Significance
Communication often is a major component of social interactions. Signaling individuals are faced with the challenge of capturing the attention of intended receivers while limiting eavesdropping by potential predators. We conducted an experiment in nature to evaluate the hypothesis that prey species can modulate the physical properties of movement-based displays in response to the presence of predators. We found that male brown anoles dramatically decreased the amplitude of their head-bob displays in the presence of a predatory lizard, resulting in less conspicuous signals. Although less conspicuous signals may be safer for the signaling individuals, they also reduce the distance from which potential mates or competitors can detect them, which might affect the territory size and reproductive success of signaling males.
Keywords: communication, signal modulation
Abstract
Signaling individuals must effectively capture and hold the attention of intended conspecific receivers while limiting eavesdropping by potential predators. A possible mechanism for achieving this balance is for individuals to modulate the physical properties of their signals or to alter the proportion of time spent signaling, depending upon local levels of predation pressure. We test the hypothesis that prey can alter their visual signaling behavior to decrease conspicuousness and potentially limit predation risk via modulation of signal properties or display rate. To do so, we conducted a manipulative experiment in nature to evaluate the possible effect of predation pressure on the physical properties of movement-based signals and on the proportion of time spent signaling by using a well-understood predator–prey system in the Bahamas, the semiarboreal lizard Anolis sagrei, and one of its main predators, the curly-tailed lizard Leiocephalus carinatus. We find that on islands onto which the predator was introduced, male anoles reduce the maximum amplitude of head-bob displays but not the proportion of time spent signaling, in comparison with control islands lacking the predator. This reduction of amplitude also decreases signal active space, which might alter the reproductive success of signaling individuals. We suggest that future studies of predator–prey interactions consider the risk effects generated by changes in signals or signaling behavior to fully determine the influence of predation pressure on the dynamics of prey populations.
The process of communication is central to many aspects of social interaction, from attracting mates to establishing territories. The major prerequisite for communication is that an individual or its signal must effectively capture and hold the attention of intended receivers (1, 2). However, communication rarely occurs in a context free from the risk of predation, and thus the presence of predators is an important selective pressure on the physical designs of signals and the behaviors associated with their display (e.g., refs. 3–9). In fact, predation pressure typically results in signals with reduced conspicuousness (i.e., the likelihood of being seen by a predator) at one or both of two timescales: (i) across generations (i.e., via evolutionary mechanisms) or (ii) within the lifetime of an individual (i.e., via behavioral mechanisms). The evolution of less conspicuous signal properties in response to predation pressure has been demonstrated across signaling modalities, including acoustic, electrical, visual, and ultrasonic signals (e.g., refs. 9, 10). Predation pressure also shapes the evolution of the behaviors associated with the production of signals, often resulting in a shift in the amount of time spent signaling throughout the day or in the use of less vulnerable display sites (11–16).
Behavioral changes favoring a reduction in the likelihood of communication-associated predation typically precede evolutionary changes (12). This process most likely is driven by the plasticity of behavioral traits, which may change within the lifetime of an individual. Therefore, elucidating the behavioral mechanisms by which organisms can decrease the conspicuousness of their signals in response to predation has become a major area of inquiry in behavioral and evolutionary ecology (5, 11, 13).
An individual can behaviorally limit the conspicuousness of its signal to a predator via two non-mutually exclusive strategies. Commonly, individuals modulate the amount of time they spend displaying throughout the day. This response decreases the amount of time an individual is vulnerable to detection by a predator or narrows a signaler’s temporal window of vulnerability (e.g., refs. 17–19). An alternative response is for an individual to modify the physical properties of its signal’s design to decrease the ability of a predator to detect or localize the signaler (e.g., ref. 20). These changes often diminish the active space of a signal, which is defined as the maximum distance at which the signal can capture the attention of a receiver. A smaller active space limits the probability of predation by shrinking the area over which a signal can be detected by a predator (i.e., narrowing the spatial window of vulnerability).
Visual signals frequently are used to study the effects of predation pressure on communication (e.g., refs. 21–23). Visual signals typically consist of both color and motion components, with motion often cited as the most salient feature of the display (24–26). Nevertheless, most studies evaluating the effects of predation pressure on the conspicuousness of visual signals have focused on coloration and, in most cases, on changes on an evolutionary timescale (e.g., 27–29). Here, we evaluate the hypothesis that the movement-based visual signaling behavior of a prey species may change via behavioral mechanisms (e.g., modulation of signal properties or proportion of time spent signaling) to decrease conspicuousness and potentially limit predation risk.
We evaluate the effect of predation pressure on the physical properties of movement-based signals and on the proportion of time spent signaling by using a well-understood predator–prey system: the small semiarboreal lizard Anolis sagrei and one of its main predators, the much larger, mostly terrestrial curly-tailed lizard Leiocephalus carinatus (see ref. 30, for photographs of this predator–prey interaction). Previous experiments showed that curly-tailed lizards may be a major selective force on A. sagrei, and their presence, even at low density, may greatly affect the demography and structural niche of A. sagrei (31–34). In response to the presence of curly-tailed lizards, male A. sagrei rarely use the ground, moving higher up in the vegetation to perches that usually are narrower and less exposed than those used by males where curly-tails are absent (32, 33). Furthermore, this shift in habitat use may occur in a short period, and the magnitude of the response seems to be proportional to changes in the activity of curly-tailed lizards (34). Finally, the perceived threat of predation is sufficiently strong that under natural conditions, if presented with a single curly-tailed lizard, individuals of A. sagrei will flee immediately at the mere sight of the predator (32).
