Significance
Antibodies are selected to bind microbial but not self-antigens, because binding to self would compete with binding microbes, shorten antibody half-life, and cause autoimmunity. Self-tolerance is actively acquired in part by discarding self-binding antibodies before the body is exposed to a microbe or vaccine. The experiments here provide evidence of an opposite mechanism, allowing antibodies that initially bind both foreign and self-antigens to acquire self/non-self discrimination during the course of an immune response through somatic hypermutation away from self-reactivity. In addition to selection for lower-affinity binding to self, antibody variants were selected with fewer binding sites available to bind self-antigen because most were occupied by N-linked carbohydrate, possibly explaining the frequent occurrence of N-linked glycosylation of antibody variable domains.
Keywords: self-tolerance, affinity maturation, clonal selection, autoimmunity
Abstract
The best-understood mechanisms for achieving antibody self/non-self discrimination discard self-reactive antibodies before they can be tested for binding microbial antigens, potentially creating holes in the repertoire. Here we provide evidence for a complementary mechanism: retaining autoantibodies in the repertoire displayed as low levels of IgM and high IgD on anergic B cells, masking a varying proportion of autoantibody-binding sites with carbohydrates, and removing their self-reactivity by somatic hypermutation and selection in germinal centers (GCs). Analysis of human antibody sequences by deep sequencing of isotype-switched memory B cells or in IgG antibodies elicited against allogeneic RhD+ erythrocytes, vaccinia virus, rotavirus, or tetanus toxoid provides evidence for reactivation of anergic IgMlow IgD+ IGHV4-34+ B cells and removal of cold agglutinin self-reactivity by hypermutation, often accompanied by mutations that inactivated an N-linked glycosylation sequon in complementarity-determining region 2 (CDR2). In a Hy10 antibody transgenic model where anergic B cells respond to a biophysically defined lysozyme epitope displayed on both foreign and self-antigens, cell transfers revealed that anergic IgMlow IgD+ B cells form twice as many GC progeny as naïve IgMhi IgD+ counterparts. Their GC progeny were rapidly selected for CDR2 mutations that blocked 72% of antigen-binding sites with N-linked glycan, decreased affinity 100-fold, and then cleared the binding sites of blocking glycan. These results provide evidence for a mechanism to acquire self/non-self discrimination by somatic mutation away from self-reactivity, and reveal how varying the efficiency of N-glycosylation provides a mechanism to modulate antibody avidity.
Following somatic recombination of Ig variable (V), diversity (D), and joining (J) gene elements, each B lymphocyte makes a different antibody displayed on the plasma membrane as B-cell antigen receptors (BCRs). Selection of antibodies to avoid binding self-antigens is currently known to follow mechanisms conforming to Burnet’s clonal selection hypothesis, whereby antibodies that bind self are discarded during B-cell formation by receptor editing, where the B cell undergoes a second Ig gene recombination, or by clonal deletion of the B cell itself before the self-binding antibody can be tested for binding to microbial antigens (1, 2). An alternative theoretical possibility raised by Jerne and by Diaz and Klinman (3, 4) is that B cells bearing self-reactive antibodies might somatically mutate away from self-reactivity, although this possibility has not been experimentally addressed.
Approximately one-quarter of the preimmune B-cell repertoire display self-reactive antibodies on their cell surface primarily containing a constant region segment of the IgD isotype, with only a small proportion of their BCRs containing the IgM constant region isotype. This IgD+ IgMlow subset has the phenotypic, biochemical, and functional characteristics of B cells that have become anergic with intrinsically suppressed ability to proliferate or secrete antibodies in response to most stimuli (5–9). Here we investigate the possibility that display of autoantibodies on IgD+ IgMlow anergic B cells allows somatic mutation of the antibody away from self-reactivity, first by studying the patterns of mutations in human antibodies using the IGHV4-34 gene, and second by analyzing recurrent mutations in the mouse Hy10 antibody against lysozyme that are selected when anergic B cells are induced to form germinal centers by a foreign antigen with the same lysozyme epitope as a self-antigen.
Results
Human IGHV4-34 Antibody Variants.
In humans, antibodies using the IGHV4-34*01 variable element are displayed as high IgD and low IgM on 7% of circulating naïve B cells that are anergic to BCR stimulation (10). IGHV4-34 antibodies are autoantibodies that agglutinate self-erythrocytes at low temperatures (cold agglutinins) by binding self-carbohydrate I/i antigens composed of repeating N-acetyllactosamine units on the surface of erythrocytes and B cells. This is due to an Ala–Val–Tyr hydrophobic patch in framework region 1 not present in other IGHV4 family elements, is independent of complementarity-determining region (CDR)3H or light-chain sequence, and is abolished if the AVY residues are individually mutated (11–14) (Fig. 1A). To extend earlier evidence that IGHV4-34+ IgD+ IgMlow anergic B cells are recruited into physiological germinal center (GC) responses against foreign antigens (15), we performed a Blastn search of the National Center for Biotechnology Information (NCBI) nonredundant nucleotide database using the IGHV4-34*01 sequence. The search revealed 14 human antibodies with a hypermutated IGHV4-34*01 sequence that had been elicited in normal individuals by repeated immunization either with allogeneic RhD+ erythrocytes (16), rotavirus (17), vaccinia virus (18), or tetanus toxoid (19) (Fig. 1A).
Fig. 1.
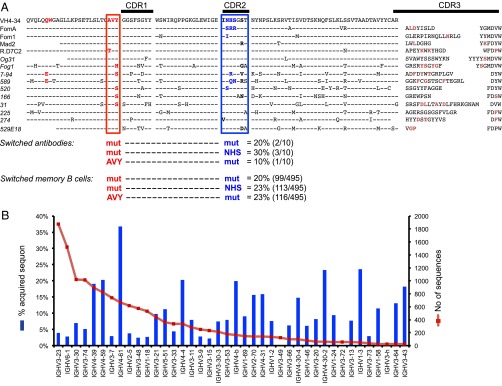
Evidence for mutation away from self-reactivity in IGHV4-34 antibodies. (A) Antibodies using IGHV4-34 from normal donors. The germ-line IGHV-34*01 amino acid sequence is shown at the top. In red are the residues of the hydrophobic patch that cause binding to self-antigens on the surface of B cells and erythrocytes, notably I/i carbohydrates, with the AVY sequence boxed. In blue and boxed is the germ-line NHS N-glycosylation sequon in CDR2. Sequons flanking residues that may modulate glycosylation efficiency analogous to Hy10 are also shown in bold. Aligned beneath are the corresponding sequences of specific antibodies (switched IgG antibodies are italicized) elicited by immunization with a foreign antigen, revealed by an IGHV-34*01 Blastn search of the NCBI nonredundant nucleotide database and analyzed using IMGT/V-QUEST. Identity to germ line is denoted by a dash, and substituted residues in CDR3 are in dark red. The percentage of switched antibodies with mutations that inactivate the hydrophobic patch AVY sequence or the core NHS glycosylation sequon, or both, is shown below for the switched antibodies of known specificity and in massively parallel cDNA sequences from memory B cells. Antibody specificities and GenBank accession numbers are as follows: anti-RhD (16): FomA (X64153), Fom1 (X64152), Mad2 (X64159), R.D7C2 (A49385), Og31 (X64156), Fog1 (X64150); anti-rotavirus (17): 7-94 (AF453121); anti-vaccinia (18): 589 (HQ378397), 520 (HQ378388), 166 (HQ378337), 31 (HQ378326), 225 (HQ378353), 274 (HQ378366); and anti-tetanus toxoid (19): 529E18 (JN111017). (B) Repertoire of mutated IGH cDNAs in IgD− CD27+ memory B cells in blood of normal donors, analyzed by 454 massively parallel sequencing, showing the number of sequences for each IGHV gene and the percentage that have acquired an N-glycosylation sequon. Excluded are IGHV genes with germ-line sequons (IGHV4-34, IGHV1-8, IGHV5-a) or with less than 20 reads.
