Abstract
Collagen-apatite (Col-Ap) scaffolds have been widely employed for bone tissue engineering. We fabricated a Col-Ap scaffold with a unique multi-level lamellar structure consisting of co-aligned micro and macro pores. The basic building blocks of this scaffold are bone-like mineralized collagen fibers developed via a biomimetic self-assembly process in a collagen-containing modified simulated body fluid (m-SBF). This biomimetic method preserves the structural integrity and great tensile strength of collagen by reinforcing the collagen hydrogel with apatite nano-particles. Unidirectional aligned macro pores with a size of 63.8 to 344 μm are created by controlling the freezing rate and direction. The thickness of Col-Ap lamellae can be adjusted in the range 3.6 to 23 μm depending on the self-compression time. Furthermore, the multi-level lamellar structure has led to a twelve-fold increase in Young's modulus and a two-fold increase in the compression modulus along the aligned direction compared to a scaffold of the same composition with an isotropic equiaxed pore structure. Moreover, this novel lamellar scaffold supports the attachment and spreading of MC3T3-E1osteoblasts. Therefore, owing to the biomimetic composition, tunable structure, improved mechanical strength, and good biocompatibility of this novel scaffold, it has great potential to be used in bone tissue engineering applications.
Introduction
In recent years, bone tissue engineering involving a scaffold, cells and biological signals has attracted widespread attention and represents a promising approach for the repair and regeneration of damaged bone. 1 A porous scaffold serves as a vehicle for bioactive molecules to adhere to, and a 3-D matrix for cells to attach, proliferate, and differentiate. It plays a critical role in directing new bone formation. 2-4 An ideal scaffold should possess a 3-D structure with interconnected pores to facilitate cellular activities such as vascularization and transport of nutrients and metabolic waste while maintaining sufficient mechanical strength to support cell adhesion and physiological loading. In addition, the scaffold should have a suitable surface chemistry and morphology to promote cell colonization and a controllable degradation rate concurrent with new bone ingrowth. 5 Existing scaffolds have limitations such as low permeability, weak mechanical strength and poor osteointegration, therefore, the need to develop an ideal scaffold is still urgent.
Concerning the selection of scaffold materials, collagen, a major component of extracellular matrix (ECM), has attracted the attention of many researchers because of its abundance, low antigenicity, and excellent cell signalling properties. 6-9 The main limitation of reconstituted collagen scaffolds has been related to their low mechanical strength and fast degradation rate. 10 Studies have demonstrated that the stiffness of a collagen matrix is directly related to the collagen fibrillar density and fibrillogenesis 11, 12, 16. Techniques based on plastic compression have been developed to expel the fluid within a collagen hydrogel thereby increasing the fibrillar density of scaffold. 8, 11-18 The kinetics of collagen fibrillogenesis are carefully controlled by pH, temperature, and collagen concentration in order to form collagen bundles with increased diameter, which also contributes significantly to the improvement of scaffold mechanical strength. 19 Dense collagen scaffolds mimicking the ECM fibrillar density and microstructure exhibit superior mechanical properties and excellent biocompatibility, which have great potential for tissue engineering applications, such as skin grafts, cornea epithelial reconstructs, and bone regeneration. 11, 13, 14, 20
Another possible approach to overcome the mechanical limits and tailor the degradation rate of the scaffold is the addition of apatite as an inorganic reinforcing component. 21-26 The microstructure of the collagen-apatite (Col-Ap) composite, which is mainly referred to as the organization of the collagen fibers and crystal phase of apatite, plays an important role in early bone formation upon implantation.27, 28 Recent studies have focused on the fabrication of Col-Ap scaffolds mimicking the hierarchical structure of bone at the different length scales. The biomimetic fabrication approach involving the self-assembly of collagen fibers and in situ apatite precipitation has attracted the attention of many researchers. Kikuki et al. prepared Col-Ap composites consisting of collagen fibers and apatite nano-particles by simultaneous titration of a Ca(OH)2 suspension, a solution of H3PO4, and collagen. 29 As compared to a scaffold prepared by physically mixing of collagen and apatite particles, co-precipitation can be used to control the composition and nano-structure of the scaffold, although they may not completely recapitulate the 3-D morphology of the ECM in bone at the macro- and micro-scale. 30
Other than composition and microstructure, the 3-D macro porous structure plays a key role in bone regeneration. 31 The freeze-drying technique consists of freezing an aqueous suspension followed by sublimation of the solidified phase and has been used widely to obtain porous structures. 7, 32-34 The pore size and shape is controlled by the freezing rate and ice growth direction. A scaffold fabricated under a non-directional and a slow freezing rate demonstrates an isotropic, equiaxed pore structure.7 In contrast, directional freezing could create columnar ice crystals with the major axis aligned in the dominant direction of heat transfer, thereby producing scaffolds with a preferential pore orientation. 34-36 A porous structure is desirable to support the cell viability, while high porosity decreases the mechanical property of the scaffold. A unidirectional aligned structure should maximize the mechanical strength along the pore direction. 37 Therefore, there has been significant interest in enhancing the mechanical strength of porous materials via the control of pore orientation. In the current study, we fabricated a mineralized collagen hydrogel using a simple biomimetic fabrication technique involving the use of modified simulated body fluid (m-SBF) containing collagen. SBF is a solution that has inorganic ion concentrations similar to those of human blood plasma. Qu et al. developed a m-SBF solution with calcium and phosphate ion concentrations three times as high as those in the original SBF . 3x m-SBF was used for the preparation of mineralized collagen fibrils. 38, 39 Then, we developed a novel method by combining the self-compression of a mineralized collagen hydrogel and controllable freeze casting to fabricate a biomimetic Col-Ap hybrid scaffold for bone tissue regeneration. While attempts have been made to increase the mechanical stability of the composite hydrogel using plastic compression, the prepared scaffold usually exhibits a dense structure.11 This is the first paper that demonstrates a 3-D macro porous scaffold while maintaining the biomimetic surface properties and bone-like structure at the micro-scale. Other unique properties of the scaffold include the control of the pore size and pore orientation over a wide range from submicron to millimeter dimensions.