In anoles, including A. sagrei, social interactions are mediated by visual displays, which combine abrupt movements of the head (head bobs) with expansion and retraction of a colorful throat fan, termed a “dewlap” (35–37). These signals are given spontaneously by males to advertise their presence as they patrol their territories, as well as being directed toward other individuals during courtship and the settlement of territorial disputes (38–40). Theoretical predictions, supported by empirical data, have shown that the physical properties of head-bob displays in A. sagrei, particularly the square wave-like temporal pattern generated by rapid changes in head position, are highly conspicuous to visually oriented receivers, even when those receivers are inattentive (41, 42). The amplitude of these movements determines the maximum distance from which the sensory system of a conspecific is stimulated effectively by the signal—what is termed the “active space.” For a territorial species such as A. sagrei, the active space may affect an individual’s ability to repel rivals and attract mates from a distance (40, 43). Thus, in the context of social interactions, selection should favor signals that maximize active space. However, curly-tailed lizards, like anoles, are visually oriented, communicate using head-bob displays that move along a vertical axis, and must detect prey that move in rapid bursts, suggesting that the visual systems of curly-tailed lizards also are likely to be sensitive to sudden linear movements (44–46) (also, see ref. 42 for a discussion of visual motion detection). As a result, male Anolis lizards in the presence of curly-tailed lizards may face contrasting selection pressures, because displays that maximize the conspecific active space (i.e., displays with high-amplitude head movements) should be beneficial with regard to intraspecific interactions but detrimental with regard to predator–prey interactions.
In this study, we used a replicated experimental design conducted under natural conditions to address the effects of the presence of L. carinatus on the signals (head-bob displays) of A. sagrei. We predict that the amplitude of spontaneous head bobs, and thus conspicuousness, should be lower for populations in which L. carinatus is present. Furthermore, we also expect to find a difference in the proportion of time spent signaling between populations, with a decreased proportion of time allocated to signaling in populations exposed to L. carinatus. Finally, we evaluate whether changes in signal properties might affect the efficacy of head bobs during social interactions by using an empirically derived motion detection model for A. sagrei to test for differences in the conspecific active space of signals.
Materials and Methods
Study Site and Experimental Design.
We examined lizard populations on nine small islands (mean vegetated area, 280 ± 64 m2) in the Snake Creek region of Great Abaco Island, Bahamas. These islands are characterized by a rocky substrate and dominated by relatively sparse shrubbery typically less than 2 m in height (see ref. 31 for a general description of the vegetation on similar islands from this area). The islands used for this study are a subset of those used for a larger experiment started in May 2008, which evaluates the impact of a top predator, L. carinatus, across multiple levels of the food web. In the latter experiment, 14 islands sustaining natural populations of A. sagrei were selected and divided into seven pairs matched by area, vegetation profile, and A. sagrei population size. For each pair, one island was randomly selected to introduce L. carinatus (experimental islands) and the other was left alone (control islands). L. carinatus occurs on nearby larger islands and is known to colonize the smaller islands (31). Only adult L. carinatus were used as colonizers, and the number relocated to each island was proportional to the population size of A. sagrei. For this study, we sampled five experimental and four control islands chosen because of their accessibility (the remote location of some islands made them impractical for the intense monitoring of this project).
Data Collection.
Focal observations were conducted from May 18 to June 11, 2011, to characterize general aspects of the behavior of male A. sagrei. Observations were conducted from 0700 to 1900 hours on 240 males, for a total of 77.1 h of observations. We visited each of the nine islands a minimum of seven times (range, 7–11 visits), walking systematically through each island until a lizard was located. The individual then was filmed with a portable mini-DV camcorder (Canon ZR-960) for ∼20 min or until it moved out of sight (; range, 5.1–22.7). Videos were analyzed later in the laboratory to extract relevant data. We calculated the mean perch height for each individual as the mean of all perches used during the observation period. These values were estimated during filming and then confirmed or amended when necessary using a tape measure immediately following the observation. Changes in perch height occurred when a lizard moved to a new perch (i.e., branch or trunk) or moved along the same perch a distance that exceeded the body length of the lizard. Proportion of time spent signaling was calculated as the proportion of time an individual gave head-bob displays and/or dewlap flashes during the observation period.
To evaluate whether the presence of curly-tailed lizards had an impact on the physical properties of the head-bob displays of A. sagrei, we filmed spontaneous broadcast displays (i.e., nondirected displays given to advertise the presence of the signaler) of 39 adult male A. sagrei using a Canon GL2 mini-DV camcorder. Each individual was filmed only once, and we avoided resampling individuals by identifying each lizard with a unique mark that was applied before the study. We visited eight of the nine islands on at least four separate occasions, for a minimum of eight total hours per island (see Table S1 for detailed sampling efforts). We visited one island only twice, for 5.5 total hours, because we successfully filmed all known adult males during those trips. Spontaneous displays were recorded during the same period of dates and times of day as described above for the behavioral data. Before filming, we secured the camcorder to a tripod ∼4 m from and approximately at the same height as the focal anole. For each individual, we recorded the perch height and perch diameter of the display site. Following each display, we placed a ping pong ball of known size at the site of the display to serve as a standard that could be used later to convert all movement distances to real units.
Video Analysis.
We imported all video footage into the video editing application iMovie, in which we trimmed and converted clips of each visual display to a format appropriate for the motion analysis software VideoPoint 2.5, which allows users to superimpose a Cartesian coordinate system onto a sequence of video frames. First, we plotted the position of the snout of the lizard (i.e., a landmark) on every frame (30 frames per second). We then measured the pixel length of the standard (i.e., the ping pong ball) and converted the units of a Cartesian coordinate system overlaid onto all frames of the video clip from pixels to real units (millimeters). Next, we rotated the coordinate system such that the vertical (y) axis was aligned with the apparent axis of maximal head motion (i.e., maximum amplitude). Because this alignment is achieved “by eye,” an underestimate of maximum head amplitude might occur. To reduce the likelihood of this error, we also rotated the coordinate system 5° to the right and 5° to the left of our original alignment and then compared the maximum head amplitudes given by all three alignments (rotation typically resulted in a shift in calculated amplitude of less than 0.5 mm). We used data obtained from the alignment that yielded the largest maximum head amplitude. We also counted the number of individual head bobs (i.e., a movement of the head up and then back down) in each display.