Four of the identified antibodies were RhD-specific agglutinating IgM antibodies used in blood typing, and indeed the IgM response to foreign RhD is dominated by IGHV4-34*01 antibodies (16, 20). Three of the anti-RhD IgM antibodies retain the AVY sequence and are known to retain self-reactivity measured by agglutination of I/i antigen-bearing erythrocytes (20). Experimentally introduced mutations in the AVY motif that inactivate self-I/i agglutination also abolish RhD-mediated agglutination by an IGHV4-34 IgM antibody (13), indicating that mutation away from self-reactivity would come at the cost of losing foreign RhD reactivity. Nevertheless, the AVY self-reactivity sequence has been somatically mutated in one of the IgM anti-RhD antibodies and in a high-affinity IgG anti-RhD antibody, Fog1, isolated from the same donor as two of the IgM antibodies (FomA and Fom1). The AVY sequence was also inactivated in IgG antibodies against rotavirus and vaccinia. Overall, the AVY motif was mutated in 5 of the 10 IGHV4-34 IgG antibodies, consistent with earlier observations that this sequence is mutated more frequently than would be expected by chance in switched B cells of unknown specificity in surgically resected tonsils (12). To extend this analysis to normal memory B cells, we analyzed a total of 78,074 VDJH sequences obtained by massively parallel sequencing of cDNA from sorted peripheral blood memory B cells from 13 normal donors (21, 22). Nonsynonymous AVY substitutions occurred in 43% of the 3,030 IGHV4-34 memory B-cell sequences (Fig. 1A).
N-Linked Glycosylation Sequons in Human Antibody V Regions.
IGHV4-34*01 is also unique among the family of IGHV4 elements because it has acquired during germ-line evolution an Asn–X–Ser/Thr sequon promoting N-linked glycosylation of CDR2 (Fig. 1A). Attachment of bulky oligosaccharides to CDR2 can interfere with binding to antigen (23), and a fraction of immune IgG appears to be hemiglycosylated on one of the two antigen-binding sites, preventing formation of multivalent immune complexes (24). Among the foreign antigen-elicited antibodies or memory B-cell cDNA IGHV4-34*01 sequences with an inactivated AVY motif, 40% and 47%, respectively, had also somatically inactivated the CDR2 glycosylation sequon (Fig. 1A). We speculate that inactivation of the sequon could increase accessibility of their binding sites to the eliciting foreign antigens, although this possibility will need to be tested experimentally in future biophysical studies.
Almost all other germ-line human VH elements do not contain an Asn–X–Ser/Thr sequon, although sequons can be generated by somatic hypermutation (25, 26). In deep-sequenced cDNA from human CD27+ IgD− memory B cells, N-glycosylation sequons had been acquired by somatic mutation in 20–35% of sequences using IGHV4-61, IGHV4-39, and IGHV4-59 but in only 2–4% of those using IGHV3-23, IGHV6-1, IGHV3-7, IGHV2-5, IGHV3-48, IGHV1-18, and IGHV3-15 (Fig. 1B). The low frequency in IGHV3-23, IGHV3-48, and IGHV3-15 is notable because these contain hypermutation hotspots in CDR2 enabling the germ-line sequence to be converted into a glycosylation sequon in the majority of follicular lymphomas using these V elements (26). These results are consistent with independent estimates that 20% of normal human antibodies carry N-linked glycans in their V domain (23, 24, 26–28).
Activation of Anergic B Cells to Form Germinal Centers in Mice.
The results above are consistent with the possibility that antibodies can be selected in GCs to mutate away from self-reactivity. This is the simplest explanation for frequent inactivation of AVY sequences in switched IGHV4-34 antibodies, and has been inferred previously (12). However, frequent inactivation of the AVY motif could also result from a mutation hotspot or selection for better binding to foreign antigens (29), and these alternative explanations cannot be excluded because the selecting foreign and self-epitopes are heterogeneous and not well-defined biophysically. To resolve this question experimentally, we analyzed anergic IgD+ IgMlow B cells expressing an antibody, Hy10, recognizing a highly characterized epitope on hen egg lysozyme (HEL) (30–32) under conditions where the same epitope was displayed on foreign and self-antigens, or only on foreign antigen as a control.
B cells expressing the Hy10 antibody were obtained from MD4:ML5 double-transgenic mice making HEL protein as a circulating self-protein at concentrations of ∼10−9 M. These concentrations are higher than the KD of the Hy10 antibody, so that >50% of BCR occupancy by circulating self-HEL protein induced an IgD+ IgMlow anergic state (5, 33). As controls, IgD+ IgMhi naïve B cells unexposed to self-HEL were obtained from MD4 single-transgenic mice. HEL-specific B cells (105) were injected into nontransgenic mice, diluting them to a frequency <0.01% of the recipient repertoire. The recipients were immunized with HEL or a lower-affinity variant with two mutations in antibody contact residues, HEL2X, coupled to the surface of sheep red blood cells (SRBCs) to enable the anergic or naïve B cells to bind the foreign immunogen and collaborate with SRBC-specific T-follicular helper (TFH) cells required for GC formation. Flow cytometric and immunofluorescence analysis 5–6 d later yielded the surprising result that anergic cells usually produced more GC progeny than naïve counterparts (Fig. 2 A–C). This was opposite the decreased accumulation of plasmablasts from anergic B cells previously observed during extrafollicular reactions driven by naïve helper T cells that did not support GC differentiation (34–36) or induced with SRBC-primed T cells in irradiated recipients (33).
Fig. 2.
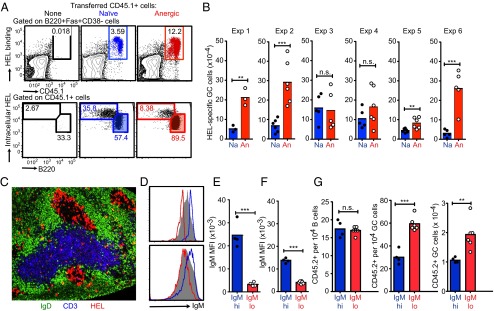
IgMlow anergic B cells paradoxically make more GC progeny. (A–C) CD45.1-marked HEL-specific B cells (105) that were anergic (An) or naïve (Na) were injected into normal C57BL/6 recipient mice together with 108 HEL-SRBCs or HEL2X-SRBCs. (A) Representative flow cytometric analysis of recipient spleen cells 5 d after immunization with HEL2X-SRBCs, gating on Fas+ CD38− GC B cells to enumerate percentage CD45.1+ donor-derived (Upper) or gating on CD45.1+ donor B cells (Lower) and measuring the percentage differentiated into B220low plasma cells with high intracellular HEL-binding antibody or into GC cells. (B) Arithmetic means and data from individual recipients immunized with HEL2X-SRBCs (exp 1–4) or HEL-SRBCs (exp 5 and 6). Statistical analysis was by t test: **P ≤ 0.01, ***P ≤ 0.001; n.s., not significant. (C) Representative immunofluorescence staining of a spleen cryosection from the recipient of anergic cells on day 5 after immunization with HEL2X-SRBCs, locating most HEL-binding progeny (red) in GCs. (D–G) Mature CD23+ B cells from C57BL/6 mice were stained with F(ab′) anti-IgM, and cells in the lowest (IgMlo; red histograms) or highest (IgMhi; blue histograms) quartile of cell-surface IgM were sorted and injected into B6.SJL-CD45.2 congenic recipients, and the recipients were immunized with SRBCs. (D) Distribution of surface IgM on the sorted populations compared with unsorted CD23+ B cells (gray-filled histogram) on day 0 (Upper) and compared with CD45.1+ recipient B220+ B cells 6 d after transfer (Lower). (E and F) Surface IgM mean fluorescence intensity (MFI) on the sorted populations from separate donors at the time of transfer (E) and in separate recipients after 6 d (F). (G) Frequency of CD45.2+ donor-derived cells among B220+ Fas− GL7− B cells (Left) or among B220+ Fas+ GL7+ GC cells (Center) in individual recipients, and the total number of donor-derived GC cells (Right). Statistical analysis was done as above.
