Abstract
In an effort to enhance patient safety in Opioid Treatment Programs (OTPs), the Substance Abuse and Mental Health Services Administration (SAMHSA) convened a multi-disciplinary Expert Panel on the Cardiac Effects of Methadone. Panel members reviewed the literature, regulatory actions, professional guidances, and OTPs’ experiences regarding adverse cardiac events associated with methadone.
The Panel concluded that, to the extent possible, every OTP should have a universal Cardiac Risk Management Plan (incorporating clinical assessment, ECG assessment, risk stratification, and prevention of drug interactions) for all patients, and should strongly consider patient-specific risk minimization strategies (such as careful patient monitoring, obtaining ECGs as indicated by a particular patient’s risk profile, and adjusting the methadone dose as needed) for patients with identified risk factors for adverse cardiac events. The Panel also suggested specific modifications to informed consent documents, patient education, staff education, and methadone protocols.
BACKGROUND AND PURPOSE
Drug-induced long QT syndrome occurs with a wide range of medications, including methadone.1,2 Prolongation of the QT interval serves as a surrogate marker for the risk of developing Torsades de Pointes (TdP), a relatively rare and potentially lethal ventricular arrhythmia. The association between methadone, prolonged QT interval, and TdP came into sharp focus in 2006, when the U.S. Food and Drug Administration (FDA) issued a physician safety alert regarding fatalities and cardiac arrhythmias associated with methadone3 see Appendix B of this report). This was followed by a warning in the manufacturer’s product labeling.4 Warnings about the association between methadone and cardiac arrhythmias also are catalogued in Thompson Reuters’ MICROMEDEX5 and a website that dynamically archives QT-prolonging drugs.6
Methadone has a long history as an effective treatment for opioid addiction and thus is in wide use in Opioid Treatment Programs (OTPs) in the U.S. It therefore is of concern that a number of recent reports suggest that methadone prolongs the QT interval when used at doses higher than 60–120 mg/day, which often are required in such treatment.6–9 Also of concern is evidence of a significant knowledge gap among OTP medical staff regarding the risk of QT prolongation and TdP associated with methadone.10
The prevalence of QT interval prolongation or TdP in patients who are treated with methadone has not been firmly established,11 though marked prolongation of the corrected QT (QTc) interval appears to occur in only 2% of OTP patients.12 To address the topic, the Substance Abuse and Mental Health Services Administration (SAMHSA) convened a multi-disciplinary Expert Panel on the Cardiac Effects of Methadone and charged it with evaluating the available evidence and formulating recommendations to enhance the cardiac safety of patients in OTPs. The Panel also reviewed the context for the situation. This included a discussion of methadone’s unequivocal effectiveness in treating opioid addiction, current regulatory actions and recommendations from private sector organizations, guidance documents from other countries, and OTP providers’ awareness of QT and TdP.
Panel members are cardiologists with backgrounds in addiction medicine, electrophysiologists, addiction treatment specialists, medical educators and researchers, and experts in drug development. Ex officio members include representatives of scientific and regulatory authorities (including the National Institute on Drug Abuse [NIDA], and the FDA) and stakeholder organizations that share a commitment to assuring the safety of patients in opioid addiction treatment.
The principles that guided the Panel’s approach included a recognition that methadone has been associated with a reduction in overall mortality1,2 has few therapeutic alternatives, and is cost-effective. Therefore, the Panel operated on the premise that methadone must remain widely available in the U.S. for the treatment of opioid addiction.
The purpose of the Panel’s work, as reflected in this report, is solely to provide a workable template for OTP administrators and clinical staff who are attempting to develop and implement cardiac safety standards for methadone induction and maintenance treatment in Federally-certified OTPs.
SAMHSA encourages providers to consider the Panel’s report and to take action to the extent that they are clinically, administratively and financially able to do so. However, nothing in the report is intended to create a legal standard of care for any OTP, or an accreditation requirement, or to interfere with the judgment of individual clinicians who are responsible for delivering patient care.
METHODS
Panel members reviewed the available information at an initial meeting in December 2007, after which a draft document was prepared by the Panel’s writing group. A second meeting of the Panel was convened in July 2008 and a third in June 2009.
Literature Search
As part of the Panel’s review, a comprehensive literature search was performed via MEDLINE and EMBASE (covering articles published between 1966 and July 2008) for publications addressing the cardiac effects of methadone. English-language manuscripts were reviewed, as were official opioid treatment guidelines published in Canada and the United Kingdom, background articles on QT prolongation and TdP provided by Panel members, and the findings of relevant meetings.
Members of the Panel also reviewed information from regulatory authorities, considered the challenges involved in applying cardiac safety recommendations within OTPs, examined data regarding physician awareness of the cardiac effects of methadone, and evaluated contextual materials regarding the cardiac effects of the methadone derivative levo-alpha-acetyl-methadyl (LAAM), which is no longer marketed.13 The objective of this process was to broadly synthesize available evidence regarding the cardiac effects of methadone, then to distill that information into a practical framework for improving cardiac safety in OTPs.
Field Review
In 2009, SAMHSA distributed more than 470 copies of the draft document to stakeholder organizations and to individual experts for field review. Copies also were distributed to participants in workshops at the 2009 AATOD and ASAM annual meetings.
The field review draft was marked “Not for Reproduction or Distribution.” Responses were requested by May 31, 2009. A total of 51 responses were received (an 11% response rate). All of the comments and suggested changes were compiled and circulated to the Panel for consideration at a meeting in July 2009.
As part of the review process, the draft document was submitted to a law firm specializing in professional liability matters, with a request for a determination as to whether the language of the document would tend to increase the exposure of OTPs and individual clinicians to legal action and, if so, for suggested language modifications that would minimize such exposure. The law firm complied with these requests and their suggested language has been incorporated herein.14
Finally, the draft and the supporting literature were sent to a health care statistician, who was asked to determine whether the articles on which the Panel based its findings and conclusions met standard tests for statistical significance. The results of that examination also are reflected in this draft.15
This version of the report incorporates comments received through the field review, as well as from literature published subsequent to preparation of the earlier drafts, and revisions agreed on at the Panel’s June 2009 meeting. It supersedes any previous drafts.
Background
Methadone Pharmacology
Methadone is a potent full mu and delta opioid agonist with good oral bioavailability and a long duration of action. Its actions may mimic those of the endogenous opioids, enkephalins, and endorphins and affect the release of other neurotransmitters, such as acetylcholine, norepinephrine, substance P, and dopamine.16 This accounts for its analgesic and antitussive properties, respiratory depression, sedation, decrease in bowel motility, increase in biliary tone, hormone regulation and increase of prolactin and growth hormone release, miotic pupils, nausea, and hypotension.17
Methadone also is a non-competitive antagonist at the N-methyl-D-aspartate (NMDA) receptor. There is a great deal of interest in this receptor because it may be linked to pain processing and spinal neural plasticity, although its exact role and how it can be manipulated awaits further clarification.18
On the other hand, methadone’s high inter-individual variability and potential adverse effects – particularly respiratory depression and death – make a fundamental knowledge of its pharmacokinetics and pharmacodynamics imperative to the safe use of this important medication.19
Pharmacokinetic studies have shown that oral methadone has a delayed onset of action. Following oral administration, time to achieve peak plasma drug concentration is 2.5 hours for methadone in solution and three hours for methadone in tablet form. Oral bioavailability is about 85% (range: 67–95%), or three times that of morphine. Although there are no data on tissue distribution for methadone in humans, the distribution to various tissues has been shown to be extensive in animal models. This is consistent with the high volume of distribution in humans (4.2–9.2 L·kg−1 in opioids addicts and 1.7–5.3 L·kg−1 in chronic pain patients.20–25
Methadone is biotransformed in the liver by the cytochrome P450-related enzymes (primarily by the 3A4 and—to a lesser extent—the 2D6 and 1A2 systems) to two N-demethylated biologically inactive metabolites, which undergo additional oxidative metabolism.26 At physiological pH, 86% of methadone is bound to plasma proteins, predominately to α1-acid glycoprotein (AAG). AAG is an acute-phase reactive protein and the plasma level fluctuates with various physiologic and pathologic conditions such as stress, opioid addiction, cancer, and concomitant administration of certain medications.
The clinical implication of increased levels of AAG is that such an individual may be protected from the toxic effects of a dose of methadone, as compared to a healthy casual user of methadone who would not have elevated levels of AAG. Unlike morphine, methadone is biotransformed rather than conjugated in the liver. At daily doses less than 55 mg, the majority of the metabolites are cleared via the fecal route. Methadone is metabolized by the type I cytochrome P450 group of enzymes. The main enzyme responsible for N-demethylation of methadone is CYP3A4, with lesser involvement from CYP1A2. CYP2B6, and CYP2D6.18
Current evidence suggests that CYP2B6 may play a very significant role in metabolism as well. The main product of N-demethylation, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), is inactive. The activities of these cytochrome enzymes, especially CYP3A4, can be induced or inhibited by other drugs or by the methadone itself, accounting for the large individual variations in methadone pharmacology (refer to Appendix D). While the primary metabolite of methadone is inactive, methadol and normethadol are two minor metabolites produced in small amounts that have similar pharmacologic activity to methadone.25 Renal excretion is variable and is pH dependent. At a urine pH above 6, renal clearance is only 4% of the total drug elimination. When urine pH drops below 6, the unchanged methadone excreted by the renal route is approximately 30% of the total administered dose. Despite this, methadone does not accumulate in patients with renal failure and is poorly removed by hemodialysis. The renal excretion of the primary metabolite, EDDP, is not pH-dependent.
Methadone undergoes a biphasic pattern of elimination: slow distribution or α-elimination phase (8–12 hours) and a ß-elimination phase (30–60 hours). The α-elimination correlates with the duration of analgesia that is typically six to eight hours. The plasma level in the ß-elimination phase is sub-analgesic but is sufficient to prevent withdrawal symptoms.24
QT Interval and Torsades de Pointes
The QT interval is a measure of the time between the start of the Q wave and the end of the T wave in the heart’s electrical cycle. The QT interval is dependent on the heart rate in an obvious way (the faster the heart rate, the shorter the QT interval) and must be adjusted to aid interpretation. Normal values for the QT interval are between 0.30 and 0.44 (0.45 for women) seconds. Prolonged QT interval has been defined as > 450 milliseconds for men and > 460 – 470 milliseconds for women.27,28
QT interval can be measured by different methods, such as the threshold method, in which the end of the T wave is determined by the point at which the component of the T wave merges with the isoelectric baseline or the tangent method in which the end of the T wave is determined by the intersection of a line extrapolated from the isoelectric baseline and the tangent line which touches the terminal part of the T wave at the point of maximum downslope.29
The QT interval generally is corrected for its natural dependence on heart rate using Bazett’s formula:30 QTc = QT interval (in milliseconds) divided by the square root of the preceding RR interval (in seconds). Although this formula is likely to over-correct in the setting of high heart rates,31 it is nevertheless a reasonable method for screening purposes, with the proviso that patients remain supine for approximately 5 minutes prior to ECG acquisition.
Prolongation of the QT interval occurs when there is an interruption in the normal balance and flow of ions in the myocardium, increasing the time it takes for repolarization to occur.32 A QT interval of 390–420 milliseconds in men or 400–440 milliseconds in women generally is considered (Figure 1).33
Figure 1.