Predicting the Conspecific Signaling Active Space.
Both the visual system and motion perception of anoles in general and A. sagrei in particular are well characterized. Behavioral experiments show that movements in a particular range of visual angles (0.2–0.8°) are more likely to capture the attention of anoles, including A. sagrei, than movements below or above that range; in other words, movements between 0.2° and 0.8° appear to maximally stimulate the motion detectors of receivers (25, 40, 42). The visual angle (θ) of a movement is the angle at which that movement subtends the eye of a viewer and is given by
| [1] |
where S is the amplitude of the movement and D is the distance between the moving object and the eye of the viewer (47).
Using Eq. 1, we calculated the distance (Do) at which the head movement of greatest amplitude for each display has a visual angle of 0.2°. At a distance beyond Do, the head movement generates a suboptimal response by the motion detectors of an anoline viewer of the display. Therefore, Do provides an estimate of the maximum distance for which the sensory system of an inattentive anoline receiver will be stimulated optimally by the head movement of a signaler and may be considered a metric for the conspecific active space of a signal (25, 40, 42, 48). This conspecific active space is the maximum straight-line distance from which another anole is predicted to detect a display.
Statistical Analysis.
We calculated the mean value for each island for each of the following variables: mean perch height, proportion of time spent signaling, maximum amplitude of head-bob displays, and number of head bobs per display. Data for proportion of time spent signaling was arcsine square-root transformed. After confirming that all four variables were distributed normally by using the Shapiro–Wilk normality test, we compared control and experimental treatments using one-tailed, unequal variance t tests (Welch’s two-sample t tests). We used two-tailed Pearson correlations to test for associations between the maximum amplitude of head-bob displays and both perch height and diameter within each of the treatments. All tests were performed using R statistical programming language V 2.15.0 (49) at α = 0.05.
Ethical Note.
All aspects of this study were approved by the Institutional Animal Care and Use Committee of Duke University.
Results
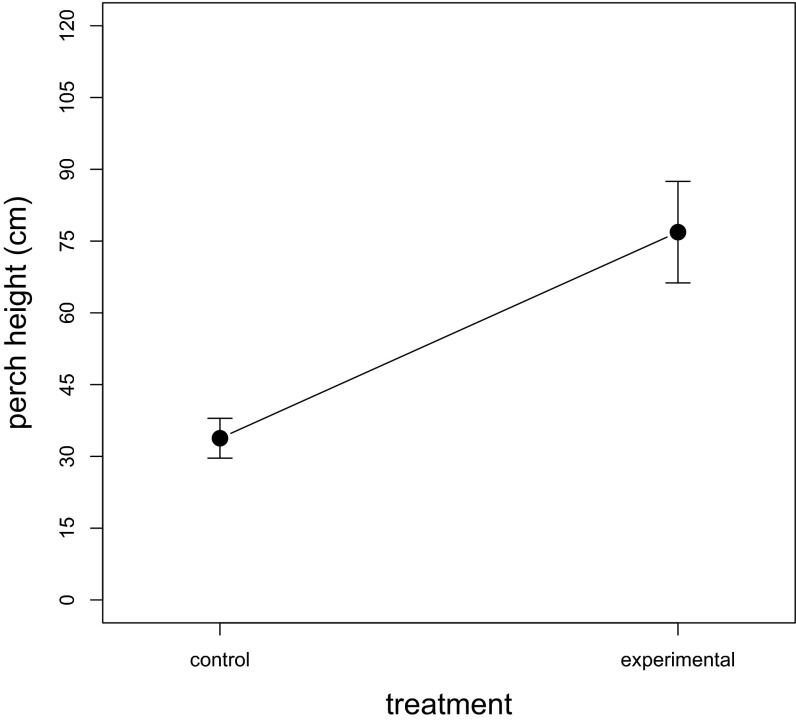
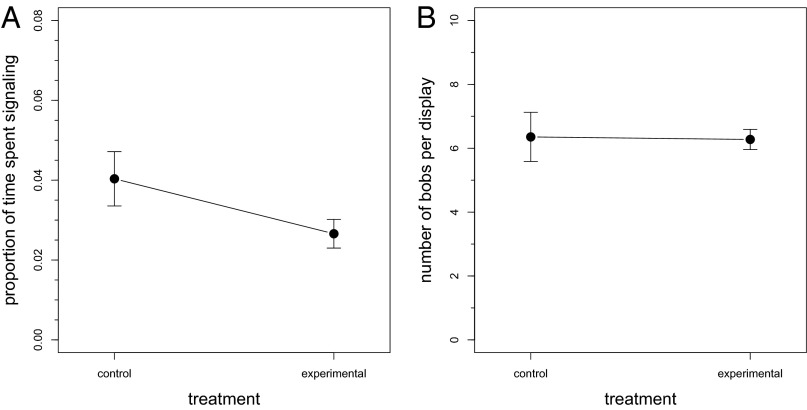
Male A. sagrei perched over twice as high above the ground on islands where L. carinatus was present than on control islands (; t = −3.76, P = 0.01, n = 9; Fig. 1). However, males from the control and experimental treatments did not differ significantly in the proportion of time spent signaling (control: ; experimental: , t = 1.74, P = 0.07, n = 9; Fig. 2A) or in the number of head bobs per display (control: bobs/display; experimental: bobs/display, t = 0.01, P = 0.46, n = 9; Fig. 2B).
