Abstract
Background
Obesity is a risk factor for venous disease. We tested the associations between adipokines and the presence and severity of venous disease.
Methods
Participants for this analysis were drawn from a cohort of 2,408 employees and retirees of a university in San Diego who were examined for venous disease using duplex ultrasonography. From this cohort, a case-control study sample of all 352 subjects with venous disease and 352 age-, sex- and race-matched subjects without venous disease were included in this analysis. All subjects completed health history questionnaires, had a physical examination with anthropometric measurements and venous blood analyzed for adipokines.
Results
After adjustment for age, sex and race, those with venous disease had significantly higher levels of body mass index (BMI), leptin and interleukin-6. Levels of resistin and tumor necrosis factor-alpha were also higher but of borderline significance (0.05 < p < 0.10). Compared to the lowest tertile and with adjustment for age, sex, race and BMI, the 2nd and 3rd tertiles of resistin (Odds Ratios: 1.9 & 1.7 respectively), leptin (1.7 & 1.7) and tumor necrosis factor-alpha (1.4 & 1.7) were associated with increasing severity of venous disease. Conversely, a 5 kg/m2 increment in BMI was associated with an increased odds (1.5) for venous disease, which was independent of the adipokines included in this study.
Conclusions
Both obesity and adipokines are significantly associated with venous disease. These associations appear to be independent of each other suggesting potentially different pathways to venous disease.
INTRODUCTION
Chronic venous disease is relatively common in general clinical practice and can be associated with significant morbidity among those afflicted. The disease ranges from dilation of cutaneous venules (“telangiectasias”) to varicose veins to deep venous thrombosis (DVT) and, potentially, pulmonary thromboembolism. Individuals with a history of DVT may develop post-thrombotic syndrome (PTS), which resembles chronic venous disease clinically. Clinical features of PTS include pain, pruritus, swelling, eczematous skin changes, development of varicose veins and leg ulcerations.[1]
Little is known about the potential risk factors for the development of chronic venous disease. However, several studies have shown obesity to be a risk factor,[2–4] as well as associated with the severity of chronic venous disease.[4, 5] An important epidemiologic consideration for understanding the link between increased adiposity and chronic venous disease is whether the pathophysiology involves inflammatory changes associated with obesity. In this regard, recent research has identified various cytokines that are released from adipose tissue.[6–8] These adipokines are physiologically active, affect metabolic processes and have been associated with cardiovascular disease morbidity.[9–13] Examples of adipokines include interleukin-6, adiponectin, leptin, resistin and tumor necrosis factor-alpha. The aim of this study was to test the hypothesis of significant associations between the adipokines and the presence and severity of chronic venous disease that are independent of the degree of obesity (i.e. body mass index) and other relevant risk factors.
METHODS
Participants
The San Diego Population Study consists of 2,408 men and women randomly selected from employees and retirees the University of California, San Diego [14, 15]. This population is comprised of 1) current employees and retirees who were selected at random within sex, ethnic and age-decade strata, 2) the spouse/significant other of those listed in 1) and who may or may not be a UCSD employee or retiree themself, 3) volunteers and 4) the spouse/significant other of the volunteers. Volunteers learned of the study by word of mouth from other UCSD employees. Participants self-defined their race/ethnicity as: “White, not Hispanic”, “Hispanic/Chicano”, “African American”, “Asian/Pacific Islander”, “Native American”, or “Other”.
Information on personal and family health history and anthropometric measures was collected and a physical examination performed. Thrombosis history was defined during a structured interview as self-report of superficial or deep vein thrombosis or pulmonary embolus that had been treated with anticoagulants. Superficial vein thrombosis was defined as a positive response to a history of a “blood clot, phlebitis or inflamed vein” in a superficial leg vein. Serious leg injury was defined as fracture, burn, gunshot or stab wound, or crush injury. Prior surgery was defined for analysis as lying for surgery for longer than one hour. Venous surgery was defined as sclerotherapy injection, vein stripping or ligation or other surgery for a “problem with veins in your legs”. Using a questionnaire that was based on an empirical literature review of commonly reported symptoms in venous disease, participants were asked in standardized fashion whether they had leg symptoms of aching, heaviness, itching, tired, swelling, or cramping either in the past or currently.