Enhanced GC differentiation was also exhibited by the corresponding subset of IgD+ IgMlow B cells in the normal preimmune repertoire. Mature CD23+ B cells with the lowest and highest quartiles of cell-surface IgM were sorted from normal B6 mice (Fig. 2D) and injected into congenic B6.CD45.1 recipients together with SRBCs. Six days later, most of the CD45.2+ donor B cells were not stimulated by the SRBC immunogen and persisted either as IgMlow or IgMhi naïve B cells (Fig. 2 D–F) and at comparable frequencies (Fig. 2G, Left), indicating that their difference in cell-surface IgM is a stable trait and not simply a maturation marker. A subset of the transferred IgMlow or IgMhi CD45.2+ B cells were stimulated to differentiate into Fashi GL7+ GC cells, in competition with the much more numerous host-derived CD45.1+ GC cells. IgMlow B cells nevertheless formed twice as many GC cells as IgMhi B cells (Fig. 2G). The bias toward GC differentiation in self-reactive B cells may result from decreased BCR signaling to NF-κB and consequent poor induction of Ebi2 and Irf4 in anergic B cells (37), because induction of these genes favors plasma cell differentiation and disfavors GC differentiation (38–40).
Mouse Hy10 Antibody Variants Selected by Self-Antigen.
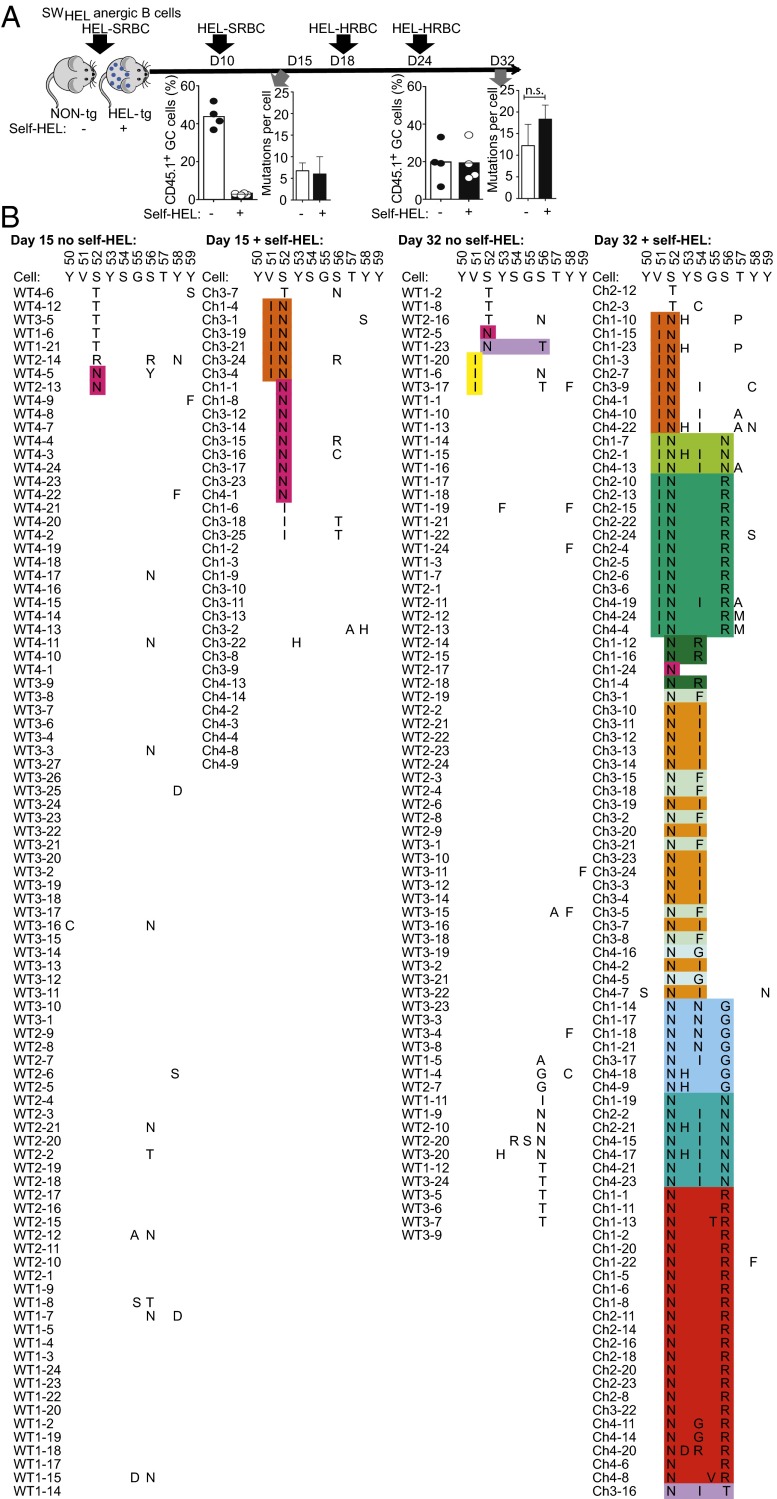
VH hypermutation and selection in the GC-differentiated progeny of anergic cells were studied by performing cell transfers as above but with anergic IgD+ IgMlow B cells from SWHEL:ML5 double-transgenic mice that express Hy10 with the VDJH exon integrated into the normal Igh locus (41). HEL was coupled to the foreign carrier and was also expressed as self-HEL in half the recipients, so that the SWHEL B cells would encounter identical epitopes on foreign and self-antigens and any selection could not be attributed to conventional affinity maturation to the foreign antigen. Analogous to the repeated immunizations with RhD+ erythrocytes used to raise the human antibodies analyzed above, the mice were boosted with repeated injections of HEL-coupled erythrocytes on days 10, 18, and 24 (Fig. 3A). The last two boosters were with HEL coupled to serologically non–cross-reactive horse red blood cells (HRBCs) to minimize immunogen clearance by SRBC-binding antibodies that develop by 14 d, and to parallel the stimulation of broadly neutralizing antibodies to conserved influenza hemagglutinin stalk epitopes by serologically disparate virus strains (42). Anergic SWHEL B cells formed large numbers of GC progeny on day 15 in immunized nontransgenic recipients, where the GC progeny were free to accumulate without ongoing binding of self-HEL (Fig. 3A). In HEL-transgenic recipients, where the GC B cells concurrently encountered both self-HEL and foreign HEL-SRBCs, accumulation of GC progeny was greatly diminished during the early phase of the GC reaction on day 15. This was consistent with previous evidence that binding soluble or self-HEL triggers BCR down-regulation, migration to the base of the GC dark zone, and apoptosis of GC cells (43, 44). By contrast, self-HEL did not inhibit GC accumulation later in the response.
