Significance
ATP-binding cassette (ABC) exporters transport substrates by an alternating access mechanism that is driven by ATP binding and hydrolysis. The general mechanism is a motion from an inward to an outward state, with a different intertwining of the half-transporters in both states. In this study we determined the function and crystal structure of the ABC exporter McjD that exports the antibacterial peptide microcin J25. Our structure represents a novel nucleotide-bound, outward-occluded state. It does not possess subunit intertwining and shows a well-defined binding cavity that is closed to all sides, consistent with it being an intermediate between the inward- and outward-facing state. Our structure provides valuable insights in a transition state of an ABC exporter.
Keywords: antimicrobial peptide ABC exporter, membrane transporter crystal structure, substrate binding, transport mechanism, microcin immunity protein
Abstract
Enterobacteriaceae produce antimicrobial peptides for survival under nutrient starvation. Microcin J25 (MccJ25) is an antimicrobial peptide with a unique lasso topology. It is secreted by the ATP-binding cassette (ABC) exporter McjD, which ensures self-immunity of the producing strain through efficient export of the toxic mature peptide from the cell. Here we have determined the crystal structure of McjD from Escherichia coli at 2.7-Å resolution, which is to the authors’ knowledge the first structure of an antibacterial peptide ABC transporter. Our functional and biochemical analyses demonstrate McjD-dependent immunity to MccJ25 through efflux of the peptide. McjD can directly bind MccJ25 and displays a basal ATPase activity that is stimulated by MccJ25 in both detergent solution and proteoliposomes. McjD adopts a new conformation, termed nucleotide-bound outward occluded. The new conformation defines a clear cavity; mutagenesis and ligand binding studies of the cavity have identified Phe86, Asn134, and Asn302 as important for recognition of MccJ25. Comparisons with the inward-open MsbA and outward-open Sav1866 structures show that McjD has structural similarities with both states without the intertwining of transmembrane (TM) helices. The occluded state is formed by rotation of TMs 1 and 2 toward the equivalent TMs of the opposite monomer, unlike Sav1866 where they intertwine with TMs 3–6 of the opposite monomer. Cysteine cross-linking studies on the McjD dimer in inside-out membrane vesicles of E. coli confirmed the presence of the occluded state. We therefore propose that the outward-occluded state represents a transition intermediate between the outward-open and inward-open conformation of ABC exporters.
Microcins are gene-encoded antibacterial peptides of low molecular weight (<10 kDa), produced by Enterobacteriacea (1). They are secreted under conditions of nutrient exhaustion through dedicated ATP-binding cassette (ABC) exporters and exert potent antibacterial activity against closely related species (2). Microcin J25 (MccJ25) is a plasmid-encoded, ribosomally synthesized, and posttranslationally modified 21-aa antimicrobial peptide (3). Its 3D structure shows a unique lasso topology (4–6), with the C-terminal tail threading through an N-terminal eight-residue macrolactam ring, where it is locked by bulky amino acid side chains, thus forming a compact interlocked structure called the lasso fold (Fig. S1). This extraordinarily stable structure is apportioned into two regions: a loop involved in uptake of the microcin into sensitive bacteria and a ring/tail region that interacts with the cytoplasmic target of the antimicrobial peptide (1, 7, 8). MccJ25 enters the target cell using the siderophore receptor FhuA (9), and inside the cell it inhibits the bacterial RNA polymerase (7, 8, 10). Four genes are required for the biosynthesis and export of MccJ25 (11). The lasso topology is acquired by modification of a linear 58-aa precursor peptide (McjA) by two dedicated enzymes (McjB and McjC) (12). The ABC transporter McjD ensures efficient export of the toxic mature peptide out of the cell and simultaneously serves as a self-immunity strategy for the producing strain (11). Homologs of McjD and MccJ25-like defense systems can be identified in several genomes of bacterial pathogens (13).
ABC exporters form a large superfamily of transmembrane proteins responsible for the translocation across the membrane of a large diversity of substrates, ranging from small ions to amino acids, sugars, lipids, or peptides, using the energy of ATP hydrolysis. Some ABC exporters contribute to multidrug resistance. Bacterial ABC exporters are dimers, with each monomer composed of a transmembrane domain (TMD) consisting of six TM helices, which forms the translocation pathway across the membrane bilayer and ensures the substrate specificity, and a nucleotide-binding domain (NBD) where binding and hydrolysis of ATP take place. Biochemical and modeling studies, and the crystal structures of the Escherichia coli lipid A transporter MsbA (14), the Staphylococcus aureus exporter Sav1866 (15), and others suggest that ABC exporters extrude their substrates out of the cell via an alternating access mechanism. However, the current structures do not explain how the transition between inward-open and outward-open conformations occurs mechanistically. Here we have determined the high-resolution structure at 2.7-Å resolution of the E. coli immunity-conferring ABC exporter McjD that is responsible for the export of the lasso peptide MccJ25. It displays a new conformation, outward-occluded and without intertwining of the TMDs, which is intermediate between the outward-open and inward-open state. In addition, the structure defines a clear binding cavity that can accommodate one MccJ25 molecule. Our functional data in detergent solution and proteoliposomes demonstrate that McjD mediates MccJ25 transport in an ATP-dependent fashion and that in the absence of Mccj25, the protein can mediate the transport of typical substrates of multidrug transporters.
Results
Functional Characterization.