A case-control study population was drawn from the 2,408 participants. We selected hierarchical case groups (Groups 1 – 5) with increasing severity of peripheral venous disease based on a priori definitions using clinical and ultrasound characteristics (see below). Group classification did not consider a history of venous thrombosis. Presence of varicose veins (VV) and telangectasias were not considered in the case group definitions, nor was superficial functional disease (SFD) in the absence of other findings. For example, an individual with SFD but no trophic changes of the skin (TCS) or edema was excluded. Each participant was classified based on his or her worst leg. There were 725 participants who did not meet criteria for being a case or control and they were excluded from all analyses.
The control group (“Group 1”) was selected from 1,313 participants with no SFD or deep functional disease (DFD) on ultrasound; no edema, VV or TCS on physical exam; and no reports of leg aching. There were 370 subjects classified with chronic venous disease (Groups 2 – 5). Among the cases, the lowest severity group (“Group 2”) consisted of 125 participants with DFD on ultrasound and TCS, aching or edema. The next group (“Group 3”) consisted of 137 participants with SFD on ultrasound and either TCS or edema (n=113) or TCS and a normal ultrasound (n=24). “Group 4” consisted of 59 participants with DFD on ultrasound and leg aching or edema but no TCS. The last group (“Group 5”) consisted of 49 participants with DFD on ultrasound and TCS, regardless of symptoms.
Since eighteen cases did not have an adequate blood sample for analysis, the total case group consisted of 352 participants. Therefore, we selected 352 controls from the overall control group (n = 1,313) who were frequency (group) matched to cases on sex, 10-year age group and ethnicity. Forty-three potential controls who were previously involved in a study of peripheral artery disease[16] were excluded from selection as they had limited blood samples[16].
Clinical Examination
Duplex ultrasound was used with standardized methods to provide quantitative information on the degree of valvular insufficiency (reflux time), flow velocity and the degree and location of obstruction.[17] The Acuson model 128 duplex ultrasonography (Siemens Corporation, Mountain View CA) with a 5-MHz transducer was used as previously published.[14] Valvular insufficiency was defined as reflux or Valsalva reflux duration of >= 0.5 seconds. Partial and complete venous obstruction was assessed by the degree of compressibility of the venous walls, with normal defined as complete compressibility. Superficial venous functional disease and DFD were defined as reflux on ultrasound or abnormal compression in superficial and deep veins, respectively. We classified 26 legs with a clinical history of vein stripping as SFD if the examination was normal. The classification of normal, SFD, or DFD was mutually exclusive and hierarchical.[14]
Visible venous disease corresponded to the Clinical Etiologic Anatomic Pathologic (CEAP) classification categories: C0 (none), C1 (telangectasias or reticular veins), C2 (varicose veins; VV), C3 (edema), C4 (pigmentation, eczema, lipodermatosclerosis or atrophie blanche), C5 (healed venous ulcer), C6 (active venous ulcer). Categories were collapsed into hierarchical categories of normal, telangectasias (C1), VV (C2), edema (C3) or trophic changes of skin (C4–6). Edema was considered separately.
Laboratory Methods
Blood was drawn and centrifuged within 3 hours, plasma was stored at −70 degrees Celsius. Participants were not necessarily fasting (13% fasted >= 8 hours, 30% >= 4 hours, 49% >= 3 hours and 72% >= 2 hours), but the distribution of time since eating was the same in the two groups (3.8 hours vs. 3.8 hours, p = 0.92). Interleukin-6 levels were determined by ELISA (R&D Systems, Minneapolis, MN) while adiponectin, leptin, resistin and tumor necrosis factor- alpha were measured using Bio-Rad Luminex flow cytometry (Millepore, Billerica, MA). Average analytical coefficients of variation across several control samples for these analytes ranged from 6.0–13.0%. The analysis of the blood samples was conducted at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT).
Statistical Analysis
Data were first examined for systematic missing data and the presence of outliers. For the outliers above or below assay detection limits, rather than exclude these subjects, we imputed values. For those below the detectable limit, we assigned a value half way between 0 and the lowest detectable value. For those above, we assigned a value 5% above the highest detectable value. Analysis of variance was used to compare study groups on baseline characteristics and adipokine levels, and to compute age, sex, and race adjusted means. Odds ratios for factors related to both prevalent venous disease, as well as increasing severity of venous disease across the study groups, were computed using unconditional binary and ordinal logistic regression, respectively.[18] In these models, we adjusted for age, sex and race to account for any residual confounding not accounted for by matching. Odds ratios were computed separately for tertiles of the adipokine (based on the distributions in the controls), as well as for 1 standard deviation unit increases. Analyses were conducted using SAS v.9.1 (Cary, NC: SAS Institute Inc.).