Fig. 3.
Recurring VH CDR2 mutations selected by self-antigen. (A) Anergic CD45.1-marked HEL-specific B cells (105) from SWHEL x ML5 donor mice were transferred into non-Tg (no self-HEL) or ML5 HEL-Tg (+self-HEL) C57BL/6 recipients together with HEL-SRBCs, and recipients were boosted on the indicated days with HEL-SRBCs or HEL-HRBCs. The % CD45.1+ cells among B220+ Fas+ GL7+ GC B cells in individual recipients on day 15 or 32 is shown. Single CD45.1+ B220+ Fas+ GL7+ cells were sorted into microtiter plates for Hy10 VDJH exon sequencing, yielding the mean number of VDJH nucleotide substitutions in B cells from individual recipients and the SD of this mean between recipients. (B) Amino acid changes in CDR2 in single sorted B cells after 15 or 32 d in recipients with or without self-HEL. Cells from different mice are denoted by the prefix Ch1–Ch4 for the four HEL-transgenic mice on day 15 and day 32, and WT1–4 for the four non-Tg mice at each time point. From each of the mice analyzed, the individual cells are numbered with a suffix from 1 to 25. The Hy10 sequence for CDR2 residues 50–59 is shown above. Substitutions in individual cells are shown, clustered by sequence similarity. The different colors denote recurring combinations of CDR2 mutations, often found in several independent recipient mice, as indicated by the Ch1–Ch4 prefix.
VDJH exon sequencing of single SWHEL GC cells early and late in the response revealed that they accumulated as many VDJH mutations per cell when exposed to self-HEL in the GCs as in the controls (Fig. 3A), but recurring CDR2 amino acid substitutions became prevalent only when self-HEL was present (Fig. 3B). By day 15, 42% of Hy10-expressing GC cells had acquired a Ser52Asn substitution, and 40% of these had also acquired a flanking Val51Ile mutation. By day 32, 93% had acquired S52N and 95% of these paired S52N with flanking V51I, S56R, or various S54 substitutions. In the absence of self-HEL exposure during the GC reaction only 1–2% of GC cells carried S52N and none paired S52N with V51I or S56R, demonstrating that the CDR2 mutations were selected in response to self-antigen and not by the foreign antigen on its own.
The recurrently substituted residues S52, S54, and S56 each contact HEL, with S52 located at the center of the contact surface, whereas V51 is immediately beneath (Fig. 4A). The fact that many B cells on day 15 had only acquired S52N (Fig. 3B) implied it was selected first, followed by the flanking CDR2 mutations. This was confirmed within individual recipients by reconstructing mutation genealogies using rare (nonhotspot) synonymous mutations as clonal markers (Fig. 4B). Hy10 IgG1 antibodies with the recurrent CDR2 mutations were expressed and tested for binding the foreign immunogen HEL-SRBCs by flow cytometry. At any fixed IgG1 concentration, ∼75% less S52N antibody bound than wild-type Hy10, demonstrating that this single mutation decreased either the affinity or avidity of binding to the foreign immunogen (Fig. 4C). Pairing S52N with V51I partly restored binding (Fig. 4C). Binding of wild-type Hy10 to HEL-SRBCs was blocked by free HEL monomer at the concentration that circulates in ML5 mice (5), whereas much higher [HEL] monomer was required to block binding of the mutated antibodies to the foreign antigen (Fig. 4D).
Fig. 4.
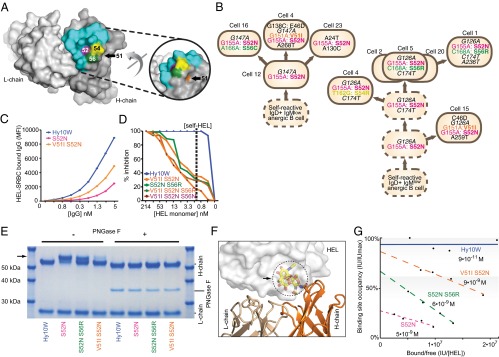
Mutation away from self-reactivity by modulating glycosylation, affinity, and avidity. (A) Location of mutated VH residues relative to the H-chain contact surface with lysozyme in blue, from Protein Data Bank (PDB) ID code 3D9A (32). (B) Representative GC B-cell genealogies in individual recipients on day 15 (Left; from mouse number Ch3) and day 32 (Right; from mouse number Ch1). Clonally related cells were identified by shared silent mutations in nonhotspot VH nucleotides (italicized black). Recurrent nonsynonymous nucleotide and corresponding amino acid substitutions are colored; other mutations are shown in black. Sequenced cells are numbered as in Fig. 2, and inferred precursors are indicated by dashed circles. (C and D) IgG1 antibodies with the wild-type H-chain sequence (Hy10W) or the indicated CDR2 substitutions were expressed in CHO cells, and binding to HEL-SRBCs was detected by fluorescent anti-IgG antibody and flow cytometry. MFI, geometric mean fluorescence intensity of IgG fluorescence on gated single erythrocytes. (C) Binding in the absence of competing soluble HEL. (D) Percent inhibition of binding to HEL-SRBCs of 0.5 nM IgG in the presence of varying concentrations of HEL monomer in solution. The dashed line denotes the mean concentration of self-HEL in the extracellular fluid of ML5 mice. (E) Hy10 IgG1 antibodies with the wild-type H-chain sequence (Hy10W) or the indicated CDR2 substitutions were expressed in 293T cells, purified, and analyzed by SDS/PAGE under reducing conditions and stained with Coomassie blue. Where indicated, N-linked carbohydrates were removed from the antibodies by treatment with PNGase F (+). The arrow indicates mobility shift due to VH glycosylation. (F) Molecular model of V-region glycosylation, with carbohydrate (arrow) occupying the HEL antigen-binding site. The model was generated using the structure of Hy10W bound to HEL (cartoon and transparent surface; PDB ID code 3D9A), with a single N-linked branched NAG-FUC-NAG trisaccharide modeled onto Kabat position 52 of the variable heavy domain (colored sticks; glycan-derived from PDB ID code 3CA1). (G) Biolayer interferometry measurements of binding of HEL antigen in solution to immobilized Hy10 IgG antibodies. Plotted is binding site occupancy (% Hy10W maximum) versus Octet IU intensity units per antigen concentration (nm/M).
N-Linked Glycosylation of the Antigen-Binding Sites in Mutated Hy10.
The primary S52N mutation created a potential N-glycosylation sequon within CDR2, N52–Y53–S54. SDS/PAGE confirmed that S52N resulted in a second N-linked carbohydrate on 72% of H chains (Fig. 4E), in addition to the expected glycan in the IgG1 C region. Pairing S52N with S56R or V51I decreased the fraction of V-region glycosylated H chains to 43% and 12%, respectively. Substitution of flanking residues thus modulates the efficiency of carbohydrate attachment to N–X–S/T sequons in antibody V regions, consistent with observations in other proteins (45). V51I or S56R were acquired by 42% of the S52N-bearing cells on day 32, and another 54% acquired an S54 substitution eliminating the sequon itself (Fig. 3B), so that 95% of the B cells that had initially acquired glycosylation of their antigen-binding sites had cleared the majority of sites with a second CDR2 mutation.
