/(SICI)1521-4141(199903)29:03<1051::AID-IMMU1051>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 42.Klonowski KD, et al. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20(5):551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 43.Clark RA, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176(7):4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.