RESULTS
The characteristics of the study population stratified by case status are provided in Table 1. After adjustment for age, sex and race, those with venous disease were not different than those without venous disease across many sociodemographic and comorbidity variables except body mass index (29.1 vs 27.5 kg/m2, respectively; p < 0.01). However, those with venous disease had higher levels of leptin (21.9 vs. 17. 3 ng/ml, p < 0.01) and interleukin-6 (2.37 vs. 1.93 pg/ml, p = 0.01), while also having levels of resistin (16.1 vs. 15.0 ng/ml, p = 0.09) and tumor necrosis factor-alpha (2.91 vs. 2.66 pg/ml, p = 0.07) that were higher and of borderline statistical significance (0.05 < p < 0.10). As expected, those with a history of venous disease were more likely to have a history of serious leg injury, treatment for venous thrombosis, history of any venous surgery and a family history of either venous thrombosis or venous ulcer.
Table 1.
Characteristics of Cases and Noncases
| Characteristic |
Controls [Group 1] |
All Cases [Groups 2 – 5] |
Group 2 | Group 3 | Group 4 | Group 5 | p value†† |
|---|---|---|---|---|---|---|---|
| Participant factors | N = 352 | N = 352 | N = 119 | N = 132 | N = 53 | N = 48 | |
| Age, years* | 56.8 (1.2) | 57.2 (1.2) | 55 (1.5) | 59.6 (1.4) | 54.5 (1.9) | 59.1 (1.9) | 0.30 |
| Sex, male† | 97 (27.6) | 97 (27.7) | 39 (32.7) | 34 (26.0) | 9 (15.2) | 15 (31.7) | 0.58 |
| Ethnicity, White† | 243 (69.1) | 243 (69.1) | 81 (67.7) | 88 (66.3) | 40 (75.9) | 34 (69.2) | 0.60 |
| African-American† | 33 (9.2) | 33 (9.2) | 9 (7.2) | 12 (8.9) | 4 (8.2) | 8 (16.7) | 0.12 |
| Hispanic† | 37 (10.6) | 37 (10.6) | 11 (9.1) | 17 (13.3) | 6 (11.2) | 3 (6.9) | 0.63 |
| Asian† | 39 (11.0) | 39 (11.0) | 19 (16.0) | 15 (11.5) | 2 (4.7) | 3 (7.1) | 0.08 |
| BMI, kg/m2* | 27.5 (0.5) | 29.1 (0.5) | 27.7 (0.6) | 29.4 (0.6) | 30.3 (0.8) | 30.6 (0.8) | <0.01 |
| Ever Smoking† | 171 (48.7) | 151 (43.0) | 51 (43.0) | 52 (40.0) | 28 (54.2) | 20 (42.2) | 0.92 |
| Hypertension† | 117 (33.3) | 129 (36.7) | 39 (33.2) | 52 (39.3) | 20 (37.4) | 18 (37.2) | 0.44 |
| Diabetes† | 39 (10.9) | 42 (11.9) | 10 (8.5) | 19 (14.5) | 8 (14.1) | 5 (11.1) | 0.48 |
| Congestive Heart Failure† | 1 (0.14) | 3 (0.9) | 0 (0) | 2 (1.2) | 1 (1.1) | 0 (0) | 0.34 |
| Serious leg injury† | 44 (12.5) | 69 (19.6) | 23 (19.6) | 18 (14.2) | 17 (33.0) | 11 (23.6) | 0.02 |
| Surgery for >1 hour† | 295 (83.7) | 292 (83.0) | 96 (81.3) | 108 (81.5) | 47 (89.6) | 41 (86.1) | 0.16 |
| Treated venous thrombosis† | 0 (0) | 18 (5.1) | 2 (2.0) | 0 (0) | 4 (5.7) | 12 (26.0) | <0.01 |
| Any venous surgery† | 0 (0) | 48 (13.7) | 10 (8.9) | 16 (12.1) | 9 (16.6) | 13 (26.2) | <0.01 |