S52 lies at the center of the buried surface between Hy10 and HEL (30), and modeling studies predict a bulky carbohydrate here would preclude antigen binding (Fig. 4F). Consistent with that prediction, biosensor measurement of binding to and dissociation from immobilized IgG of varying concentrations of soluble HEL monomer revealed that the maximum amount bound to immobilized S52N IgG was ∼30% of wild type (Fig. 4G). For the sites that could bind, HEL dissociated 70 times faster from S52N (kd 3 × 10−3 s−1) than from wild-type Hy10 (kd 2 × 10−5 s−1), reducing the overall KD from ∼1 × 10−10 M for the wild-type binding site to 5 × 10−9 M for S52N (differences between on-rates were minor for all of the analyzed mutants). Pairing S52N with S56R or V51I preserved the lower affinity (KD 6–9 × 10−9 M, kd 2–3 × 10−3 s−1) but increased the sites available to bind, consistent with only a minority of VH regions occupied by glycan. When single-chain variable fragments (scFvs) were expressed in bacteria, where N-linked glycosylation cannot occur, biosensor analysis confirmed that S52N did not result in any difference in binding site availability but lowered affinity 100-fold, whereas pairing S52N with V51I or S56R had little additional effect on either measure (Table 1).
Table 1.
Biolayer interferometry measurements of binding of HEL antigen in solution to immobilized Hy10 antibodies produced as glycosylated IgG in mammalian cells and aglycosylated scFv in bacteria
| Antibody | KD, M | ka, M−1 s−1 | kd, s−1 | Occupancy, % |
| Hy10 (IgG) | ||||
| WT | 9 × 10−11 | 2.60 × 10−5 | 2.30 × 10−5 | 100 |
| S52N | 5 × 10−9 | 5.46 × 10−5 | 2.80 × 10−3 | 27 |
| S52N/S56R | 6 × 10−9 | 4.34 × 10−5 | 2.81 × 10−3 | 53 |
| V51I/S52N | 9 × 10−9 | 2.41 × 10−5 | 2.17 × 10−3 | 76 |
| Hy10 (scFv) | ||||
| WT | <1 × 10−10 | 1.71 × 10−5 | <10−5 | 100 |
| S52N | 2 × 10−9 | 4.63 × 10−5 | 7.11 × 10−4 | 109 |
| S52N/S56R | 5 × 10−9 | 2.31 × 10−5 | 1.12 × 10−3 | 99 |
| V51I/S52N | 4 × 10−9 | 2.65 × 10−5 | 9.88 × 10−4 | 96 |
Discussion
Evolutionary selection for germ-line V, D, and J elements that have a propensity to bind foreign antigens is likely to bring with it propensity to bind self-antigens (46), as illustrated by the AVY hydrophobic patch in IGHV4-34 promoting binding to foreign RhD on allogeneic erythrocytes and to self-I/i on syngeneic erythrocytes (13). The observations here provide two lines of evidence that autoantibodies carried on anergic B cells can be “redeemed” in response to foreign antigen by hypermutation and selection in germinal centers to remove self-reactivity, instead of being discarded by clonal deletion or receptor editing. First, human antibodies using the IGHV4-34 germ-line gene uniformly bind self-antigens on erythrocytes, rendering B cells carrying these antibodies anergic, but almost half the mutated IgG antibodies or memory B-cell sequences using this V gene have acquired somatic mutations in the AVY motif responsible for binding to self-erythrocytes. Second, anergic mouse B cells preferentially formed germinal centers in cell-transfer studies, and those carrying the well-characterized Hy10 antibody to hen egg lysozyme mutated away from self-reactivity by acquiring CDR2 mutations when lysozyme was present as a self-antigen and as a foreign antigen in the GC. N-linked glycosylation of CDR2 in a fraction of the H chains appears to provide a mechanism to modulate autoantibody avidity by occupying a varying proportion of antibody-binding sites with glycan.
In the studies with Hy10, a single somatic mutation—S52N—masked self-reactivity by causing the majority of binding sites to become occupied with an N-linked glycan. S52N also diminished self-reactivity by decreasing the affinity of the residual unglycosylated sites by ∼100-fold. Acquisition of S52N was followed by various flanking secondary mutations that partly or completely prevented N-glycosylation, resulting in antibodies whose binding sites were now available to bind antigen but with a greatly reduced affinity (KD 5–9 × 10−9 M). The reduced affinity was less than the circulating HEL concentration (10−9 M), so that only a small proportion of sites would be occupied by self-HEL at any one time, and the antibodies were now free to bind the multivalent HEL-SRBC antigen without competitive inhibition by the circulating monomeric self-HEL. Despite identity of the HEL-epitope on self- and foreign antigens, increasing the single-site dissociation rate by ∼100-fold enabled the monomeric self-HEL and polymeric array of foreign HEL on SRBCs to be discriminated by epitope valency and binding cooperativity. This parallels the discrimination of sparsely arrayed self-glycans and densely arrayed foreign glycans by mannose-binding protein (47) or by a hypermutated antibody to HIV gp120 (48).
The high starting affinity of Hy10 for HEL has experimental advantages and limitations. A chief advantage is that Hy10 affinity has already been optimized by repeated immunization of the mouse that yielded the Hy10 hybridoma, making it possible here to study selection by self-antigen of affinity-lowering mutations without concurrent selection by foreign antigen for affinity-raising mutations. The lack of selection for affinity-raising mutations presumably explains the absence of recurring mutations in SWHEL B cells responding to HEL-SRBCs alone in nontransgenic control recipients. Because S52N became frequent only in B cells responding to HEL-SRBCs in the presence of self-HEL, its acquisition was driven by the presence of self-HEL and was not the result of a mutation hotspot or an indirect effect of selection for increased affinity for the foreign antigen. These three alternative explanations are difficult to disentangle in the case of recurrent mutations found in VH4-34+ antibodies or in other physiological antibody responses.
A limitation of the high affinity is that it could be argued that the findings with Hy10 may not be generalizable to physiological antibody responses, because most preimmune antibodies would have much lower affinity for self-antigens and their binding sites would not be as highly occupied by self-antigen. However, comparable competitive inhibition would be expected when antibodies in the preimmune repertoire start out with low affinity (e.g., KD 10−6 M) for serum glycoproteins such as IgG, because IgG is present at concentrations of greater than 10−6 M. Likewise, the concentration of I/i glycoproteins in serum and on self-erythrocytes is likely to be very high, and they compete for binding of unmutated VH4-34+ antibodies to RhD, vaccinia virus, or tetanus toxoid.
Selection in GCs for decreased antibody affinity and avidity is surprising because antibody hypermutation in GCs has primarily been considered to underpin increases in affinity (49), driven by B-cell competition to capture and present antigen to TFH cells (50). When an antibody recognizes self- and foreign epitopes that differ in tertiary structure, such as VH4-34 antibody recognition of self-I/i versus foreign RhD or vaccinia, decreased affinity for self could arise as a passive by-product of positive selection for better structural fit and higher binding affinity against the foreign epitope (29). This type of passive selection against self-reactivity is nevertheless excluded in the Hy10 experiments with HEL because (i) it is not observed in control recipients where the GC B cells only encountered foreign HEL and (ii) the mutations selected in the presence of self-HEL also decrease binding to the foreign HEL measured either free in solution or conjugated to SRBCs.
Although the findings here demonstrate acquisition of self/non-self discrimination by antibody mutation away from self-reactivity, future studies will need to investigate how lower affinity for self was selected. Positive selection by TFH cells could actively select for lower-affinity binding because GC B cells whose BCRs are mostly occupied by self-HEL or self-I/i carbohydrates will have fewer binding sites available to capture HEL-SRBCs or RhD antigens and present fewer peptides to TFH cells. Progeny with mutated antibodies of lower affinity for self, and consequently less self-occupancy, might paradoxically capture more foreign antigen and receive more help rather than less. This would ensure that secreted antibody binding to foreign antigen is not outcompeted by self-antigen, nor rapidly cleared from the circulation by binding self-antigens.
