Abstract
Persistent gene silencing is crucially required for the successful therapeutics of short interfering RNA (siRNA). Here, we describe a nanoparticle based delivery system which assembled by layering siRNAs between protease degradable polypeptides to extend the therapeutic window. These tightly packed nanoparticles are efficiently taken up by cells by endocytosis, and the fabricated siRNAs are gradually released following intracellular degradation of the polypeptide layers. During cell division, the particles are distributed to the daughter cells. Due to the slow degradation through the multiple layers, the particles continuously release siRNA in all cells. Using this controlled release construct, the in vivo gene silencing effect of siRNA is consistent for an ultra-long period of time (>3 weeks) with only a single treatment.
Keywords: siRNA, gene silencing, gold nanoparticles, in vivo, poly-L-lysine
1. Introduction
Short interfering RNAs (siRNAs)-mediated gene regulation in mammalian cells was first discovered about two decades ago and siRNA has since shown immense potential in treating various diseases by silencing abnormally up-regulated genes.[1–4] However, delivering the highly charged siRNA to the targeted cells and enabling the specific target gene silencing effect to persist remains challenging.[5] Although huge efforts have been invested to develop effective non-viral siRNA delivery systems, including polyplexes, micelleplexes, and exosome nanoparticles,[6, 7] various physiological limitations still hinder the successful clinical translation.[8, 9] The main drawbacks of gene silencing using synthetic siRNA are transient silencing due to the biodegradation of siRNA, siRNA dilution upon cell division, and its nonrenewable-nature.[10] To obtain a consistent silencing effect in vivo, it usually requires repetitive administration of the agent.[11] However, such repeated injections have resulted in a substantial impediment to patient treatment, as evidenced by decreased enrollment in clinical trials and decreased patient compliance.[12] Complete and prolonged gene silencing with a single treatment would offer enormous benefits for chronic diseases, scaling down the administration frequency in dosing schedule.[13]
To achieve long-lasting controlled release and gene silencing effects, various groups have developed novel delivery systems for slow and sustained release of the oligonucleotide or siRNA.[14–18] Several trials have also extended into primary T lymphocytes and human stem cells for prolonged gene silencing.[19, 20] Although promising cell culture results were obtained in those studies, the actual in vivo efficiency remains to be demonstrated. Viral vectors have shown great efficiency in in vivo siRNA delivery,[21] however, introducing a foreign gene into patients is also a clinical concern.[22, 23] Recently we have developed a fabrication method to prepare multilayer siRNA delivery nano-vector and a long-lasting nanoparticle -based fluorescent label for extended cell imaging.[24, 25] Onto an inert gold nanoparticles (AuNPs), negatively charged siRNAs were layered between the oppositely charged polypeptide layers which are sensitive to proteases. The initial results with this multi-layered siRNA delivery vector suggest several advantages over other non-viral delivery systems, including uniform size, efficient cell translocation, and enhanced siRNA stability. In this study, we further optimized this nanodelivery system and determined its exceptional gene silencing effect was persistent for more than three weeks in vivo.
2. Results and Discussion
To prepare the multilayered siRNA-coated AuNPs (sRAuNPs), the negatively charged AuNPs (size: 40 nm) in water were dropped into the positively charged poly-L-lysine (PLL) solution (average Mw = 22.5 KDa; Figure 1a). After a 30-min incubation at room temperature with shaking and several washes with sterilized water, the positively charged PLL-coated AuNPs were added to the negatively charged siRNA solution. After incubation, free unbound siRNAs were removed by centrifugation and an additional PLL layer was added so the resulting positively charged AuNPs (sR1P) had one layer of siRNA and two layers of PLL. By repeating this procedure again, AuNPs (sR2P) with two layers of siRNA and three layers of PLL were successfully fabricated by electrostatic interactions. A zigzag pattern of the surface zeta-potential of each layer supported the success of the layering (Figure 1b). The final particle size, determined by dynamic light scattering (DLS) measurement, was found to be approximately 150 nm (Figure 1b).
Figure 1.