| Family history venous thrombosis† | 0 (0) | 9 (2.6) | 0 (0) | 4 (3.0) | 1 (2.0) | 4 (8.1) | <0.01 |
| Family history venous ulcer† | 0 (0) | 3 (0.9) | 0 (0) | 3 (2.3) | 0 (0) | 0 (0) | 0.13 |
| Adipokines | |||||||
| Resistin, ng/ml* | 15 (0.9) | 16.1 (9.4) | 15.8 (1.1) | 15.6 (1.1) | 16.9 (1.5) | 17.2 (1.5) | 0.07 |
| Adiponectin, ug/ml* | 18.2 (2.1) | 18.8 (2.1) | 19.8 (2.6) | 19.4 (2.5) | 14.6 (3.3) | 18.5 (3.4) | 0.50 |
| Leptin, ng/ml* | 17.3 (2.1) | 21.9 (2.1) | 15.5 (2.5) | 24.4 (2.4) | 27.5 (3.3) | 25.1 (3.3) | <0.01 |
| TNFa, pg/ml* | 2.66 (0.21) | 2.91 (0.22) | 2.39 (0.26) | 2.83 (0.25) | 3.66 (0.33) | 3.75 (0.34) | <0.01 |
| Il6, pg/ml* | 1.97 (0.24) | 2.37 (0.25) | 1.91 (0.3) | 2.18 (0.28) | 3.61 (0.39) | 3.15 (0.4) | <0.01 |
Adjusted for age (continuous), sex and race;
Comparing Across Case and Control Groups (Ordinal)
Mean (SEM),
Freq (%)
Group Definitions:
Group 1 (controls): No superficial functional disease (SFD) or deep functional disease (DFD) on ultrasound; no edema, varicose veins (VV), trophic changes of the skin (TCS), or leg aching.
Group 2: DFD on ultrasound and no TCS, aching or edema.
Group 3: SFD on ultrasound plus TCS or edema, or TCS and a normal ultrasound.
Group 4: DFD on ultrasound and aching or edema but no TCS.
Group 5: DFD on ultrasound and TCS, regardless of symptoms.
Table 1 also shows the characteristics of the cases and the controls by severity of venous disease. With increasing severity of venous disease there was a trend for higher levels of body mass index. There was also a trend for a higher prevalence of a history of serious leg injury, treatment for venous thrombosis, any venous surgery, and a family history of venous thrombosis. With increasing severity of venous disease there were trends for higher levels of leptin, tumor necrosis factor alpha and interleukin-6, but not adiponectin. There was a suggestion of a trend for resistin, which was of borderline statistical significance (p = 0.07).
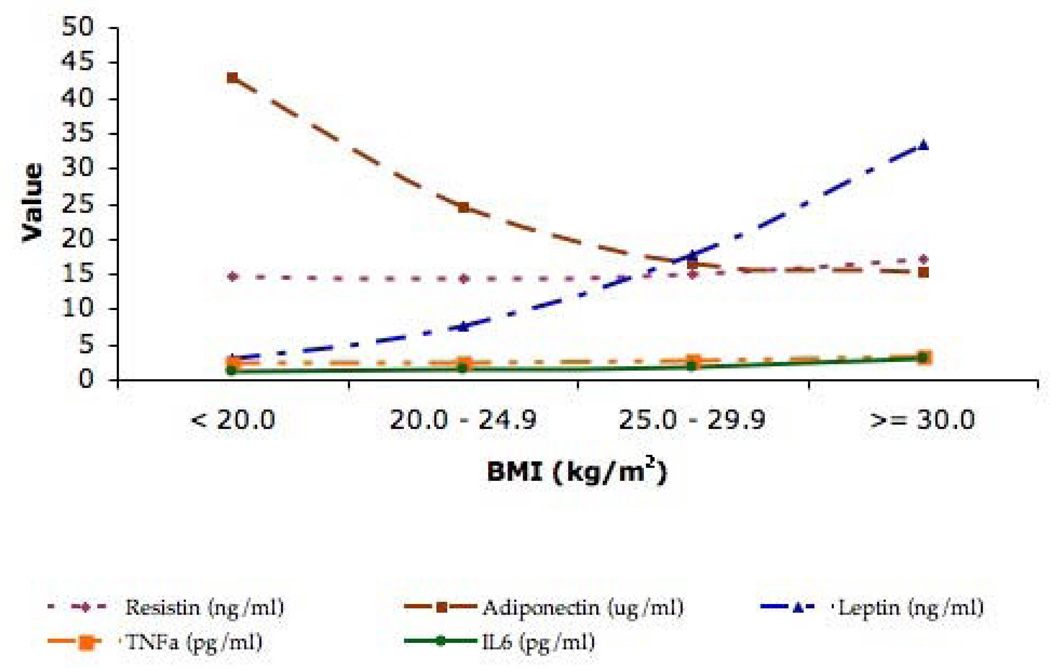
To determine the associations between the different adipokines and body mass index, we computed age, sex and race adjusted mean values across four mutually exclusive categories of BMI (< 20.0, 20.0 – 24.9, 25.0 – 29.9, >= 30.0 kg/m2) among the entire cohort. The results are depicted in Figure 1. With increasing BMI, there were corresponding increases in resistin, leptin, tumor necrosis factor alpha and interleukin-6 while adiponectin decreased across these levels of BMI. Although the largest changes were found for leptin and adiponectin, all of the trends were statistically significant.