Materials and methods
Materials
All chemicals were of analytical chemical grade. Type I collagen was extracted from rat tails, purified based on the protocol reported by Rajan et al. 40
Preparation of freeze-dried Col-Ap hydrogel
A biomimetic Col-Ap hydrogel was synthesized using a collagen containing m-SBF. The m-SBF was prepared as reported previously. 38, 39, 41 The concentration of collagen in m-SBF was adjusted to 2 g/L to achieve an apatite content of 35 wt% in the scaffold. The pH of the m-SBF solution was adjusted to 7 by addition of HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) and NaOH. The Col-Ap gel was prepared using a two-temperature process. Briefly, the solution was incubated in a sealed vial at 25 °C for 1 hr, and the temperature was then increased at a rate of 0.5 °C/min to 40 °C and held at 40 °C for 22.5 hr.
The Col-Ap hydrogel was allowed to undergo unconfined self-compression at room temperature for different time periods (5, 20 and 45 min). Hydrogels were named as Col-Ap-x, with × representing the self-compressing time. Then the Col-Ap gel in a custom-made mold was placed in a chamber pre-cooled to −25 °C. The frozen hydrogel was then lyophilized in a freeze dryer (Free Zone®, Labconco, USA). The freeze-dried scaffolds were subsequently cross-linked with 1 wt% N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide (EDC) hydrochloride for 24 h. The scaffolds were then rinsed thoroughly in distilled water, followed by 5% glycine solution, rinsed again with distilled water, and finally freeze-dried a second time. To investigate the effect of freezing temperature on the pore structure, a Col-Ap-20 hydrogel was frozen unidirectionally at three freezing temperatures: −25 °C, −80 °C and −196°C. Moreover, Col-Ap-20 was frozen non-unidirectionally at a constant cooling rate of 0.4 °C/min from room temperature to −25 °C. As a control, Col-Ap scaffold with an equiaxed structure were prepared. 42 Briefly, Col-Ap precipitates were prepared using m-SBF containing 2 g/L collagen under constant stirring. The precipitates were collected, centrifuged, and then frozen at a constant cooling rate of 0.4 °C/min from room temperature to −25 °C. The scaffold was then freeze-dried, cross-linked by EDC, and then rinsed with a 5% glycine solution followed by distilled water.
Characterization
A small amount of the Col-Ap hydrogel from m-SBF containing collagen at a concentration of 2 g/L was placed on a copper mesh and observed using transmission electron microscopy (JEOL JEM-2010) at an accelerating voltage of 120 kV. The hydrogel was first fixed with formalin, dehydrated stepwise in ethanol, and then observed using field emission scanning electron microscopy (JEOL JSM-6335F) at 5 kV. The surface morphology of the freeze-dried Col-Ap scaffold was also imaged using FESEM. Pure collagen and Col-Ap composite scaffolds were examined using Fourier-transform infrared spectroscopy (Perkin Elmer) in absorption mode over a range of 4000-500 cm−1 at a resolution of 4 cm−1 and taking the average of 32 scans. X-ray diffraction (BRUKER AXS D5005) was also performed on collagen and the Col-Ap scaffold at a step size of 0.02° and a scan rate of 1 °/min with CuKα radiation (λ=1.54056 nm). Thermogravimetric analysis (TGA) was carried out to determine the amount of apatite in the Col-Ap scaffolds. Scaffolds with a weight of 20 mg were examined using a TG analyzer (TGA-1000, Rheometric Scientific) and the measurements were recorded from 30 to 1000 °C with a heating rate of 10 °C/min in air.
To enable X-ray tomography, scaffolds were stained with 1% (w/v) iodine in 100% ethanol overnight. 43 Scaffolds were then clamped and placed on the instrument stage (MicroXCT-400, Xradia). 1500 views were acquired at an angle of −103° to 103° with an exposure time of 4 s, source power of 8 W, a voltage of 55 kV and a 20× objective. Tomography images were reconstructed with XM Reconstructor (Xradia) and viewed with the 3-D viewer plugin for NIH ImageJ.
The lamellar spacing and lamellar thickness were determined from 20 FESEM images of cross-sections in the plane parallel to the direction of freezing. Twenty-five measurements were taken randomly in each image and then the mean interlamellar spacing was calculated. The porosity of the scaffolds was calculated by gravimetry.44 The densities used for collagen and apatite were 1.32 and 3.16 g/cm3, respectively.
Rheological measurement
The in situ time sweep was conducted on Col-Ap gels on an ARG2 rheometer (TA instruments) in a cuvette at a given frequency of 0.5 Hz with a controlled strain of 0.5%. In all cases, 19.2 ml m-SBF containing 2 g/L collagen was adjusted to pH 7 and then added to a cuvette. The measurements were conducted under three temperature conditions: 25 °C, 25 °C and then 40 °C, and 40 °C. The time-sweep measurements were run for a time period of 24 hr.
Mechanical test
Unconfined compression tests were performed on dry scaffolds using a DMA 2980 Dynamic Mechanical Analyzer (TA instrument Inc., New Castle, DE). For each group of scaffolds, four cubic specimens, 4 mm in diameter and 4 mm thick, were cut at different locations in the scaffold. All compression tests were performed both perpendicular and parallel to the plane of pore orientation at a uniform stress rate of 0.5 N/min up to a maximum stress of 18 N. The compression modulus was calculated for each compression test from the slope of the linear elastic regime at which the stiffness was reduced by 3% from the maximum stiffness. The uniaxial tensile test was also performed on DMA 2980 (n=4). The samples were cut into strips (15 mm × 5 mm) with a thickness of 0.5 mm. The tensile modulus was calculated from the slope of the stress-strain curve over a strain range of 5-10 %.