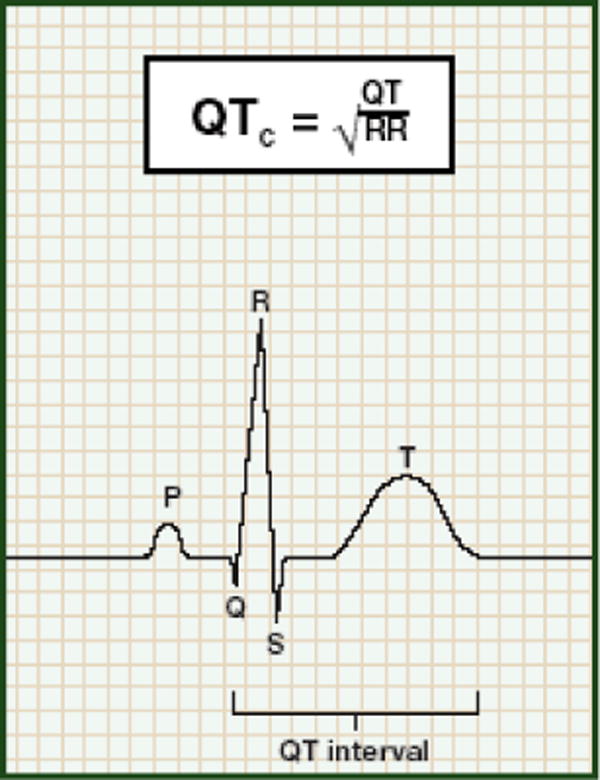
Prolonged QT Interval on Electrocardiogram
Causation of the prolonged QT interval involves the human ether-a-go-go-related gene (hERG) and the subunit of the voltage-gated potassium channels (found predominantly in the myocardium) for which it encodes.34 These channels are the predominant facilitators of the delayed-rectifier potassium ion currents (IK), which cause repolarization. Abnormalities in these channels have been shown to lead to prolonged action potentials that are expressed as long QT intervals on the electrocardiogram.35
The health risks associated with a prolonged QT interval are not clear. Although individuals with a prolonged QT interval often are asymptomatic, some may develop palpitations, syncope, seizures, or cardiac arrest.36 QT interval prolongation can also lead to the potentially fatal reentrant arrhythmia known as Torsades de Pointes (TdP).33
Torsades de Pointes is a French term that literally means “twisting of the points.” TdP is a potentially lethal form of ventricular arrhythmia named for its hallmark feature: polymorphic QRS complexes that appear to twist around the isoelectric line.
However, the mere presence of QT interval prolongation may not lead to TdP.37 Some medications, such as amiodarone, are commonly associated with a prolonged QT interval but rarely with TdP.38 Conversely, TdP can occur in individuals whose QT interval is within the normal range.34
Torsades usually occurs in bursts that are not sustained; thus, the rhythm strip usually shows the patient’s baseline QT prolongation. Although frequently self-terminating, TdP may degenerate into ventricular fibrillation and can lead to sudden death.
QT prolongation is the mandatory substrate for development of TdP and is the most commonly scrutinized pharmacologic adverse effect evaluated during the safety phase of drug development and post-marketing surveillance (Figure 2).31,39
Figure 2.
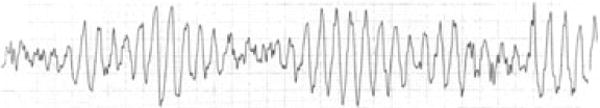
Torsades de Pointes
Drug-induced QT prolongation or TdP often result from a confluence of factors. Roden40 compiled a list of factors that may increase the likelihood of a patient developing QT interval prolongation and subsequent progression to TdP, such as female gender, hypokalemia, a history of drug interactions, underlying cardiac conditions, unrecognized congenital long QT syndrome (LQTS), and predisposing DNA polymorphisms. While these risk factors have not been studied in patients receiving methadone, they should be taken into consideration when treating this specific population.
Pre-existing QT prolongation appears to be a particularly important risk factor for drug-induced arrhythmia. In a meta-analysis of 1,288 subjects from 181 clinical trials of the antiarrhythmic drug sotalol, in which the incidence of TdP was 2%, an increased QT at baseline (mean QT 455 ± 11 milliseconds in those who experienced TdP versus 428 ± 1 millisecond in those who did not, p<0.003) was the most consistent predictor of TdP.41
The DIAMOND-HF study – a multivariate analysis of those receiving the QT-prolonging drug dofetilide – produced similar findings,42 with a baseline QT greater than the upper quartile value of 479 milliseconds associated with a relative risk for death of 1.9 (95% CI 1.0–3.6, p=0.05).
While many drugs prolong the QT interval, drug-induced TdP is associated with a much smaller number of medications and often is accompanied by an additional predisposing risk factor31 such as bradycardia.43,44 Women have a slightly longer QT interval than men and are at greater risk for arrhythmia due to QT-prolonging compounds.45 Threshold values of 430 milliseconds for men and 450 milliseconds for women have been proposed as the upper limit of normal.46 Despite these variable definitions, the international regulatory guidance for drug development suggests a gender-independent categorical threshold for QT prolongation of 450 milliseconds.47
The chiral nature of the methadone molecule also may be important in understanding QT interval prolongation associated with methadone. In addition to CYP3A4, methadone is metabolized by CYP2B6 and CYP2D648–50 Stereoselectivity of CYP2B6 toward S-methadone has been demonstrated in vitro51 and in vivo.52,53 It may be that slow metabolizers of CYP 2B6 who possess a *6/*6 genotype have difficulty metabolizing S-methadone but not R-methadone. Eap and colleagues53 demonstrated that S-methadone blocks the hERG channel more potently than does R-methadone, and that slow metabolizers of CYP 2B6 with a *6/*6 genotype were more likely to have a prolonged QT interval than did patients without the *6/*6 genotype (extensive metabolizers). This is clinically significant, as approximately 6% of Caucasians and African Americans possess the *6/*6 genotype.52,53
Although there is disagreement over the exact degree of risk posed by drug-related QT prolongation, it is generally accepted that significant risk of TdP occurs at measurements over 500 milliseconds.54 The paucity of long-term studies of QT-prolonging drugs in large populations makes it difficult to assign a relative risk to a QT >500 milliseconds, but in patients with the long QT syndrome, a QT >500 milliseconds was associated with an odds ratio for syncope or sudden death of 4.22 (95% CI, 1.12 to 15.80; P=0.033), presumably due to TdP.55
Rationale for Screening
The reason for the variability in QT intervals among patients treated with methadone is not completely clear, which complicates the process of screening for risk. Ehret and colleagues56 posited that some other factors might have contributing roles, as they found that long QT intervals seemed to be associated with the use of CYP3A4 inhibitors, low potassium concentrations, and abnormal hepatic function. They concluded that the occurrence of ECG changes is more likely in patients receiving methadone, and other physiological factors are likely to contribute to the manifestation of abnormal cardiac function.
Other limitations of QT interval screening include selection of a methodology for rate correction at extremes of heart rate; choosing between fully manual, semi-automatic or automated QT measurements; and the limited predictive value of QT prolongation for arrhythmia risk at an individual level. Despite these limitations, QT interval screening is the current standard for assessing cardiac drug safety in the major domains of medicine: clinical trials, cardiology practice, drug development, and regulatory assessment for drug withdrawal and/or manufacturers’ labeling changes.57 This type of screening does not necessarily require a specialist and has been judged appropriate for primary care settings.28
Advice from Relevant AGENCIES AND Organizations
There are no current, widely accepted recommendations from U.S. authorities for the prevention and treatment of methadone-induced QT interval prolongation. As noted earlier, the FDA issued a physician safety alert regarding fatalities and cardiac arrhythmias associated with methadone,3 which was followed by a “black box warning” in the manufacturer’s product labeling.4 Although the revised product label suggests careful monitoring of patients with prolonged QT intervals, it does not specify what form of monitoring is appropriate.
Thompson Reuters’ MICROMEDEX is somewhat more concrete in suggesting ECG monitoring of patients with known cardiac conduction abnormalities or those at increased risk for such abnormalities.5
Relevant advice from the American Association for the Treatment of Opioid Dependence (AATOD) and from Canadian and British health authorities is summarized in Appendix B.
RESULTS OF THE LITERATURE REVIEW
The literature linking methadone to QT prolongation and TdP was organized into the following categories: experimental (in vitro) studies, clinical case series, forensic/toxicologic studies, cross-sectional investigations, and prospective cohort studies or randomized trials. In addition, the published studies were scrutinized for the relationship between methadone dose or serum concentration and cardiac repolarization. An abbreviated discussion of the prinicipal findings follows (also refer to Appendix D).
Experimental Studies
As noted earlier, the most common cause of drug-induced QT prolongation and TdP is blockade of the human cardiac ether-a-go-go-related gene (hERG), which encodes the rapid component of the delayed-rectifier potassium ion current (Ikr).34 Blockade of this cardiac ion channel prolongs the terminal portion of the cardiac action, potentially causing delayed repolarization, which manifests as QT interval prolongation on the surface ECG.
Methadone has been shown to be a potent inhibitor of the hERG channel, capable of achieving 50% in vitro inhibition (IC50%) of Ikr at concentrations between 1–20 μM, depending on experimental conditions – a level that can be attained in clinical practice (Katchman, McGrory et al., 2002).53,58 The ratio of the IC50% to maximal serum concentration (Cmax) is a better predictor of arrhythmia risk and is identical for methadone and LAAM, but an order of magnitude more potent than for buprenorphine, another opioid approved for use in addiction treatment.
Major inter-individual variability in serum levels for any given dose due to variable hepatic clearance makes a priori prediction of QT effect problematic.48 Specifically, methadone is metabolized by the cytochrome P450 system, and inhibitors of this enzyme can significantly raise plasma area-under-curve measurements. Additionally, unsuspected polymorphisms in the gene for the hERG channel occur in 2% of the healthy population and may be associated with increased sensitivity to hERG channel blockade by methadone or similar compounds.59
Beyond its impact on cardiac repolarization via blockade of hERG channels, methadone possesses additional properties that may predispose patients to development of TdP. For example, risk of TdP increases in the setting of bradycardia – an effect that has been confirmed clinically.60 Methadone appears to exhibit negative chronotropic effects through two key mechanisms: calcium channel antagonism61,62 and anti-cholinesterase properties.63–65
Clinical Case Series
As early as 1973, a group of heroin-addicted patients were evaluated for predisposing risk factors for sudden cardiac death.66,67 The authors observed QT prolongation in a number of patients on methadone who also were using illicit drugs. At the time, there was no clinical evidence that methadone possessed cardiac properties.
Only three decades later was the association between very high doses of methadone and TdP documented in a North American case series of 17 patients who experienced TdP.66 The patients’ mean ± S.D. daily dose of methadone was 397 ± 283 mg, and their mean ± S.D. QT interval was 615 ± 77 milliseconds. None of the patients died, but an implantable pacemaker or defibrillator was placed in 14 patients. The researchers concluded that doses of methadone greater than 60 mg a day can lead to TdP. However, because of the small sample size, lack of a control group, and absence of testing for congenital LQTS, they cautioned that their research did not establish a causal relationship between methadone use and TdP. (In a later publication, Krantz, Kutinsky et al. further analyzed the same data and determined that methadone use was the only variable predictive of QT interval increases and that the increases were dose-dependent.68)
Since that time, a growing body of evidence has emerged to suggest a clinical association between methadone, QT prolongation and TdP69–87
For example, Justo and colleagues88 performed a meta-analysis of 14 case reports (totaling 40 opioid-dependent patients) of methadone-associated TdP to identify factors associated with methadone-related QT interval prolongation and TdP. Only patients being treated in methadone programs for opioid addiction were included. The mean ± S.D. QT interval during or immediately after TdP was 598 ± 75 milliseconds. The mean ± S.D. daily methadone dose was 231 ± 201 mg (range, 60–1000 mg daily). The most common risk factor identified was a methadone dose greater than 60 mg a day (n = 39, 98%). Other common risk factors included the use of other medications known to increase serum methadone levels or trigger TdP (n = 22, 55%), HIV infection (n = 16, 40%), hypokalemia (n = 14, 35%), female sex (n = 13, 33%), cirrhosis or renal failure (n = 11, 28%), and heart disease (n = 9, 33%). The mean number of risk factors was 3.5 per patient, with all patients having at least 1 risk factor and 85% having 2 or more.