Figure 1.
Mean Levels of the Different Adipokines across Distinct Categories of Body Mass Index
Adjusted for age, sex and race
The odds for the presence of venous disease for each of the adipokines are shown in Table 2. After adjustment for age, sex and race, the highest tertile of resistin, leptin and interleukin-6 were associated with the presence of chronic venous disease while the association with tumor necrosis factor – alpha was of borderline significance. With additional adjustment for body mass index, the associations for resistin and interleukin – 6 remained statistically significant. Additionally, and in general, the magnitude of the associations between the second tertile of the different adipokines and chronic venous disease were similar to those for the third tertile except for interleukin-6, which was markedly smaller.
Table 2.
The Odds for Both the Presence and Increasing Severity of Venous Disease for Different Adipokines
| PRESENCE OF VENOUS DISEASE | INCREASING SEVERITY OF VENOUS DISEASE | |||||
|---|---|---|---|---|---|---|
| Adipocytokine (tertile cutpoints) | Odds Ratios (95% CI) | Odds Ratios (95% CI) | Odds Ratios (95% CI) | Odds Ratios (95% CI) | ||
| Resistin (11.4, 17.0 ng/ml) | Tertile 2 vs 1 | Tertile 3 vs 1 | 1-SD Increment | Tertile 2 vs 1 | Tertile 3 vs 1 | 1-SD Increment |
| Model 1 | 1.7 (1.2–2.6) | 1.9 (1.3–2.9) | 1.15 (0.98–1.34) | 1.9 (1.3–2.7) | 1.8 (1.3–2.6) | 1.16 (1.01–1.33) |
| Model 2 | 1.7 (1.1–2.5) | 1.9 (1.3–2.8) | 1.10 (0.93–1.29) | 1.9 (1.3–2.8) | 1.7 (1.2–2.5) | 1.11 (0.96–1.28) |
| Adiponectin (17.8, 31.4 ug/ml) | ||||||
| Model 1 | 1.2 (0.8–1.7) | 1.0 (0.7–1.6) | 1.03 (0.87–1.22) | 1.1 (0.8–1.6) | 1.0 (0.7–1.5) | 1.00 (0.85–1.17) |
| Model 2 | 1.3 (0.9–1.9) | 1.3 (0.9–2.1) | 1.15 (0.96–1.37) | 1.2 (0.8–1.8) | 1.4 (0.9–2.1) | 1.12 (0.95–1.32) |
| Leptin (5.1, 13.9 ng/ml) | ||||||
| Model 1 | 1.9 (1.3–2.9) | 2.6 (1.7–4.0) | 1.35 (1.12–1.64) | 2.0 (1.4–3.0) | 3.1 (2.0–4.6) | 1.43 (1.22–1.66) |
| Model 2 | 1.6 (1.1–2.5) | 1.6 (0.9–2.7) | 1.06 (0.86–1.31) | 1.7 (1.1–2.6) | 1.7 (1.1–2.8) | 1.14 (0.96–1.37) |
| Tumor Necrosis Factor-α (2.11, 3.27 pg/ml) | ||||||
| Model 1 | 1.5 (1.0–2.3) | 1.5 (1.0–2.3) | 1.17 (0.98–1.40) | 1.7 (1.2–2.4) | 2.0 (1.4–3.0) | 1.39 (1.19–1.63) |
| Model 2 | 1.4 (0.9–2.1) | 1.3 (0.8–1.9) | 1.09 (0.92–1.30) | 1.4 (1.0–2.1) | 1.7 (1.2–2.5) | 1.28 (1.10–1.49) |
| Interleukin-6 (0.93, 1.74 pg/ml) | ||||||
| Model 1 | 1.5 (1.0–2.2) | 2.5 (1.6–3.8) | 1.24 (1.04–1.47) | 1.5 (1.0–2.2) | 2.7 (1.8–4.0) | 1.39 (1.20–1.61) |
| Model 2 | 1.3 (0.9–2.0) | 1.8 (1.1–2.8) | 1.09 (0.91–1.30) | 1.3 (0.9–2.0) | 1.8 (1.2–2.9) | 1.21 (1.03–1.41) |
Model 1: Age (continuous), sex, race; Model 2: Model 1 + body mass index (continuous)