However, selection by TFH cells does not readily account for self-antigen–driven acquisition of S52N in Hy10, because the mutation blocked most antigen-binding sites with glycan to diminish sites available to take up foreign HEL-SRBCs. An active negative effect of self-antigen itself (44) provides an alternative explanation for the acquisition of S52N. In experiments where HEL was injected at the height of a GC response by Hy10-expressing B cells, it triggered the GC cells to concentrate in the dark zone away from TFH cells and to down-regulate surface BCRs within 2 h, followed by a wave of apoptosis after 4 h (43). BCR engagement by foreign HEL-SRBC fragments presented in the GC light zone may also trigger migration back to the dark zone (50), but once in the dark zone the GC cells would get relief from this BCR signal to regain surface BCRs and the capacity to migrate back to the light zone. By contrast, self-antigen that is circulating or on the membrane of all cells would continue to stimulate the BCRs once the cells have moved to the dark zone, signaling them to stay removed from TFH cells and down-regulating their surface BCRs to deprive the cells of a tonic PI3-kinase survival signal (51). Masking the antigen-binding sites with glycan may relieve this continuous BCR signal from self-antigen. Recurrent selection for acquired V-region N-glycosylation sequons in follicular lymphomas (26) might also be driven by masking of self-reactivity to increase the pool of surface BCRs delivering PI3-kinase survival signals. Glycosylated BCRs may also be recognized by lectins on follicular dendritic cells or macrophages in the germinal center to deliver a survival advantage (26).
A need for some preimmune antibodies to mutate away from self-reactivity provides an alternative explanation for the phenomenon of repertoire shift during the maturation of antibody responses, when certain antibody specificities that ultimately dominate responses to haptens (52–57) or RhD alloantigens (16, 20) or bind conserved neutralizing epitopes of viruses (58–62) are minimally represented in the primary wave of plasma cells but gradually emerge after repetitive stimulation by foreign antigen. Inherited defects that prevent hypermutation away from self-reactivity by antimicrobial antibodies may explain the surprising prevalence of autoantibodies in human AICDA deficiency, where GCs are formed but hypermutation is crippled (63). Structural constraints that make it difficult for mutations to remove self-binding without also losing binding to the eliciting foreign antigen, as has been shown for VH4-34 antibodies against RhD (13) and for the 2F5 broadly neutralizing antibody against HIV (61), may explain the difficulty eliciting high titers of certain broadly neutralizing antibodies against viruses (60–62).
Materials and Methods
Human Memory B-Cell Repertoire Analysis by Massively Parallel Sequencing.
CD19+ CD27+ IgD− peripheral blood mononuclear cells were isolated from 13 healthy donors (18–86 y) after obtaining written consent as approved by the Guy's Hospital Research ethics committee, London. cDNA was synthesized, and Ig genes were PCR-amplified by a seminested, isotype-specific reaction and sequenced on the 454 GS FLX Titanium Sequencer as previously described (21, 22). The accuracy of the method as a whole was determined using analysis of results from a control Ig gene and was less than one error per 300 bp, or one per 1,300 bp if indels (a known issue with the sequencing platform) were discounted. Sequences were assigned an isotype and aberrant PCR products were removed after the data was checked for quality based on presence and orientation of sequence motifs, together with minimum- and maximum-size thresholds based on plausible limits determined by germ-line Ig organization as previously described (21, 22). Sequences that passed these initial quality control criteria but whose reads were too short to span the CDR3 region were excluded as uninformative. For the remaining sequences, the IGHV, IGHD, and IGHJ gene usage and amino acid sequence of each region of the variable region were determined using IMGT/V-QUEST (64) allowing for indels. This provides a second round of quality control, with only sequences that generated a V-QUEST output and spanned the CDR3 region being analyzed further. The amino acid sequence of the VDJ region was defined on the basis of the V-QUEST output, and sequon motifs were identified using regular expression searches for N[^P][ST] (N followed by any amino acid other than a P, followed by an S or T). Data were combined and subsequent analyses were performed in Excel (Microsoft). A total of 78,074 sequences were obtained from memory B cells. Of these, 3,030 were IGHV4-34 genes that had an IGHV region greater than 74 bp, and 14,475 were mutated sequences from CD27+ IgD− memory cells using genes other than IGHV4-34, IGHV5a, or IGHV1-8 (the genes that have a sequon in germ-line configuration).
Adoptive Transfers.
Recipients were C57BL/6 nontransgenic or ML5 HEL-transgenic mice. Donors were MD4, MM4, or SWHEL C57BL/6.SJL-Ptprca (CD45.1) congenic mice. Mice were bred and housed under specific pathogen-free conditions at the Australian National University (ANU), and all procedures were approved by the ANU Animal Ethics and Experimentation Committee. Spleen cells containing 105 HEL-binding B cells were i.v. injected together with 2 × 108 SRBCs conjugated to HEL or HEL2X and boosted by i.v. injection of 2 × 108 HEL-SRBCs or HEL-HRBCs, and recipient spleen cells were analyzed 5–32 d later by flow cytometry, single-cell sorting, H-chain sequencing, and cryosection staining as described previously (65, 66). For responses by nontransgenic B cells, B6 spleen cells were stained with antibody to CD23 (BioLegend) and Alexa647-conjugated F(ab′) fragments of goat anti-mouse IgM antibody (Jackson ImmunoResearch) and sorted into IgMlow and IgMhi quartiles. Preliminary studies established that this combination of antibodies did not alter the survival of stained B cells after injection into congenic B6.CD45.1 recipients. Sorted B cells (1.5 × 106) were injected with SRBCs (2 × 108) into the lateral tail vein of individual B6.CD45.1 recipients, and 6 d later spleen cell suspensions were analyzed by flow cytometry as above.
Expression, Purification, and Deglycosylation of Hy10 IgG Variants.
Plasmid DNA (pcDNA3; Invitrogen) containing the unmodified Hy10 light-chain gene or wild-type, S52N, V51I/S52N, or S52N/S56R versions of the Hy10 IgG1 heavy-chain gene were cotransfected at a 4:1 light-to-heavy ratio into CHO or HEK293F cells (Invitrogen). Transfections were performed using the Lipofectamine 2000 reagent (Invitrogen) per the manufacturer’s instructions, and recombinant antibodies were purified from culture supernatants at day 7 after transfection using Protein G Sepharose 4 Fast Flow (GE Healthcare Life Sciences). Eluted IgG samples were dialyzed into PBS using SnakeSkin dialysis tubing with a 3.5-kDa molecular weight cutoff (Thermo Scientific). Ten micrograms of IgG was treated with PNGase F per the manufacturer’s instructions (New England Biolabs) and first incubated 10 min at 94 °C in the presence of denaturing buffer, followed by addition of PNGase F in Nonidet P-40 and G7 (50 mM sodium phosphate pH 7.5) buffers overnight at 37 °C. This process was replicated without PNGase F as a negative control. Treated samples were analyzed by SDS/PAGE on precast 4–12% bis-Tris gels (NuPAGE; Invitrogen), visualized with InstantBlue protein stain (Expedeon), and subjected to densitometry analyses.
Affinity Measurements.