Cell culture
MC3T3-E1 osteoblast cells were cultured in DMEM containing 4.5 g/L glucose, L-glutamine, and sodium pyruvate (Cellgro 10-013-CV, Corning) supplemented with 10 % FBS (Corning) and 1% penicillin-streptomycin (Corning). Frozen cells were expanded and trypsinized (Corning) before seeding each scaffold with one million cells in 15 μL of culture medium. Cells were allowed to settle for 30 min before adding an additional 1 mL of culture medium. Seeded constructs were maintained in a humidified incubator at 37 °C and 5% CO2 with daily medium changes. To determine cell number in the scaffold, we divided the total mass of DNA in the scaffold by the mass of DNA in a mouse cell (5.5 pg). DNA content was examined at one and four days after seeding (n=3) by freezing scaffolds in lysis buffer containing 10 mM tris-HCl, 0.1% Triton X-100, 1 mM EDTA, and 0.4 mg/mL proteinase-K (Invitrogen). Samples were then thawed and the solution was held overnight at 55 °C to activate proteinase-K digestion of the collagenous phase of the scaffold. The concentration of DNA in the resulting solution was measured with a fluorescent probe (Picogreen, Invitrogen) and plate reader (Synergy H1, BioTek). Cell seeded scaffolds after one day of incubation were also examined in 3D with 2-photon microscopy (Ultima IV, Prairie Technologies). First, scaffolds were fixed in 10 % formalin, permeabilized with 0.1 % Triton X-100 in PBS, and stained with Hoescht (Invitrogen) and Rhodamine-Phalloidin (Invitrogen). Then scaffolds were cut in half and imaged both in cross-section and from a top view. Image stacks were viewed with NIH ImageJ.
Statistical analysis
Results are presented as mean ± standard deviation. Statistically significant differences were determined by paired t-test. Statistical significance was accepted at p < 0.05.
Results and discussion
Effect of temperature on the gelation process
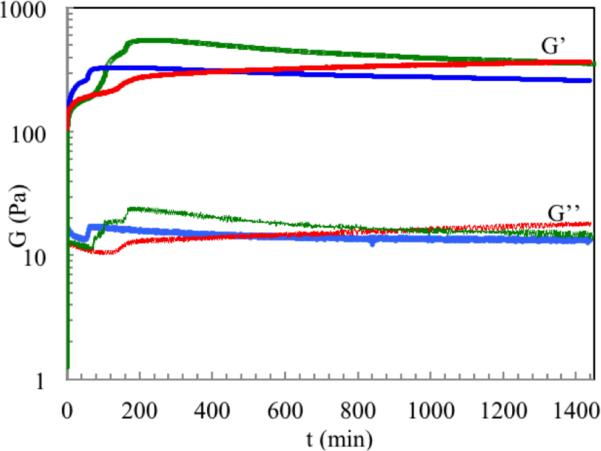
Gelation of 2.0 g/L collagen at different temperatures was studied by rheology (Figure 1). The storage modulus (G’) and loss modulus (G”) were monitored as a function of time to investigate the self-assembly and mineralization of collagen in m-SBF. In all cases, both G’ and G” increased sharply until reaching the first plateau. Under the two-temperature process, a peak modulus was observed after the temperature increased from 25 to 40 °C. The Col-Ap hydrogel formed at a constant temperature of 40 °C exhibited the lowest modulus and a clear phase separation as shown in Figure 2 a.
Figure 1.
Gelation behavior monitored by measuring the time-dependence of the elastic (G’) and viscous modulus of Col-Ap solution incubated at 25°C for 24 hr (red); 25°C for 1 hr and then 40 °C for 22.5 hr (green) and 40 °C (blue) for 24 hr.
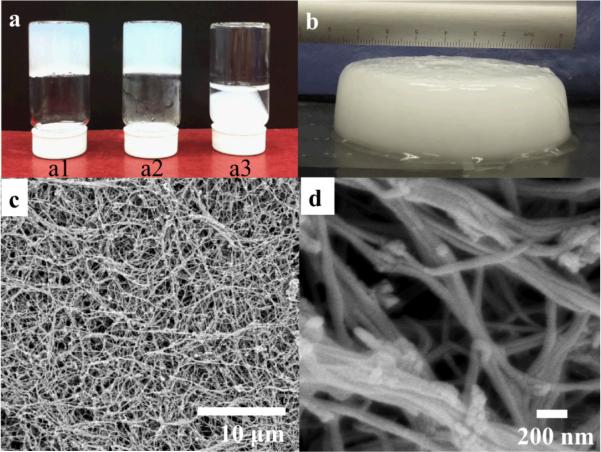
Figure 2.
Photograph of hydrogel prepared at 25°C for 24 hr (a1); 25 °C for 1 hr and then 40 °C for 22.5 hr (a2) and 40 °C for 24 hr (a3), Photograph of hydrogel used for self-compression and freeze/freeze drying prepared at 25 °C for 1 hr and then 40 °C for 22.5 hr (b), FESEM image of hydrogel prepared at 25 °C for 1 hr and then 40 °C for 22.5 hr dehydrated by ethanol solution and then super critical point dried (c & d).
Characterization of microstructures
The scaffold was prepared by freeze casting of a mineralized collagen hydrogel as shown in Figure 2. The hydrogel exhibits an opaque structure consisting of water and mineralized collagen fibers. The sample in Figure 2c & d was prepared by ethanol dehydration and critical pointing drying, which preserves the fibrils morphology in the hydrogel. It shows that the hydrogel constitutes of an interwoven network of long mineralized collagen fibers. The length of collagen fibers ranges from micrometer scale to centimeter scale. The diameter of a single collagen fiber is approximately 100 nm and collagen bundles formed by fusion of collagen fibers were observed. The large void space between the collagen fibers was filled by water in the hydrogel stage, indicating the collagen hydrogel is mainly composed of water.