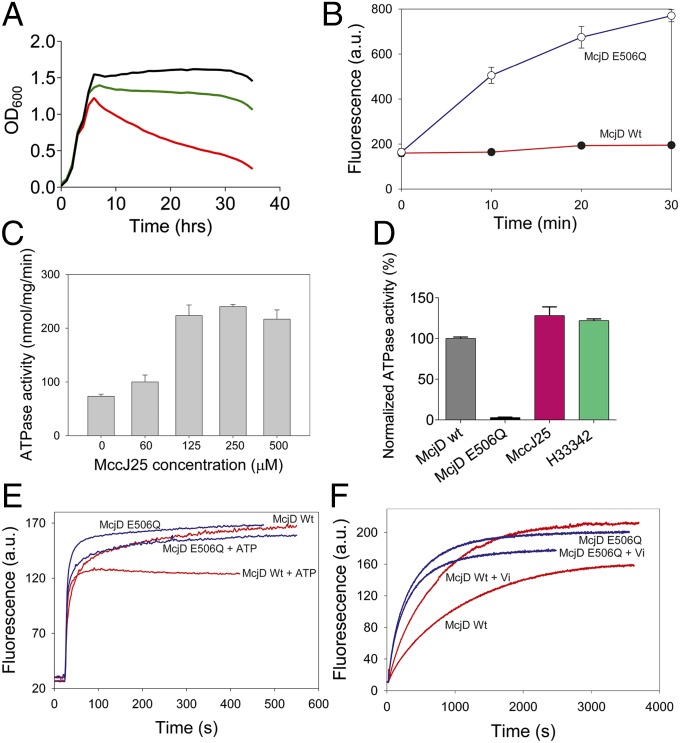
Growth curves of E. coli strains producing MccJ25 with or without McjD showed that the immunity of cells to MccJ25 required the expression of McjD, whereas sensitivity to MccJ25 was observed in the absence of McjD expression (Fig. 1A). McjD-mediated resistance was also observed for MccJ25 added to growth medium (Fig. S1). Transport measurements in these cells with fluorescent, BODIPY-labeled MccJ25 demonstrated that the MccJ25 resistance is due to reduced peptide accumulation and active extrusion by the McjD transporter (Fig. 1B). The interaction between McjD and MccJ25 was further examined using purified McjD in detergent solution and proteoliposomes (Figs. S2 and S3). Binding studies using microscale thermophoresis (MST) showed that McjD bound MccJ25 with a KD of 104 ± 52 μM (Fig. S4). McjD exhibited a basal ATPase activity in the absence of added transport substrate (Fig. S1); the basal activity of McjD was measured at different ATP concentrations and displayed a Km of 169.3 ± 6.7 µM and a Vmax of 44.4 ± 0.5 nmol min−1 mg protein−1. A Walker B mutant, E506Q, was used as a control to subtract ATP background hydrolysis and showed significantly reduced activity, 2% relative to the wild-type McjD. The ATPase activity of McjD was inhibited by the specific inhibitors adenosine 5′-(β,γ-imido)triphosphate (AMP-PNP) and ortho-vanadate, with an IC50 of 39.5 ± 0.6 μM and 61.6 ± 1.1 μM, respectively (Fig. S5). The ATPase activity of McjD reconstituted in proteoliposomes was stimulated by MccJ25 in a concentration-dependent manner (Fig. 1C). The ATPase activity of detergent purified McjD could also be stimulated by MccJ25 at slightly lower levels compared with the reconstituted protein (Fig. 1D). Surprisingly, the McjD ATPase activity was also stimulated by Hoechst 33342 (Fig. 1D), which is a typical substrate for multidrug transporters such as Sav1866 and MsbA (16, 17). Our observations on McjD-mediated Hoechst 33342 transport in proteoliposomes, and McjD-mediated Hoechst 33342 and ethidium transport in intact cells, underscore the relevance of structural comparisons between McjD and these ABC exporters (Fig. 1 E and F and Fig. S1).
Fig. 1.
Functional characterization of McjD. (A) Growth of cytotoxic MccJ25-expressing E. coli requires coexpression of ABC exporter McjD (green, production of MccJ25 plus McjD; red, MccJ25 minus McjD; black, without MccJ25 production). (B) Active efflux of fluorescent BODIPY-labeled MccJ25 from cells results in reduced MccJ25 accumulation for McjD compared with the inactive E506Q McjD with equal expression (Fig. S3). (C) Stimulation of purified McjD-ATPase by MccJ25 in proteoliposomes. (D) Ligand-stimulated ATPase activity of McjD in detergent solution. (E) Hoechst 33342 transport in proteoliposomes containing an equal amount of McjD or the E506Q mutant. ATP-dependent Hoechst 33342 transport from the membrane into the acidic lumen causes quenching of dye fluorescence for McjD. (F) Hoechst 33342 efflux in intact cells. Inhibition of McjD with ortho-vanadate (Vi) increases Hoechst 33342 accumulation for McjD but not E506Q-McjD. Fluorescence traces are typical for data obtained in three independent experiments using independent batches of cells and proteoliposomes. Error bars are shown for all measurements (mean ± SEM; n = 3); when error bars are not visible, they are contained within the symbols.
Overall McjD Structure.
We determined the structure of McjD in complex with the ATP analog AMP-PNP at 2.7-Å resolution (Table S1) by molecular replacement using the Salmonella typhimurium MsbA (14) and S. aureus Sav1866 (15) as search models. The quality of the electron density allowed us to build the entire sequence, and the structure was refined to an Rfactor of 24.7% and Rfree of 26.6%. Clear electron density was also observed for AMP-PNP, Mg2+, and two detergent molecules (Fig. S6). The overall architecture of McjD is similar to Sav1866 and MsbA multidrug ABC exporters (Fig. 2A); the structure is a homodimer, and each monomer is composed of an N-terminal TMD (6 TM helices) and a C-terminal NBD. The transporter is 124 Å long, 55 Å wide, and 51 Å deep. The dimer interface at the TMDs is formed between TM2 and TM5/TM6 from one monomer, with the equivalent TM5/TM6 and TM2 from the opposite monomer, burying a surface area of ∼7,100 Å2 per subunit. The TM helices are connected by extracellular (ECL) and intracellular (ICL) loops; ICL1 (between TM2 and TM3) and ICL2 (between TM4 and TM5) form the coupling helices that interact with the NBD to transmit ATP binding and hydrolysis-induced conformational changes to the TMDs. For this purpose ICL1 contacts both NBDs, whereas ICL2 only interacts with the NBD from the opposite monomer, as observed for other ABC exporters. The overall conformation of the McjD NBD is very similar to that of Sav1866 and MsbA. Because McjD was cocrystallized with the ATP analog AMP-PNP and MgCl2, the NBDs are dimerized in the ATP-bound state with the P-loop and ABC signature motifs involved in the binding of the nucleotide at each nucleotide-binding site.
