What are the cellular and molecular mechanisms that regulate new vessel formation and maturation? This question identifies several fundamental issues of vascular biology related to the development of the vasculature via vasculogenesis and angiogenesis. Early investigations of vasculature were largely descriptive (1, 2). The advent of methods for the isolation and maintenance of vascular cells in tissue culture led to assay systems aimed at recreating the initial events of the angiogenic process, including endothelial cell protease production, migration and proliferation. However, less attention has been directed to the later stages of vascularization, such as investment of vessels with mural cells (pericytes or smooth muscle cells), production of basement membrane, induction of vessel bed specializations or the process of vascular regression. The final stages of vessel formation might also be referred to as “remodeling” and appear to define a process through which a forming vasculature becomes a stable, mature vessel bed. These steps may also be taken advantage of to develop antitumor strategies, as suggested by Benjamin et al. in this issue (3).
What regulates the phenotypic changes that occur between forming and mature vessels? A number of clinical and experimental observations support the concept that the association between the vascular tube and the mural cells mediates vessel stabilization or maturation. Ultrastructural studies of vascularization during wound healing revealed a temporal correlation between pericyte investment of the capillary and the cessation of vessel growth. This observation led the authors of this study to state: “The incorporation of pericytes within the basement membrane of proliferating capillaries is proposed as the mechanism for inhibition of capillary proliferation” (4).
Clinical observations are consistent with a role for mural cells in stabilizing vessels. The vessel proliferation that characterizes proliferative diabetic retinopathy is preceded by a stage characterized by the selective loss of pericytes (5). Similarly, the new vessels that form as a complication of the retinopathy of prematurity arise from immature microvessels that have yet to be invested by pericytes (6). Thus, the physical association between the nascent vascular tube and mural cells appears to signal vessel stabilization.
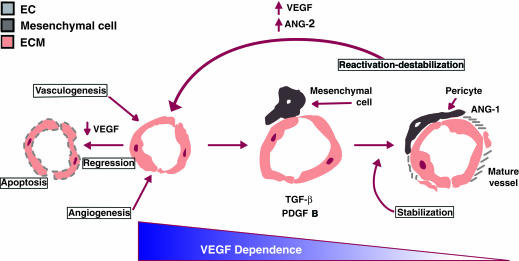
Earlier work from Stone and Keshet indicates that vessels are dependent upon exogenous survival factors, e.g., VEGF (7) (Fig. 1), for a critical period of time during their development. The current study by Benjamin et al. in this issue (3) further suggests that the association of forming vessels with the mural cells marks the end of this period of growth factor dependence. Two aspects of vessel stabilization are addressed in this study. The first is that in the absence of associated pericytes or smooth muscle cells, the nascent endothelial cell tube is unstable and is prone to regression. The second is the notion that the nascent endothelial tube requires VEGF for survival. In normal development, the time for overlap of these events is terminated by the arrival of the mural cells. The tumor vasculature, on the other hand, appears to be trapped in a cycle where high levels of growth factors can induce and sustain immature vessels in the relative absence of mural cells. The chronic immaturity of tumor vessels has led Dvorak to characterize a tumor as a “wound that never heals” (8). At the same time, these features make tumor vessels viable targets for antitumor therapies. Benjamin et al. (3) demonstrate that removal of growth factors leads not only to the cessation of new vessel growth, but also to regression of the immature tumor vasculature (3).
Figure 1.
Vessel maturation: vessel development proceeds from a stage of growth-factor dependence where loss of a survival factor leads to apoptosis. Vessel stabilization is marked by investment with mural cells, local activation of TGF-β, and basement membrane production. The angiopoietins are clearly implicated, though their precise roles are yet to be determined.