Through a search of the FDA’s MedWatch system, Pearson and Woosley89 identified 5,503 methadone-related adverse events reported to the FDA between 1969 and 2002. Of these, 59 were related to QT interval prolongation or TdP. Of the 59 cases, 28 resulted in hospitalization and 5 resulted in death. Interestingly, 56 of the cases were reported in the last two years of the review. The authors proposed several explanations for this, including the fact that the arrhythmias can be confirmed only through ECG monitoring, which was not routinely conducted in the earlier years of methadone treatment. They also found that the abnormal cardiac rhythm events reported occurred over a large range of doses. (It is important to note that only a fraction of drug-related serious adverse events are voluntarily reported to MedWatch, so the true number of arrhythmia episodes attributable to methadone is not known.)
Patel, Singh et al.90 described 8 methadone-maintenance patients who presented with aborted sudden death or TdP and required placement of implantable cardioverter defibrillators. The patients were receiving high doses of methadone (mean 204 + 173 mg/day) and were followed longitudinally for a mean of 27 months. Six of the 8 patients continued methadone therapy; one died, and 3 others received electroshock for recurrent TdP. This case series suggests that methadone-treated patients who experience TdP are at substantial risk for recurrent arrhythmia if methadone is continued, particularly at higher doses.
Toxicologic Studies
Chugh and colleagues91 conducted a study of patients who had sudden cardiac death and were assessed by a medical examiner. Case subjects included those with therapeutic blood methadone concentrations (<1 mg/L) on postmortem toxicological evaluation; controls included subjects who did not take methadone. Cases in which higher methadone levels were present were presumed to be overdoses and were excluded, as were cases in which recreational drug use was determined to be the cause of death. A total of 22 patients with sudden cardiac death and therapeutic methadone levels were identified and compared with 106 patients in the control group. Based on autopsy findings, it was determined that a structural cardiac abnormality that could have caused sudden cardiac death (e.g., coronary artery disease, severe left ventricular hypertrophy, hypertrophic cardiomyopathy) was present in only 23% of the methadone patients, compared with 60% of patients in the control group (p = 0.002). Rates of use of other medications (such as benzodiazepines, antidepressants, anticonvulsants, antihistamines, muscle relaxants) were similar in both groups. Although it was not possible to determine how many of the study subjects had died from respiratory depression unrelated to a cardiac event, the authors concluded that the results of the study supported the presence of cardiac risk associated with methadone given at therapeutic levels.
It should be emphasized that this study is only inferential, because central nervous system depression with resulting anoxia also can cause arrhythmias. However, the findings are consistent with methadone’s potent proarrhythmic effects in vitro and the statistical reality that a small but significant proportion of arrhythmic events will be fatal.92,93
Cross-Sectional Studies
Cross-sectional studies were available for four ambulatory and one inpatient cohort. Maremmani and associates94 conducted a restrospective analysis of 83 patients being treated with methadone maintenance for their heroin addiction for at least six months. At the time of ECG recording, all patients had urine toxicology negative for opioids, cocaine, and amphetamines, and none were taking other medications associated with QT interval prolongation. Eighty-three percent of the patients had QT interval prolongation greater than the reference value for persons of the same sex and age, though only two patients had QT intervals exceeding 500 milliseconds. No baseline ECGs or plasma methadone levels were obtained. The mean daily dose of methadone was 87 mg (range, 10–600 mg daily), but no correlation was found between the QT interval and the methadone dose.
Ehret and colleagues56 examined ECG changes in a retrospective study of 527 former heroin users. Of these, 280 were excluded for not having adequate records available, for abusing methadone, or for having severe heart disease. The remaining 247 individuals were separated into two groups: methadone maintenance patients (n = 167) and patients not receiving any pharmacotherapy (n = 80). Twenty-seven patients in the methadone maintenance group had a QT interval of 500 milliseconds or longer compared with no patients in the control group (p < 0.001). The relationship between dose and QT interval was weakly associated, but a higher daily methadone dose was associated with significantly greater QT interval prolongation (p < 0.01). In this study, the lowest methadone dosage found to increase the QT interval above 500 milliseconds was 30 mg per day. In the methadone-treated group, 6 patients experienced TdP. These patients were taking 40–200 mg per day and had a QT interval of 430–750 milliseconds. Of note, 2 of the patients who experienced TdP had QT intervals within the normal range.
The largest cross-sectional comparative study performed to date analyzed 393 methadone-treated patients and 43 buprenorphine-maintained patients in Copenhagen.95 The authors observed QT prolongation (>440 milliseconds) in 32% of methadone-treated patients, but in no buprenorphine-maintained patients. Of the methadone-treated patients, all of whom were receiving doses > 100 mg/day, 8 had a QT > 500 milliseconds. Similar findings were documented in a cohort of chronic pain patients (n=104) receiving methadone, 33% of whom had QT prolongation, defined as > 430 in men and > 450 in women.96
Prospective and Randomized Trials
In the largest prospective cohort study to date, Martell and colleagues97 evaluated 167 new entrants into methadone maintenance treatment. ECGs were blinded to dose and time interval. Oral methadone induction resulted in a significant increase in the mean QT interval, averaging 12.4 + 23 milliseconds at 6 months, which persisted (10.7 + 30 milliseconds) at 12 months. A similar increase in QT interval dispersion (9.5 + 18.6 milliseconds, p<0.0001), which is a marker for heterogeneous cardiac repolarization, also was observed from baseline to 6 months in the study cohort.98
Wedam and colleagues99 conducted a randomized controlled trial of 220 patients with opioid dependence to evaluate the changes in QT intervals of patients previously enrolled in a randomized, blinded study of methadone, levomethadyl, and buprenorphine. Patients’ ECG data were analyzed, and QT threshold values of 470 and 490 milliseconds were selected for men and women, respectively. Controlling for the use of other medications, hypokalemia, and renal insufficiency, the investigators found that 28% of patients in the levomethadyl group, 23% of patients in the methadone group, and none in the buprenorphine group exceeded this threshold during the 16-week study (p < 0.001). Twenty-one percent of the levomethadyl-treated patients and 12% of the methadone-treated patients had a QT interval increase from baseline of >60 milliseconds at some point in the study. This difference was significant when compared with buprenorphine (p < 0.001). There was no significant difference observed in the QT interval of patients receiving methadone versus levomethadyl when using Bazett’s formula. However, the researchers found a progressive prolongation in the QT interval of patients receiving methadone even when their dose remained stable (p = 0.01). This trend was not significant among patients treated with levomethadyl, nor was there a significant difference in interval prolongation between men and women.
Evidence of Dose-Dependent Effects
A number of studies have attempted to quantify the magnitude of the relationship between methadone dose and delayed repolarization. For example, a 2007 article by Atkinson and colleagues reported QT normalization when the methadone was discontinued or the dose reduced.71
In a series of patients with TdP, oral methadone dose was modestly correlated with the absolute QT interval recorded at the time the patient presented with arrhythmia.68 With intravenous methadone, a significant linear relationship between QT and methadone dose was noted.100 The correlation became more robust in the subset of patients who also were using cocaine (r=+0.4, p=0.03). This is consistent with a synergistic effect of combined methadone and cocaine on hERG channel blockade.101
In the Copenhagen study, Fanoe and colleagues95 reported on the effects of methadone and buprenorphine on QT interval in 450 heroin-addicted patients. The authors obtained ECGs approximately 24 hours after the last dose of methadone and found a significant association between QT interval and methadone dose in both sexes. The association existed regardless of the correction formula used (p < 0.001). There was no association between QT interval and buprenorphine dose. Overall, 32% of patients treated with methadone had a QT interval greater than 440 milliseconds. There was no significant association between QT interval and age or length of time in treatment. The investigators also asked the patients about histories of syncope over the prior year and found that patients receiving higher doses of methadone reported more episodes of syncope. Syncope also was found to be related to longer QT intervals. Limitations of the study included lack of baseline ECGs and the absence of information regarding other medications being used by the study subjects.
Peles and colleagues102 published the results of a study of 138 patients (71% male) on methadone maintenance for heroin dependence for a mean of 4.4 years (range, 0.3–10.7 years). ECGs were performed and serum methadone levels were measured for all patients approximately 24 hours after their last methadone dose. The mean ± S.D. methadone dosage was 170.9 ± 50.3 mg daily (range, 40–290 mg daily), with 80.4% of patients receiving greater than 120 mg daily. The mean ± serum methadone concentration was 708.2 ± 363.1 Ngami (range, 110–2350 Ngami). The mean ± S.D. QT interval was 418.3 ± 32.8 milliseconds (range, 330–520 milliseconds). During the month before the study, 29.7% of the patients had urine toxicology positive for opiates and 22.5% had toxicology positive for cocaine. Neither the methadone dose nor the serum methadone concentration correlated with QT interval. Of the three patients who had QT intervals greater than 500 milliseconds, two had died by the time of the two-year follow-up, though neither death was attributed to cardiac causes. None of the 19 patients with QT intervals of 450–500 milliseconds had any cardiac problems.
With regard to serum levels, Martell and colleagues prospectively demonstrated that the QT change from baseline to 12 months after initiation of methadone was significantly correlated with both trough and peak serum methadone concentrations.97 Similar relationships have been observed with the methadone derivative, LAAM.103 Taken in the aggregate, the available literature supports a dose-dependent effect of methadone and its derivative, LAAM, on cardiac repolarization. This creates a safety/efficacy paradox, given that higher doses of methadone may reduce illicit drug use, yet place patients at greater risk for TdP.104
Summary
Based on evidence published in the peer-reviewed literature,67–85 the Panel concluded that the relationship between methadone and QT prolongation is causal. Moreover, available evidence suggests that both oral and intravenous methadone hydrochloride have an independent association with QT prolongation.
Prolongation of the QT to >500 milliseconds is thought to confer significant risk with respect to arrhythmias. In all but one study of patients enrolled in methadone maintenance treatment, a QT >500 milliseconds was seen in 2% of those enrolled. Wedam and colleagues99 found a higher incidence, which they estimated at 10%. If one accepts the more conservative estimate of 2%, one would predict that an estimated 5,000 of the 250,000 subjects currently enrolled in opioid treatment programs are in need of interventions for cardiac risk reduction. An additional 40,000 to 60,000 OTP patients likely have a QT between 450 and 500 milliseconds, and may have some lesser (but nevertheless elevated) risk. Undercurrent illnesses or use of additional QT prolonging agents may substantially prolong the QT in these subjects and expose them to arrhythmia, so increased vigilance is warranted.
CONTEXT AND COnclusions
Through the steps described earlier, the Expert Panel arrived at a series of conclusions, which are summarized below. The following factors were considered by the Panel members in reaching their conclusions.