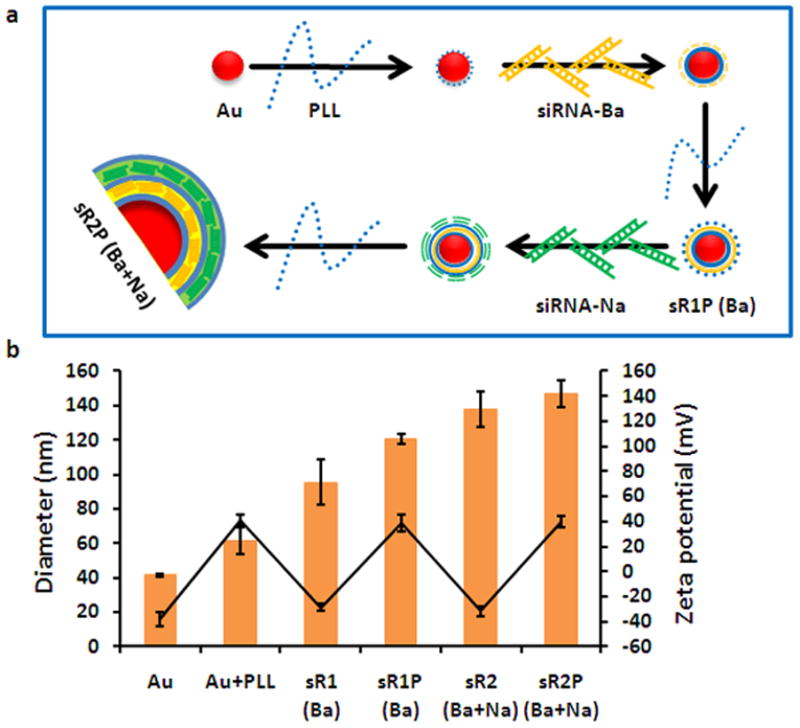
Preparation of the sRAuNPs. (a) The process of preparing multilayered siRNA-coated AuNPs by electrostatic interaction. (b) The average size (orange bar) and zeta potential (black circle) of multilayered sRAuNPs.
Two reported siRNA sequences, termed siLuc-Na and siLuc-Ba,[1, 26] against two different regions of the luciferase gene were used because maximal gene silencing effect has been reported with simultaneously applied multiple siRNAs.[27, 28] sRAuNPs were fabricated with one or two siRNAs. sR2P (Ba + Na) was prepared with siLuc-Ba on the first layer and siLuc-Na on the second layer, and the order of siRNA in sR2P(Na + Ba) was reversed. A scramble siRNA (siLuc-Sc), which has the same length but randomly sequenced of siLuc-Na, was included as a negative control.
To demonstrate the success of packing and intracellular delivery of sRAuNPs, the two siRNAs, siLuc-Ba and siLuc-Na, were labeled with cy5 and cy3 fluorescent reporters, respectively. Various sRAuNPs (1.58 × 108 particles), including sR1P (Ba-cy5), sR1P (Na-cy3), and sR2P (Ba-cy5 + Na-cy3), were incubated for 24 h with MDA-MB231-luc2 cells. The presence of siRNA in cells was investigated using fluorescence microscopy (Figure 2). Because a cy3 or cy5 reporter was anchored to each siRNA, the fluorescence images using cy3 and/or cy5 channels reveal the location of the released siRNA. We observed a spotty fluorescence signal diffused into the cytoplasm (Figure 2b–d). The images of both cy5 and cy3 fluorescence signals after treatment with sR2P (Ba + Na) clearly indicated the presence of two kinds of siRNA (Figure 2d). These cellular-uptake data indicate that sRAuNPs enter cells without a transfection agent. Once they are internalized, the siRNA could be freed from the particles by intracellular proteolysis.
Figure 2.
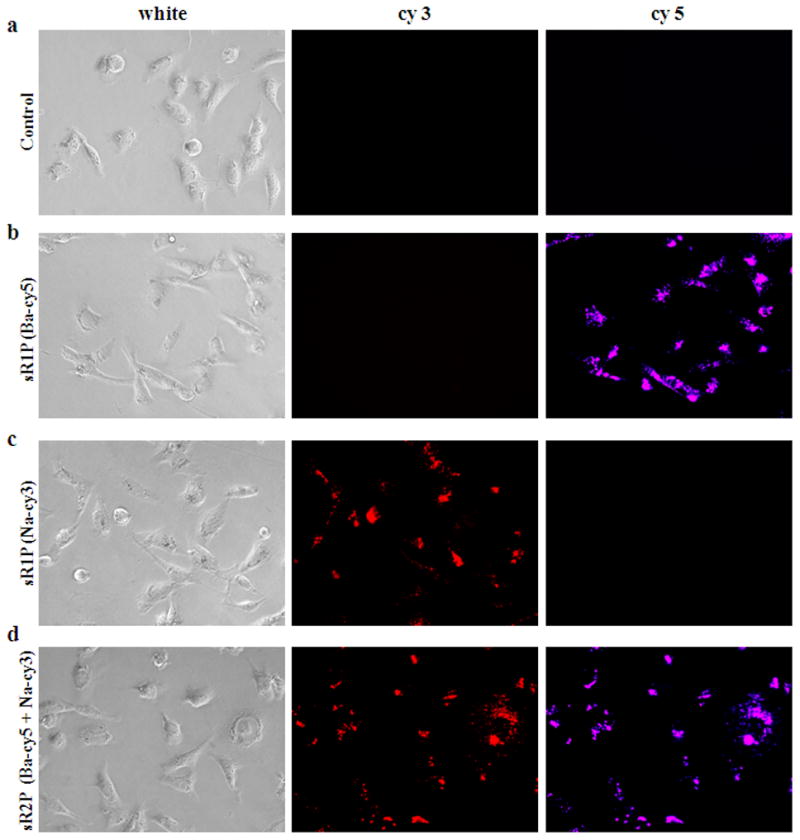
Cellular uptake of multilayered sRAuNPs. Images of released siRNA from various sRAuNPs were visualized using fluorescence microscopy with different filters after 24 h incubation in the absence (a) or presence of sR1P (Ba-cy5) (b), sR1P (Na-cy3) (c), and sR2P (Ba-cy5 + Na-cy3) (d) in MDA-MB231-luc2 cells.