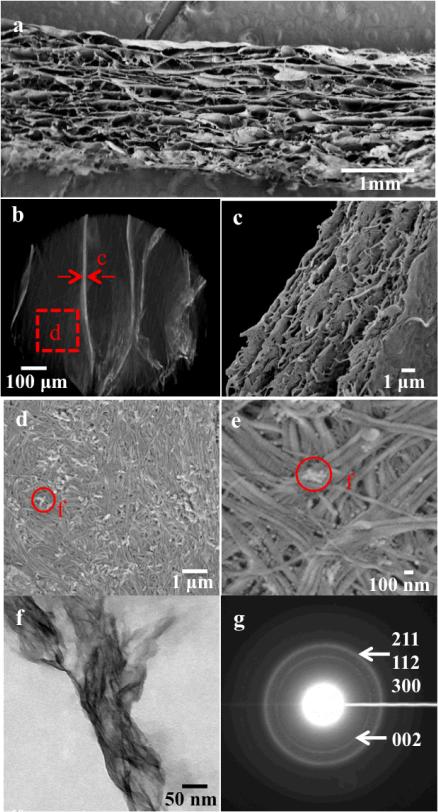
The scaffold exhibits a unique multi-level lamellar structure in which micro and macro pores were co-aligned (Figure 3). At the macro-structure level, the freeze-dried hydrogel consists of unidirectional macro-pores throughout the entire scaffold (Figure 3a & b). At the micro and sub-micro-structure level, each lamellae is comprised of aligned micro-lamellae with a thickness of several hundred nanometers (Figure 3 c). Moreover, the surface is biomimetic, consisting of hierarchical organized mineralized bundles (Figure 3 d & e). The typical collagen banding pattern was observed in the SEM image at a high magnification (Figure 3 e). TEM images of the Col-Ap hydrogel showed typical needle-like and prism-shaped apatite crystals of about 10 nm in width and 50-100 nm in length had formed throughout the cross-section of collagen fibers, similar to those found in natural bone.45 The electron diffraction pattern exhibits rings typical of low crystalline apatite structure (Figure 3 g). The apatite phase in natural bone also has low crystallinity.
Figure 3.
FESEM images of a scaffold prepared by freezing Col-Ap-20 hydrogel uni-directionally at -25 °C (a, c-e), microCT image of 3D reconstruction of the Col-Ap scaffold (b), TEM image of Col-Ap precipitates demonstrating needle-like apatite crystallites throughout the cross-section of collagen fibers (f), high magnification of selected area electron diffraction patterns exhibiting typical ring pattern of low crystalline apatite structure (g). (The Miller indices for each corresponding ring are labeled in brackets)
Figure 4 a shows the FT-IR spectra obtained from collagen and freeze-dried Col-Ap-20 scaffold. The broad band at 3500 cm−1 is associated with water in the scaffold. The peak around 1670 cm−1 arises from the C=O stretch of amide I, while those at 1540 cm−1 and 1200 cm−1 arise from N-H deformation of amide II and C-N stretch of amide III, respectively. The band at 1630 cm−1 corresponds to the O-H vibration overlapping with the peak of amide I. In the Col-Ap scaffold, the bands between 500 and 600 cm−1 are due to the anti-symmetric bending vibration of the PO4−3 groups. Bands in the region 1200-965 cm−1 were identified as the stretching vibrations of the PO4−3 groups. The carbonate peak at 875 cm−1 suggests the formation of carbonated apatite, which is similar to the mineral composition of nature bone. 38
Figure 4.
FT-IR (a) and XRD (b) of collagen and freeze dried Col-Ap-20 scaffold.
Figure 4 b shows XRD patterns of pure collagen and the Col-Ap scaffold. A broad diffused peak at about 20° was attributed to collagen. In the Col-Ap scaffold, the major peak at 2θ=31.8-32.2° corresponds to an overlap of three major planes (211), (112) and (300) of apatite. The diffraction peak was broad, implying that the apatite in Col-Ap scaffold is poorly crystallized.
Control of pore structure
Effect of compression time
The Col-Ap hydrogel contains a large volume of water. As a result, the collagen hydrogel compresses under its own weight, squeezing out water. This process is called self-compression. 17, 18 After the gel was removed from the vial, a continuous mass loss due to water expulsion was observed. By leaving the gel in the air for five, 20 and 45 minutes, the collagen fibrillar density in the gel reached 2.2, 3.9, and 5.5 g/L, respectively (Table 1). Figure 5 shows FESEM images of scaffolds obtained by freezing the Col-Ap gel at a freezing temperature of −25 °C for different self-compression times. With an increase of self-compression time from five min to 45 min, lamellar spacing decreased from 344±33 μm to 143±40 μm and wall thickness increased from 3.6±1.0 μm to 23.2±10.2 μm (Table 1). Col-Ap scaffolds prepared using Col-Ap gels with a higher collagen concentration (5.5 g/L) exhibited larger lamellae thickness (23.2 μm) compared with those (3.6 μm and 7.8 μm) prepared using Col-Ap gels with lower collagen concentrations (2.2 g/L and 3.6 g/L).
Table 1.
Pore structure of Col-Ap scaffolds prepared by freezing of Col-Ap-x hydrogels unidirecionally at −25 °C. *Significantly different from Col-Ap-5 scaffold; # Significantly different from Col-Ap-20 scaffold (p<0.05).
| Hydrogel | Compression time (min) | Collagen fibrillar density (g/ml) | Lamellar spacing ± SD (μm) | Wall thickness ± SD (μm) |
|---|---|---|---|---|
| Col-Ap-5 | 5 | 2.2 | 344 ±32.7 | 3.6 ± 1.0 |
| Col-Ap-20 | 20 | 3.9 | 173 ±45.4* | 7.8 ± 1.2* |
| Col-Ap-45 | 45 | 5.5 | 143 ±40.1* | 23±10*# |
Figure 5.
FESEM images of cross-section parallel to the freezing direction of scaffolds fabricated by freezing Col-Ap hydrogel at a freezing temperature of −25 °C but with a compression time of 5 min (a), 20 min (b) and 45 min (c), respectively.
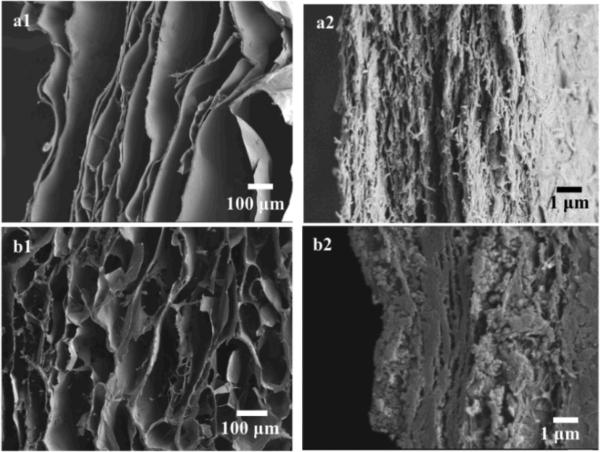
Effect of freezing conditions
As shown in Figure 6, the Col-Ap scaffold prepared by unidirectional freezing of Col-Ap-20 hydrogel exhibited anisotropic pore structure in which pores were aligned along the direction of ice growth. An image of the scaffold prepared by unidirectional freezing of Col-Ap-20 hydrogel at −196 °C is not shown. With a decrease of freezing temperature from −20 to −196 °C, a decrease of lamellar spacing from 173 μm to less than 63.8 μm was observed (Table 2). The wall thickness was not significantly different in the three scaffolds prepared by freezing Col-Ap-20 at different freezing temperatures. In contrast, the pore structure of Col-Ap scaffold fabricated by freezing the hydrogel non-directionally seems less anisotropic. More connections between two adjacent lamellae were observed.