Fig. 1.
Mean perch height of male A. sagrei in both control (nonpredator) and experimental (predator) treatments. Values are the mean and one SEM for each treatment; n = 9.
Fig. 2.
Behavior of male A. sagrei in both control (nonpredator) and experimental (predator) treatments. (A) Proportion of time spent signaling. (B) Number of head bobs per display. Values are the mean and one SEM for each treatment; n = 9.
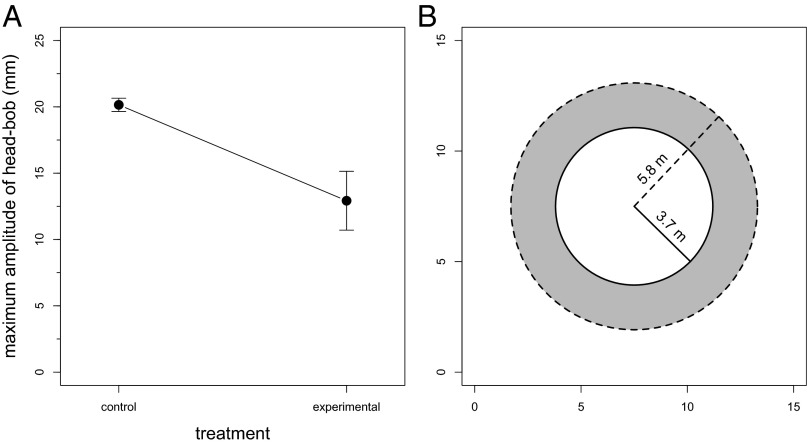
The mean maximum amplitude of head bobs from experimental islands was 7.2 mm smaller than that of control islands (experimental: ; control: ; t = 3.18, P = 0.01, n = 9; Fig. 3A). Under the relatively simple assumption that the vegetation is not obstructing the receiver’s view of the signaling lizard, this difference in amplitude corresponds to a difference in active space (i.e., the maximum range of a signal) of more than 2 m between treatments ( vs. ; Fig. 3B).
Fig. 3.
Change in the physical properties of head bob displays given by male A. sagrei in response to the presence of L. carinatus and its effects on the signaling active space. (A) Maximum amplitude of head-bob displays given in both control (nonpredator) and experimental (predator) treatments. Values are the mean and one SEM for each treatment; n = 9. (B) Illustration of the differences in conspecific active signaling space of A. sagrei on predator and nonpredator islands. The active space of a typical head-bob display from a lizard on a predator island (solid line; 3.7 m) is smaller than the active space of a typical display from a lizard on a nonpredator island (dashed line; 5.8 m). Circles demarcate the area within which an anoline receiver would be maximally stimulated by a typical signal given by a lizard on a predator island (solid circle) and a typical signal given by a lizard on a nonpredator island (dashed circle), assuming that the signaling lizard is located at the center of the circle. The x- and y-axes are used simply to scale the two circles in meters.
To examine the possibility that differences in amplitude were influenced by differences in the perch height or diameter selected by the signaling individuals, we tested for a correlation between both perch height and perch diameter and maximum head amplitude. Perch height was not correlated with the maximum amplitude of head-bob displays in either treatment (control: r = 0.02, P = 0.94, n = 20; experimental: r = −0.04, P = 0.87, n = 19). Similarly, perch diameter was not correlated with display amplitude in either treatment (control: r = −0.08, P = 0.73, n = 20; experimental: r = −0.17, P = 0.49, n = 19).
Discussion
Animals change the physical parameters of their signals (i.e., “signal modulation”) in response to changes in environmental conditions, social interactions, or the presence of predators (50–56). Surprisingly, modulation of the physical properties of movement-based visual signals, which often are considered the most salient components of visual displays, has not been documented as a response to predation threat, despite evidence for predation-associated modulation of signals in other sensory modalities (e.g., refs. 26, 57, 58). For example, it was shown that Gallus gallus reduces the auditory frequency of its call, and potentially its vulnerability to localization by predators, in response to the presence of a potential predator (20).
Previous studies documented that male anoles modulate the physical properties of their head bobs to increase visibility to conspecifics under varying social and environmental conditions (e.g., refs. 59–62). However, in this study, modulation of head-bob amplitude may serve to decrease visibility to predators. Moreover, we confirm previous reports that anoles respond to predators by altering their habitat use in demonstrating that the mean perch height of male A. sagrei during focal observations was more than twice as great in the presence of curly-tailed lizards than in their absence (Fig. 1). These findings are congruent with previous studies that indicate that A. sagrei individuals perceive the presence of L. carinatus as an increase in predation risk (32–34).
Our results demonstrate that anoles do, indeed, modulate motion signals in response to predation pressure, probably to decrease signal conspicuousness to curly-tailed lizards. We found that male A. sagrei on islands with predators produced head bobs with much-reduced maximum amplitudes (up to 60%) in comparison with males on control islands (Fig. 3A). This result is notable not only because it indicates that motion-signal modulation occurs in response to predators, but also because it suggests that modulation is not limited to courtship or alarm calls. The displays given by A. sagrei may be categorized as spontaneous broadcast signals, which often are used to advertise presence or territory ownership. Also, although it has been shown that anoles may give directed head-bob displays to approaching predators as pursuit deterrent signals (63, 64), we never witnessed an individual of L. carinatus in the vicinity of the anoles while they displayed in this study. Thus, in our case, it is highly unlikely that the observed changes in the amplitude of the head bobs resulted from prey–predator communication or interspecific territoriality.