An initial gene-silencing screening experiment was performed by incubating MDA-MB231-luc2 cells with sR1P (Na), sR1P (Ba), sR2P (Na + Na), sR2P (Ba + Ba), sR2P (Na + Ba), and sR2P (Ba + Na) for 2 days (Figure 3a, b). A control sR2P prepared with two layers of scrambled siRNA, termed as sR2P (Sc + Sc), was also used as a negative specificity control. After incubation, the particles were washed away, and MDA-MB231-luc2 cell luminescence was measured immediately after addition of luciferin. It was determined that the luminescence was decreased to approximately 54 % by sR1P (Na) (siLuc-Na 0.2 nmole; 1.58 × 108 particles) and 48 % by sR1P (Ba) (siLuc-Ba 0.2 nmole; 1.58 × 108 particles) when the luminescence of untreated cells was set as 100 %. With the same number of particles (1.58 × 108), the luminescence intensities of sR2P (Na + Na), sR2P (Ba + Ba), sR2P (Na + Ba), and sR2P (Ba + Na) were reduced to 46 %, 44 %, 38 %, and 30 %, respectively. As shown in Figure 3c and d, similar results were observed with LNCaP-luc2 cell lines (sR1P (Na): 50 %/sR1P (Ba): 49 %/sR2P (Na + Na): 46 %/sR2P (Ba + Ba): 40 %/sR2P (Na + Ba): 33 %/sR2P (Ba + Na): 22 %). When the specific siRNA was replaced with the scrambled siRNA (total siLuc-Sc 0.4 nmole), the silencing effect was insignificant. These data reveal the sequence specific silencing effect of the luciferase gene in two different cancer cell lines and show both sequences have similar inhibition effects. For comparison, commercially available and widely used Lipofectamine 2000, which is used in nucleic acid delivery, was tested in both cell lines. However, the gene silencing effect was not as remarkable as those treated with sRAuNPs even in the presence of same amount of siRNA (each siRNA 0.2 nmole; Supporting Information Figure S1). Additionally, similar luciferase signals were observed in cells treated with Lipofectamine 2000 alone and Lipofectamine 2000 formulated with siRNA. From these initial short-term inhibition results, sR2P (Ba + Na) was selected as the construct to be used in long-term gene silencing studies.
Figure 3.
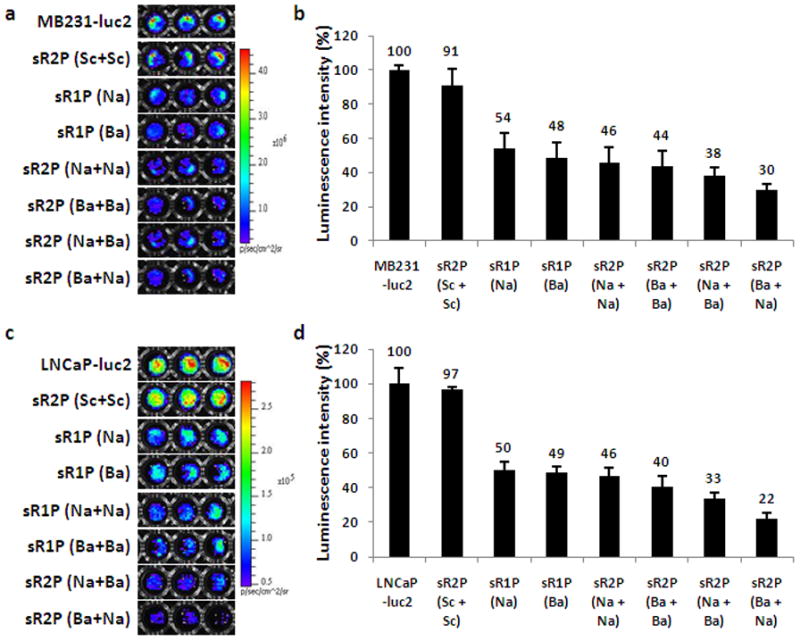
In vitro gene-silencing effect of multilayered sRAuNPs. The luminescence signal and the luminescence intensity (photons s−1) in MDA-MB231-luc2 cells (a, b) or LNCap-luc2 (c, d) after incubation for 2 days with various sRAuNPs. The luminescence intensity of each cell line without treatment was set as 100 %. The results are representative of three independent experiments.