Use of methadone for the treatment of opioid addiction in the United States is confined to Federally certified OTPs, most of which are freestanding outpatient clinics. The Expert Panel discussed specifics of OTP care that could present barriers to cardiac risk assessment or lead to unintentional consequences when such guidelines are applied. Panel members agreed that any steps recommended must preserve patients’ access to care.
Most OTPs are not licensed, staffed or funded to provide cardiac screening services. Therefore, most OTPs would have to change their intake and outreach procedures to include screening for cardiac risk, which may result in the need to arrange alternative treatments or delay initiation of care. The Expert Panel supports prompt access to treatment for the disease of addiction and recommends that every effort be made to treat patients appropriately and safely.
Compared to the range of medications available to treat chronic pain, OTP physicians’ choice of therapeutic agent is limited by Federal regulations to oral methadone, naltrexone, or sublingual formulations of buprenorphine and buprenorphine/naloxone.
An optimal strategy for identifying and reducing QT-associated risk has not yet been established. Screening for risk is the current standard of care. In patients who have dose-related QT prolongation, it remains unknown how much additional risk of relapse is associated with reducing the methadone dose. However, higher methadone maintenance doses are associated with better treatment retention and outcomes.
Protocols that call for patient ECGs and review of cardiac risk may require consultation with a cardiologist or other physician, yet such services may not be reimbursable under existing contracts.
There is compelling evidence that the majority of physicians who direct treatment in OTPs are not fully aware of methadone’s association with adverse cardiac events. In a survey of medical directors of all accredited OTPs in the U.S., only 41% (95% CI, 37–45) of 692 physicians who responded were aware of methadone’s QT-prolonging properties. Only 24% (95% CI, 21–27) recognized the potential risk for TdP.10
The Panel’s conclusions are intended to assist clinicians and program administrators in developing cardiac safety standards for methadone induction and maintenance treatment in Federally certified OTPs. However, the conclusions provide only general guidance and are not intended to supplant clinical judgment in treating individual patients, nor do they represent Federal requirements or accreditation standards.
Conclusions Regarding Clinical Procedures
Expert Panel members agreed that, to the extent possible, every OTP should have a cardiac risk management plan that incorporates the following elements:
Clinical Assessment
The assessment conducted at intake should include a complete medication history; personal and family history of structural heart disease (including long QT syndrome, sudden cardiac death, myocardial infarction, heart failure); any personal history of arrhythmia and/or syncope; and use of QT-prolonging drugs, including prescribed medications and illicit drugs such as cocaine.
ECG Assessment
The Expert Panel considered whether routine ECGs to measure the QT interval should be performed on every patient within 30 days of admission. After extensive discussion and review of the evidence, the Panel members and ex officio members could not reach agreement on the merits of such a statement, largely because of concerns over the level of resources involved in implementing routine ECGs and the absence of clear evidence for the effectiveness of the practice in achieving meaningful reductions in methadone-associated cardiac events. Of those who expressed an opinion, nine were in favor of routine screening within the first 30 days, while four felt such routine screening is not necessary. (Voting in favor: Drs. Kotz, Krantz, Martin, Mehta, and Stimmel [Panel members]; Mr. Bowman and Drs. Haigney, Khan and O’Keeffe [Ex Officio members]. Voting against: Drs. Kreek, McCarroll, Payte and Taylor [Panel members].) Therefore, routine screening of every patient within 30 days of admission to treatment is not part of the consensus-driven conclusions presented here. The Panel did agree that a baseline ECG at the time of admission and within 30 days should be performed on patients with significant risk factors for QT prolongation, including a history of cardiac arrhythmia or prolonged QT interval; symptoms suggestive of arrhythmia, such as episodes of syncope, dizzy spells, palpitations or seizures; medication history; family history of premature death, or any other historical information suggestive of a possible cardiac arrhythmia. Additional ECGs should be performed annually or whenever the methadone dose exceeds 120 mg/day.
In addition to scheduled ECGs, any patient who experiences unexplained syncope or generalized seizures should have an ECG. If marked QT prolongation is documented, TdP should be suspected and the patient hospitalized for monitoring (through telemetry).
For most OTP patients, it is likely that automated (computer-generated) measurements of QT interval provide reasonable estimates of arrhythmia risk. However, if there is uncertainty about the presence of significant QT prolongation in a particular patient, it is prudent to repeat the ECG and/or to have the tracing interpreted by a cardiologist.
Risk Stratification
If the QT interval is more than 450 but less than 500 milliseconds, methadone may be initiated or continued, accompanied by a risk-benefit discussion with the patient and more frequent monitoring. For methadone-maintained patients with marked QT prolongation (>500 milliseconds), strong consideration should be given to adoption of a risk minimization strategy (such as reducing the methadone dose, eliminating other contributing factors, transitioning the patient to an alternative treatment such as buprenorphine, or discontinuing methadone treatment).
Routine echocardiography to assess for structural heart disease is not indicated in OTPs, nor is genetic testing for congenital long QT syndrome. That having been said, unexplained symptoms of syncope or seizures that emerge during therapy require urgent evaluation.
Drug Interactions
Physicians should be aware that interactions between methadone and other medications also have QT-prolonging properties, as does concurrent use of drugs that slow the elimination of methadone (see Appendix C).
Conclusions Regarding Administrative Procedures
Cardiac risk that is related to methadone should be incorporated into the informed consent document presented to patients at intake.
Conclusions Regarding Patient Education
Patients should receive educational materials that explain, in lay language, cardiac risk and its relationship to QT interval. Part of this enhanced education involves the use of a consent form that addresses cardiac concerns.
Conclusions Regarding Staff Education
OTP medical directors and clinical staff should be educated about the risks posed by prolonged QT interval and trained in assessing patients for risk of TdP and other cardiac problems.
Conclusions Regarding the Use of Methadone
The Panel affirms that methadone can be used with reasonable assurance that it is effective and that its benefits exceed its risks, providing that the potential for QT prolongation is recognized, that patients receive ECG screening at indicated intervals, and that appropriate clinical action is taken in the presence of significant QT prolongation.
Implementation Issues
The Panel acknowledges that acting on the conclusions presented here will pose challenges to many OTPs. Identifying clinically relevant QT prolongation remains difficult, given the variability of ECG machine measurements and difficulty in defining the precise risk a prolonged QT portends for any given individual. The Panel recognizes that OTPs will be challenged to integrate cardiac arrhythmia risk assessment into the care of opioid-addicted patients without reducing access to vital addiction treatment services. The Panel also is aware that not all methadone maintenance treatment providers will be capable of administering an ECG to every patient in all the circumstances suggested above.
OTPs and other providers are encouraged to consider these conclusions to the extent that they are practically and/or financially capable of doing so. Nothing in this report is intended to create a legal standard of care for any OTP, or to interfere with clinical judgment in the practice of medicine.
Acknowledgments
The authors thank Anthony Campbell, R.Ph., D.O., and Bonnie B. Wilford, M.S., for project management, Lilian Hockensmith and Mary A. Kelly, M.S.L.S., for research support, Ellen Dreyer, R.N. and Gwen Littman, M.D. for editorial support, and Adriana Padget for administrative support.
FUNDING SOURCE
The Substance Abuse and Mental Health Services Administration (SAMHSA) funded the work of the Panel. All members of the Expert Panel had access to the information, participated in its interpretation, and had an opportunity to review and modify the report.
APPENDIX A: SAMHSA Expert Panel on Cardiac Effects of Methadone
* Joined the Panel subsequent to preparation of the 2009 Field Review draft document.
PANEL CHAIR
Barry Stimmel, M.D., FASAM
Dean Emeritus of Graduate Medical Education
Mt. Sinai School of Medicine
1 Gustave L. Levy Place, Box 1076
New York, NY 10029-6500
Phone: 212-241-6694
Email: barry.stimmel@mssm.edu
PANEL MEMBERS
Thomas Killip, M.D.
Beth Israel Medical Center
Milton and Carroll Petrie Division
First Avenue at 16th Street
New York, NY 10003
Phone: 212-420-4010
Email: tkillip@bethisraelny.org, thomaskillip@aol.com
Margaret Kotz, D.O.
Director, Addiction Recovery Services and Professor of of Psychiatry,
Case Western Reserve University School of Medicine
1100 Euclid Avenue
Cleveland, Ohio 44106-1704
Phone: 216-844-1704
Email: Margaret.Kotz@UHhospitals.org
Mori J. Krantz, M.D.
Associate Professor of Medicine
Division of Cardiology
University of Colorado School of Medicine, and Director, Colorado Prevention Center
Denver Health Medical Center
777 Bannock Street, MC 0960
Denver, CO 80204
Phone: 303-436-7818
Email: MKrantz@dhha.org
* Mary Jeanne Kreek, M.D.
Professor and Head
The Laboratory of the Biology of Addictive Diseases
The Rockefeller University
1230 York Ave., Box 171
New York, NY 10065
Phone: 212-327-8490
Email: Kreek@rockefeller.edu
Judith Martin, M.D.
Medical Director
BAART Turk Street Clinic
433 Turk Street
San Francisco, CA 94102
Phone: 415-928-7800 x 212
Email: jmartin@baartprograms.com or jmrtn@earthlink.net
Brian A. McCarroll, D.O., M.S.
Medical Director
BioMed Behavioral Healthcare, Inc.
22900 Eastremick Drive
Sterling Hts, MI 48036
Phone: 586-783.4802 (office)
Email: bamccarroll@pol.net
Davendra Mehta, M.D., PhD,
Professor of Medicine, and
Director, Electrophysiology Section
Mt. Sinai School of Medicine
1 Gustave L. Levy Place
New York, NY 10029-6500
Phone: 212-241-7272
Email: davendra.mehta@mssm.edu
* J. Thomas Payte, M.D.
13501 Ranch Road 12
Suite 103, # 321
Wimberley, Texas 78676
Phone: 512-722-3618
Email: jtpayte@jtpayte.com
Trusandra Taylor, M.D., M.P.H.
Medical Director
JEVS Human Services
5820 Old York
Philadelphia, PA 19141
Phone: 215-276-8400
Email: trusandra@msn.com
EX OFFICIO MEMBERS
Paul Bowman, CMA
New England Director
National Association of Methadone Advocates
144 Quincy Shore Dr., Unit 129
North Quincy, MA 02171
Email: bostonnama@yahoo.com
* Amina Chaudhry, M.D., M.P.H.
Medical Advisor
Division of Pharmacologic Therapies
Center for Substance Abuse Treatment
1 Choke Cherry Road, Room 2-1071
Rockville, MD 20857
Phone: 240-276-2701
Email: Amina.Chaudhry@samhsa.hhs.gov
Jennifer Fan, Pharm.D., J.D.
CDR, U.S. Public Health Service
Public Health Advisor
Division of Pharmacologic Therapies
Center for Substance Abuse Treatment
1 Choke Cherry Road, Room 2-1071
Rockville, MD 20857
Phone: 240-276-1759
Email: Jennifer.Fan@samhsa.hhs.gov
Mark C. P. Haigney, M.D., FAHA
Director of Cardiology and
Professor of Medicine
Uniformed Services University of the Health Sciences (USUHS)
4301 Jones Bridge Road, A3060
Bethesda, MD 20814
Phone: 301-95-3826
Email: mhaigney@usuhs.mil
* Roberta Kahn, Ph.D.