Although recent evidence suggests that endothelial cell–mural cell contact and VEGF-dependence are pivotal junctures during vessel maturation, questions regarding the molecular basis of vessel maturation remains unanswered. Using tissue culture models, we have previously shown that endothelial cells recruit mural cell precursors via the secretion of PDGF-BB. Consistent with this prediction, mice null for PDGF B lack pericytes in some microvascular beds (10). Furthermore, coculture studies reveal that endothelial cell–mural cell contact leads to the activation of TGF-β (9, 11), a pleiotrophic growth factor that has been shown to mediate differentiation of a variety of cell types. In the context of the microvasculature, we speculate that TGF-β functions at multiple steps leading to vessel stabilization, including inhibition of endothelial cell proliferation and migration, as well as induction of pericyte/smooth muscle cell differentiation (12) (Fig. 1). Once tube formation occurs, flow commences and local oxygen levels increase, resulting in a decrease in VEGF levels. The reduction in VEGF is coincident with pericyte/smooth muscle cell association. In addition, TGF–β can alter integrin profiles and stimulate basement membrane production and accumulation (for review) (13).
In support of this idea, ultrastructural studies document that a basement membrane is deposited only after endothelial cell–mural cell association has occurred (4). Thus, the presence of basement membrane may provide a long-term signal that supports vessel stabilization. Furthermore, recent evidence suggests that the angiopoietins play a role in vessel stabilization (for review) (14). Angiopoietin-1 is associated with developing vessels and its absence leads to defects in vessel remodeling. On the other hand, angiopoietin-2, which antagonizes the actions of angiopoietin-1, appears to play a role in the destabilization of existing vessels. Although the precise role played by each of these factors is not clear, their existence suggests yet another level of control during vessel maturation (Fig. 1). Thus, vessel stabilization results from a balance between stimulators (such as VEGF) and inhibitors (such as TGF-β), all acting in the context of the vessel microenvironment.
The idea that cell–cell interactions mediate differentiation and stabilization of developing tissues is not restricted to the vasculature. Similar interactions have been described in the developing kidney (15), lung (16), mammary gland (17), and heart (18) where there are heterotypic interactions between epithelial and mesenchymal cells, associated in many cases, with the local production of members of the TGF-β superfamily. An additional parallel can be drawn to the developing nervous system, where heterotypic interactions between an innervating neuron and its target cell effect the survival and/or differentiation of both cell types (19). Thus, heterotypic cell–cell interactions may be a universal mechanism for the local regulation of cell-type specification and differentiation.
Whereas the earliest studies of the vasculature described cell behavior in situ, and the past two decades have focused on dissecting the function of isolated vascular cells in culture, we are now at a transition point which recognizes the complexity of the microvasculature (20). Most importantly, the importance of heterotypic cell–cell interactions, local control of growth factor expression (e.g., hypoxic regulation of VEGF, local activation of TGF-β), and the signaling potential of the basement membrane are acknowledged and being investigated. As a result, new questions continue to arise.
Is vessel assembly controlled similarly in all vascular beds? Studies involving PDGF B-deficient mice suggest there are tissue-specific differences, since not all microvascular beds in these mice lacked pericytes.
What is the relevance of the fact that different microvessel beds have different levels of coverage by pericytes? Many prostatic vessels have few pericytes, retinal and brain microvessels have extensive coverage, lymphatic vessels have no mural cells at all, and pericytes presence in tumor vessels is variable.
What is the nature of the heterotypic cell–cell interactions between endothelial cells and mural cells? Is there a role for gap junctions and/or signaling via adhesion molecules? Which diffusible factors are involved in this communication (e.g., angiopoietins, VEGFs, PDGFs, TGF-family members, etc.)?
What is the role of growth factors in the adult vasculature? Why is there continued production of VEGF in adult tissues if mature vessels are VEGF-independent? Are there other survival factors? Does TGF-β play a role in vessel maturation? What is the role of the basement membrane?
Understanding the cellular and molecular basis of vessel maturation is central to delineating the full “life cycle” of blood vessels and lays the groundwork for developing effective pro-angiogenic and anti-angiogenic therapies.