Fig. 2.
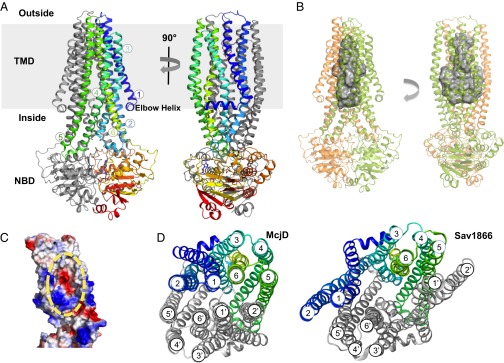
Structure of the outward occluded conformation of McjD. (A) The McjD structure is viewed in the plane of the membrane. The membrane is shown in gray. The transmembrane helices of one subunit are numbered. Bound AMP-PNP is shown as sticks. (B) The cavity of McjD is shown as gray surface. (C) The electrostatic surface calculation of McjD cavity displays positive and negative charges to bind MccJ25. The cavity is outlined with a broken yellow line. The surfaces are colored from blue (positively charged regions) to red (negatively charged regions). Hydrophobic regions are shown as white. (D) Periplasmic view of the outward occluded McjD (Left) and “winged” outward-open Sav1866 (pdb 2ONJ) (Right) structures show clear differences in helix packing and accessibility to the interior chamber. The NBDs have been omitted for clarity.
McjD Ligand Binding Cavity.
The 12 TM helices form a large cavity, ∼5,900 Å3, that is occluded from both sides of the membrane (Fig. 2 B–D). The cavity is aligned by ionizable (six Arg) residues, as well as polar and hydrophobic residues (Fig. 2C). The cavity of McjD is approximately 40 Å long, with its widest point at 21 Å and the narrowest point at 10 Å, perpendicular to the membrane. In the absence of direct structural information about the McjD–MccJ25 complex, we constructed a model to identify potential residues that coordinate peptide binding. The NMR structure of MccJ25 shows that the lasso peptide is 23 Å long with a loop length of 10 Å, and is 18 Å wide at the lariat ring (4–6). In the current conformation of McjD, we could only position MccJ25 in one orientation without further manipulation. The volume of MccJ25 from the NMR structure was calculated to be 1784 Å3; it thus could fit inside the McjD cavity with some minor steric hindrance (Fig. S7); the lariat ring of MccJ25 is a very rigid structure, and it could only be positioned facing toward the wider and more charged cytoplasmic side of the cavity. The cavity at the periplasmic face is more narrow and hydrophobic and thus can accommodate and bind residues in the loop region of MccJ25 (Phe10, Ile13, Ile17, and Phe19). We have recently shown that MccJ25 loses its β-strand structure at the loop region upon binding to the outer membrane receptor FhuA (9). The less structured and wider loop conformation allows MccJ25 to interact with the FhuA barrel wall and extracellular loops for internalization. This new conformation of MccJ25 could also be accommodated within the McjD cavity. On the basis of the position of MccJ25 into the McjD cavity, we constructed point mutations along the cavity to identify residues involved in the binding of MccJ25. Unlike wild-type McjD, the presence of MccJ25 did not enhance the ATPase activity of the mutants. Furthermore, the binding affinity of the McjD mutants for MccJ25 was reduced by almost 10-fold (Fig. S4). These single point mutations are unlikely to completely abolish MccJ25 binding, but the absence of ligand-induced ATPase activity and reduced binding affinity support the proposition that the residues lining the McjD cavity are involved in the binding and transport of MccJ25.
Inspection of the McjD binding cavity revealed elongated electron density close to the bottom of the ligand-binding cavity that is formed between the two McjD monomers (Fig. S6); the high resolution allowed us to model and refine this density as nonyl-glucopyranoside (derived from the crystallization buffer). The residues in this region can bind inhibitors, as shown by the low-resolution inward-facing structure of the mammalian multidrug resistance ABC transporter P-glycoprotein in the presence of the cyclic peptide inhibitors QZ59-SSS and QZ59-RRR (18). The two nonyl-glucopyranoside detergent molecules in the McjD structure are bound in a similar location as the P-glycoprotein inhibitors. However, the ATPase activity of purified McjD in detergent solution and in proteoliposomes was not significantly stimulated or inhibited by nonyl-glucopyranoside (Fig. S8). Furthermore, McjD expression in intact cells was not associated with enhanced resistance to nonyl-glucopyranoside compared with cells expressing nonactive E506Q-McjD (Fig. S8); McjD expression does confer resistance on cells to MccJ25 under these conditions (Fig. 1A and Fig. S1C). We therefore conclude that nonyl-glucopyranoside is not a substrate or inhibitor of McjD. Under the current crystallization conditions, the detergent contributes to the stability of the McjD dimer in this occluded state. The crystal packing may further stabilize the current conformation, even though we do not observe significant crystal contacts along the TMs (Fig. S6).
Cysteine Cross-Linking.