MDA-MB231-luc2 cells were treated for 2 days with sR2P (Ba + Na) in a 6-well plate (each siRNA 2.0 nmole; 1.58 × 109 particles). The cells were washed and aliquoted into separate 96-well plates for the time course study (up to 10 days). As shown in Figure 4a and b, the efficiency of gene silencing after treatment with sR2P (Ba + Na) on day 1 was 14 % when the initial luminescent intensity of control cells was set as 100 %. This significant gene silencing effect was sustained for at least 10 days (48 %). In contrast, the luminescent signal increased with time as the control untreated cells divided. The luminescent signal of the untreated cells was approximately 400 %, but the signal of the treated cells was less than 50 % of the original signal. The experiment was forced to end at day 10, because of the physical constraint of the wells.
Figure 4.
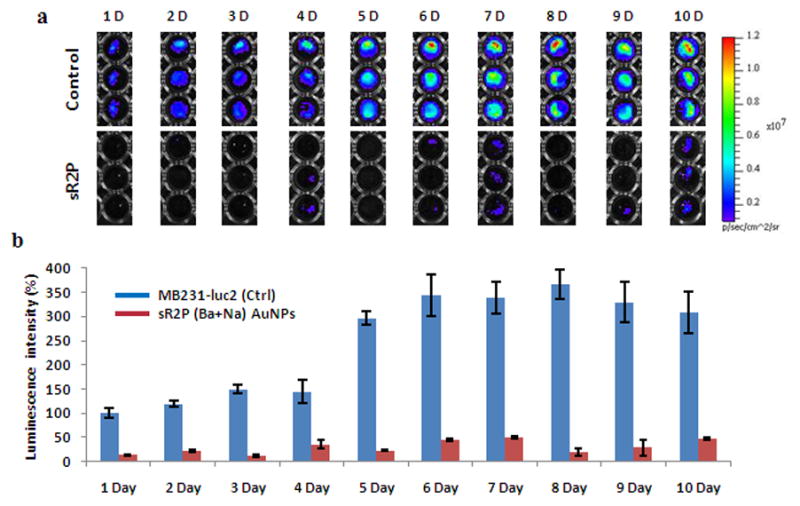
Prolonged gene-silencing effect of multilayered sRAuNPs. (a, b) The prolonged gene silencing effect was also evaluated by measuring the luminescence signal for up to 10 days after treatment with sR2P (Ba + Na) in MDA-MB231-luc2 cells. The luminescence intensity without treatment at day 1 was set as 100%. The results are representative of three independent experiments.
Although toxicity of surface modified AuNPs decorated with other polycationic polymers has been reported,[29, 30] cytotoxicity of our prepared sRAuNPs was not observed. As shown in Supporting Information Figure S2, no significant toxicity was detected in any sRAuNPs-treated MDA-MB231-luc2 or LNCaP-luc2 cells, indicating silencing of the luciferase gene does not cause of non-specific cytotoxicity. In contrast, some toxicity was observed with Lipofectamine 2000 in both cell lines and less than 80 % cells were viable.
This 10-day experiment indicated that gene silencing was effective even after cell division. With an average doubling time of 31 h for MDA-MB231 cells,[31] the sRAuNPs must effectively inhibit both the mother and daughter cells, otherwise the total luminescence should rise quickly with time. Because the unprotected siRNA is subjected to rapid nuclease degradation in cells, a persistent gene silencing effect in the daughter cells should be the result of gradually released siRNAs. Presumably, the loaded particles were re-distributed evenly to the cells during division, and the multi-layered assembly and charge-charge interaction might contribute to the prolonged gene silencing. Re-distribution of the loaded particles during cell division has recently confirmed using a similar particle which was labeled with layers of fluorescence tags.[25] The intracellular fluorescent signal was found evenly distributed to all cells and persisted for 21 days.
The in vivo gene silencing potential was evaluated further by inoculating MDA-MB231-luc2 cells (3.5 × 106) and sR2P (Ba + Na) treated MDA-MB231-luc2 cells (3.5 × 106) in both flanks nude mice. The real-time gene silencing effect was investigated non-invasively by comparing the luminescence between the inoculated tumors (Figure 5a). The observed gene silencing was extremely effective throughout the tested period. The relative inhibition efficiency at day 1 was 23 % and was sustained until day 20 (39 %). Maximal inhibition was observed at day 7 (9 %). All mice tested revealed similar gene silencing efficiencies at all indicated time points (Supporting Information Figure S3), except at day 20, one mouse showed large variation (Figure 5b). As expected, there were no differences in tumor size between the untreated and sRAuNP, which targeting luciferase gene, treated tumors (Figure 5c). This data clearly show the inhibition of luciferase intensity is not caused by cytotoxic effect. These long-term inhibition results further suggest that the loaded siRNAs were able to silence its target gene in primary treated cells as well as their daughter cells for an extended period.