Adipokine Tertile Sample sizes:
Resistin: Tertile 1 = 189, Tertile 2 = 240, Tertile 3 = 256
Adiponectin: T1 = 215, T2 = 230, T3 = 240
Leptin: T1 = 182, T2 = 238, T3 = 265
Tumor Necrosis Factor - alpha: T1 = 202, T2 = 240, T3 = 243
Interleukin-6: T1 = 175, T2 = 195, T3 = 254
Ordinal logistic regression was used to determine the magnitude and significance of the associations between the different adipokines and increasing severity of chronic venous disease (Table 2). Severity ranged from Group 1 (lowest) to Group 5 (highest). With adjustment for age, sex and race, and compared to the lowest tertile, the 2nd and 3rd tertiles of resistin, leptin, tumor necrosis factor-alpha and interleukin-6 were associated with increasing severity of chronic venous disease. With additional adjustment for BMI (model 2), the associations for tumor necrosis factor-alpha and interleukin-6 were attenuated but both upper tertiles in the former remained significantly associated with increasing severity of chronic venous disease while only the highest tertile of interleukin-6 was significant. Moreover, the additional adjustment for BMI did not change the significance of the associations between resistin or leptin and severity of chronic venous disease, although the odds ratio for the third tertile of resistin, as well as both upper tertiles of leptin, were modestly attenuated. Replacing BMI with waist circumference in these models did not materially change the results. Similarly, further adjustment for family history of venous ulceration did not affect those associations. Adiponectin was not associated with severity of chronic venous disease in any of the models.
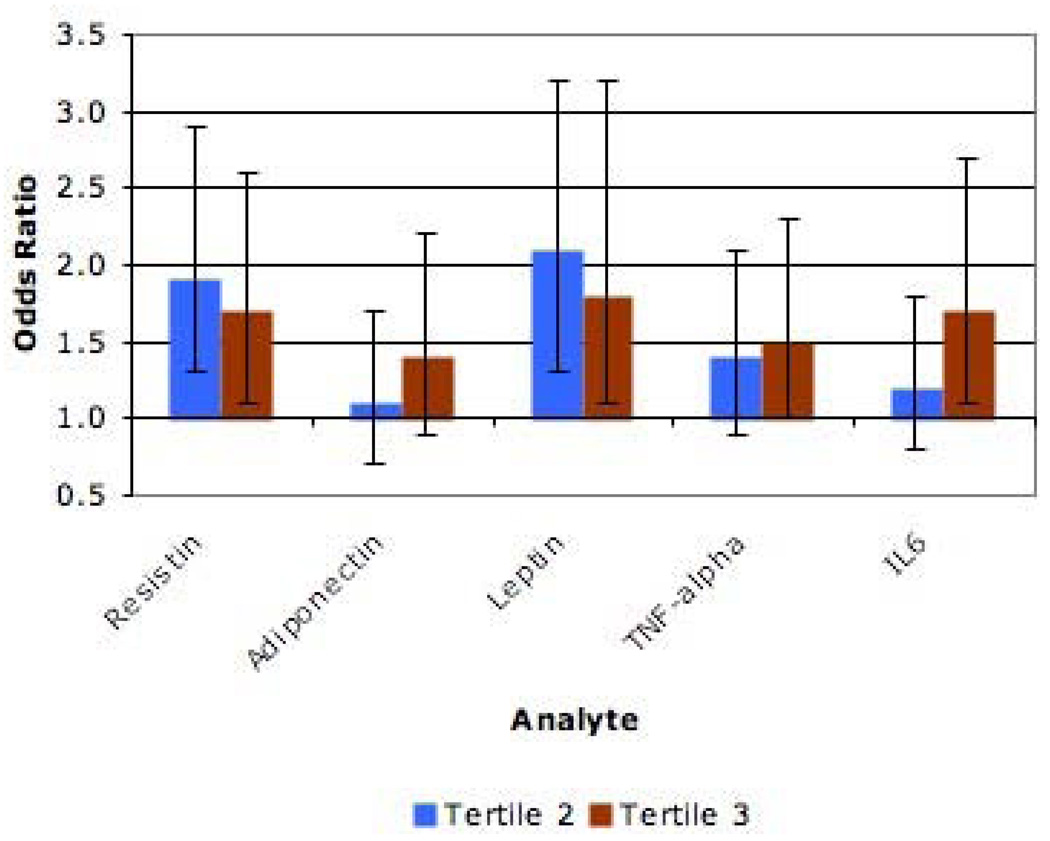
We also conducted multivariable analysis where all of the adipokines were included in the same model (Figure 2). In this model and after additional adjustment for age, sex, race and body mass index, both tertiles of resistin and leptin remained associated with increasing severity of chronic venous disease while only the third tertiles of tumor necrosis factor – alpha and interleukin – 6 were associated. As before, replacing BMI with waist circumference did not materially change the results. To summarize, when compared to models where the adipokines were analyzed separately, the results from the model that contained all of the adipokines were essentially unchanged for resistin, attenuated for both tertiles of leptin and interleukin – 6 and attenuated for one of the tertiles for adiponectin and tumor necrosis factor – alpha. Of all of the adipokines, leptin had the largest associations with the severity of chronic venous disease.