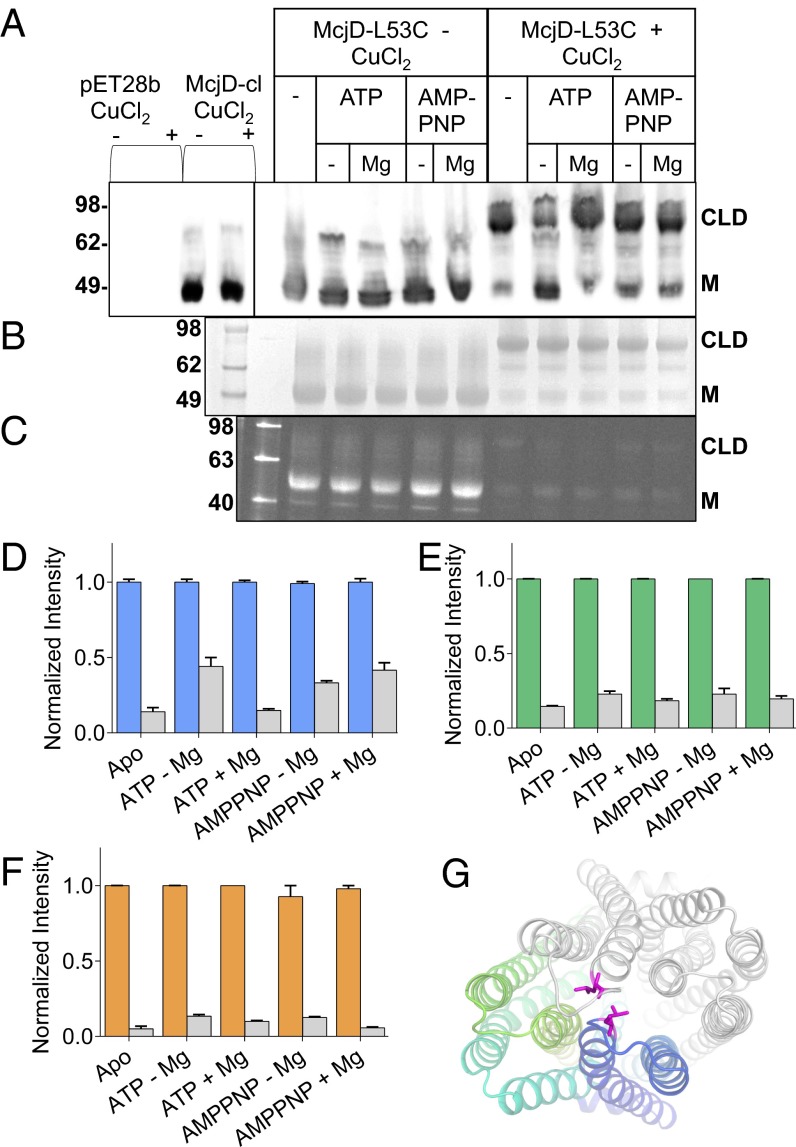
In the presence of AMP-PNP, both the MsbA and Sav1866 structures adopt a “winged” outward-open conformation. Because the McjD structure in the presence of AMP-PNP is in an occluded state, we used a predictive cysteine-cross approach to further characterize this new conformation. As the wild-type protein contains four cysteines, we first constructed a Cys-less McjD protein (McjD-cl). This mutant is functional because it shows a basal ATPase and ethidium efflux activity comparable to wild-type protein and mediates cellular resistance to MccJ25 (Fig. S1). Next we introduced a single cysteine at position 53 (McjD-L53C), which allows intermolecular cross-linking between the extracellular loops connecting TM1-TM2 and TM1’-TM2’ in the ATP-bound McjD dimer in a manner that is specific for the occluded state and that excludes the “winged” outward-facing state. ISOVs containing McjD-L53C and detergent purified protein were preincubated with nucleotides for 10 min before the addition of the oxidizing agent CuCl2 (SI Materials and Methods). McjD-cl did not display any cross-linking. In addition, the apo McjD-L53C preparation did not contain significant amounts of preformed cross-linked dimer in the absence of CuCl2. In the presence of the oxidizing agent CuCl2 and AMP-PNP or ATP ± Mg2+, intermolecular cross-linking in the McjD dimer verified the existence of the nucleotide-bound occluded state in both E. coli membranes and detergent solution (Fig. 3). Apo McjD can be cross-linked as effectively as the AMP-PNP–bound state because the L53C residues are also in close proximity in an inward-facing, MsbA-based model of McjD. Because the new McjD conformation was crystallized in the absence of MccJ25, we also tested the effect of MccJ25 addition on intermolecular cross-linking in the McjD L53C dimer in E. coli membrane vesicles. Preincubation of McjD with MccJ25 did not alter the cross-linking behavior in the absence of nucleotides (Fig. S1), suggesting that, upon binding of MccJ25 to inward-facing McjD, the TMs do not undergo major conformational changes.
Fig. 3.
Predictive cross-linking of McjD. The reaction conditions for each lane are indicated above the gels. McjD-L53C was preincubated with appropriate nucleotides for 10 min before cross-linking reactions; cross-linking of McjD-L53C was performed in the presence of 1 mM CuCl2 for 30 min at room temperature. (A) Western-blot analysis of McjD-L53C cross-linked in ISOVs. Nucleotide dependence of intermolecular cysteine cross-linking between the McjD monomers at position 53 is consistent with the formation of an outward-facing occluded state in the ATP-bound state. CLD denotes the formation of the cross-linking dimer and M the monomer in the absence of CuCl2. Leu53Cys mutation is located in the loop that connects TM 1 and 2 that form the occluded structure; the formation of the cross-linking dimer in the presence of nucleotides verifies that the nucleotide-bound occluded state can also exist in the plasma membrane of E. coli. (B) Coomassie-stained SDS/PAGE gel of cross-linked detergent purified McjD. (C) The degree of cross-linking of detergent-purified McjD was estimated in the presence of 7-diethylamino-3-(4'-maleimidylphenyl)-4-methylcoumarin (cpm) that becomes fluorescent upon binding to free cysteines. Formation of the cysteine cross-links resulted in reduced available free cysteines for labeling by the cpm; a small degree of nonspecific binding is observed for the dimeric species by cpm. (D) The relative intensity for each band was estimated by densitometry of the Western blot, (E) Coomassie-stained, and (F) in-gel fluorescence gel; the signal is displayed as fold change in intensity relative to the non–cross-linked reactions. In the absence of CuCl2, the fluorescent signal intensity is high for the monomeric species, M, whereas formation of the CLD has resulted in a very reduced signal. Error bars are shown for all densitometry measurements (mean ± SEM; n = 3). A very small percentage of monomeric species is still present when the cross-linking reactions are terminated. Gray bars represent fraction of monomeric species relative to the total protein present. (G) View from the periplasm of the Leu53 side chain position. Coloring scheme as in Fig. 2A. The L53 side chain is shown as magenta stick.