Division of Pharmacotherapies & Medical Consequences of Drug Abuse
National Institute on Drug Abuse
6001 Executive Blvd., Room 4133
Bethesda, MD 20892
Email: rkahn@nida.nih.gov
Robert Lubran, M.S., M.P.A.
Director, Division of Pharmacologic Therapies
Center for Substance Abuse Treatment
1 Choke Cherry Road, Room 2-1071
Rockville, MD 20857
Phone: 240-276-2714
Email: Robert.Lubran@samhsa.hhs.gov
Charles O’Keeffe
Professor, Department of Epidemiology and Community Health
Virginia Commonwealth University
MCV Campus, McGuire Hall
1112 East Clay Street
Richmond, VA 23298-0524
Phone: 804-828-6246
Email: cbokeeffe@vcu.edu
Bob Rappaport, M.D.
Director, Division of Anesthesia, Analgesia, and Rheumatology Products
Food and Drug Administration
10903 New Hampshire Avenue, Bldg. 22
Silver Spring, MD 20993
Email: Bob.Rappaport@fda.hhs.gov
GUEST
* Marc N. Gourevitch, M.D.
Dr. Adolph and Margaret Berger Professor of Medicine and Professor of Psychiatry, and
Director, Division of General Internal Medicine
New York University Langone Medical Center
Old Bellevue 6, A616
550 First Ave., OBV-616
New York, NY 10016
Phone: 212-263-8553
Email: Marc.Gourevitch@nyumc.org
CSAT PROJECT OFFICER
Anthony Campbell, R.Ph., D.O.
CDR, U.S. Public Health Service
Medical Advisor
Division of Pharmacologic Therapies
Center for Substance Abuse Treatment
1 Choke Cherry Road, Room 2-1071
Rockville, MD 20857
Phone: 240-276-2702
Email: Anthony.Campbell@samhsa.hhs.gov
JBS PROJECT DIRECTOR
Bonnie B. Wilford, M.S.
Project Director and Senior Principal
JBS International, Inc.
210 Marlboro Ave., Suite 31, PMB 187
Easton, MD 21601
Phone: 410-770-4866
Email: BBWilford@aol.com
APPENDIX B: Relevant Advice from Private-Sector Organizations and Government Agencies
American Association for the Treatment of Opioid Dependence (AATOD; United States)
AATOD’s guidelines on cardiac risk, issued in 2009, include the following general and specific steps:
OTPs should develop comprehensive cardiac arrhythmia risk management plans that specify the threshold and frequency for ECG screening and monitoring.
A personal medical history of long QT syndrome, cardiac conduction defects, arrhythmias, syncope episodes, seizures, palpitations, dizziness and lightheadedness, and family history of long QT syndrome, cardiac conduction defects, arrhythmias, syncope episodes, seizures and sudden or unexpected death should be part of a medical assessment prior to admission to an OTP.
Electrolytes – in particular hypokalemia, hypomagnesaemia and patients on medications that can induce these conditions (diuretics and laxatives) – should be included in the medical assessment conducted at admission.
Patient records should note any history of clinically significant bradycardia or cardiac disease (such as CHF and reduced ventricular function).
All prescribed medications should be reviewed prior to induction onto methadone treatment. Particular attention should be given to medications that are substrates of CYP3A4 or CYP3D26 and those that block HERG channel currents, as well as over-the-counter agents, herbal preparations, and dietary supplements.
Toxicology screens should be reviewed for the presence of illicit drugs, particularly those that add to cardiac risk such as cocaine and amphetamines.
Medically fragile patients (including the elderly; patients with advanced heart, liver or kidney disease; patients with advanced HIV/AIDs; patients who are taking opioid analgesics for chronic pain; and patients with a history of poor, extensive or rapid metabolism of methadone) should be closely monitored.
Patient consent to methadone treatment should include information about the cardiac risk associated with continued use of illicit drugs, particularly drugs diluted with quinine.
Physicians who work in OTPs (as well as those in pain management) should be educated about the risk of QTc/TdP in methadone-maintained patients.105
College of Physicians and Surgeons of Ontario (CPSO; Canada)
In its Methadone Maintenance Guideline,18 the CPSO suggests that an ECG be performed when the methadone dose exceeds 150 mg/day. It recommends a repeat ECG when the dose approaches 180–200 mg.
The Canadian guideline further proposes tapering the methadone dose and referring the patient to a cardiologist if the QTc interval exceeds 470 milliseconds.
Food and Drug Administration (FDA; United States)
The issues described in this communication have been addressed in product labeling. FDA ALERT [11/2006]: Death, Narcotic Overdose, and Serious Cardiac Arrhythmias “FDA has reviewed reports of death and life-threatening adverse events such as respiratory depression and cardiac arrhythmias in patients receiving methadone. These adverse events are the possible result of unintentional methadone overdoses, drug interactions, and methadone’s cardiac toxicities (QT prolongation and Torsades de Pointes). Physicians prescribing methadone should be familiar with methadone’s toxicities and unique pharmacologic properties. Methadone’s elimination half-life (8–59 hours) is longer than its duration of analgesic action (4–8 hours). Methadone doses for pain should be carefully selected and slowly titrated to analgesic effect even in patients who are opioid-tolerant. Physicians should closely monitor patients when converting them from other opioids and changing the methadone dose, and thoroughly instruct patients how to take methadone. Healthcare professionals should tell patients to take no more methadone than has been prescribed without first talking to their physician.”
Medicines and Healthcare Products Regulatory Agency (MHRA; United Kingdom)
MHRA recommends monitoring patients on methadone doses greater than 100 mg per day. However, the MHRA recommendation does not define the monitoring approach to be used.106 The UK guideline on clinical management of drug abuse and addiction incorporates the MHRA approach and suggests that patients be informed of the reasons cardiac monitoring is recommended,107 as follows:
“A2.1 Drug-induced prolongation of the QT interval. The QT interval is measured on an ECG* from the beginning of the QRS complex (caused by contraction of the ventricular mass) until the end of the T wave (caused by the return of the ventricular mass to the resting state). The QT corrected (QTd) interval is the QT interval (in milliseconds) corrected for heart rate using a standard formula (for example, Bazett’s formula: QTc (ms) = QT (ms) / RR½ – QT divided by the square root of the R-R interval). QTc calculators are available on the internet.
“The QTc interval is a useful indicator of risk of polymorphic ventricular tachycardias, or torsade de pointes which can be fatal. QTc interval prolongation beyond normal limits (440 ms for men and 470 ms for women) is associated with increased risk of cardiac arrhythmias and sudden death, especially above 500 ms.108
“Various psychotropic medications have recently been identified as causing QT prolongation and sudden death. In the past decade this has become the most common reason for a drug to be withdrawn from the market. In the drug treatment field, this was the reason for levacetylmethadol (LAAM or ORLAAM) being withdrawn.”12
APPENDIX C: Drugs that May Interact with Methadone to Elevate Cardiac Risk
A limited number of clinical studies have investigated interactions between methadone and specific drugs; therefore, some interactions are predicted based on lower levels of evidence, such as case reports, laboratory experiments, or pharmacologic principles. The various levels of evidence are denoted in the table as follows:
Level 1: Interaction demonstrated via published clinical studies and/or by the well-established and specific pharmacology of methadone metabolism.
Level 2: Based on published clinical case series reports and/or laboratory investigations in animals or tissues (in vitro).
Level 3: Proposed in the literature, but predicted from general pharmacologic principles and/or sporadic anecdotal cases.
| Generic Name | TRADE NAME (EXAMPLES) | MECHANISM OF ACTION | LEVEL OF EVIDENCE |
|---|---|---|---|
| amiodarone | Cordarone, Nexterone, Amiadarone HCl injection | Proposed due to CYP450 inhibition. | Level 3 |
| amitriptyline | Elavil | Possible increased TCA toxicity; uncertain effect on methadone. | Level 2 |
| clarithromycin | Biaxin | Strong CYP3A4 inhibition. | Level 3 |
| cocaine and crack | — | Methadone elimination accelerated | Level 2 |
| desipramine | Norpramin | Possible increased TCA toxicity; uncertain effect on methadone. | Level 2 |
| doxepin | Sinequan, Prudoxin, Zonalon | Possible increased TCA toxicity; uncertain effect on methadone. | Level 2 |
| erythromycin | Benzamycin, EES, Emgel, Erythrocin, Ilosone, Ilotycin | Strong CYP3A4 inhibition. | Level 3 |
| fluoxetine | Prozac, Serafem | Variable CYP450 enzyme inhibition. | Level 2 |
| imipramine | Tofranil | Possible increased TCA toxicity; uncertain effect on methadone. | Level 2 |
| ketoconazole | Extina, Nizoral, Xolegel, | Predicted due to CYP3A4 inhibition. | Level 3 |
| nortriptyline | Pamelor | Possible increased TCA toxicity; uncertain effect on methadone | Level 2 |
| paroxetine | Asimia, Paxil | Variable CYP450 enzyme inhibition. | Level 2 |
| lansoprazole, amoxicillin and clarithromycin | Prevpac | CYP3A4 inhibition (contains clarithromycin). | Level 3 |
| protriptyline | Vivactil | Possible increased TCA toxicity; uncertain effect on methadone | Level 2 |
| sertraline | Zoloft | Variable CYP450 enzyme inhibition. | Level 2 |
| tricyclic antidepressants | Aventyl | Possible increased TCA toxicity; uncertain effect on methadone. | Level 2 |
SOURCES: Adapted from Leavitt SR, Toombs JD & Kral L. Methadone-drug interactions. Pain Treatment Topics Jan. 20, 2006; Table 5; and Leavitt SR, Bruce RD, Eap CB, Kharasch E, Kral L, McCance-Katz E & Payte JT. Methadone-drug interactions. 3rd ed. Pain Treatment Topics Nov. 2005, pp. 18–24.