Figure 5.
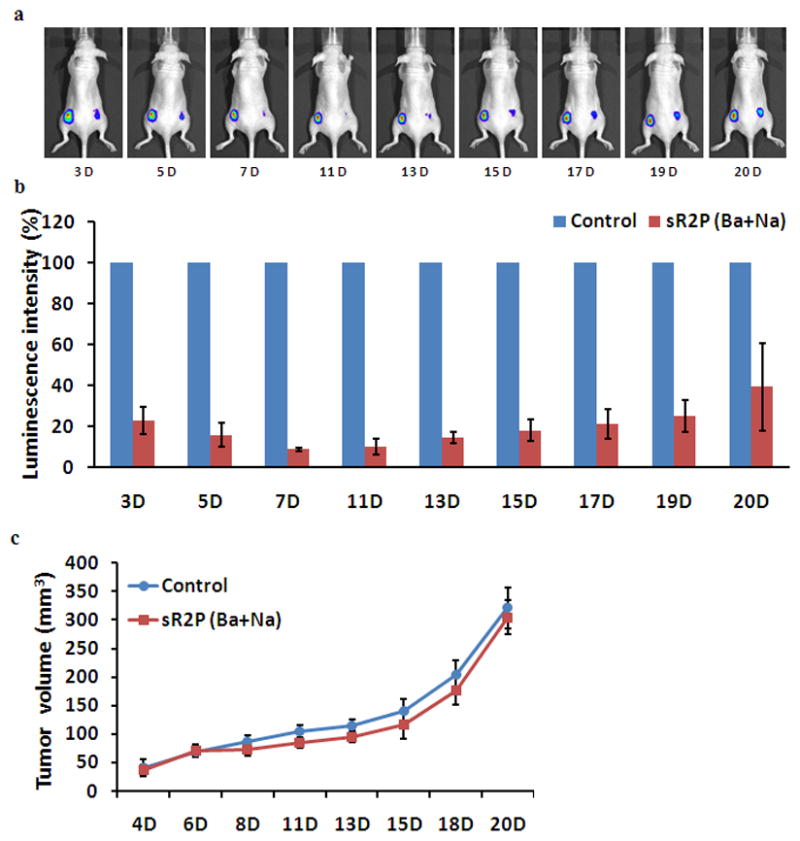
In vivo gene-silencing effect of multilayered sRAuNPs. The luminescence signal (a) and the luminescence intensity (b) of non-treated (left flank) or sR2P (Ba + Na) treated MDA-MB231-luc2 cells (right flank) were collected up to 20 days after implantation by IVIS-200. The luminescence intensity (photons s−1) of non-treated cells (left flank) at each time point was set as 100%. (c) Comparison of the tumor size of control versus the sR2P (Ba + Na) treated group during 20 days. Data represent the tumor volume (mean ± SEM) for each group.
3. Conclusion
In summary, an ultra-long siRNA gene silencing effect was achieved both in vitro and in vivo using a functionalized nano-reservoir, which is consisted of multiple siRNA and degradable polypeptide layers. By adopting a layering deposition technology,[24, 32–34] we successfully fabricated sRAuNPs by electrostatic interaction, which contain multi-layers of protease degradable PLL and synthetic siRNA. The positively charged sRAuNPs were efficiently taken up by cells and once inside the cells, the PLL was gradually degraded by intracellular proteases, resulting in the continuous release of siRNA. These intracellular sRAuNPs act as siRNA reservoirs, providing siRNA to inhibit its target genes. Upon cell division, the nano-reservoirs were distributed to daughter cells and continued to release siRNA for an extended period of time. Due to extraordinary persistent gene silencing effect, functionalized multilayered sRAuNPs are expected to have great potentials in the areas of cancer therapy, in case luciferase siRNA replaced with siRNA of onco-genes which are predominantly overexpressed in various tumors. Although this in vivo siRNA effect was performed using pretreated cells, this simple experiment demonstrated that a well-defined nanoconstruct can achieve ultra-long gene silencing effects in vivo. Currently, we are optimizing the fabrication for systemic treatment.
4. Experimental Section
Chemicals and materials
All siRNA and poly-L-lysine (PLL) (Mw = 15,000~30,000 g mol−1) were obtained from Sigma-Aldrich (St. Louis, MO). Bare AuNPs (40 nm) particles were purchased from BB International (Cardiff, UK), Lipofectamine 2000 was from Invitrogen (Carlsbad, CA), D-Luciferin was from Regis Technologies (Morton Grove, IL), Matrigel (phenol red-free) was from BD Biosciences (San Jose, CA) and CellTiter aqueous one solution was from Promega (Madison, WI).
