Bread wheat (hexaploid Triticum aestivum) provides an extraordinary 10,000-y story of a new species, established by early farmers, selecting for simple agronomical traits to facilitate efficient and plentiful grain harvest. The genetic changes underlying wheat domestication over thousands of years, however, included not just a collection of beneficial single gene mutations, but also introgressions and whole genome duplication. The hexaploidization event occurred spontaneously in nature, but the resulting wild species did not survive; it is known only in its domesticated form. Evolutionary bottleneck(s) reduced genetic variation of the species, and it was introduced broadly outside its native geographical range and habitat. Nevertheless, modern breeding programs delivered high-yield elite cultivars, which are planted in most major wheat-producing areas of the world. In the face of quickly declining arable land expansion and challenges from climate change, the following question arises: where can we find the next wave of increase in yield and global production of wheat? In PNAS, Kempe et al. (1) describe molecular engineering of an elegant male sterility–fertility restoration system for the exploration of heterosis (hybrid plant vigor) in wheat. In the future, this system could facilitate introduction of hybrid seeds on a large scale.
Emmer wheat, one of the eight founder crops domesticated in the Middle East about 10,000 y ago, was instrumental in spawning the Agricultural Revolution. The transition of wild to cultivated emmer initiated the extensive genetic restructuring of domesticated wheat, primarily involving mutations that resulted in the transition of types with natural seed dispersal mechanisms (brittle spikes) to types with a nonbrittle rachis (2). The initial transition from the wild habitat to cultivated fields also involved selection for free-threshing seeds, nondormant seeds, uniform and rapid germination, erect plants, and increased grain size. It was a domesticated derivative of emmer that hybridized with goatgrass about 8,000 y ago (3), probably southeast of the Caspian Sea, that resulted in the first hexaploid bread wheat (4).
During most of the last 10,000 y, farmers grew wheat in heterogenic stands consisting of mixed genotypes and ploidy levels. The first bread wheat captured genetic variation from the two progenitors, but subsequent hybrid swarms allowing for gene flow and increased genetic variation occurred in such stands. During this time, the geographical range of wheat expanded dramatically as it acquired adaptation to extreme environments and additional competitive traits such as increased tillering, increased height, and wide leaves (5).
Only in the last 100 y has wheat been grown in vast monocultures where modern plant breeding practices have led to the development of high performance varieties grown over large acreages. As a result of plant breeding, these modern varieties contain superior allelic combinations that have led to traits such as increased yield, erect leaves, increased disease and pest resistance, lodging resistance, reduced height, improved harvest index, and enhanced response to fertilizers (5). However, reduced genetic diversity has diminished the potential of wheat to evolve further and adapt to changing environments. Although the frequency of most adapted alleles has increased, other potentially adaptive alleles have been lost (6).
To meet the demand of nine billion people expected by 2050, estimated annual wheat production needs to increase at a rate of about 1.6%/y, but it has only increased at a rate of less than 1%/y in recent decades. These increases will need to be made without an expansion of arable land, in a changing global climate that will yield increased temperatures, CO2, and ozone levels, increased frequency of droughts and other extreme weather events, and alterations in virulence of pathogens and pests, and also enhanced pressure to use less synthetic fertilizers, pesticides, and fossil fuels. Fortunately, numerous avenues exist for the improvement of wheat productivity. Agronomic practices can be improved by developing more efficient and environment-friendly fertilizers and methods of weed and pest control. The genetic diversity of wild wheat relatives can be harnessed to identify and deploy new adaptive alleles and genes for pest resistance, agronomic performance, and enhanced end-use quality. Plant physiology may be manipulated to potentially increase photosynthetic capacity and radiation use efficiency for increased wheat yields (7). The use of modern and molecular tools such as high-throughput phenotyping and genotyping can maximize progeny screening and selection leading to more efficient production of superior varieties (8). Whole genome DNA sequences will provide a solid framework for the development of new molecular strategies (9). Biotechnology and genetically modified (GM) wheat, when globally accepted, would provide another means to potentially achieve advances. Finally, the capture of heterosis by the development of wheat hybrids may provide yet another method to meet the growing demand.