Figure 6.
FESEM images of cross-section parallel to the freezing direction of Col-Ap scaffold fabricated by freezing the Col-Ap-20 hydrogel unidirectionally at −80 °C (a); FESEM images of scaffold fabricated by freezing the Col-Ap-20 hydrogel non-directionally at −20 °C (b).
Table 2.
Pore structure of Col-Ap scaffold fabricated by freezing Col- Ap-20 under different freezing temperatures. *Significantly different from scaffold prepared by unidirectionally freezing of Col-Ap-20 hydrogel at -20 °C (p<0.05).
| Freezing temperature (°C) | Freezing method | Lamellar spacing ± SD (μm) | Wall thickness ± SD (μm) |
|---|---|---|---|
| −20 | unidirectional | 173 ± 45.3 | 7.8 ± 1.2 |
| −80 | unidirectional | 146 ± 41.5 * | 8.2 ± 1.9 |
| −196 | unidirectional | 63.8 ± 32.1 * | 6.4 ± 2.1 |
| −20 | Non-unidirectional | 142 ± 59.7 | 7.2 ± 1.4 |
Mechanical property of Col-Ap scaffolds
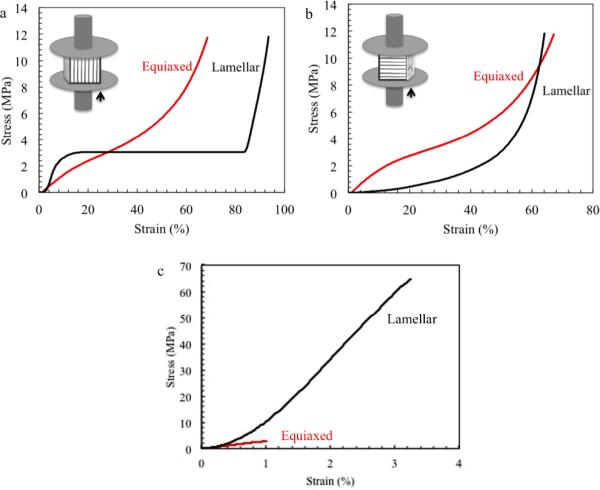
To investigate the effect of pore structure and fiber morphology on mechanical properties of the scaffold, unconfined compression and tensile tests were conducted on both scaffolds with multi-level lamellar pores (Lamellar Scaffold) and with equiaxed pores (Equiaxed Scaffold). The stress-strain behavior of Lamellar Scaffold and Equiaxed Scaffold under unconfined compression is shown in Figure 7. For the Lamellar Scaffold, the compression modulus in the direction of the lamellae was 20 fold higher compared to that in the direction perpendicular to the lamellae (Table 3). In the lamellae direction the stress increased gradually with increasing strain until a plateau was reached. After the plateau, the stress increased sharply. In contrast, in the direction perpendicular to the lamellae, no plateau was observed. The stress-strain behaviour of the scaffold with equiaxed pore structure was isotropic. There was no significant difference between the compression modulus of the Equiaxed Scaffold in the two testing directions. The compression modulus of Lamellar Scaffold along the pore direction was two times the magnitude of that of the Equiaxed Scaffold.
Figure 7.
Representative unconfined compressive stress-strain curves of Col-Ap scaffolds (a & b). Representative stress-strain curve under uniaxial tensile strength (c).
Table 3.
Compressive modulus of Lamellar Scaffold and Equiaxed Scaffold. *Significantly different from compression modulusparallel to the lamellae; # Significantly different from Lamellar Scaffold (p<0.05). (n=4)
| Sample | Apatite content (wt%) | Freezing condition | Porosity % | Lamellar spacing or pore size (μm) | Compression modulus (MPa)-parallel to the lamellae | Compression modulus (MPa)-perpendicular to the lamellae |
|---|---|---|---|---|---|---|
| Lamellar Scaffold | 35 | −25 °C, unidirectionally | 94 ± 5.8 | 173 ± 45.4 | 36.4 ± 10.0 | 1.87 ± 0.32 * |
| Equiaxed Scaffold | 35 | −25 °C, nondirectionally | 92 ± 4.7 | 82.3 ±15.1 | 18.0 ± 1.72 # | 17.4 ± 3.35 # |
A linear relationship between tensile stress and strain was observed in both Lamellar Scaffold and Equiaxed Scaffold (Figure 7 c). The Young's modulus of the Lamellar Scaffold (3298 MPa) was twelve times as high as that of the Equiaxed Scaffold (265.0 MPa), shown in Table 4. Moreover, the Lamellar Scaffold exhibits higher tensile strength and strain at failure compared to those of the Equiaxed Scaffold.
Cell attachment in scaffolds after in vitro incubation
The morphology of MC3T3-E1 osteoblast cells on the Lamellar Scaffold is shown in Figure 8. The cells were well attached to both scaffolds. Cell attachment in the center of the Lamellar Scaffold was also observed and is shown in Figure S1. Moreover, cells were spread throughout the Lamellar Scaffold (Figure S2). Cells appear to be elongated at the micrometer scale (Figure 8 a2). Figure 8c summarizes cell attachment on both scaffold types. At day one, approximately 17% of the original cells remain attached to the Lamellar Scaffold, while about 7% of the seeded cells are attached to the Equiaxed Scaffold. However, there was no significant difference in cell number between Lamellar Scaffold and Equiaxed Scaffold at day one. At day four, cell number remains constant in both scaffold types. Due to reduced deviation, the cell number on the Lamellar Scaffold was significantly higher than that on the Equiaxed Scaffold at day four.
Figure 8.