Discussion
Comparison with Other ABC Exporters.
McjD was cocrystallized with the ATP analog AMP-PNP and MgCl2. The two NBDs are in the ATP-bound state in which the P-loop and ABC signature motifs tightly interact with the nucleotide. ATP binding and hydrolysis at the NBD site trigger conformational changes at the TMDs, and these changes are transmitted through the two coupling helices ICL 1 and 2, that link the NBDs to the TMs. ICL1 contacts both NBDs, whereas ICL2 only interacts with the NBD from the opposite monomer, as with other ABC exporters. The overall conformation of the McjD NBDs is very similar to that of Sav1866.
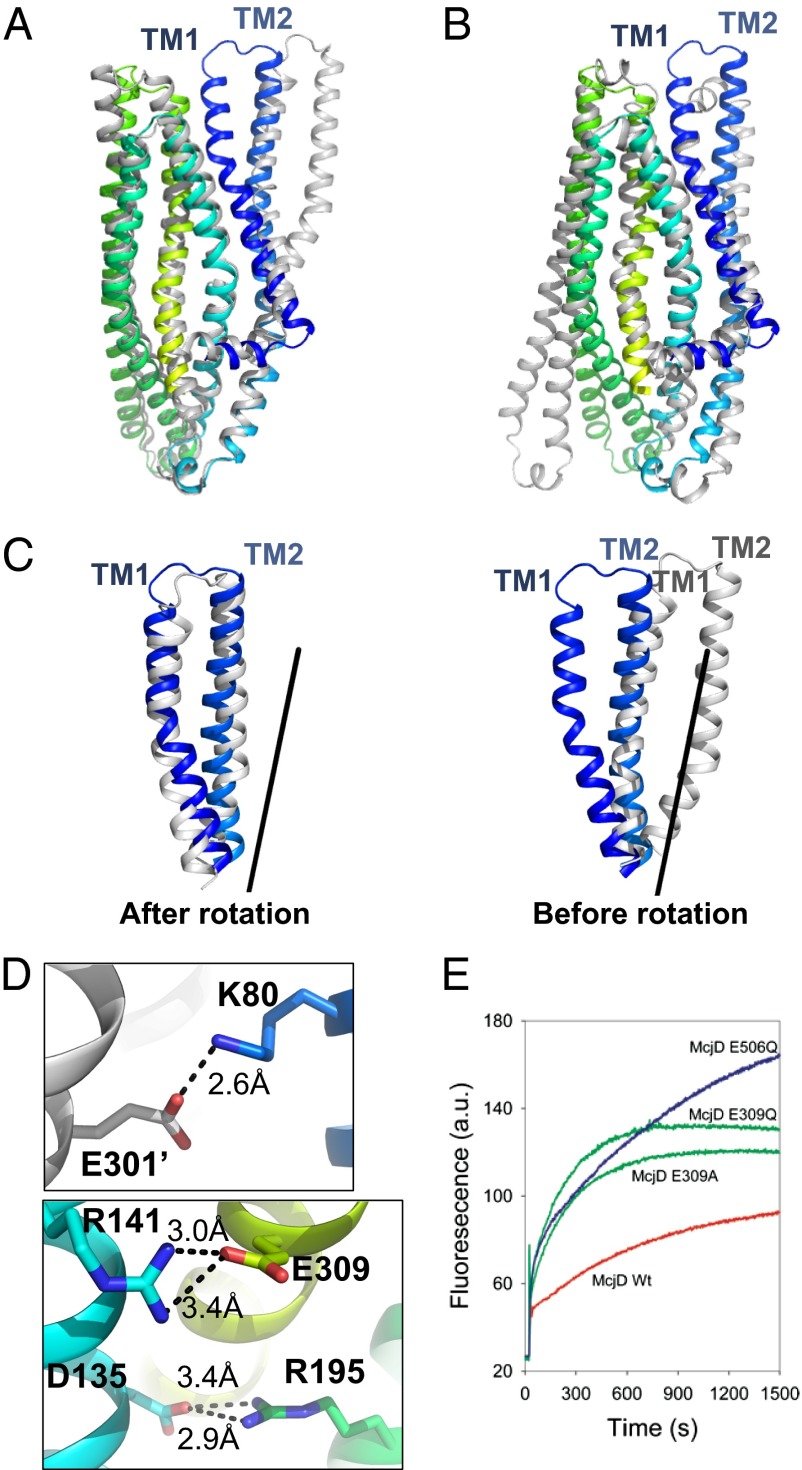
The most striking feature of McjD is that it is occluded on both sides of the membrane. Comparison of McjD with the outward open structures of Sav1866 and MsbA reveals that both the cytoplasmic and periplasmic sides of the McjD dimer are occluded, unlike the Sav1866 and MsbA structures, which are open at the periplasmic side (Figs. 2D and 4 A and B). A model of MsbA and Sav1866 based on McjD results in an occluded cavity of similar size as found in McjD. McjD can be aligned with the outward-open Sav1866 and MsbA at the TM region (330 Ca atoms) with an rmsd of 2.3 Å and 2.2 Å, respectively. The Sav1866 and MsbA dimer structures contain two “wings” formed by the TMs 1 and 2 of one subunit and TMs 3–6 of the other subunit. In McjD, TMs 1 and 2 have rotated symmetrically by 26° along Leu29 and Gln90 and shifted by 6 Å at ECL1 relative to Sav1866 (Fig. 4C). As a result, TM1 is located in the vicinity of TM6 of the same monomer and TM1 of the adjacent monomer. TM2 is close to TM5 from the adjacent monomer; TM5 bends by 25° at Leu264 toward TM2. This configuration of TMs causes the occlusion at the periplasmic side and is consistent with the observations on cysteine cross-linking at position L53C in the apo and AMP-PNP bound conformations of McjD (Fig. 3 and Fig. S1 F and G). Another feature of McjD is the presence of three salt bridges between Lys80 and Glu301 (from the opposite monomer), Arg141 and Glu309, and Arg195 and Asp135; these salt bridges seem to stabilize packing of TM2 to TM6 (of the opposite monomer), TM3 to TM6, and TM3 to TM4 in our occluded state (Fig. 4D). For comparison, the outward-open Sav1866 is stabilized by salt bridges between Glu78 from TM2 and Arg295 from TM6 of the opposite monomer. We have previously shown that Glu314 in the ABC multidrug transporter LmrA from Lactococcus lactis, which is equipositional to Glu309 in McjD, is important for LmrA-mediated efflux of ethidium and other substrates (19). McjD shares 19% sequence identity at the TMD (residue 1–342) with LmrA. We investigated the role of Glu309 for McjD activity by mutating this residue to Gln and Ala. Our ethidium transport data in whole cells (Fig. 4E) are reminiscent of those obtained for LmrA (19) and show a low residual ethidium efflux activity for the mutants compared with wild-type protein. Therefore, the stabilization of the TMs in the McjD dimer by the salt bridges supports the important role of our occluded state in the propagation of the transport cycle. We also investigated whether McjD can adopt an outward-open state by performing the Leu53Cys cross-linking experiment at pH 4.5 (Fig. S1 H and I), where salt bridges in the McjD dimer can be disrupted through protonation of relevant carboxylates. In the absence of nucleotides, a substantial population of McjD formed a cross-linked dimer under these conditions, whereas dimer formation was not observed in the presence of ATP or AMP-PNP. These results suggest that McjD can sample an outward-open conformation similar to Sav1866.