Appendix D: Summary of Major Studies* of Methadone and Prolonged QT Syndrome
| AUTHORS | JOURNAL | TYPE OF STUDY |
NUMBER | STUDY POPULATION (MMTP OR PAIN) |
OTHER MEDICATIONS EVALUATED OR PRESENT |
OTHER DRUGS OF ABUSE EVALUATED |
OTHER MEDICAL CONDITIONS EVALUATED |
MAJOR FINDINGS | NOTES, INCLUDING LIMITATIONS |
|---|---|---|---|---|---|---|---|---|---|
| Chugh et al., 2008 | American Journal of Medicine | 22 cases compared with 106 controls | All deaths in Portland metropolitan
area over 4 years with ‘therapeutic’ levels of
methadone present, compared with non-methadone
cases. 55% were taking methadone for pain control. |
Yes | Persons with evidence of methadone overdose or other recreational drug use excluded | Structural heart disease only | Among methadone cases, evidence of structural heart disease in 23%, compared with 60% of controls | There is no single cut-off level at which methadone becomes toxic for all persons. | |
| Cruciani et al., 2005 | Journal of Pain and Symptom Management | Cross-sectional | 104 total | Both MMTP and pain | Yes (not included in a multivariate analysis) | No | Excluded with congenital long QT
syndrome, implanted pacemaker, atrial fibrillation, or wide QRS
complex on prior ECG Serum electrolytes measured |
33% had QTc prolongation
(defined as QTc>430 ms for males and 450 ms for
females) No patient had QTc>500ms Significant dose response was seen in men on methadone <12 months |
No control group Small, exploratory study |
| Ehret et al., 2006 | Archives of Internal Medicine | Case-control | 167 methadone cases 80 control |
Hospitalized IDU on methadone versus control IDU not on methadone | Yes, but not specified | Yes | HIV Hepatitis C Hepatitis B Structural heat disease | 16.2% of methadone patients
had QTC ≥ 500ms compared to 0% of
controls. 3.6% of those in the methadone group had TdP. QTc was weakly but significantly correlated with methadone dose. In a multivariate regression analysis, QT prolongation was associated with higher methadone dose, lower potassium, lower prothrombin time, and co-medication with CYP3A4 inhibition. |
No formal assessment of structural
heart disease Other substance use was self-reported |
| Fanoe et al., 2007 | Heart | Cross-sectional | 450 (52%) of treatment population in Copenhagen | OTP (methadone versus buprenorphine) | No | Yes Cocaine, cannabis, illicit opioids, illicit benzodiazepines (but not included in the multivariate analysis) | Persons with atrial fibrillation or
flutter, bundle branch block, bigeminy, or pacemaker were
excluded -serum potassium was checked |
Methadone dose was significantly
correlated with QTc for both men and women. No association was found between buprenorphine dose and QT interval. In a multivariate analysis, methadone dose was associated with prolonged QT interval, and serum potassium was negatively associated with prolonged QT. Odds of self-reported syncope also were 1.2 times higher when methadone dose was increased by 50mg. |
Buprenorphine group was younger and had shorter duration of therapy |
| Kornick et al., 2003 | International Association for the Study of Pain | Prospective (chart review) over 20 months | N=47 (iv
methadone) N=35 (morphine) |
Cancer pain | Unknown if other medications were
present. According to article, information was
collected. Morphine. |
Morphine | None | Methadone in combination with chlorobutanol is associated with QT interval prolongation. | IV methadone, which contains chlorobutanol, was evaluated. Chlorobutanol alone can affect QT interval. Small sample size. |
| Krantz et al., 2003 | Pharmaco-therapy | Retrospective case series analysis | N=17 | 9 patients were receiving methadone for opioid dependency (6 were from Colorado); 8 patients were receiving methadone for chronic pain from one center. All patients had been hospitalized from 1996–2001 with torsades de pointes. | Olanzapine, fluoxetine, levacetylmethadol, nelfinavir, amitriptyline | Cocaine, alcohol | Structural heart disease | A relationship was found between daily methadone dose and the QT interval. | Small study population, retrospective study design, and inherent selection bias. |
| Maremmani et al., 2005 | European Addiction Research | Cross-sectional | 83 | MMTP program in Italy. In MMTP for at least 6 months Stable dose for at least 4 months |
No other medications known to prolong
QT on board Reported all had normal electrolytes and nutritional status (values not provided) |
At time of ECG, all negative for
morphine, cocaine metabolites, and amphetamines All self reported “habitual” alcohol use (not defined) |
Not reported Reported all had normal electrolytes and nutritional status (values not provided) |
83.1% had QT more prolonged
than age and sex matched controls Only 2 subjects (2.4%) had QT>500ms No relationship observed between QT and methadone dose |
Small sample size No data on plasma levels |
| Martell et al., 2005 | American Journal of Cardiology | Prospective | 160 | MMTP | Antidepressants; Ca+ Channel Blockers; Antiretrovirals; Diuretics; Phenytoin | Cocaine; ETOH; tobacco | Hepatitis C; HIV infection | Positive correlation between serum Methadone conc. and magnitude of QT prolongation | Effect of medical conditions or prescription medications on QT prolongation could not be totally excluded |
| Peles et al., 2006 | Society for the Study of Addiction | Prospective. Patients’ medical charts were also reviewed retrospective-ly for prescribed medications in the period before the study was performed. Cross sectional. |
N=138 | MMT (must be in treatment for at least 100 days in addition to being on steady methadone doses for at least 14 days). Physicians encouraged patients receiving high methadone doses (over 120 mg/day) to participate.) | Benzodiazepines, opiates, amphetamines. Other medications present: ursodeoxycholic acid, spironolactone, colchicine, salbutamol, theotrime, ipratropium bromide, trazodone, metformin, thyroxin sodium, clonazepam, amoxicillin, fluoxetine, diazepam, fluvoxamine, insulin, penfluridol, enoxaparin, escitalopram, amitriptyline, melatonin, haloperidol, biperiden, propanolol, aspirin, mirtazapime sodium, valproate, acetylsalicylic acid, simvastatin, ramipril, isosorbide. | Benzodiaze-pines, opiates, cocaine, cannabis, amphetamines. | HCV, HIV | No correlation between QT interval and methadone doses and serum levels; however, significant correlation between methadone dose and QT interval were found in patients who were urine positive for cocaine. | Even though the study was cross-sectional, a greater proportion of “high-dose” patients (120 mg/day) were included, limiting the generalizability of the finding. Study population limited to one program in Tel Aviv, Israel; not multi-center. No baseline ECGs. |
| Wedam et al., 2007 | Archives of Internal Medicine | Randomized controlled trial | 220 | MMTP | N/A | Alcohol; heroin; cocaine | N/A | Compared to levomethadyl and methadone, buprenorphine is associated with less QT prolongation | Absence of placebo arm to assess random incidence of QT prolongation |
*Excludes case studies and case series.
Footnotes
DISCLOSURE OF FINANCIAL INTERESTS
Consistent with requirements of the Accreditation Council on Continuing Medical Education (ACCME), members of the Expert Panel and project staff were asked to report any relevant financial relationships. The following Panel members, ex officio members, guests, and staff reported no known or potential conflicts of interest: Mr. Bowman, Dr. Campbell, Dr. Fan, Dr. Haigney, Dr. Kahn, Dr. Killip, Dr. Kreek, Mr. Lubran, Dr. Martin, Dr. Payte, Dr. Rappaport, Dr. Stimmel, Dr. Taylor, and Mrs. Wilford.
Dr. Gourevitch reported research support from Alkermes, Inc. Dr. Kotz reported honoraria paid to her spouse by Abbott Laboratories and Forrest Laboratories. Dr. Krantz reported conference support from Reckitt-Benckiser, Inc. Dr. McCarroll reported honoraria from Reckitt-Benckiser, Inc. Dr. Mehta reported honoraria from Medtronic and Boston Scientific. Dr. O’Keeffe reported consulting fees from Catalyst Pharmaceutical Partners, Inc. and Reckitt-Benckiser, Inc., and an ownership interest in Kingston Pharmaceuticals, Inc.
DISCLAIMER
The views, opinions, and content of this document are those of the Expert Panel members and other referenced sources and do not necessarily reflect the views, opinions, or policies of the Substance Abuse and Mental Health Services Administration (SAMHSA) or any other part of the U.S. Department of Health and Human Services (DHHS).
The conclusions of the Panel have not been adopted by SAMHSA as either Treatment Improvement Protocols (TIPs) or certification standards. Accordingly, the contents of this report do not constitute Federal guidelines.
NOTE
This report is intended to enhance patient care, but it does not supplant clinical judgment in the care of individual patients. Moreover, the advice given here may not apply to all patients or clinical situations.
References
- 1.Center for Substance Abuse Treatment (CSAT) Methadone-Associated Mortality: Report of a National Assessment, May 2003. Rockville, MD: CSAT, Substance Abuse and Mental Health Services Administration; 2004. [Accessed at http://www.csdp.org/research/methadone.samhsa204.pdf on February 16, 2011.] [Google Scholar]
- 2.Center for Substance Abuse Treatment (CSAT) Methadone Mortality: A Reassessment – Report of the Meeting and Follow-up Activities, July 2007. Rockville, MD: CSAT, Substance Abuse and Mental Health Services Administration; 2007. pp. 32–36. [Accessed at http://www.dpt.samhsa.gov/pdf/Methadone_Report_10%2018%/2007_Brief%20w%20attch.pdf on February 16, 2011.] [Google Scholar]
- 3.Food and Drug Administration (FDA) Public Health Advisory: Methadone Use for Pain Control May Result in Death and Life-Threatening Changes in Breathing and Heart Beat. 2006 Nov 27; [Accessed at http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafety/SafetyInformationforPatientsandProviders/DrugSafetyInformationforHealthcareProfessionals/PublicHealthAdvisories/ucm/24346.htm on February 16, 2011.]
- 4.Roxane Laboratories. Dolophine Hydrochloride CII (Methadone Hydrochloride Tablets, USP) Columbus OH 43216: [Accessed at http://www.accessdata.fda.gov/drugsatfda_docs/label/2006/006134s0281lbl.pdf on February 16, 2011.] [Google Scholar]
- 5.Thompson Reuters MICROMEDEX®. [Accessed at www.thomsonhc.com. February 22, 2011.]
- 6.Center for Education and Research on Therapeutics (CERT) Drugs with risk of Torsades de Pointes. The University of Arizona; [Accessed at http://www.azcert.org/medical-pros/drug-lists/list-01.cfm, on February 16, 2011.] [Google Scholar]
- 7.Ancherson K, Clausen T, Gossop M, et al. Prevalence and clinical relevance of corrected QT interval prolongation during methadone and buprenorphine treatment: A mortality assessment study. Addiction. 2009;104(6):993–99. doi: 10.1111/j.1360-0443.2009.02549.x. [DOI] [PubMed] [Google Scholar]
- 8.Wallner C, Stollberger C, Lhavin A, et al. Electrocardiographic abnormalities in opiate addicts. Addiction. 2008 Dec;103(12):1994. doi: 10.1111/j.1360-0443.2008.02333.x. [DOI] [PubMed] [Google Scholar]
- 9.George S. Methadone-associated QT prolongation and Torsades de Pointes. Br J Hosp Med (London) 2007;68:24. doi: 10.12968/hmed.2007.68.4.221. [DOI] [PubMed] [Google Scholar]
- 10.Krantz MJ, Rowan S, Schmittner J, et al. Physician awareness of methadone’s cardiac effects: A national survey. J Addict Dis. 2007;26:79–85. doi: 10.1300/J069v26n04_10. [DOI] [PubMed] [Google Scholar]
- 11.Athanasos P, Farquharson AL, Compton P, Psaltis P, Hay J. Electrocardiogram characteristics of methadone and buprenorphine maintained subjects. J Addict Dis. 2008;27(3):31–35. doi: 10.1080/10550880802122596. [DOI] [PubMed] [Google Scholar]
- 12.Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney MCP. QTc Interval Screening in Methadone Treatment. Ann Intern Med. 2009;150:387–395. doi: 10.7326/0003-4819-150-6-200903170-00103. [DOI] [PubMed] [Google Scholar]
- 13.Food and Drug Administration (FDA) MedWatch Safety Information and Adverse Event Reporting Program, Safety Information: ORLAAM (levomethadyl hydrochloride acetate) withdrawn from market March 2001. [Information updated June 2009; Accessed at http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/UCM/53332.htm on February 16, 2011.]
- 14.Hodgson Russ Associates. Personal communication. Jun 14, 2009.