Two-photon images of MC3T3-E1 on the Lamellar Scaffold (a & b). Images were taken in the direction perpendicular to the pore direction (a) and along the pore direction (b). Cell number in the Lamellar Scaffold and the Equiaxed Scaffold at day one and day four (c).
Mechanism of multi-level lamellar geometry
The present study provides a simple method to prepare a biomimetic Col-Ap scaffold with a multi-level lamellar structure for bone tissue engineering. The procedure is based on a biomimetic fabrication technique using m-SBF containing both inorganic ions and collagen molecules. The Col-Ap hydrogel consisted of interconnected mineralized collagen fibers with great structural integrity. Using a strategy combining time-dependent self-compression with controllable unidirectional freezing, we are able to achieve a 3-D porous structure consisting of co-aligned micro and macro lamellar pores. The scaffold with a multi-level lamellar structure exhibits greater tensile strength and higher compression strength in the direction of lamellae compared to scaffold with an equiaxed pore structure.
We report a biomimetic approach to synthesize a mineralized collagen hydrogel using m-SBF containing collagen. Compared to conventional co-precipitation method, this novel biomimetic approach is easy to operate and is able to preserve the structural integrity of collagen fibers. In a conventional co-precipitation method, constant stirring is necessary to enhance the diffusion of calcium and phosphate ions and facilitate precipitation of apatite. 26, 29 The fiber length is usually at the micrometer scale. 29 Mineralized fibers are then collected by centrifugation and cross-linked by covalent bonds in the later freeze drying and chemical crosslinking process. 46 Here, a novel biomimetic approach is reported to prepare a mineralized collagen hydrogel, which is the basic building block of our Col-Ap scaffold. Collagen was added to m-SBF and its concentration was adjusted to prepare a Col-Ap hydrogel with different apatite: collagen ratios. 42 The kinetics of collagen fibrillogenesis and mineralization are simply controlled by initial pH and temperature in a static m-SBF solution. Initially, neutralization of the pH and an increase of temperature trigger the assembly of triple-helical collagen molecules into collagen fibrils and in situ precipitation of apatite onto the collagen matrix. A stiffer hydrogel has been formed by incubating the solution at 25°C for 1 hr and then 40 °C compared to hydrogels formed at a constant temperature of either 25°C or 40°C for 24 hr (Figure 1). Using this two-temperature process, the diameter of collagen fiber bundles is also increased, which may further strengthen the collagen matrix. 19 The XRD and FTIR results prove that the scaffold contains poorly crystalline carbonated apatite resembling the biological apatite in bone. 11 The apatite particles disperse homogenously throughout the collagen matrix (Figure 2c) without interfering with the collagen triple helical structure and the structural integrity.
A possible mechanism of multi-level lamellar geometry is shown in Figure 9. The co-alignment of micro- and macro pores may be attributed to the combined effect of self-gravity driven compression and lyophilization. The fresh hydrogel has a soft texture and is mechanically unstable after removal from the sample vial. The compression force applied by self-gravity raises the chemical potential of water inside the hydrogel. 15 As a result, water was removed from the gel and the resulting collagen density (Ct) of the Col-Ap hydrogel is time-dependent (Figure 9a & b). During the above unconfined self-compression process, the fluid within the scaffold leaves the surface of each lamella, inducing a shear stress which leads to the alignment of mineralized collagen fibrils within the compacted lamellar scaffold. 15 This simple self-compression method increases the fibrillar concentration, thereby improving the mechanical strength of the hydrogel. Additionally, it creates a series of stacked micro-lamellae aligned in the transverse direction.
Figure 9.
Schematic illustration of the technique used for the preparation of prepare lamellar Col-Ap scaffold. C0: initial collagen concentration in the hydrogel; Ct: collagen concentration in the hydrogel after self-compression for a certain period of time (t); Cf: collagen concentration in the frozen gel. A1A2 shows the direction of heat flux that is from the external of the mold to the center of Col-Ap hydrogel.
To control the orientation and size of macro pores, unidirectional freezing under different freezing rates has been applied in a house-made mold as shown in Figure 9c. The compressed hydrogel with a fibrillar concentration of Ct is frozen in the mold, which controls the temperature gradient in the transverse direction. The ice front, identified as the solid-liquid interface, propagates along the direction of heat flux radially from the outer surface towards the central region (along A1 to A2 direction as shown in Figure 9). The mineralized collagen fibrils are pushed by the advancing ice front. Therefore the Col-Ap concentration in the diminishing liquid increases to a higher concentration (Cf). 33 Meanwhile, the shear stress applied by the ice front on the hydrogel network further increases the degree of the alignment of nanolayers in each lamella. The dendritic surface topography in the solidification direction confirms that aligned macro pores are created by freezing the hydrogel in a single direction (Figure 9c). The spacing between two adjacent lamella depends on the degree of supercooling, which is described as the difference between the ice front temperature and the equilibrium temperature. In this study, the freezing temperature was varied to tailor the degree of supercooling.34 When the freezing temperature was decreased from −25 to −196 °C, the average lamellar spacing was decreased from 173 to 63.8 μm. In contrast, under slow and unidirectional freezing conditions, more connections between two adjacent lamella were formed (Figure 6 b). The pores became more round as the hydrogel was frozen in a random direction and coarsening was enhanced under a slow freezing rate.
It has been reported that an increased collagen concentration in the hydrogel generates a microenvironment more favorable to mineral deposition. 9 Vigier et al. reported increased new bone formation in a collagen matrix with high fibrillar density in vivo. 20 However, these studies on compression induced high fibrillar density were focused on 2-D dense films with a thickness of a few hundred micrometers. Limitations are nevertheless encountered in the case of a 3-D scaffold, because it requires a combination of biomimetic surface morphology to maintain a high affinity with cells, sufficient mechanical strength to support new bone formation, and the preservation of the 3-D porous structure to facilitate the transportation of cells, nutrient and metabolic waste.