Preparation and characterization of multilayered sRAuNPs
Using layer by layer (LbL) fabrication method, multilayers of siRNA and PLL were successfully deposited on Au surface. The sequences of siLuc-Ba[26] against luciferase are sense strand: 5′-UUAAUCAGAGACUUCAGGCGGUdTdT-3′, antisense strand: 5′-ACCGCCUGAAGUCUCUGAUUAAdTdT-3′, siLuc-Na[1] are sense strand: 5′-CGUACGCGGAAUACUUCGAdTdT-3′, antisense strand: 5′ UCGAAGUAUUCCGCGUACGdTdT-3′, and of control nonsnese siRNA (siLuc-ctrl) are sense strand: 5′-AGCUUCAUAAGGCGCAUGCdTdT-3′ and antisense strand: 5′-GCAUGCGCCUUAUGAAGCU-3dTdT-3′). AuNPs solution (3.15 × 109 particles in 0.7 mL) was added dropwisely onto a PLL solution (0.5 mL of 5 mg mL−1) in pure water. After incubating for 30 min in the dark with gentle shaking, the solution was centrifuged for 30 min at 16,100 g using a micro centrifuge (Eppendorf, Hauppauge, NY). The supernatant was removed, and the gel-like deep red pellet was re-suspended with pure water and centrifuged for 30 min at 16,100 g. PLL coated AuNPs were stored in pure water after additional wash. Next polyelectrolyte layer was deposited by adding PLL coated AuNPs (in 0.5 mL pure water) to siRNA solution (4.0 nmole, 0.5 mL). The reaction solution was incubated in the dark for 30 min with gentle shaking, followed by three washes. The deposition procedures were repeated to have total 5 layers of polyelectrolytes (3 layers of PLL and 2 layers of siRNA). Sizes and zeta potentials of AuNPs in water were measured by Zetasizer Nano-ZS (Malvern, Worcestershire, UK) according to the manufacturer’s instruction.
Cell lines
The human breast cancer cell line stably expressing firefly luciferase (MDA-MB231-luc2) and the human prostate cancer cell line stably expressing firefly luciferase (LNCaP-luc2) were purchased from Caliper (Alameda, CA). MDA-MB231-luc2 cell line were cultured in DMEM medium (Mediatech Inc., Manassas, VA), while LNCaP-luc2 cell line were cultured in RPMI 1640 medium (Thermo Scientific, Rockford, IL) and both cell lines were supplemented with 2 mM L-glutamine, 100 U mL−1 penicillin, 100 mg mL−1 streptomycin, and 10 % heat-inactivated fetal bovine serum (Sigma-Aldrich) in a humidified atmosphere of 5 % CO2 at 37 ºC.
Live cell imaging for cellular uptake of sRAuNPs
A cy5 fluorochrome was tagged on the 5′ end of the sense siLuc-Ba and a cy3 fluorochrome was tagged on the 5′ end of the sense siLuc-Na. Fluorescence images of live cells were acquired using a fluorescence microscopy system (Olympus, Tokyo, Japan). Briefly, MDA-MB231-luc2 cells were seeded on a 96-well black clear-bottom culture plate (Corning Life Sciences, Pittston, PA) at a density of 5.0 × 103 cells per well. After 1 day, the culture medium was replaced with sR1P (Ba-cy5), sR1P (Na-cy3) or sR2P (Ba-cy5 + Na-cy3) AuNPs (1.58 × 108 particles) containing medium, and further cultured for 24 h. Cells were then washed twice with phosphate buffered saline (PBS) and cultured in the phenol red-free medium and imaged with a fluorescence microscopy.
Cytotoxicity assessment of sRAuNPs
A cell proliferation assay was performed to assess the cytotoxicity of Lipofectamine 2000 (Invitrogen) and the preparations of sRAuNPs. Briefly, MDA-MB231-luc2 cells were collected by trypsinization, counted, and plated in a 96-well culture plate at a density of 5 × 103 (or 2.5 × 104 of LNCaP-luc2) cells per well. One day later, sRAuNPs (1.58 × 108 particles) or Lipofectamine 20000 (0.2 μL) were added and the cells were further cultured for 48 h. For Lipofectamine 20000, due to toxicity issue, fresh complete medium were replaced after incubation with Lipofectamine 2000 for 4 h and further cultured for additional 44 h. At day 3, 20 μL CellTiter solution (Promega) was added to each well after replacing with fresh medium and incubated for an additional 3 h, and then the absorbance of the solution was measured at 490 nm using a Spectramax M2 plate reader (Molecular Devices).