- 15.Pullum T. Personal communication. Department of Statistics, University of Texas at Austin; Jun 15, 2009. [Google Scholar]
- 16.Inturrisi CE. Pharmacology of methadone and its isomers. Anesthesiology. 2005;71(7–8):435–437. [PubMed] [Google Scholar]
- 17.Anderson IB, Kearney TE. Use of methadone. West J Med. 2000;172(1):43–46. doi: 10.1136/ewjm.172.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.College of Physicians of Ontario (CPSO) Methadone Maintenance Guidelines. Toronto, Ontario: The College; Nov, 2004. [Google Scholar]
- 19.Fishman SM, Wilsey B, Mahajan G, et al. Methadone reincarnated: Novel clinical applications with related concerns (review) Pain Med. 2002;3(4):339–348. doi: 10.1046/j.1526-4637.2002.02047.x. [DOI] [PubMed] [Google Scholar]
- 20.Anggaard E, Gunne L-M, Holmstrand J, et al. Disposition of methadone in methadone maintenance. Clin Pharmacol Ther. 1974;17:258–266. doi: 10.1002/cpt1975173258. [DOI] [PubMed] [Google Scholar]
- 21.Dole VP, Kreek MJ. Methadone plasma level: Sustained by a reservoir of drug in tissue. Proc Natl Acad of Sci U S A. 1973;70:10. doi: 10.1073/pnas.70.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inturrisi CE, Verebely K. The levels of methadone in the plasma in methadone maintenance. Clin Pharmacol and Ther. 1972;13:633–637. doi: 10.1002/cpt1972135part1633. [DOI] [PubMed] [Google Scholar]
- 23.Kling MA, Carson RE, Borg L, et al. Opioid receptor imaging with positron emission tomography and [18F]cyclofoxyl in long-term, methadone-treated former heroin addicts. J Pharmacol Exper Ther. 2000;295:1070–1076. [PubMed] [Google Scholar]
- 24.Kreek MJ. Plasma and urine levels of methadone. N Y State J Med. 1973;73:2773–2777. [PubMed] [Google Scholar]
- 25.Peng PW, Tumber PS, Gourlay D. Review article: Perioperative pain management of patients on methadone therapy. Can J Anaesth. 2005;52(5):513–523. doi: 10.1007/BF03016532. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan HR, Smits SE, Due SL, et al. Metabolism of d-methadone: Isolation and identification of analgesically active metabolites. Life Sciences. 1972;11:1093–1104. [Google Scholar]
- 27.Committee for Proprietary Medicinal Products (CPMP) Points to consider: The assessment of the potential for QT interval prolongation by non-cardiovascular medicinal products. The European Agency for the Evaluation of Medicinal Products. 1997 Dec; [CPMP/986/96] [Google Scholar]
- 28.Al-Khatib SM, Allen LaPointe NM, Kramer JM, et al. What clinicians should know about the QT interval. JAMA. 2003;289:2120–27. doi: 10.1001/jama.289.16.2120. [DOI] [PubMed] [Google Scholar]
- 29.Panicker GK, Karnad DR, Joshi R, et al. Comparison of QT measurement by tangent method and threshold method. Ind Heart J. 2006;58:487–88. [Google Scholar]
- 30.Bazett HC. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–70. [Google Scholar]
- 31.Food and Drug Administration (FDA) Center for Drug Evaluation and Research, Food and Drug Administration. Rockville, Maryland: FDA, Department of Health and Human Services; 2005. Guidance for Industry: E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. [Google Scholar]
- 32.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–53. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 33.Merri M, Benhorin J, Alberti M, et al. Electrocardiographic quantitation of ventricular repolarization. Circulation. 1989;80:1301–08. doi: 10.1161/01.cir.80.5.1301. [DOI] [PubMed] [Google Scholar]
- 34.Stringer J, Welsh C, Tommasello A. Methadone-associated QT interval prolongation and torsades de pointes. Am J Health Syst Pharm. 2009 May 1;66(9):825–33. doi: 10.2146/ajhp070392. [DOI] [PubMed] [Google Scholar]
- 35.Fenichel RR, Malik M, Antzelevitch C, et al. Drug-induced torsades de pointes and implications for drug development. J Cardiovasc Electrophysiol. 2004;15:475–95. doi: 10.1046/j.1540-8167.2004.03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–69. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 37.Montanez A, Ruskin JN, Hebert PR, et al. Prolonged QTc interval and risks of total and cardiovascular mortality and sudden death in the general population: a review and qualitative overview of the prospective cohort studies. Arch Intern Med. 2004;164:943–48. doi: 10.1001/archinte.164.9.943. [Erratum, Arch Intern Med. 2004; 164:1796.] [DOI] [PubMed] [Google Scholar]
- 38.Yoshida H, Sugiyama A, Satoh Y, et al. Comparison of the in vivo electrophysiological and proarrhythmic effects of amiodarone with those of a selective class III drug, sematilide, using a canine chronic atrioventricular block model. Circ J. 2002;66:758–62. doi: 10.1253/circj.66.758. [DOI] [PubMed] [Google Scholar]
- 39.Redfern WS, Carlsson L, Davis AS, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and Torsades de Pointes for a broad range of drugs: Evidence for a provisional safety margin in drug development. Cardiovas Res. 2003;58(1):32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- 40.Roden DM. Long QT syndrome: reduced repolarization reserve and the genetic link. J Intern Med. 2006;259:59–69. doi: 10.1111/j.1365-2796.2005.01589.x. [DOI] [PubMed] [Google Scholar]
- 41.Soyka LF, Wirtz C, Spangenberg RB. Clinical safety profile of sotalol in patients with arrhythmias. Am J Cardiol. 1990;65(Suppl):74A–81A. doi: 10.1016/0002-9149(90)90207-h. [DOI] [PubMed] [Google Scholar]
- 42.Brendorp B, Elming H, Jun L, et al. for the DIAMOND Study Group QT interval as a guide to select those patients with congestive heart failure and reduced left ventricular systolic function who will benefit from antiarrhythmic treatment with dofetilide. Circulation. 2001;103:1422–27. doi: 10.1161/01.cir.103.10.1422. [DOI] [PubMed] [Google Scholar]
- 43.Kurita T, Ohe T, Shimizu W, et al. Early after depolarization in a patient with complete atrioventricular block and Torsades de Pointes. Pacing Clin Electrophysiol. 1993;16:33–38. doi: 10.1111/j.1540-8159.1993.tb01532.x. [DOI] [PubMed] [Google Scholar]
- 44.Wesley RC, Jr, Turnquest P. Torsades de Pointes after intravenous adenosine in the presence of prolonged QT-syndrome. Am Heart J. 1992;123:794–96. doi: 10.1016/0002-8703(92)90525-z. [DOI] [PubMed] [Google Scholar]
- 45.Ebert SN, Liu XK, Woosley RL. Female gender as a risk factor for drug-induced cardiac arrhythmias: Evaluation of clinical and experimental evidence. J Womens Health. 1998;7:547–57. doi: 10.1089/jwh.1998.7.547. [DOI] [PubMed] [Google Scholar]
- 46.Garson A., Jr How to measure the QT interval – What is normal? Am J Cardiol. 1993;72:14B–16B. doi: 10.1016/0002-9149(93)90034-a. [DOI] [PubMed] [Google Scholar]
- 47.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–22. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 48.Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet. 2002;41:1153–93. doi: 10.2165/00003088-200241140-00003. [DOI] [PubMed] [Google Scholar]
- 49.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of hepatic and intestinal cytochrome P450 3A and 2B6 in the metabolism, disposition, and miotic effects of methadone. Clin Pharmacol Ther. 2004;76:250–69. doi: 10.1016/j.clpt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Gerber JG, Rhodes RJ, Gal J. Stereoselective metabolism of methadone N-demethylation by cytochrome P450 2B6 and 2C19. Chirality. 2004;16:36–44. doi: 10.1002/chir.10303. [DOI] [PubMed] [Google Scholar]
- 51.Crettol S, Deglon JJ, Besson J, et al. Methadone enantiomer plasma levels, CYP2B6, CYP2C19, and CYP2C9 genotypes, and response to treatment. Clin Pharmacol Ther. 2005;78:593–604. doi: 10.1016/j.clpt.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Crettol S, Deglon JJ, Besson J, et al. ABCB1 and cytochrome P450 genotypes and phenotypes: influence on methadone plasma levels and response to treatment. Clin Pharmacol Ther. 2006;80:668–81. doi: 10.1016/j.clpt.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 53.Eap CB, Crettol S, Rougier JS, et al. Stereoselective block of hERG channel by (S)-methadone and QT interval prolongation in CYP2B6 slow metabolizers. Clin Pharmacol Ther. 2007;81:719–28. doi: 10.1038/sj.clpt.6100120. [DOI] [PubMed] [Google Scholar]
- 54.Bednar MM, Harrigan EP, Ruskin JN. Torsades de Pointes associated with nonantiarrhythmic drugs and observations on gender and QT. Am J Cardiol. 2002;89:1316–19. doi: 10.1016/s0002-9149(02)02337-8. [DOI] [PubMed] [Google Scholar]
- 55.Brink PA, Crotti L, Corfield V, et al. Phenotypic variability and unusual clinical severity of congenital long-QT syndrome in a founder population. Circulation. 2005;112:2602–10. doi: 10.1161/CIRCULATIONAHA.105.572453. [DOI] [PubMed] [Google Scholar]
- 56.Ehret GB, Voide C, Gex-Fabry M, et al. Drug-induced long QT syndrome in injection drug users receiving methadone: High frequency in hospitalized patients and risk factors. Arch Intern Med. 2006;166:1280–87. doi: 10.1001/archinte.166.12.1280. [DOI] [PubMed] [Google Scholar]
- 57.DePonti F, Poluzzi E, Cavalli A, et al. Safety of non-antiarrhythmic drugs that prolong the QT interval or induce Torsades de Pointes: An overview. Drug Saf. 2002;25:263–286. doi: 10.2165/00002018-200225040-00004. [DOI] [PubMed] [Google Scholar]
- 58.Katchman AN, McGroary KA, Kilborn MJ, et al. Influence op opioid agonists on cardiac human ether-a-go-go-related gene K(+) currents. J Pharmacol Exper Ther. 2002;303:688–694. doi: 10.1124/jpet.102.038240. [DOI] [PubMed] [Google Scholar]
- 59.Ackerman MJ, Tester DJ, Jones GS, et al. Ethnic differences in cardiac potassium channel variants: Implications for genetic susceptibility to sudden cardiac death and genetic testing for congenital long QT syndrome. Mayo Clin Proceedings. 2003;78:1479–87. doi: 10.4065/78.12.1479. [DOI] [PubMed] [Google Scholar]
- 60.De Bels D, Staroukine M, Devriendt J. Torsades de Pointes due to methadone. Ann Intern Med. 2003;139:E156. doi: 10.7326/0003-4819-139-2-200307150-00021-w2. [DOI] [PubMed] [Google Scholar]
- 61.Seyler DE, Borowitz JL, Maickel RP. Calcium channel blockade by certain opioids. Fundam Appl Toxicol. 1983;3:536–42. doi: 10.1016/s0272-0590(83)80101-8. [DOI] [PubMed] [Google Scholar]