Here, we describe a scaffold with a multi-level lamellar porous structure and biomimetic surface. The surface is comprised of hierarchically organized mineralized collagen fiber bundles. Good cell attachment was observed on the surface because the microenvironment in terms of fibrillar density and organization of this novel scaffold is similar to natural bone.15, 46 It has been reported that the unidirectional pore structure supports cell spreading and promotes the ingrowth of surrounding tissues.35,47 Moreover, improved mechanical strength was also found in scaffolds with a multi-level lamellar structure. Harley et al. reported that the compression modulus increased linearly with decreased porosity.48 However, the geometric effect of pore structure and collagen fibrillogenesis on the compression modulus and Young's modulus remain unclear. It has been found that the Young's modulus and compression modulus are maximized along the aligned pore direction in the scaffold with a multi-level lamellar structure.
The scaffold with a multi-level lamellar structure exhibited a stress-strain response similar to that of cancellous bone. 49 In cancellous bone, the compression curve is demonstrated as an initial linear region followed by a plateau. The plateau indicated that the scaffold struts started to collapse. Then the load increased exponentially when the structure started to compact. The compression modulus of the scaffold with a lamellar pore structure along the pore direction is two times the magnitude compared to the scaffold with conventional equiaxed pore structure. The Young's modulus of the scaffold with multi-level lamellar structure is twelve times the magnitude of the scaffold with equiaxed pore structure.
Hierarchically organized materials are attracting much attention because the increased level of organization offers a high active surface area and improved mechanical properties. To the best of our knowledge, this is the first report of a hierarchical process to engineer a three-dimensional tissue engineering scaffold from nanometer scale to millimeter scale. At the nanometer scale, the scaffold is comprised of mineralized collagen fiber bundles similar to natural bone. Moreover, the scaffold consisting of hierarchical aligned submicron-pores may lead to improved permeability of oxygen and nutrients, and faster removal of metabolic waste compared to conventional uniaxial pore structure or equiaxed pore structure because of the presence of additional porosity in each lamella. It was reported that one major challenge in the development of bone scaffolds has been the limitation of cell migration and tissue ingrowth attributed to the insufficient oxygen and nutrient supply. We anticipate that this structure can be used to better facilitate cell viability that may result in improved bone regeneration. At the macro level, the Col-Ap scaffolds have unidirectional pores with a size from 64 to 344 μm, which is sufficient for cell migration and vascularization. 50 In vivo tests will be conducted in future work to determine the suitability of this scaffold as a bone tissue engineering implant.
Conclusions
A method was developed to prepare a biomimetic Col-Ap scaffold with a multi-level lamellar structure for bone tissue regeneration. A mineralized collagen hydrogel consisting of bone-like mineralized fiber bundles was prepared by a simple biomineralization technique using collagen containing m-SBF. A multi-level lamellar structure consisting of co-aligned micro and macro pores was developed by combining a time-dependent self-compression process with controllable freeze casting. Aligned pores with a size ranging 64 to 344 μm were created by controlling the freezing conditions. The thickness of each lamella, comprised of aligned micro lamellae, could be adjusted in the range of 3.6 to 23.2 μm depending on the self-compression time. The bone-like microstructure and multi-level lamellar porous structure resulted in a twelve-fold increase in Young's modulus and a two-fold increase in compression modulus along the aligned direction compared to conventional scaffold with equiaxed pore structure. Moreover, this lamellar scaffold supported osteoblast attachment and spreading in vitro. Therefore, with its inherent hierarchical biomimetic structure and improved anisotropic mechanical strength, this scaffold has a great potential for use in bone tissue engineering applications.
Supplementary Material
Acknowledgements
The research was supported by the National Science Foundation (CBET 1133883) and the National Institute of Health (1R21AR059962-01A1). The authors thank Dr. Montgomery Shaw and Dr. Anson W K Ma for helpful discussion. The authors also thank Dr. Lichun Zhang for technical assistance with the TEM study, Mr. Kevin Kavanagh and Ms. Teresa L. Samuels for supplying rat tails to the project.
References
- 1.Dvir T, Timko BP, Kohane DS, Langer R. Nat. Nanotech. 2011;6:13. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szpalski C, Barbaro M, Sagebin F, Warren SM. Tissue. Eng. B. Rev. 2012;18:258. doi: 10.1089/ten.TEB.2011.0440. [DOI] [PubMed] [Google Scholar]
- 3.Roman J, Cabanas MV, Pena J, Vallet-Regi M. Sci Technol Adv Mat. 2011;12:045003. doi: 10.1088/1468-6996/12/4/045003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutmacher DW. Biomaterials. 2000;21:2529. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 5.Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biomaterials. 2006;27:3413. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 6.Davidenko N, Gibb T, Schuster C, Best SM, Campbell JJ, Watson CJ, et al. Acta. Biomater. 2012;8:667. doi: 10.1016/j.actbio.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 7.Haugh MG, Murphy CM, O'Brien FJ. Tissue. Eng. C. Methods. 2010;16:887. doi: 10.1089/ten.TEC.2009.0422. [DOI] [PubMed] [Google Scholar]