An important aspect of the anole–Leiocephalus predator–prey interaction is that curly-tailed lizards, like anoles, are visually oriented animals (45, 46, 65). Although the properties of motion detection in Leiocephalus have not been measured explicitly, increasing the distance between a moving object and the eye of a receiver reduces the apparent size of the movement until at some point, it no longer is detectable (66). Consequently, a reasonable expectation is that reduction in the amplitude of A. sagrei head bobs would have the effect of making those displays less conspicuous to curly-tailed lizards (i.e., shortening the maximum distance of detection). In such a scenario, reducing the amplitude of head bobs likely would decrease the active space of signals to the point at which the signaler would be likely to detect (and flee or hide from) a moving predator from a much greater distance than the predator could detect the signal, thereby limiting the risk of unforeseen attacks.
One particular advantage of modulation is that it may take place immediately and, if needed, be adjusted along a relatively fine scale, because the physical properties of a signal are under control of motor neurons. The ability of individuals to modify their signaling display at such a fine temporal scale might provide an effective mechanism to balance the tradeoff between effective social communication and predation pressure. Fine-scale modulation has been documented in Anolis gundlachi during social interactions under natural conditions, in which males are capable of altering the amplitude of head-bob displays to effectively stimulate the sensory systems of intended receivers located at varying distances (62).
The fitness impact of predation on prey species traditionally has been measured by mortality rate. However, an emerging view is that predation also may affect the individual fitness of prey indirectly through so-called risk effects that contribute to a reduction in reproductive success (reviewed in refs. 67, 68). Based on our understanding of the natural history of anoles, including their social dynamics and the contribution of territory size to male reproductive success (69–71), the observed changes in the physical properties of head-bob displays in response to the presence of L. carinatus are likely to decrease the fitness of territorial males in multiple ways. Our signal-detection modeling approach reveals a 35% reduction in the conspecific active space of anoles in the presence of predators (Fig. 3B). One obvious effect of such a reduction in active space might be a decrease in the ability of territorial males to attract distant females and successfully repel males before potentially dangerous close-range encounters, perhaps leading to smaller territory sizes and fewer females with which to mate. This influence of changes in signal active space on territory size (and possibly mate attraction) has been demonstrated in red-winged blackbirds (Agelaius phoeniceus) (48, 58, 72) and spring peepers (Hyla crucifer) (73). Such changes might then have cascading effects on sexually selected traits and mating strategies.
We predicted that male A. sagrei would respond to the presence of L. carinatus by not only decreasing display amplitude, but also by decreasing the proportion of time spent signaling. However, we found no statistical difference in the proportion of time spent signaling (although proportion of time spent signaling is 67% higher on control islands than on islands on which Leiocephalus were present, a difference with a P value of 0.07 in a one-tailed comparison; Fig. 2A). Because our sample size is relatively small, it is possible that the lack of a significant difference reflects a lack of statistical power. Given that the difference in number of head bobs per display, which might serve as another means of altering an individual’s temporal window of vulnerability, clearly is nonsignificant (Fig. 2B) and assuming that the lack of difference we detected in proportion of time spent displaying is real, then A. sagrei does not follow the same trend found in other studies that find a decrease in proportion of time allocated to signaling to be one of the responses exhibited by prey species to a perceived increase in predation threat (e.g., refs. 6, 74–76). For example, male túngara frogs reduce their calling rate, defined as the proportion of time spent calling, in response to the presence of frog-eating bats (77), and male Trinidadian guppies shorten the duration of courtship displays in the presence of predatory fish (16). It should be noted that most studies reporting decreases in signaling focused on mating displays (e.g., refs. 16, 77); however, A. sagrei head-bob displays not only are given as part of courtship displays, but also commonly are used for many aspects of anole social interactions.
In this case, two non-mutually exclusive explanations might account for the failure of A. sagrei to conform to the general pattern seen in other species (78). First, as we discussed above, the decrease in signal amplitude and its effect on the signal conspicuousness may reduce the likelihood of predation, such that altering the proportion of time spent signaling would impart little or no additional benefit. In other words, males have adopted one of two effective antipredator strategies. Second, the importance of holding a territory and attracting mates might select against a decrease in the amount of time allocated to signaling. In anoles, including A. sagrei, males patrol their territories while producing displays that function to advertise their position to nearby rivals and females and to repel potential intruders (79–81). As we discussed above, the reduced amplitude of displays limits the active space over which the display can be detected, but within that active space, males still can attract nearby mates or repel nearby rivals by not decreasing the proportion of time spent signaling. Therefore, it is possible that a decrease in the proportion of their daily activity allocated to display might not be advantageous, even in the presence of predators, because such a strategy might hamper the ability of males to hold a territory.
Replicated large-scale manipulations, conducted under natural conditions with natural populations, have provided some of the strongest evidence that predation is a major selective force shaping the evolution of social signals (see ref. 29 and references therein). We have demonstrated that these effects include modulation of head-bob displays by A. sagrei. Because these signals are used in a variety of contexts related to social interactions, the indirect effects of such modulation might have great consequences, altering not only selection pressures, but possibly the social dynamics and population structure of prey species. Recent studies of predator–prey interactions show that elucidating the importance of risk effects on prey populations is crucial for understanding the evolutionary forces shaping predator–prey interactions (67, 68, 82). We propose that changes in social signals also be included in these studies, because those changes have the potential to change the population dynamics of prey species.
Supplementary Material
Acknowledgments
We thank B. Pinder, M. Campano, A. Les, and D. Owen for assistance in the field; J. A. Endler and M. J. Ryan for helpful comments that greatly improved this manuscript; the National Science Foundation (NSF) for funding (DEB-0949357) and for an NSF Graduate Research Fellowship (to D.S.S.); and the Bahamas Ministry of Agriculture and the Bahamas Environment, Science, and Technology Commission of the Ministry of the Environment for permission to conduct this research.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 9026.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407190111/-/DCSupplemental.