For HEL-SRBC binding studies, culture supernatants were analyzed by ELISA to determine IgG concentration, diluted to 0.5 nM IgG in PBS buffer with varying [HEL], incubated for 2 h before addition to HEL-SRBCs, washed, developed with fluorescent anti-IgG antibodies, and analyzed by flow cytometry as described previously (65). For biolayer interferometry measurements (ForteBio), purified samples of wild-type or mutant Hy10 were biotinylated at a 5:1 molar ratio using EZ-Link NHS-PEO4-Biotinylation reagent followed by desalting on a Zeba Spin column (Thermo Scientific). Streptavidin biosensors were preblocked with 1% BSA in PBS for 1 h and incubated with biotinylated antibody. The coupling of antibody to sensors was quantified by biolayer interferometry. Measurements were conducted using hen egg lysozyme (Sigma-Aldrich) diluted in PBS buffer.
Supplementary Material
Acknowledgments
We thank the staff of the Australian Phenomics Facility and the John Curtin School of Medical Research Flow Cytometry Facility. This work was supported through the National Health and Medical Research Council (NHMRC) Program, Project and Development Grants (to R.B., D.C., and C.C.G.), the Human Frontiers Science Program (D.K.D.-W.), Australian Research Council Discovery Grants (to D.C.), an NHMRC Australia Fellowship (to C.C.G.), NHMRC Research Fellowships (to R.B. and D.C.), and an Endeavour Postgraduate Award from the Australian Government (to Z.S.).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 9022.
References
- 1.Burnet FM. The Clonal Selection Theory of Acquired Immunity. Cambridge, UK: Cambridge Univ Press; 1959. [Google Scholar]
- 2.Goodnow CC, Ohashi PS. Immunological tolerance. In: Paul WE, editor. Fundamental Immunology. 7th Ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013. pp. 765–794. [Google Scholar]
- 3.Jerne NK. The somatic generation of immune recognition. Eur J Immunol. 1971;1(1):1–9. doi: 10.1002/eji.1830010102. [DOI] [PubMed] [Google Scholar]
- 4.Diaz M, Klinman NR. Relative roles of somatic and Darwinian evolution in shaping the antibody response. Immunol Res. 2000;21(2-3):89–102. doi: 10.1385/IR:21:2-3:89. [DOI] [PubMed] [Google Scholar]
- 5.Goodnow CC, Crosbie J, Jorgensen H, Brink RA, Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989;342(6248):385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- 6.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 7.Duty JA, et al. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J Exp Med. 2009;206(1):139–151. doi: 10.1084/jem.20080611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quách TD, et al. Anergic responses characterize a large fraction of human autoreactive naive B cells expressing low levels of surface IgM. J Immunol. 2011;186(8):4640–4648. doi: 10.4049/jimmunol.1001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zikherman J, Parameswaran R, Weiss A. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 2012;489(7414):160–164. doi: 10.1038/nature11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cappione A, III, et al. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005;115(11):3205–3216. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potter KN, Hobby P, Klijn S, Stevenson FK, Sutton BJ. Evidence for involvement of a hydrophobic patch in framework region 1 of human V4-34-encoded Igs in recognition of the red blood cell I antigen. J Immunol. 2002;169(7):3777–3782. doi: 10.4049/jimmunol.169.7.3777. [DOI] [PubMed] [Google Scholar]
- 12.Zheng NY, et al. Human immunoglobulin selection associated with class switch and possible tolerogenic origins for C delta class-switched B cells. J Clin Invest. 2004;113(8):1188–1201. doi: 10.1172/JCI20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorpe SJ, et al. Anti-D and anti-i activities are inseparable in V4-34-encoded monoclonal anti-D: The same framework 1 residues are required for both reactivities. Transfusion. 2008;48(5):930–940. doi: 10.1111/j.1537-2995.2007.01624.x. [DOI] [PubMed] [Google Scholar]
- 14.Richardson C, et al. Molecular basis of 9G4 B cell autoreactivity in human systemic lupus erythematosus. J Immunol. 2013;191(10):4926–4939. doi: 10.4049/jimmunol.1202263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman CJ, Mockridge CI, Hamblin TJ, Stevenson FK. Tracking of the V4-34 (VH4-21) gene in human tonsil reveals clonal isotype switch events and a highly variable degree of somatic hypermutation. Clin Exp Immunol. 1996;105(2):360–368. doi: 10.1046/j.1365-2249.1996.d01-769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bye JM, et al. Germline variable region gene segment derivation of human monoclonal anti-Rh(D) antibodies. Evidence for affinity maturation by somatic hypermutation and repertoire shift. J Clin Invest. 1992;90(6):2481–2490. doi: 10.1172/JCI116140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weitkamp JH, et al. Infant and adult human B cell responses to rotavirus share common immunodominant variable gene repertoires. J Immunol. 2003;171(9):4680–4688. doi: 10.4049/jimmunol.171.9.4680. [DOI] [PubMed] [Google Scholar]
- 18.Lantto J, et al. Capturing the natural diversity of the human antibody response against vaccinia virus. J Virol. 2011;85(4):1820–1833. doi: 10.1128/JVI.02127-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poulsen TR, Jensen A, Haurum JS, Andersen PS. Limits for antibody affinity maturation and repertoire diversification in hypervaccinated humans. J Immunol. 2011;187(8):4229–4235. doi: 10.4049/jimmunol.1000928. [DOI] [PubMed] [Google Scholar]
- 20.Thorpe SJ, et al. Cold agglutinin activity is common among human monoclonal IgM Rh system antibodies using the V4-34 heavy chain variable gene segment. Transfusion. 1997;37(11-12):1111–1116. doi: 10.1046/j.1537-2995.1997.37111298088038.x. [DOI] [PubMed] [Google Scholar]
- 21.Wu YC, et al. High-throughput immunoglobulin repertoire analysis distinguishes between human IgM memory and switched memory B-cell populations. Blood. 2010;116(7):1070–1078. doi: 10.1182/blood-2010-03-275859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu YC, Kipling D, Dunn-Walters DK. The relationship between CD27 negative and positive B cell populations in human peripheral blood. Front Immunol. 2011;2:81. doi: 10.3389/fimmu.2011.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright A, Tao MH, Kabat EA, Morrison SL. Antibody variable region glycosylation: Position effects on antigen binding and carbohydrate structure. EMBO J. 1991;10(10):2717–2723. doi: 10.1002/j.1460-2075.1991.tb07819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borel IM, Gentile T, Angelucci J, Margni RA, Binaghi RA. Asymmetrically glycosylated IgG isolated from non-immune human sera. Biochim Biophys Acta. 1989;990(2):162–164. doi: 10.1016/s0304-4165(89)80029-7. [DOI] [PubMed] [Google Scholar]
- 25.Dunn-Walters D, Boursier L, Spencer J. Effect of somatic hypermutation on potential N-glycosylation sites in human immunoglobulin heavy chain variable regions. Mol Immunol. 2000;37(3-4):107–113. doi: 10.1016/s0161-5890(00)00038-9. [DOI] [PubMed] [Google Scholar]
- 26.Zhu D, et al. Acquisition of potential N-glycosylation sites in the immunoglobulin variable region by somatic mutation is a distinctive feature of follicular lymphoma. Blood. 2002;99(7):2562–2568. doi: 10.1182/blood.v99.7.2562. [DOI] [PubMed] [Google Scholar]
- 27.Abel CA, Spiegelberg HL, Grey HM. The carbohydrate contents of fragments and polypeptide chains of human gamma-G-myeloma proteins of different heavy-chain subclasses. Biochemistry. 1968;7(4):1271–1278. doi: 10.1021/bi00844a004. [DOI] [PubMed] [Google Scholar]
- 28.Sox HC, Jr, Hood L. Attachment of carbohydrate to the variable region of myeloma immunoglobulin light chains. Proc Natl Acad Sci USA. 1970;66(3):975–982. doi: 10.1073/pnas.66.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin J, Beuscher AE, IV, Andryski SE, Stevens RC, Schultz PG. Structural plasticity and the evolution of antibody affinity and specificity. J Mol Biol. 2003;330(4):651–656. doi: 10.1016/s0022-2836(03)00631-4. [DOI] [PubMed] [Google Scholar]