Acknowledgments
P.A. D’Amore is a Jules and Doris Stein Research to Prevent Blindness Professor. D.C. Darland and P.A. D’Amore are supported by NIH-EY05318, EY05985 and CA45548.
References
- 1.Clark ER, Clark EL. Microscopic observations on the growth of blood capillaries in the living mammal. Am J Anat. 1939;64:251–299. [Google Scholar]
- 2.Ausprunk DH, Knighton DR, Folkman J. Differentiation of vascular endothelium in the chick chorioallantois: a structural and autoradiographic study. Devel Biol. 1974;38:237–248. doi: 10.1016/0012-1606(74)90004-9. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crocker DJ, Murad TM, Greer JC. Role of the pericyte in wound healing. An ultrastructural study. Exp Mol Pathol. 1970;13:51–65. doi: 10.1016/0014-4800(70)90084-5. [DOI] [PubMed] [Google Scholar]
- 5.Speiser P, Gitttelsohn AM, Patz A. Studies on diabetic retinopathy. III. Influence of diabetes on intramural pericytes. Arch Ophthalmol. 1968;80:332–337. doi: 10.1001/archopht.1968.00980050334007. [DOI] [PubMed] [Google Scholar]
- 6.Patz A. The role of oxygen in retrolental fibroplasia. Trans Am Ophth Soc. 1968;66:940–985. [PMC free article] [PubMed] [Google Scholar]
- 7.Alon T, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 8.Dvorak H, Brown L, Detmar M, Dvorak A. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Path. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 9.Hirschi K, Rohovsky SA, D'Amore PA. PDGF, TGF-β and heterotypic cell-cell interactions mediate the recruitment and differentiation of 10T1/2 cells to a smooth muscle cell fate. J Cell Biol. 1998;141:805–814. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 11.Antonelli-Orlidge A, Saunders KB, Smith SR, D'Amore PA. An activated form of transforming growth factor β is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci USA. 1989;86:4544–4548. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirschi, K.K., Rohovsky, S.A., Beck, L.H., Smith, S.R., and D'Amore, P.A. 1999. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ. Res. In press. [DOI] [PubMed]
- 13.Roberts, A., and Sporn, M. 1996. Transforming growth factor-b. In Molecular and cellular biology of wound repair. R.A.F. Clark, editor. Plenum Press, New York, NY. 275–308.
- 14.Beck L, Jr, D'Amore PA. Vascular development: cellular and molecular regulation. FASEB J. 1997;11:365–373. [PubMed] [Google Scholar]
- 15.Saxen L, Sariola H. Early organogenesis of the kidney. Pediatr Nephrol. 1987;1:385–392. doi: 10.1007/BF00849241. [DOI] [PubMed] [Google Scholar]
- 16.O'Reilly MA, Stripp BR, Pryhuber GS. Epithelial-mesenchymal interactions in the alteration of gene expression and morphology following lung injury. Microsc Res Tech. 1997;38:473–479. doi: 10.1002/(SICI)1097-0029(19970901)38:5<473::AID-JEMT3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 17.Cunha GR. Role of mesenchymal-epithelial interactions in normal and abnormal development of the mammary gland and prostate. Cancer. 1994;74:1030–1044. doi: 10.1002/1097-0142(19940801)74:3+<1030::aid-cncr2820741510>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 18.Olson EN, Sternberg E, Hu JS, Spizz G, Wilcox C. Regulation of myogenic differentiation by type beta transforming growth factor. J Cell Biol. 1986;103:1799–1805. doi: 10.1083/jcb.103.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao M, et al. Neurotrophin receptors: mediators of life and death. Brain Res Rev. 1998;26:295–301. doi: 10.1016/s0165-0173(97)00036-2. [DOI] [PubMed] [Google Scholar]
- 20.Folkman J, D'Amore PA. Blood vessel formation: What is its molecular basis? Cell. 1996;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]