References
- 1.Dusenbery DB. Sensory Ecology: How Organisms Acquire and Respond to Information. New York: Freeman; 1992. [Google Scholar]
- 2.Bradbury JW, Vehrencamp SL. Principles of Animal Communication. Sunderland, MA: Sinauer; 1998. [Google Scholar]
- 3.Tuttle MD, Ryan MJ. Bat predation and the evolution of frog vocalizations in the neotropics. Science. 1981;214(4521):677–678. doi: 10.1126/science.214.4521.677. [DOI] [PubMed] [Google Scholar]
- 4.Pocklington R, Dill LM. Predation on females or males: Who pays for bright male traits? Anim Behav. 1995;49(4):1122–1124. [Google Scholar]
- 5.Zuk M, Kolluru GR. Exploitation of sexual signals by predators and parasitoids. Q Rev Biol. 1998;73(4):415–438. [Google Scholar]
- 6.Hedrick AV. Crickets with extravagant mating songs compensate for predation risk with extra caution. Proc Biol Sci. 2000;267(1444):671–675. doi: 10.1098/rspb.2000.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woods WA, Jr, Hendrickson H, Mason J, Lewis SM. Energy and predation costs of firefly courtship signals. Am Nat. 2007;170(5):702–708. doi: 10.1086/521964. [DOI] [PubMed] [Google Scholar]
- 8.Akre KL, Farris HE, Lea AM, Page RA, Ryan MJ. Signal perception in frogs and bats and the evolution of mating signals. Science. 2011;333(6043):751–752. doi: 10.1126/science.1205623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endler JA. Natural selection on color patterns in Poecilia reticulata. Evolution. 1980;34(1):76–91. doi: 10.1111/j.1558-5646.1980.tb04790.x. [DOI] [PubMed] [Google Scholar]
- 10.Stoddard PK. Predation enhances complexity in the evolution of electric fish signals. Nature. 1999;400(6741):254–256. doi: 10.1038/22301. [DOI] [PubMed] [Google Scholar]
- 11.Burk T. Evolutionary significance of predation on sexually signalling males. Fla Entomol. 1982;65(1):90–104. [Google Scholar]
- 12.Lima SL, Dill LM. Behavioral decisions made under the risk of predation: A review and prospectus. Can J Zool. 1990;68(4):619–640. [Google Scholar]
- 13.Magnhagen C. Predation risk as a cost of reproduction. Trends Ecol Evol. 1991;6(6):183–186. doi: 10.1016/0169-5347(91)90210-O. [DOI] [PubMed] [Google Scholar]
- 14.Sih A. Predation risk and the evolutionary ecology of reproductive behaviour. J Fish Biol. 1994;45(Suppl sA):111–130. [Google Scholar]
- 15.Brunel-Pons O, Alem S, Greenfield MD. The complex auditory scene at leks: Balancing antipredator behaviour and competitive signalling in an acoustic moth. Anim Behav. 2011;81(1):231–239. [Google Scholar]
- 16.Endler JA. Predation, light intensity, and courtship behaviour in Poecilia reticulata. Anim Behav. 1987;35(5):1376–1385. [Google Scholar]
- 17.Godin J-GJ. Predation risk and alternative mating tactics in male Trinidadian guppies (Poecelia reticulata) Oecologia. 1995;103(2):224–229. doi: 10.1007/BF00329084. [DOI] [PubMed] [Google Scholar]
- 18.Koga T, Backwell PRY, Jennions MD, Christy JH. Elevated predation risk changes mating behaviour and courtship in a fiddler crab. Proc Biol Sci. 1998;265(1404):1385–1390. [Google Scholar]
- 19.Jones G, Barabas A, Elliott W, Parsons S. Female greater wax moths reduce sexual display behavior in relation to the potential risk of predation by echolocating bats. Behav Ecol. 2002;13(3):375–380. [Google Scholar]
- 20.Bayly KL, Evans CS. Dynamic changes in alarm call structure: A strategy for reducing conspicuousness to avian predators? Behaviour. 2003;140:353–369. [Google Scholar]
- 21.Endler JA. Natural and sexual selection on color patterns in poeciliid fishes. Environ Biol Fishes. 1983;9(2):173–190. [Google Scholar]
- 22.Hemmi JM, Marshall J, Pix W, Vorobyev M, Zeil J. The variable colours of the fiddler crab Uca vomeris and their relation to background and predation. J Exp Biol. 2006;209(Pt 20):4140–4153. doi: 10.1242/jeb.02483. [DOI] [PubMed] [Google Scholar]
- 23.Stuart-Fox D, Moussalli A, Whiting MJ. Natural selection on social signals: Signal efficacy and the evolution of chameleon display coloration. Am Nat. 2007;170(6):916–930. doi: 10.1086/522835. [DOI] [PubMed] [Google Scholar]
- 24.Hailman JP. Optical Signals. Bloomington, IN: Indiana Univ Press; 1977. [Google Scholar]
- 25.Fleishman LJ. Motion detection in the presence and absence of background motion in an Anolis lizard. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1986;159(5):711–720. doi: 10.1007/BF00612043. [DOI] [PubMed] [Google Scholar]