- 30.Padlan EA, et al. Structure of an antibody-antigen complex: Crystal structure of the HyHEL-10 Fab-lysozyme complex. Proc Natl Acad Sci USA. 1989;86(15):5938–5942. doi: 10.1073/pnas.86.15.5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiroishi M, et al. Structural evidence for entropic contribution of salt bridge formation to a protein antigen-antibody interaction: The case of hen lysozyme-HyHEL-10 Fv complex. J Biol Chem. 2001;276(25):23042–23050. doi: 10.1074/jbc.M100480200. [DOI] [PubMed] [Google Scholar]
- 32.Acchione M, et al. Light chain somatic mutations change thermodynamics of binding and water coordination in the HyHEL-10 family of antibodies. Mol Immunol. 2009;47(2-3):457–464. doi: 10.1016/j.molimm.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodnow CC, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334(6184):676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 34.Cooke MP, et al. Immunoglobulin signal transduction guides the specificity of B cell-T cell interactions and is blocked in tolerant self-reactive B cells. J Exp Med. 1994;179(2):425–438. doi: 10.1084/jem.179.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rathmell JC, Townsend SE, Xu JC, Flavell RA, Goodnow CC. Expansion or elimination of B cells in vivo: Dual roles for CD40- and Fas (CD95)-ligands modulated by the B cell antigen receptor. Cell. 1996;87(2):319–329. doi: 10.1016/s0092-8674(00)81349-5. [DOI] [PubMed] [Google Scholar]
- 36.Cyster JG, Goodnow CC. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity. 1995;3(6):691–701. doi: 10.1016/1074-7613(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 37.Glynne R, et al. How self-tolerance and the immunosuppressive drug FK506 prevent B-cell mitogenesis. Nature. 2000;403(6770):672–676. doi: 10.1038/35001102. [DOI] [PubMed] [Google Scholar]
- 38.Pereira JP, Kelly LM, Xu Y, Cyster JG. EBI2 mediates B cell segregation between the outer and centre follicle. Nature. 2009;460(7259):1122–1126. doi: 10.1038/nature08226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gatto D, Paus D, Basten A, Mackay CR, Brink R. Guidance of B cells by the orphan G protein-coupled receptor EBI2 shapes humoral immune responses. Immunity. 2009;31(2):259–269. doi: 10.1016/j.immuni.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nat Immunol. 2010;11(8):681–688. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- 41.Phan TG, et al. B cell receptor-independent stimuli trigger immunoglobulin (Ig) class switch recombination and production of IgG autoantibodies by anergic self-reactive B cells. J Exp Med. 2003;197(7):845–860. doi: 10.1084/jem.20022144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wrammert J, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208(1):181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shokat KM, Goodnow CC. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995;375(6529):334–338. doi: 10.1038/375334a0. [DOI] [PubMed] [Google Scholar]
- 44.Chan TD, et al. Elimination of germinal-center-derived self-reactive B cells is governed by the location and concentration of self-antigen. Immunity. 2012;37(5):893–904. doi: 10.1016/j.immuni.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Jones J, Krag SS, Betenbaugh MJ. Controlling N-linked glycan site occupancy. Biochim Biophys Acta. 2005;1726(2):121–137. doi: 10.1016/j.bbagen.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Perelson AS, Oster GF. Theoretical studies of clonal selection: Minimal antibody repertoire size and reliability of self-non-self discrimination. J Theor Biol. 1979;81(4):645–670. doi: 10.1016/0022-5193(79)90275-3. [DOI] [PubMed] [Google Scholar]
- 47.Taylor ME, Drickamer K. Structural insights into what glycan arrays tell us about how glycan-binding proteins interact with their ligands. Glycobiology. 2009;19(11):1155–1162. doi: 10.1093/glycob/cwp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calarese DA, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300(5628):2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 49.Steiner LA, Eisen HN. Sequential changes in the relative affinity of antibodies synthesized during the immune response. J Exp Med. 1967;126(6):1161–1183. doi: 10.1084/jem.126.6.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Victora GD, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143(4):592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srinivasan L, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139(3):573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berek C, Griffiths GM, Milstein C. Molecular events during maturation of the immune response to oxazolone. Nature. 1985;316(6027):412–418. doi: 10.1038/316412a0. [DOI] [PubMed] [Google Scholar]
- 53.Mäkelä O, Karjalainen K. Inherited immunoglobulin idiotypes of the mouse. Immunol Rev. 1977;34:119–138. doi: 10.1111/j.1600-065x.1977.tb00370.x. [DOI] [PubMed] [Google Scholar]
- 54.Reth M, Hämmerling GJ, Rajewsky K. Analysis of the repertoire of anti-NP antibodies in C57BL/6 mice by cell fusion. I. Characterization of antibody families in the primary and hyperimmune response. Eur J Immunol. 1978;8(6):393–400. doi: 10.1002/eji.1830080605. [DOI] [PubMed] [Google Scholar]
- 55.Linton PJ, Decker DJ, Klinman NR. Primary antibody-forming cells and secondary B cells are generated from separate precursor cell subpopulations. Cell. 1989;59(6):1049–1059. doi: 10.1016/0092-8674(89)90761-7. [DOI] [PubMed] [Google Scholar]
- 56.Naparstek Y, et al. A single germline VH gene segment of normal A/J mice encodes autoantibodies characteristic of systemic lupus erythematosus. J Exp Med. 1986;164(2):614–626. doi: 10.1084/jem.164.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hande S, Notidis E, Manser T. Bcl-2 obstructs negative selection of autoreactive, hypermutated antibody V regions during memory B cell development. Immunity. 1998;8(2):189–198. doi: 10.1016/s1074-7613(00)80471-9. [DOI] [PubMed] [Google Scholar]
- 58.Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: The two extremes of a wide spectrum. Nat Rev Immunol. 2006;6(3):231–243. doi: 10.1038/nri1783. [DOI] [PubMed] [Google Scholar]
- 59.Verkoczy L, et al. Rescue of HIV-1 broad neutralizing antibody-expressing B cells in 2F5 VH x VL knockin mice reveals multiple tolerance controls. J Immunol. 2011;187(7):3785–3797. doi: 10.4049/jimmunol.1101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haynes BF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308(5730):1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 61.Verkoczy L, et al. Induction of HIV-1 broad neutralizing antibodies in 2F5 knock-in mice: Selection against membrane proximal external region-associated autoreactivity limits T-dependent responses. J Immunol. 2013;191(5):2538–2550. doi: 10.4049/jimmunol.1300971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Y, et al. Common tolerance mechanisms, but distinct cross-reactivities associated with gp41 and lipids, limit production of HIV-1 broad neutralizing antibodies 2F5 and 4E10. J Immunol. 2013;191(3):1260–1275. doi: 10.4049/jimmunol.1300770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Durandy A, Kracker S, Fischer A. Primary antibody deficiencies. Nat Rev Immunol. 2013;13(7):519–533. doi: 10.1038/nri3466. [DOI] [PubMed] [Google Scholar]
- 64.Brochet X, Lefranc MP, Giudicelli V. IMGT/V-QUEST: The highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 2008;36(Web Server issue):W503–W508. doi: 10.1093/nar/gkn316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Phan TG, et al. High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med. 2006;203(11):2419–2424. doi: 10.1084/jem.20061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Randall KL, et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat Immunol. 2009;10(12):1283–1291. doi: 10.1038/ni.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]