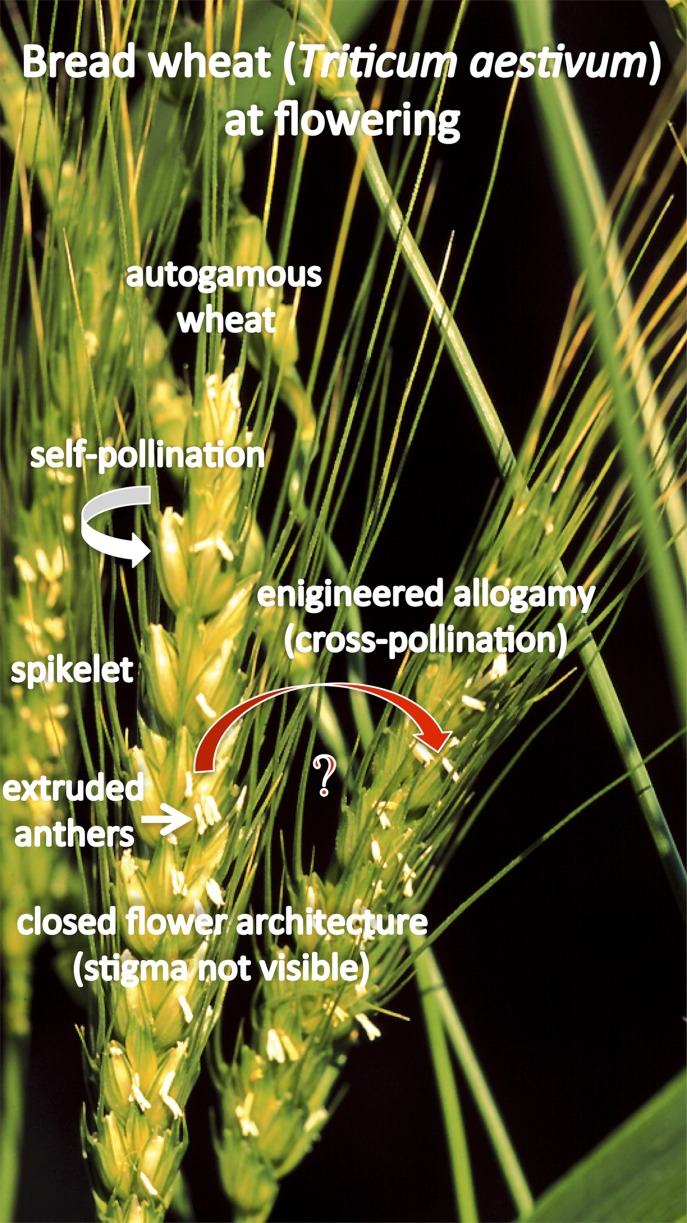
The autogamous (self-pollinating) nature of wheat makes hybrid wheat development significantly more challenging than naturally outcrossing crops (Fig. 1). Hybrid wheat has been a subject of intense focus in the last decades, especially in the 1970s and again in the 1990s. Now, it is again receiving much attention mainly due to the need to increase productivity in a changing climate. The same obstacles that hindered hybrid wheat production in the past remain today. Farmers typically save a portion of their farm-produced wheat seed for planting the following year, thus precluding the purchase of seed every year. Hybrid seed, however, cannot be saved and must be repurchased each year. The economic returns for raising wheat hybrids must outweigh the cost of purchasing seed each year despite the relatively low levels of heterosis compared with crops such as corn. From a technical standpoint, one primary obstacle has been the development of an efficient and effective male-sterile system. The wheat hybrids produced to date almost all rely on the use of chemical hybridization agents (CHAs) or cytoplasmic male sterility (CMS), but both systems have significant difficulties (1).
Fig. 1.
Heterosis (hybrid plant vigor) has not yet been systematically explored and extensively utilized for wheat yield improvement. Only 1% of wheat seeds planted worldwide are hybrid. Bread wheat is an autogamous (self-pollinating) species creating a natural barrier to hybrid seed production. Self-pollination occurs quickly primarily within the same floret (spikelet), pollen is short-lived and is shed before or when the flower starts to open, flowers have closed architecture. As a result, cross-pollination is more than an order of magnitude less frequent than self-pollination. Engineering cross-pollination (allogamy) for hybrid seed production requires modification of the reproductive system: engineering male-sterility of the female crossing partner (self-incompatibility) to prevent self-pollination and fertility restoration required for seed-producing crop plants as well as increased shedding of viable pollen and open flower architecture to allow more efficient cross-pollination. Heterotic pools of preferred crossing partners have to be established. Photograph by Jon Raupp, Kansas State University.
The report by Kempe et al. describes a successful proof-of-concept experiment aimed at developing a universal male sterility system for hybrid seed production and thus takes a big step toward addressing a major technical issue associated with the development of hybrid seed production for wheat. It uses a split-gene strategy for tapetum-specific expression of phytotoxic barnase (an RNase) to force pollen ablation and plant self-incompatibility. The split-gene feature is required for fertility restoration to allow propagation of the engineered parental lines and in plants grown from hybrid seeds, which is essential for grain crops like wheat. The two parts of the split gene encode the N-terminal and the C-terminal part of barnase, respectively, each fused to the intein domain for spontaneous ligation of the two independently expressed protein fragments to form the functional enzyme. Their coexpression occurs in the heterozygous progeny of a cross between two parent plants, each carrying a different part of the split gene. The male-sterile female crossing partner is thus established. Its pollination with an unmodified male crossing partner to produce hybrid seeds restores fertility as well, as the two split gene components segregate. Furthermore, insertion of the two split gene components at the same locus of homologous chromosomes (linkage in repulsion) eliminates recombination as a source of unwanted genotypes. It requires an additional engineering step to separate the two components of the split gene system, which initially are inserted into the genome together, but then one or the other is deleted from the locus by the action of a site-specific recombinase (integrase).