- 26.Clark DL, Uetz GW. Morph-independent mate selection in a dimorphic jumping spider: Demonstration of movement bias in female choice using video-controlled courtship behaviour. Anim Behav. 1992;43(2):247–254. [Google Scholar]
- 27.Cummings ME, Rosenthal GG, Ryan MJ. A private ultraviolet channel in visual communication. Proc Biol Sci. 2003;270(1518):897–904. doi: 10.1098/rspb.2003.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Håstad O, Victorsson J, Ödeen A. Differences in color vision make passerines less conspicuous in the eyes of their predators. Proc Natl Acad Sci USA. 2005;102(18):6391–6394. doi: 10.1073/pnas.0409228102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kemp DJ, Reznick DN, Grether GF, Endler JA. Predicting the direction of ornament evolution in Trinidadian guppies (Poecilia reticulata) Proc Biol Sci. 2009;276(1677):4335–4343. doi: 10.1098/rspb.2009.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoener TW, Slade JB, Stinson CH. Diet and sexual dimorphism in the very catholic lizard genus, Leiocephalus of the Bahamas. Oecologia. 1982;53(2):160–169. doi: 10.1007/BF00545659. [DOI] [PubMed] [Google Scholar]
- 31.Schoener TW, Spiller DA, Losos JB. Predation on a common Anolis lizard: Can the food-web effects of a devastating predator be reversed? Ecol Monogr. 2002;72(3):383–407. [Google Scholar]
- 32.Losos JB, Schoener TW, Spiller DA. Predator-induced behaviour shifts and natural selection in field-experimental lizard populations. Nature. 2004;432(7016):505–508. doi: 10.1038/nature03039. [DOI] [PubMed] [Google Scholar]
- 33.Losos JB, Schoener TW, Langerhans RB, Spiller DA. Rapid temporal reversal in predator-driven natural selection. Science. 2006;314(5802):1111. doi: 10.1126/science.1133584. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Darias M, Schoener TW, Spiller DA, Losos JB. Predators determine how weather affects the spatial niche of lizard prey: Exploring niche dynamics at a fine scale. Ecology. 2012;93(12):2512–2518. doi: 10.1890/12-0483.1. [DOI] [PubMed] [Google Scholar]
- 35.Greenberg B, Noble GK. Social behavior of the American chameleon (Anolis carolinensis Voigt) Physiol Zool. 1944;17(4):392–439. [Google Scholar]
- 36.Ruibal R. In: Lizard Ecology: A Symposium. Milstead WW, editor. Columbia, MO: Univ Missouri Press; 1965. pp. 116–140. [Google Scholar]
- 37.Scott MP. Agonistic and courtship displays of male Anolis sagrei. Breviora. 1984;479:1–22. [Google Scholar]
- 38.Jenssen TJ. Evolution of anoline display behavior. Am Zool. 1977;17(1):203–215. [Google Scholar]
- 39.Stamps JA. In: Biology of the Reptilia: Ecology and Behavior. Gans C, Winkle DW, editors. New York: Academic; 1977. pp. 265–334. [Google Scholar]
- 40.Fleishman LJ. The influence of the sensory system and the environment on motion patterns in the visual displays of anoline lizards and other vertebrates. Am Nat. 1992;139:S36–S61. [Google Scholar]
- 41.Fleishman LJ, Pallus AC. Motion perception and visual signal design in Anolis lizards. Proc Biol Sci. 2010;277(1700):3547–3554. doi: 10.1098/rspb.2010.0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pallus AC, Fleishman LJ, Castonguay PM. Modeling and measuring the visual detection of ecologically relevant motion by an Anolis lizard. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010;196(1):1–13. doi: 10.1007/s00359-009-0487-7. [DOI] [PubMed] [Google Scholar]
- 43.How MJ, Hemmi JM, Zeil J, Peters R. Claw waving display changes with receiver distance in fiddler crabs, Uca perplexa. Anim Behav. 2008;75(3):1015–1022. [Google Scholar]
- 44.Noble GK, Bradley HT. The mating behavior of lizards; its bearing on the theory of sexual selection. Ann N Y Acad Sci. 1933;35(1):25–100. [Google Scholar]
- 45.Evans LT. Tail display in an iguanid lizard. Copeia. 1953;1953:50–54. [Google Scholar]
- 46.Marcellini DL, Jenssen TA. Avoidance learning by the curly-tailed lizard, Leiocephalus schreibersi: Implications for anti-predator behavior. J Herpetol. 1991;25(2):238–241. [Google Scholar]
- 47.Endler JA. A predator’s view of animal color patterns. Evol Biol. 1978;11:319–364. [Google Scholar]
- 48.Brenowitz EA. The active space of red-winged blackbird song. J Comp Physiol. 1982;147(4):511–522. [Google Scholar]
- 49.R Development Core Team 2010. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, Vienna, Austria). ISBN 3-900051-07-0, URL http://www.R-project.org.