- 62.Lee CH, Berkowitz BA. Calcium antagonist activity of methadone, l-acetylmethadol, and l-pentazocine in the rat aortic strip. J Pharmacol Exp Ther. 1977;202(3):646–53. [PubMed] [Google Scholar]
- 63.Rendig SV, Amsterdam EA, Henderson GL, Mason DT. Comparative cardiac contractilities of six narcotic analgesics: morphine, meperidine, pentazocine, fentanyl, methadone, and l-alpha-acetylmethadol (LAAM) J Pharmacol Exp Ther. 1980;215(1):259–65. [PubMed] [Google Scholar]
- 64.Eikenburg DC, Stickney JL. Anti-cholinesterase activity of 1-alpha-acetylmethadol: relationship to bradycardia. Gen Pharmacol. 1979;10(3):195–200. doi: 10.1016/0306-3623(79)90089-2. [DOI] [PubMed] [Google Scholar]
- 65.Stickney JL. Cardiac effects of 1-alpha-acetylmethadol. 1. Chronotropic effects in vitro. Toxicol Appl Pharmacol. 1977 Apr;40(1):23–32. doi: 10.1016/0041-008x(77)90112-0. [DOI] [PubMed] [Google Scholar]
- 66.Krantz MJ, Lewkowiez L, Hays H, et al. Torsades de Pointes associated with very high-dose methadone. Ann Intern Med. 2002;137:501–04. doi: 10.7326/0003-4819-137-6-200209170-00010. [DOI] [PubMed] [Google Scholar]
- 67.Lipski J, Stimmel B, Donoso E. The effect of heroin and multiple drug abuse on the electrocardiogram. Am Heart J. 1973;86:663–68. doi: 10.1016/0002-8703(73)90344-x. [DOI] [PubMed] [Google Scholar]
- 68.Krantz MJ, Kutinsky IB, Robertson AD, et al. Dose-related effects of methadone on QT prolongation in a series of patients with Torsades de Pointes. Pharmacotherapy. 2003;23:802–05. doi: 10.1592/phco.23.6.802.32186. [DOI] [PubMed] [Google Scholar]
- 69.Pimentel L, Mayo D. Chronic methadone therapy complicated by Torsades de Pointes: A case report. J Emerg Med. 2008;34:287–90. doi: 10.1016/j.jemermed.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 70.Luthi B, Huttner A, Speck RF, et al. Methadone-induced Torsades de Pointes after stopping lopinavir-ritonavir. Eur J Clin Microbiol Infect Dis. 2007;26:367–69. doi: 10.1007/s10096-007-0293-5. [DOI] [PubMed] [Google Scholar]
- 71.Atkinson D, Dunne A, Parker M. Torsades de pointes and self-terminating ventricular fibrillation in a prescription methadone user. Anaesthesia. 2007 Sep;62(9):952–55. doi: 10.1111/j.1365-2044.2007.05129.x. [DOI] [PubMed] [Google Scholar]
- 72.Wong SC, Roberts JR. Case files of the Drexel University Medical Toxicology Fellowship: Methadone-induced QTc prolongation. J Med Toxicol. 2007;4:190–94. doi: 10.1007/BF03160939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Routhier DD, Katz KD, Brooks DE. QTc prolongation and Torsades de Pointes associated with methadone therapy. J Emerg Med. 2007;32:275–78. doi: 10.1016/j.jemermed.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 74.Iskandar SB, Abi-Saleh BS, Mechleb BK, et al. Methadone and Torsades de Pointes: Case report and review of the literature. Tenn Med. 2007;100:35–7. [PubMed] [Google Scholar]
- 75.Falconer M, Molloy D, Ingerhaug J, et al. Methadone induced Torsades de Pointes in a patient receiving antiretroviral therapy. Ir Med J. 2007;100:631–32. [PubMed] [Google Scholar]
- 76.Lamont P, Hunt SC. A twist on Torsades: A prolonged QT interval on methadone. J Gen Intern Med. 2006;21:C9–C12. doi: 10.1111/j.1525-1497.2006.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Osticherling C, Schaer B, Ammann P, et al. Methadone-induced cases of Torsades de Pointes tachycardias. Swiss Med Wkly. 2005;135:282–85. doi: 10.4414/smw.2005.10939. [DOI] [PubMed] [Google Scholar]
- 78.Krantz MJ, Garcia JA, Mehler PS. Effects of buprenorphine on cardiac repolarization in a patient with methadone-related Torsades de Pointes. Pharmacotherapy. 2005;25:611–14. doi: 10.1592/phco.25.4.611.61020. [DOI] [PubMed] [Google Scholar]
- 79.Ower K, Morley-Forsler P, Moulin D. Fluctuating QTc interval in an asymptomatic patient treated with methadone for chronic pain. J Opioid Manag. 2005;1:73–6. doi: 10.5055/jom.2005.0019. [DOI] [PubMed] [Google Scholar]
- 80.Porter BO, Coyne PJ, Smith WR. Methadone-related Torsades de Pointes in a sickle cell patient treated for chronic pain. Am J Hematol. 2005;78:316–17. doi: 10.1002/ajh.20304. [DOI] [PubMed] [Google Scholar]
- 81.Rademacher S, Dietz R, Haverkamp W. QT prolongation and syncope with methadone, doxepin, and a beta-blocker. Ann Pharmacother. 2005;39:1762–3. doi: 10.1345/aph.1G277. [DOI] [PubMed] [Google Scholar]
- 82.Almehmi A, Malas AM, Yousufuddin M, et al. Methadone-induced Torsades de Pointes in a patient with normal baseline QT interval. W V Med J. 2004;100:147–48. [PubMed] [Google Scholar]
- 83.Decerf JA, Gressens B, Brohet C, et al. Can methadone prolong the QT interval? Intensive Care Med. 2004;8:1690–1. doi: 10.1007/s00134-004-2335-0. [DOI] [PubMed] [Google Scholar]
- 84.Piguet V, Desmeules J, Ehret G, et al. QT interval prolongation in patients on methadone with concomitant drugs. J Clin Psychopharmacol. 2004;24:446–48. doi: 10.1097/01.jcp.0000132347.81455.57. [DOI] [PubMed] [Google Scholar]
- 85.Lucchini L, Barbaro G, Barbarini G. Methadone and QTc prolongation in HIV-infected patients. Am J Cardiol. 2004;94:147–48. doi: 10.1016/j.amjcard.2003.12.071. [DOI] [PubMed] [Google Scholar]
- 86.Walker P, Klein K, Kasza L. High dose methadone and ventricular arrhythmias: A report of three cases. Pain. 2003;103:321–24. doi: 10.1016/S0304-3959(02)00461-X. [DOI] [PubMed] [Google Scholar]
- 87.Gil M, Sala M, Anguera I, et al. QT prolongation and Torsades de Pointes in patients infected with human immunodeficiency virus and treated with methadone. Am J Cardiol. 2003;92:995–997. doi: 10.1016/s0002-9149(03)00906-8. [DOI] [PubMed] [Google Scholar]
- 88.Justo D, Gal-Oz A, Paran Y, Goldin Y, Zeltser D. Methadone-associated Torsades de Pointes (polymorphic ventricular tachycardia) in opioid-dependent patients. Addiction. 2006;101:1333–38. doi: 10.1111/j.1360-0443.2006.01512.x. [DOI] [PubMed] [Google Scholar]
- 89.Pearson EC, Woosley RL. QT prolongation and Torsades de Pointes among methadone users: Reports to the FDA spontaneous reporting system. Pharmacoepidemiol Drug Saf. 2005;14:747–53. doi: 10.1002/pds.1112. [DOI] [PubMed] [Google Scholar]
- 90.Patel AM, Singh JP, Ruskin J. Role of implantable cardioverter-defibrillators in patients with methadone-induced long QT syndrome. Am J Cardiol. 2008;101:209–11. doi: 10.1016/j.amjcard.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 91.Chugh SS, Socoteanu C, Reinier K, et al. A community-based evaluation of sudden death associated with therapeutic levels of methadone. Am J Med. 2008;121:66–71. doi: 10.1016/j.amjmed.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salle P, Rey JL, Bernasconi P, Quiret JC, Lombaert M. Torsades de Pointes: Apropos of 60 cases. Ann Cardil Angeiol (Paris) 1985;34(6):381–88. [PubMed] [Google Scholar]
- 93.Milon D, Daubert JC, Saint-Marc C, Goufault J. Torsades de Pointes: Apropos of 54 cases. Ann Fr Anesth Reanim. 1982;1(5):513–520. doi: 10.1016/s0750-7658(82)80094-4. [DOI] [PubMed] [Google Scholar]
- 94.Maremmani I, Pacini M, Cesaroni C, et al. QTc interval prolongation in patients on long-term methadone maintenance therapy. Eur Addict Res. 2005;11:44–49. doi: 10.1159/000081416. [DOI] [PubMed] [Google Scholar]
- 95.Fanoe S, Hvidt C, Ege P, et al. Syncope and QT prolongation among patients treated with methadone for heroin dependence in the city of Copenhagen. Heart. 2007;93:1051–55. doi: 10.1136/hrt.2006.100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cruciani RA, Sekiner R, Homel P, et al. Measurement of QTc in patients receiving chronic methadone therapy. J Pain Symptom Manage. 2005;29:385–91. doi: 10.1016/j.jpainsymman.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 97.Martell BA, Arnsten JH, Krantz MJ, et al. Impact of oral methadone on cardiac repolarization and conduction in opioid users. Am J Cardiol. 2005;95:915–18. doi: 10.1016/j.amjcard.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 98.Krantz MJ, Lowery CM, Martell BA, et al. Effects of methadone on QT interval dispersion. Pharmacotherapy. 2005;25:1523–29. doi: 10.1592/phco.2005.25.11.1523. [DOI] [PubMed] [Google Scholar]
- 99.Wedam EF, Bigelow G, Nuzzo P, et al. QT interval effects of methadone, levomethadyl acetate, and buprenorphine in a randomized trial. Arch Intern Med. 2007;167:2469–75. doi: 10.1001/archinte.167.22.2469. [DOI] [PubMed] [Google Scholar]
- 100.Kornick CA, Kilborn MJ, Santiago-Palma J, et al. QTc interval prolongation associated with intravenous methadone. Pain. 2003;105:499–506. doi: 10.1016/S0304-3959(03)00205-7. [DOI] [PubMed] [Google Scholar]
- 101.Ferreira S, Crumb JR, Carlton CG, et al. Effects of cocaine and its major metabolites on the HERG-encoded potassium channel. J Pharmacol Exp Ther. 2001;299:220–6. [PubMed] [Google Scholar]
- 102.Peles E, Bodner G, Kreek MJ, Rados V, Adelson M. Corrected-QT intervals as related to methadone dose and serum level in methadone maintenance treatment (MMT) patients: A cross-sectional study. Addiction. 2007 Feb;102(2):289–300. doi: 10.1111/j.1360-0443.2006.01668.x. [DOI] [PubMed] [Google Scholar]
- 103.Huber A, Ling W, Fradis J, et al. Comparison of the effects of methadone and LAAM on the electrocardiogram. Drug Alcohol Depend. 2001;63:S70. [Google Scholar]
- 104.Krantz MJ, Mehler PS. QTc prolongation: Methadone’s efficacy-safety paradox. Lancet. 2006;368:356–57. doi: 10.1016/S0140-6736(06)69173-3. [DOI] [PubMed] [Google Scholar]
- 105.American Association for the Treatment of Opioid Dependence (AATOD) AATOD policy on QT interval screening. New York, NY: The Association; 2009. Jan 25, [Google Scholar]
- 106.Medicines and Healthcare Products Regulatory Agency (MHRA) Current Problems in Pharmacovigilance. Vol. 31. London: The Agency; May, 2006. [Google Scholar]
- 107.Department of Health (England) and the developed administrations. Drug Misuse and Dependence: UK Guidelines on Clinical Management. London: Department of Health (England), the Scottish Government, Welsh Assembly Government and Northern Ireland Executive; 2007. [Google Scholar]
- 108.Botstein P. Is QT interval prolongation harmful? A regulatory perspective. Am J Cardiol. 1993 Aug 26;72(6):50B–52B. doi: 10.1016/0002-9149(93)90041-a. [DOI] [PubMed] [Google Scholar]