Figure 2.
Odds for Increasing Severity of Chronic Venous Disease in a Model Simultaneously Including Multiple Adipokines
Adjusted for age, sex, race and body mass index Comparing Tertiles 2 and 3 to Tertile 1 (Separately)
Table 2 also shows the association of 1 standard deviation increments of each adipokine with both the presence and severity of chronic venous disease. After adjustment for age, sex and race, 1 – SD increments in leptin and interleukin-6 were associated the presence of chronic venous disease. However, additional adjustment for BMI attenuated the associations so that they were no longer statistically significant. For increasing severity of chronic venous disease and with adjustment for age, sex and race, there were significant associations for resistin, leptin, tumor necrosis factor-alpha and interleukin-6. However, with additional adjustment for BMI the associations for resistin and leptin were no longer significant while the associations for tumor necrosis factor-alpha and interleukin-6 remained statistically significant. Further adjustment for family history of venous ulceration had no substantial impact on the results described above.
To determine if the adipokines mediated the association between the degree of obesity and severity of venous disease, we conducted multivariable ordinal logistic regression and examined the change in the odds ratio of body mass index when each adipokine was added to the model. In these analyses, each adipokine was added separately to models including age, sex, race and body mass index. With adjustment for age, sex and race, a 5 kg/m2 increment in body mass index was associated with a 50% higher odds for increasing severity of venous disease (95% CI = 1.3 – 1.8). These odds increased to 1.6 (1.4 – 1.9) with the addition of resistin and adiponectin (separately) and remained at 1.5 when leptin, tumor necrosis factor – alpha and interleukin – 6 were added (separately). The results for waist circumference were similar. Specifically, the odds for increasing severity of chronic venous disease were 30 to 50% higher (95% CI range: 1.2 – 1.6) for a 10 cm increment in waist circumference with the addition of each adipokine separately. When all of the adipokines were included in the model, a 10 cm increment in waist circumference was associated with a 20% higher odds (1.1 – 1.4) for increasing severity of chronic venous disease.
DISCUSSION
In this case-control study of employees and retirees of a large state-funded university in San Diego, several adipokines were associated with both the presence and severity of peripheral chronic venous disease independent of body mass index and family history of venous ulcer, factors related to venous disease severity in this population. Specifically, after full adjustment and compared to the lowest tertile, those in the highest tertile for resistin, leptin and tumor necrosis factor-alpha had 70% higher odds for increasing severity of venous disease while those in the highest tertile of interleukin-6 had an 80% higher odds. Additionally, those in the middle tertile of resistin, leptin and tumor necrosis factor-alpha had a higher odds of worse chronic venous disease (90, 70 and 40%, respectively). Additionally, when all of the adipokines were included in the same model, the associations were largely unchanged with leptin having the largest magnitude of the associations. These results suggest that higher levels of the adipokines studied increase the odds for venous disease, as defined here, beyond the severity or distribution (i.e. BMI vs. waist circumference) of obesity itself and that the adipokines may influence venous disease independent of one another.