- 8.Ananta M, Brown RA, Mudera V. Tissue. Eng. A. 2012;18:353. doi: 10.1089/ten.TEA.2011.0208. [DOI] [PubMed] [Google Scholar]
- 9.Marelli B, Ghezzi CE, Barralet JE, Nazhat SN. Soft. Matter. 2011;7:9898. [Google Scholar]
- 10.Hadjipanayi E, Ananta M, Binkowski M, Streeter I, Lu Z, Cui ZF, et al. J. Tissue. Eng. Regen. Med. 2011;5:505. doi: 10.1002/term.343. [DOI] [PubMed] [Google Scholar]
- 11.Marelli B, Ghezzi C,E, Barralet JE, Boccaccini AR, Nazhat SN. Biomacromolecules. 2010;11:1470. doi: 10.1021/bm1001087. [DOI] [PubMed] [Google Scholar]
- 12.Roeder BA, Kokini K, Sturgis JE, Robinson JP, Voytik-Harbin SL SL. J. Biomech. Eng. 2002;124:214. doi: 10.1115/1.1449904. [DOI] [PubMed] [Google Scholar]
- 13.Mi S, Chen B, Wright B, Connon CJ. J. Biomed. Mater. Res. A. 2010;95:447. doi: 10.1002/jbm.a.32861. [DOI] [PubMed] [Google Scholar]
- 14.Braziulis E, Diezi M, Biedermann T, Pontiggia L, Schmucki M, Hartmann-Fritsch F, et al. Tissue. Eng. C. Methods. 2012;18:464. doi: 10.1089/ten.TEC.2011.0561. [DOI] [PubMed] [Google Scholar]
- 15.Brown RA, Wiseman M, Chuo CB, Cheema U, Nazhat SN. Adv. Funct. Mater. 2005;15:1762. [Google Scholar]
- 16.Ghezzi CE, Marelli B, Muja N, Nazhat SN. Acta. Biomater. 2012;8:1813. doi: 10.1016/j.actbio.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Serpooshan V, Quinn TM, Muja N, Nazhat SN. Acta. Biomater. 2013;9:4673. doi: 10.1016/j.actbio.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 18.Serpooshan V, Quinn TM, Mujaa N, Nazhat SN. Soft. Matter. 2011;7:8. [Google Scholar]
- 19.Yang YL, Motte S, Kaufman LJ. Biomaterials. 2010;31:5678. doi: 10.1016/j.biomaterials.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 20.Vigier S, Catania C, Baroukh B, Saffar JL, Giraud-Guille MM, Colombier ML. Tissue. Eng. A. 2011;17:889. doi: 10.1089/ten.TEA.2010.0336. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Chang J. J. Biomed. Mater. Res. A. 2008;85:293. doi: 10.1002/jbm.a.31397. [DOI] [PubMed] [Google Scholar]
- 22.Deshpande AS, Beniash E. Crystal. Growth. Design. 2008;8:3084. doi: 10.1021/cg800252f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoyer B, Bernhardt A, Heinemann S, Stachel I, Meyer M, Gelinsky M. Biomacromolecules. 2012;13:1059. doi: 10.1021/bm201776r. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Azais T, Robin M, Vallee A, Catania C, Legriel P, et al. Nat. Mater. 2012;11:724. doi: 10.1038/nmat3362. [DOI] [PubMed] [Google Scholar]
- 25.Yang HS, La WG, Bhang SH, Lee TJ, Lee M, Kim BS. Tissue. Eng. A. 2011;17:2153. doi: 10.1089/ten.TEA.2010.0702. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Liao SS, Cui FZ. Chem. Mater. 2003;15:3221. [Google Scholar]
- 27.Boonrungsiman S, Gentleman E, Carzaniga R, Evans ND, McComb DW, Porter AE, et al. Proc. Natl. Acad. Sci. USA. 2012;109:14170. doi: 10.1073/pnas.1208916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scaglione S, Giannoni P, Bianchini P, Sandri M, Marotta R, Firpo G, et al. Sci Rep. 2012:274. doi: 10.1038/srep00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kikuchi M, Itoh S, Ichinose S, Shinomiya K, Tanaka J. Biomaterials. 2001;22:1705. doi: 10.1016/s0142-9612(00)00305-7. [DOI] [PubMed] [Google Scholar]
- 30.Porter JR, Ruckh TT, Popat KC. Biotech. Progress. 2009;25:1539. doi: 10.1002/btpr.246. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Ma PX. Ann. Biomed. Eng. 2004;32:477. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 32.Deville S. Adv. Eng. Mater. 2008;10:155. [Google Scholar]
- 33.Tuncaboylu DC, Okay O. Langmuir. 2010;26:7574. doi: 10.1021/la904369g. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, Cooper AI. Adv. Mater. 2007;19:1529. [Google Scholar]
- 35.Riblett BW, Francis NL, Wheatley MA, Wegst UGK. Adv. Funct. Mater. 2012;22:4920. [Google Scholar]
- 36.Caliari SR, Harley BA. Biomaterials. 2011;32:5330. doi: 10.1016/j.biomaterials.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meghri NM, Donius AE, Riblett BW, Martin EJ, Clyne AM, Wegst UGK. JOM(1989) 2010;62:71. doi: 10.1007/s11837-010-0112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia Z, Yu X, Wei M. J. Biomed.Mater. Res. B. 2012;100:871. doi: 10.1002/jbm.b.31970. [DOI] [PubMed] [Google Scholar]
- 39.Qu H, Xia Z, Knecht DA, Wei M. J. Am. Ceram. Soc. 2008;91:3211. [Google Scholar]
- 40.Rajan N, Habermehl J, Cote MF, Doillon CJ, Mantovani D. Nat. Protoc. 2006;1:2753. doi: 10.1038/nprot.2006.430. [DOI] [PubMed] [Google Scholar]
- 41.Yu X, Wang L, Peng F, Jiang X, Xia Z, Huang J, et al. J. Tissue. Eng. Regen. Med. 2012:31. doi: 10.1002/term.1490. doi: 10.1002/term.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia Z, Yu X, Jiang X, Brody HD, Rowe DW, Wei M. Acta. Biomater. 2013;9:7308. doi: 10.1016/j.actbio.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metscher BD. Dev. Dynam. 2009;238:632. doi: 10.1002/dvdy.21857. [DOI] [PubMed] [Google Scholar]
- 44.Al-Munajjed AA, Plunkett NA, Gleeson JP, Weber T, Jungreuthmayer C, Levingstone T, et al. J. Biomed. Mater. Res. B. 2009;90:584. doi: 10.1002/jbm.b.31320. [DOI] [PubMed] [Google Scholar]
- 45.Liu C, Han Z, Czernuszka JT. Acta. Biomater. 2009;5:661. doi: 10.1016/j.actbio.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 46.Nassif N, Seto J, Belamie E, Davidson P, Panine P, Mosser G G, et al. Chem. Mater. 2010;22:2. [Google Scholar]
- 47.Yunoki S, Ikoma T, Tsuchiya A, Monkawa A, Ohta K, Sotome S, Shinomiya K, Tanaka JJ. Biomed.Mater. Res. B. 2007;80:166. doi: 10.1002/jbm.b.30581. [DOI] [PubMed] [Google Scholar]
- 48.Harley BA, Leung JH, Silva EC, Gibson LJ. Acta. Biomater. 2007;3:463. doi: 10.1016/j.actbio.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 49.Teo WE, Liao S, Chan C, Ramakrishna S. Acta. Biomater. 2011;7:193. doi: 10.1016/j.actbio.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 50.Karageorgiou V, Kaplan D. Biomaterials. 2005;26:5474. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.