Fig. 4.
Conformational changes in McjD. The McjD transmembrane helices of one subunit are colored as in Fig. 2A. Superposition of McjD with (A) Sav1866 (outward open, gray) and (B) Vibrio cholerae MsbA (inward closed apo, gray). McjD shows structural similarities to the cytoplasmic TM region of Sav1866 and the periplasmic region of MsbA. The most significant conformational changes between McjD and Sav1866 are in TMs 1 and 2. (C) Superposition of TMs 1 and 2 of Sav1866 onto McjD (Left); the Sav1866 TMs 1 and 2 have been rotated around the axis shown (Right). (D) The salt bridges that are stabilizing the occluded conformation are shown as sticks. (Upper) Residues involved in stabilizing TM2 to TM6 (of the opposite monomer, shown in gray). (Lower) Residues that stabilize TM3 to TM6 and TM3 to TM4. The salt-bridge distances are in Å and shown as black broken lines. Color scheme as in Fig. 2A. (E) Disruption of the Glu309-R141 salt bridge by E309Q and -A mutations results in reduced ethidium bromide efflux in whole cells compared with wild-type McjD. The transport-inactive E506Q mutant is used as a control.
Our work on McjD describes a novel conformation for ABC exporters, a nucleotide-bound outward-facing occluded state. A nucleotide-bound occluded conformation has been previously reported for the type I ABC importer MBP-MalFGK2 (20) and type II ABC importer BtuCD-F (21). The cavity in MalFGK2 is not completely occluded at the TMD, but the MBP protein occludes it at the periplasmic side. In contrast, the BtuCD-F cavity is occluded at the TMD by the periplasmic and cytoplasmic gate. The existence of a conformation in which the NBDs are dimerized without opening of the TMD at their external side was also recently observed for MsbA in cysteine cross-linking and FRET studies (22). Our McjD conformation is therefore relevant for other ABC exporters.
Mechanistic Implication.
ABC exporters need to alternate between inward-facing and outward-facing conformations to transport their substrates from the cytoplasm to the periplasm in an ATP-dependent manner. The proposed mechanism for exporters is an alternating access model with subunit intertwining (14, 15); the transporter cycles between an inward- and outward-facing conformation in which the TM 4 and 5 hairpin is exchanged between the TMDs of the half-transporters in the inward-facing dimer, whereas the TM 1 and 2 hairpin is exchanged in the outward-facing dimer. In the inward conformation the substrate can bind in the open cavity between the TMDs, which in turn stimulates binding of ATP (22). The affinity of McjD for the mature MccJ25 peptide in detergent solution (apparent KD of 104 μM) might indicate that under physiological conditions substrate binding to McjD occurs from the cytoplasmic membrane in which MccJ25 accumulates. Our point mutations are associated with an almost 10-fold reduction in the experimentally determined binding affinity. In the inward-facing apo conformation the two NBDs are separated, and binding of the ATP brings the two NBDs together. Formation of the ATP-bound outward-open state disrupts the binding cavity causing release of MccJ25 at the external side of the membrane. MccJ25 would then be exported extracellularly in a TolC-dependent fashion (23). ATP hydrolysis finally resets the McjD transporter back to the inward-facing conformation.