The multicomponent modern molecular engineering concept is very well thought out and its potential applications go beyond just hybrid seed production. The system has significant features, not otherwise available for wheat. Only the female crossing partner has to be modified. Once it is available, crosses with many male partners can be tested for vigor and overall performance. The engineered parental lines can be maintained easily. There is no need for any additional steps to restore fertility. There is no need for chemical treatments. Establishing a single male-sterile female crossing line required a substantial amount of work, because of the complex multistep design, but the strategy worked as expected. Good strategies have been proposed to overcome the inefficiency. Large-scale hybrid breeding program and commercial hybrid seed production will certainly require additional improvements, but this study provides a significant launch.
What is next? The system needs to be tested on a larger scale and include field trials. It can then provide an important tool for establishing heterotic pools of genetically divergent germplasm to streamline selection of crossing partners to maximize the grain yield of hybrid lines, at the same time preventing loss of agriculturally important traits of the elite lines. Better understanding of the molecular mechanisms underlying heterosis in wheat could be sought. A well-established male sterility system could also be instrumental in the exploration of wheat genetic variation and in molecular engineering to attain more open flower architecture and more efficient shedding of viable pollen, both features important for further enhancement of cross-pollination frequency. All wheat (Triticum) species (diploid, tetraploid, and hexaploid) are autogamous, as is Aegilops tauschii (goatgrass), which donated its genome to hexaploid wheat. Aegilops speltoides, which donated its genome to tetraploid wheat and is the source of the cytoplasm for all polyploid wheats, however, is allogamous. A robust hybrid seed production system is likely to require both molecular engineering and introduction of desirable traits from wheat and its relatives.
Although the split-gene system described by Kempe et al. is a significant advancement for the science of hybrid wheat production, the technology involves genetic engineering to develop what is considered to be GM wheat, which is yet unaccepted by a significant portion of the world market. Indeed the consequences of producing hybrid wheat as described need to be considered. Will it affect the end-use products or pose a health risk to the consumer? Would escape of the “transgenes” into conventional varieties, breeding lines, germplasm, or wild relatives of wheat lead to unwanted sterility or expression of barnase or other problems? These concerns need to be given careful consideration before the technology is used to produce hybrid wheat seed on a commercial scale. However, as long as the concerns are considered on scientific bases, the work by Kempe et al. will move the field forward and provide a step toward achieving the ability to feed the world’s ever expanding population.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
See companion article on page 9097.
References
- 1.Kempe K, Rubtsova M, Gils M. Split-gene system for hybrid wheat seed production. Proc Natl Acad Sci USA. 2014;111:9097–9102. doi: 10.1073/pnas.1402836111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faris JD. Wheat Domestication: Key to Agricultural Revolutions Past and Future. In: Tuberosa R, Graner A, Frison E, editors. Genomics of Plant Genetic Resources. Houten, The Netherlands: Springer; 2014. pp. 439–464. [Google Scholar]
- 3.Huang S, et al. Genes encoding plastid acetyl-CoA carboxylase and 3-phosphoglycerate kinase of the Triticum/Aegilops complex and the evolutionary history of polyploid wheat. Proc Natl Acad Sci USA. 2002;99(12):8133–8138. doi: 10.1073/pnas.072223799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McFadden ES, Sears ER. The origin of Triticum spelta and its free-threshing hexaploid relatives. J Hered. 1946;37:81–89, 107. doi: 10.1093/oxfordjournals.jhered.a105590. [DOI] [PubMed] [Google Scholar]
- 5.Feldman M. Origin of cultivated wheat. In: Bonjean AP, Angus WJ, editors. The World Wheat Book. A History of Wheat Breeding. Paris: Lavoisier Publishing; 2001. pp. 3–56. [Google Scholar]
- 6.Charmet G. Wheat domestication: Lessons for the future. C R Biol. 2011;334(3):212–220. doi: 10.1016/j.crvi.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds M, et al. Achieving yield gains in wheat. Plant Cell Environ. 2012;35(10):1799–1823. doi: 10.1111/j.1365-3040.2012.02588.x. [DOI] [PubMed] [Google Scholar]
- 8.Paux E, Sourdille P, Mackay I, Feuillet C. Sequence-based marker development in wheat: Advances and applications to breeding. Biotechnol Adv. 2012;30(5):1071–1088. doi: 10.1016/j.biotechadv.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Choulet F, et al. The wheat black jack: Advances towards sequencing the 21 chromosomes of bread wheat. In: Tuberosa R, Graner A, Frison E, editors. Genomics of Plant Genetic Resources. Houten, The Netherlands: Springer; 2014. pp. 405–438. [Google Scholar]