In vitro gene silencing effect
For the examination of the gene silencing effect in vitro, bioluminescence measurement was performed after incubating with various multilayered AuNPs. Briefly, MDA-MB-231 cells were seeded in a 96-well black clear bottom culture plate at a density of 5 × 103 (or 2.5 × 104 of LNCaP-luc2) cells per well. One day later, different sRAuNP (1.58 × 108 particles) were added to each well and cultured for additional 48 h and replaced with fresh medium. Manufacturer’s instruction was followed for transfection with Lipofectamine 2000. Bioluminescence measurement was performed using IVIS-200 (Caliper) immediately after addition 125 μg mL−1 of D-Luciferin (Regis). To investigate in vitro long-term gene silencing effects, MDA-MB231-luc2 cells were seeded in a 6-well culture plate (BD Falcon, San Jose, CA) at a density of 2.0 × 105 cells per well. One day later, sR2P (Ba + Na) (1.58 × 109 particles) were added to each well and cultured for additional 48 h. sRAuNPs treated MDA-MB231-luc2 cells in 6-well plate were collected by trypsinization, counted, and re-plated in a 96-well black clear bottom culture plate at a density of 1.5 × 103 cells per well. Bioluminescence measurement of each well appropriate from day 1 to day 10 was performed using IVIS-200 (Caliper) immediately after addition 125 μg mL−1 of D-Luciferin (Regis).
In vivo gene silencing effect
All animal studies were performed in compliance with the approved animal protocols and guidelines of Institutional Animal Care and Use Committee at The Methodist Hospital Research Institute. To evaluate gene silencing effect in vivo, bioluminescence measurement was performed after implantation of sR2P (Ba + Na) treated or non-treated MDA-MB231-luc2 cells to female nude (nu/nu) mice. MDA-MB-231 cells were seeded in a 6-well culture plate (BD Falcon) at a density of 2.0 × 105 cells per well. One day later, sR2P (Ba + Na) (1.58 × 109 particles) were added to each well and cultured for additional 48 h. sRAuNPs treated or non-treated MDA-MB231-luc2 cells were collected by trypsinization, counted, and implanted bilaterally (3.5 × 106 cells in 200 μL of PBS including 50 μL of Matrigel) in the posterior flanks of mice. Bioluminescence imaging was carried out with a group of 3 mice. Bioluminescence of tumor-bearing mice was imaged using a small animal optical imaging system (IVIS-200, Caliper) immediately after injecting 2 mg (in 150 μL of PBS) of D-Luciferin (Regis) per mouse up to 20 days after cell implantation. Tumors were measured at the indicated time with digital calipers, and volumes were calculated according to the formula[35] as 0.5 × (length) × (width)2.
Supplementary Material
Acknowledgments
The authors thank Ms. Myung Shin Han for her technical support in animal imaging. This study was supported in part by NIH CA135312, DOD W81XWH-11-1-0442, and the Golfers Against Cancer Foundation.
References
- 1.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Nature. 2001;411:494. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 2.Zamore PD. Cell. 2006;127:1083. doi: 10.1016/j.cell.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Bumcrot D, Manoharan M, Koteliansky V, Sah DW. Nat Chem Biol. 2006;2:711. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnett JC, Rossi JJ. Chem Biol. 2012;19:60. doi: 10.1016/j.chembiol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lobovkina T, Jacobson GB, Gonzalez-Gonzalez E, Hickerson RP, Leake D, Kaspar RL, Contag CH, Zare RN. ACS Nano. 2011;5:9977. doi: 10.1021/nn203745n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gary DJ, Lee H, Sharma R, Lee JS, Kim Y, Cui ZY, Jia D, Bowman VD, Chipman PR, Wan L, Zou Y, Mao G, Park K, Herbert BS, Konieczny SF, Won YY. ACS Nano. 2011;5:3493. doi: 10.1021/nn102540y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Nat Biotechnol. 2011;29:341. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt C. Nat Biotechnol. 2011;29:93. doi: 10.1038/nbt0211-93. [DOI] [PubMed] [Google Scholar]
- 9.Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. Nat Rev Cancer. 2011;11:59. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takabatake Y, Isaka Y, Mizui M, Kawachi H, Takahara S, Imai E. Biochem Biophys Res Commun. 2007;363:432. doi: 10.1016/j.bbrc.2007.08.189. [DOI] [PubMed] [Google Scholar]