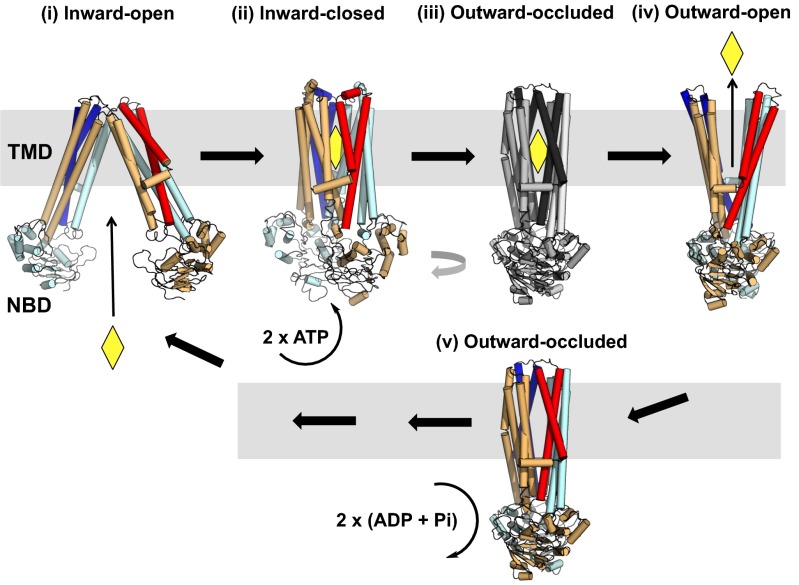
Previously published models do not explain in detail by what mechanism the transition between the two conformations occurs. The nucleotide-bound outward occluded McjD has structural similarities to the closed cytoplasmic side of outward-open Sav1866 and MsbA in which the NBDs are dimerized, and the closed periplasmic side of inward-open apo MsbA (Fig. 4 A and B). As such, it represents an intermediate state between the outward-facing conformation and the inward-facing conformation (Fig. 5). After the ligand has left the outward-open cavity, TMs 1 and 2 rotate away from TMs 3–6 of the opposite monomer to form the outward-occluded state without TMD intertwining (current McjD structure). We propose that the initial conformational changes associated with this transition are ATP-independent.
Fig. 5.
Proposed role of the outward-occluded state in the mechanism of ABC exporters. Ligand binding to the inward-open (apo) conformation (state i) facilitates transition of the transporter into the inward-closed (apo) conformation (state ii). ATP binding is associated with a transition into a hypothetical occluded conformation (state iii), and subsequent formation of a nucleotide-bound outward-open conformation (state iv). Upon release of the ligand, the ABC exporter adopts an outward-occluded conformation (state v) (current McjD structure). ATP hydrolysis resets the transporter back to the inward-facing conformation (state i). The TMs 1 and 2 from each monomer are highlighted in dark blue and red. The substrate is shown as a yellow diamond.
Transition from the outward occluded state to the inward-open conformation will require ATP hydrolysis, which disrupts the NBD dimer interface and induces intertwining of TMs 1–3, 6 from one monomer with TMs 4 and 5 from the opposite monomer. After the system reverses back to the inward conformation, a substrate-bound form of the nucleotide-bound outward occluded state might occur during the ATP-dependent transition from the inward-facing state to the outward-facing state (Fig. 5); in BtuCD-F the equivalent occluded ATP-bound state was trapped with engineered disulphide bonds (21).
In summary, to the authors’ knowledge we have generated the first high-resolution structure of an antibacterial peptide (microcin) ABC exporter. Our structure of McjD represents a novel outward-occluded intermediate between the outward-facing state and inward-facing state of ABC exporters.
Materials and Methods
Detailed materials and methods are available online (SI Materials and Methods). McjD was cloned as a fusion with a C-terminal GFP that contained a His6-tag and tobacco etch virus (TEV) site. The protein was expressed in E. coli and purified in 0.03% n-Dodecyl-β-D-maltopyranoside. The GFP-tag was removed by TEV protease before crystallization and biochemical analyses in detergent solution. For functional analyses in intact cells and proteoliposomes, non–GFP-tagged McjD was expressed in a drug-hypersensitive E. coli that lacks the major endogenous drug efflux pump AcrAB-TolC. The ATPase activity of purified proteins in detergent solution and proteoliposomes was measured in an enzymatic-coupled assay and by the malachite green method, respectively. Transport in intact cells and proteoliposomes was determined by fluorimetry. Ligand binding was assessed by MST. Cysteine cross-linking studies were performed in ISOVs and detergent-purified McjD protein. McjD crystals were grown in the presence of 10 mM AMP-PNP, 2.5 mM MgCl2, and nonyl-glucopyranoside. The structure of McjD was solved by molecular replacement using the MsbA (14) and Sav1866 (15) structures as search models. The final structure was refined to a resolution of 2.7 Å.
Supplementary Material
Acknowledgments
We thank Diamond Light Source for beam time allocation and access; Prof. Xiaodong Zhang for access to the Monolith NT.115 for MST measurements; David Charles for technical assistance; Dr. Alexander Cameron and Yusuke Sekiguchi for kindly providing pure AsbT-GFP protein for MST measurements; Christophe Goulard for purifying the MccJ25 peptide ligands used in this study; and Prof. Robert Ford and Dr. Alexander Cameron for critical reading of the manuscript. Financial support was provided by the Biotechnology and Biological Sciences Research Council (Grants BB/H01778X/1 to K.B., BB/G023425/1 to S.I., and BB/I002383/1 to H.W.v.V.) and Wellcome Trust (MPL: WT/099165/Z/12/Z to S.I.). I.M. is supported by a Ministry of Science, Technology and Innovation postgraduate scholarship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 4PL0).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320506111/-/DCSupplemental.