- 50.Potash LM. Noise-induced changes in calls of the Japanese quail. Psychon Sci. 1972;26(5):252–254. [Google Scholar]
- 51.Cynx J, Lewis R, Tavel B, Tse H. Amplitude regulation of vocalizations in noise by a songbird, Taeniopygia guttata. Anim Behav. 1998;56(1):107–113. doi: 10.1006/anbe.1998.0746. [DOI] [PubMed] [Google Scholar]
- 52.Brumm H, Voss K, Köllmer I, Todt D. Acoustic communication in noise: Regulation of call characteristics in a New World monkey. J Exp Biol. 2004;207(Pt 3):443–448. doi: 10.1242/jeb.00768. [DOI] [PubMed] [Google Scholar]
- 53.Peters RA, Hemmi JM, Zeil J. Signaling against the wind: modifying motion-signal structure in response to increased noise. Curr Biol. 2007;17(14):1231–1234. doi: 10.1016/j.cub.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 54.Anderson RC, Searcy WA, Peters S, Nowicki S. Soft song in song sparrows: acoustic structure and implications for signal function. Ethology. 2008;114(7):662–676. [Google Scholar]
- 55.Ord TJ, Stamps JA, Losos JB. Adaptation and plasticity of animal communication in fluctuating environments. Evolution. 2010;64(11):3134–3148. doi: 10.1111/j.1558-5646.2010.01056.x. [DOI] [PubMed] [Google Scholar]
- 56.Gavassa S, Roach JP, Stoddard PK. Social regulation of electric signal plasticity in male Brachyhypopomus gauderio. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013;199(5):375–384. doi: 10.1007/s00359-013-0801-2. [DOI] [PubMed] [Google Scholar]
- 57.Blumstein DT, Armitage KB. Alarm calling in yellow-bellied marmots: I. The meaning of situationally variable alarm calls. Anim Behav. 1997;53(1):143–171. [Google Scholar]
- 58.Patricelli GL, Dantzker MS, Bradbury JW. Differences in acoustic directionality among vocalizations of the male red-winged blackbird (Agelaius pheoniceus) are related to function in communication. Behav Ecol Sociobiol. 2007;61(7):1099–1110. [Google Scholar]
- 59.Stamps JA, Barlow GW. Variation and stereotypy in the displays of Anolis aeneus (Sauria: Iguanidae) Behaviour. 1973;47:67–94. [Google Scholar]
- 60.Fleishman LJ. Sensory influences on the physical design of a visual display. Anim Behav. 1988;36(5):1420–1424. [Google Scholar]
- 61.Ord TJ, Peters RA, Clucas B, Stamps JA. Lizards speed up visual displays in noisy motion habitats. Proc Biol Sci. 2007;274(1613):1057–1062. doi: 10.1098/rspb.2006.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steinberg DS, Leal M. Sensory system properties predict signal modulation in a tropical lizard. Anim Behav. 2013;85(3):623–629. [Google Scholar]
- 63.Leal M, Rodríguez-Robles JA. Signalling displays during predator–prey interactions in a Puerto Rican anole, Anolis cristatellus. Anim Behav. 1997;54(5):1147–1154. doi: 10.1006/anbe.1997.0572. [DOI] [PubMed] [Google Scholar]
- 64.Leal M. Honest signalling during prey-predator interactions in the lizard Anolis cristatellus. Anim Behav. 1999;58(3):521–526. doi: 10.1006/anbe.1999.1181. [DOI] [PubMed] [Google Scholar]
- 65.Campano M, Les AM. Leiocephalus carinatus (northern curly-tailed lizard). Diet. Herpetol Rev. 2012;43:333–334. [Google Scholar]
- 66.McIlwain JT. An Introduction to the Biology of Vision. New York: Cambridge Univ Press; 1996. [Google Scholar]
- 67.Creel S, Christianson D. Relationships between direct predation and risk effects. Trends Ecol Evol. 2008;23(4):194–201. doi: 10.1016/j.tree.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 68.Creel S. Toward a predictive theory of risk effects: Hypotheses for prey attributes and compensatory mortality. Ecology. 2011;92(12):2190–2195. doi: 10.1890/11-0327.1. [DOI] [PubMed] [Google Scholar]
- 69.Rand AS. Ecology and social organization of the iguanid lizard Anolis lineatopus. Proc US Natl Mus. 1967;122:1–79. [Google Scholar]
- 70.Andrews RM. Structural habitat and time budget of a tropical Anolis lizard. Ecology. 1971;52(2):262–270. [Google Scholar]
- 71.Trivers RL. Sexual selection and resource-accruing abilities in Anolis garmani. Evolution. 1976;30:253–269. doi: 10.1111/j.1558-5646.1976.tb00908.x. [DOI] [PubMed] [Google Scholar]
- 72.Peek FW. An experimental study of the territorial function of vocal and visual display in the male red-winged blackbird (Agelaius phoeniceus) Anim Behav. 1972;20(1):112–118. doi: 10.1016/s0003-3472(72)80181-7. [DOI] [PubMed] [Google Scholar]
- 73.Brenowitz EA, Wilczynski W, Zakon HH. Acoustic communication in spring peepers. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1984;155(5):585–592. [Google Scholar]
- 74.Morris GK. Calling display and mating behaviour of Copiphora rhinoceros Pictet (Orthoptera: Tettigoniidae) Anim Behav. 1980;28(1):42–51. [Google Scholar]
- 75.Simon VB. Not all signals are equal: Male brown anole lizards (Anolis sagrei) selectively decrease pushup frequency following a simulated predatory attack. Ethology. 2007;113(8):793–801. [Google Scholar]
- 76.Fowler-Finn KD, Hebets EA. The degree of response to increased predation risk corresponds to male secondary sexual traits. Behav Ecol. 2011;22(2):268–275. [Google Scholar]
- 77.Tuttle MD, Taft LK, Ryan MJ. Evasive behaviour of a frog in response to bat predation. Anim Behav. 1982;30(2):393–397. [Google Scholar]
- 78.Endler JA. Natural Selection in the Wild (No. 21) Princeton, NJ: Princeton Univ Press; 1986. [Google Scholar]
- 79.Stamps JA. The function of the survey posture in Anolis lizards. Copeia. 1977;1977:756–758. [Google Scholar]
- 80.Jenssen TA, Nunez SC. Spatial and breeding relationships of the lizard, Anolis carolinensis: Evidence of intrasexual selection. Behaviour. 1998;135:981–1003. [Google Scholar]
- 81.Jenssen TA, Lovern MB, Congdon JD. Field-testing the protandry-based mating system for the lizard, Anolis carolinensis: Does the model organism have the right model? Behav Ecol Sociobiol. 2001;50(2):162–172. [Google Scholar]
- 82.Sih A, et al. Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos. 2010;119(4):610–621. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.