- 11.Merritt WM, Lin YG, Spannuth WA, Fletcher MS, Kamat AA, Han LY, Landen CN, Jennings N, De Geest K, Langley RR, Villares G, Sanguino A, Lutgendorf SK, Lopez-Berestein G, Bar-Eli MM, Sood AK. J Natl Cancer Inst. 2008;100:359. doi: 10.1093/jnci/djn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka T, Mangala LS, Vivas-Mejia PE, Nieves-Alicea R, Mann AP, Mora E, Han HD, Shahzad MM, Liu X, Bhavane R, Gu J, Fakhoury JR, Chiappini C, Lu C, Matsuo K, Godin B, Stone RL, Nick AM, Lopez-Berestein G, Sood AK, Ferrari M. Cancer Res. 2010;70:3687. doi: 10.1158/0008-5472.CAN-09-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raemdonck K, Vandenbroucke RE, Demeester J, Sanders NN, De Smedt SC. Drug Discov Today. 2008;13:917. doi: 10.1016/j.drudis.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bulut S, Erkal TS, Toksoz S, Tekinay AB, Tekinay T, Guler MO. Biomacromolecules. 2011;12:3007. doi: 10.1021/bm200641e. [DOI] [PubMed] [Google Scholar]
- 15.Vandenbroucke RE, De Geest BG, Bonne S, Vinken M, Van Haecke T, Heimberg H, Wagner E, Rogiers V, De Smedt SC, Demeester J, Sanders NN. J Gene Med. 2008;10:783. doi: 10.1002/jgm.1202. [DOI] [PubMed] [Google Scholar]
- 16.Mehrotra S, Lee I, Chan C. Acta biomaterialia. 2009;5:1474. doi: 10.1016/j.actbio.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujimoto H, Kato K, Iwata H. Analytical and bioanalytical chemistry. 2010;397:571. doi: 10.1007/s00216-010-3648-1. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Kovtun A, Mendoza-Palomares C, Oulad-Abdelghani M, Fioretti F, Rinckenbach S, Mainard D, Epple M, Benkirane-Jessel N. Biomaterials. 2010;31:6013. doi: 10.1016/j.biomaterials.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 19.Mantei A, Rutz S, Janke M, Kirchhoff D, Jung U, Patzel V, Vogel U, Rudel T, Andreou I, Weber M, Scheffold A. Eur J Immunol. 2008;38:2616. doi: 10.1002/eji.200738075. [DOI] [PubMed] [Google Scholar]
- 20.Rosner M, Siegel N, Fuchs C, Slabina N, Dolznig H, Hengstschlager M. Nat Protoc. 2010;5:1081. doi: 10.1038/nprot.2010.74. [DOI] [PubMed] [Google Scholar]
- 21.Suckau L, Fechner H, Chemaly E, Krohn S, Hadri L, Kockskamper J, Westermann D, Bisping E, Ly H, Wang X, Kawase Y, Chen J, Liang L, Sipo I, Vetter R, Weger S, Kurreck J, Erdmann V, Tschope C, Pieske B, Lebeche D, Schultheiss HP, Hajjar RJ, Poller WC. Circulation. 2009;119:1241. doi: 10.1161/CIRCULATIONAHA.108.783852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, Sorensen R, Forster A, Fraser P, Cohen JI, de Saint Basile G, Alexander I, Wintergerst U, Frebourg T, Aurias A, Stoppa-Lyonnet D, Romana S, Radford-Weiss I, Gross F, Valensi F, Delabesse E, Macintyre E, Sigaux F, Soulier J, Leiva LE, Wissler M, Prinz C, Rabbitts TH, Le Deist F, Fischer A, Cavazzana-Calvo M. Science. 2003;302:415. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 23.Guo X, Huang L. Acc Chem Res. 2012;45:971. doi: 10.1021/ar200151m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SK, Han MS, Asokan S, Tung CH. Small. 2011;7:364. doi: 10.1002/smll.201001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SK, Han MS, Tung CH. Small. 2012;8:3315. doi: 10.1002/smll.201200751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang K, Elledge SJ, Hannon GJ. Nat Methods. 2006;3:707. doi: 10.1038/nmeth923. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Shi Z, Liu W, Jules J, Feng X. BMC Biotechnol. 2006;6:50. doi: 10.1186/1472-6750-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peter ME. Oncogene. 2010;29:2161. doi: 10.1038/onc.2010.59. [DOI] [PubMed] [Google Scholar]
- 29.Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Angew Chem Int Ed Engl. 2010;49:3280. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Read ML, Singh S, Ahmed Z, Stevenson M, Briggs SS, Oupicky D, Barrett LB, Spice R, Kendall M, Berry M, Preece JA, Logan A, Seymour LW. Nucleic Acids Res. 2005;33:e86. doi: 10.1093/nar/gni085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe N, Okochi E, Mochizuki M, Sugimura T, Ushijima T. Cancer Res. 2001;61:7739. [PubMed] [Google Scholar]
- 32.Jewell CM, Lynn DM. Adv Drug Deliv Rev. 2008;60:979. doi: 10.1016/j.addr.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reum N, Fink-Straube C, Klein T, Hartmann RW, Lehr CM, Schneider M. Langmuir. 2010;26:16901. doi: 10.1021/la103109b. [DOI] [PubMed] [Google Scholar]
- 34.Suma T, Miyata K, Anraku Y, Watanabe S, Christie RJ, Takemoto H, Shioyama M, Gouda N, Ishii T, Nishiyama N, Kataoka K. ACS Nano. 2012;6:6693. doi: 10.1021/nn301164a. [DOI] [PubMed] [Google Scholar]
- 35.Kim K, Chadalapaka G, Pathi SS, Jin UH, Lee JS, Park YY, Cho SG, Chintharlapalli S, Safe S. Mol Cancer Ther. 2012;11:1852. doi: 10.1158/1535-7163.MCT-12-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