References
- 1.Duquesne S, Destoumieux-Garzón D, Peduzzi J, Rebuffat S. Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat Prod Rep. 2007;24(4):708–734. doi: 10.1039/b516237h. [DOI] [PubMed] [Google Scholar]
- 2.Baquero F, Bouanchaud D, Martinez-Perez MC, Fernandez C. Microcin plasmids: A group of extrachromosomal elements coding for low-molecular-weight antibiotics in Escherichia coli. J Bacteriol. 1978;135(2):342–347. doi: 10.1128/jb.135.2.342-347.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rebuffat S, Blond A, Destoumieux-Garzón D, Goulard C, Peduzzi J. Microcin J25, from the macrocyclic to the lasso structure: Implications for biosynthetic, evolutionary and biotechnological perspectives. Curr Protein Pept Sci. 2004;5(5):383–391. doi: 10.2174/1389203043379611. [DOI] [PubMed] [Google Scholar]
- 4.Rosengren KJ, et al. Microcin J25 has a threaded sidechain-to-backbone ring structure and not a head-to-tail cyclized backbone. J Am Chem Soc. 2003;125(41):12464–12474. doi: 10.1021/ja0367703. [DOI] [PubMed] [Google Scholar]
- 5.Wilson KA, et al. Structure of microcin J25, a peptide inhibitor of bacterial RNA polymerase, is a lassoed tail. J Am Chem Soc. 2003;125(41):12475–12483. doi: 10.1021/ja036756q. [DOI] [PubMed] [Google Scholar]
- 6.Bayro MJ, et al. Structure of antibacterial peptide microcin J25: A 21-residue lariat protoknot. J Am Chem Soc. 2003;125(41):12382–12383. doi: 10.1021/ja036677e. [DOI] [PubMed] [Google Scholar]
- 7.Destoumieux-Garzón D, et al. The iron-siderophore transporter FhuA is the receptor for the antimicrobial peptide microcin J25: Role of the microcin Val11-Pro16 beta-hairpin region in the recognition mechanism. Biochem J. 2005;389(Pt 3):869–876. doi: 10.1042/BJ20042107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Semenova E, Yuzenkova Y, Peduzzi J, Rebuffat S, Severinov K. Structure-activity analysis of microcinJ25: Distinct parts of the threaded lasso molecule are responsible for interaction with bacterial RNA polymerase. J Bacteriol. 2005;187(11):3859–3863. doi: 10.1128/JB.187.11.3859-3863.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathavan I, et al. Structural basis for hijacking siderophore receptors by antimicrobial lasso peptides. Nat Chem Biol. 2014;10(5):340–342. doi: 10.1038/nchembio.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathavan I, Beis K. The role of bacterial membrane proteins in the internalization of microcin MccJ25 and MccB17. Biochem Soc Trans. 2012;40(6):1539–1543. doi: 10.1042/BST20120176. [DOI] [PubMed] [Google Scholar]
- 11.Solbiati JO, et al. Sequence analysis of the four plasmid genes required to produce the circular peptide antibiotic microcin J25. J Bacteriol. 1999;181(8):2659–2662. doi: 10.1128/jb.181.8.2659-2662.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duquesne S, et al. Two enzymes catalyze the maturation of a lasso peptide in Escherichia coli. Chem Biol. 2007;14(7):793–803. doi: 10.1016/j.chembiol.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Maksimov MO, Pelczer I, Link AJ. Precursor-centric genome-mining approach for lasso peptide discovery. Proc Natl Acad Sci USA. 2012;109(38):15223–15228. doi: 10.1073/pnas.1208978109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward A, Reyes CL, Yu J, Roth CB, Chang G. Flexibility in the ABC transporter MsbA: Alternating access with a twist. Proc Natl Acad Sci USA. 2007;104(48):19005–19010. doi: 10.1073/pnas.0709388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443(7108):180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 16.Reuter G, et al. The ATP binding cassette multidrug transporter LmrA and lipid transporter MsbA have overlapping substrate specificities. J Biol Chem. 2003;278(37):35193–35198. doi: 10.1074/jbc.M306226200. [DOI] [PubMed] [Google Scholar]
- 17.Velamakanni S, Yao Y, Gutmann DA, van Veen HW. Multidrug transport by the ABC transporter Sav1866 from Staphylococcus aureus. Biochemistry. 2008;47(35):9300–9308. doi: 10.1021/bi8006737. [DOI] [PubMed] [Google Scholar]
- 18.Aller SG, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323(5922):1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shilling R, et al. A critical role of a carboxylate in proton conduction by the ATP-binding cassette multidrug transporter LmrA. FASEB J. 2005;19(12):1698–1700. doi: 10.1096/fj.04-3558fje. [DOI] [PubMed] [Google Scholar]
- 20.Oldham ML, Khare D, Quiocho FA, Davidson AL, Chen J. Crystal structure of a catalytic intermediate of the maltose transporter. Nature. 2007;450(7169):515–521. doi: 10.1038/nature06264. [DOI] [PubMed] [Google Scholar]
- 21.Korkhov VM, Mireku SA, Locher KP. Structure of AMP-PNP-bound vitamin B12 transporter BtuCD-F. Nature. 2012;490(7420):367–372. doi: 10.1038/nature11442. [DOI] [PubMed] [Google Scholar]
- 22.Doshi R, van Veen HW. Substrate binding stabilizes a pre-translocation intermediate in the ATP-binding cassette transport protein MsbA. J Biol Chem. 2013;288(30):21638–21647. doi: 10.1074/jbc.M113.485714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delgado MA, Solbiati JO, Chiuchiolo MJ, Farías RN, Salomón RA. Escherichia coli outer membrane protein TolC is involved in production of the peptide antibiotic microcin J25. J Bacteriol. 1999;181(6):1968–1970. doi: 10.1128/jb.181.6.1968-1970.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.