Overall, one of every three patients with a history of deep-vein thrombosis of the lower extremities will develop chronic venous disease that varies from minor signs to severe manifestations such as chronic pain, intractable edema, and leg ulceration.[19] The pathophysiology of this disease is thought to include venous hypertension due to persistent outflow obstruction or valvular incompetence, as well as abnormal lymphatic function.[20] In this regard, increasing abdominal adiposity results in higher intra-abdominal pressure[5], which can result in venous stasis[21] and has been implicated as a contributing factor to the development of chronic venous disease.
Previous clinical studies support the hypothesis of a link between increasing adiposity and chronic venous disease. For example, Danielsson and colleagues found a significant association between obesity and the severity of chronic venous disease defined as both skin changes and incompetent valve (reflux) disease.[5] Moreover, obese individuals are more likely to develop more severe forms of chronic venous insufficiency.[22] For instance, in the parent study for these analyses, waist circumference was significantly and independently associated with the presence of severe chronic venous disease in both men and women.[23] However, the association may not be limited to those classified as obese since a study of young women found that a BMI greater than 22 was associated with over a 4-fold higher risk for incident chronic venous disease.[24] Given this, we speculated that there might be other factors associated with adiposity that result in a higher risk for chronic venous disease (e.g. adipokines).
Adipokines are polypeptides produced by adipose tissue that have potent autocrine, paracrine, and endocrine functions. They include leptin, adiponectin and resistin, as well as cytokines of the immune system, such as tumor necrosis factor – alpha and interleukin – 6. Leptin, adiponectin and interleukin 6 are secreted by adipose tissue itself while resistin and tumor necrosis factor – alpha are produced by the stromovascular fraction.[25–28] Plasma levels of these adipokines increase with higher levels of adiposity except for adiponectin which is inversely related to visceral fat accumulation. All of these adipokines contribute to the chronic inflammatory state that characterizes higher levels of adiposity.[27] For example, leptin influences monocyte activation, phagocytosis, and cytokine production.[29] Similarly, interleukin - 6 alters regulates hepatic production of fibrinogen and C-reactive protein, is procoagulant and stimulates adhesion of circulating leucocytes to the vascular endothelium.[30]
Beyond their effects on inflammation, these cytokines also contribute to the metabolic consequences of obesity to include insulin resistance and subsequently, the metabolic syndrome and diabetes mellitus.[27] In contrast to the substantial literature linking these two conditions to arterial diseases such as atherosclerosis, studies are sparse on how these diseases affect the venous system. However, epidemiologic studies indicate an increased risk for both deep venous thrombosis and venous thromboembolism among those with the metabolic syndrome or diabetes[31–33] and it is postulated that the increased risk is due to systemic effects on coagulation and fibrinolysis.[34] Whether the associations of adipokines with chronic venous disease are partially or completely mediated by the metabolic consequences of insulin resistance and glucose dysregulation is currently unknown.
In our study, the association between the adipokines and chronic venous disease was independent of body mass index or waist circumference suggesting that adipokines may affect venous function independent of the mechanical consequences of increased intra-abdominal pressure. To further test this hypothesis, we conducted analyses examining the association between the degree of obesity (vis-à-vis body mass index or waist circumference) and the severity of venous disease that included adjustments for the different adipokines. In these analyses, the magnitudes and significances of the associations between venous disease and increasing increments of either body mass index or waist circumference disease was not materially influenced by the addition of any of the adipokines to the models. These results suggest that the relationship between obesity and chronic venous disease is not mediated by the adipokines. Accordingly, we believe this indicates that there may be two pathways by which adipose tissue is linked to venous disease: 1) mechanical effects of increased intra-abdominal pressure and 2) inflammatory/metabolic effects of adipokines. Given the paucity of research in this area, more studies are definitely needed to confirm these results.
The strengths of our study include the population studied and careful ascertainment of clinical, imaging and biochemical risk factors. Limitations include the study design, reliance on self-report of previous venous thrombosis to classify participants and lack of information on duration of venous disease. The case-control design limits claims to causality and does not allow for strict assessments of mediation. However, we have used the latter term to describe the analyses on the association between body mass index and chronic venous disease since adipokines are elaborated from adipose tissue and have “downstream” effects. Importantly, we are currently conducting a prospective study of incident venous disease that is a follow-up of the San Diego Population Study and will expand upon the findings presented above. As for self-report of venous thrombosis, it is possible that some individuals experienced previous thrombosis and did not report it. However, since thrombosis is relatively rare and typically associated with significant clinical symptoms resulting in clinical examination, we believe the impact of this on our findings is likely small.
In conclusion, the results of the current study indicate that higher levels of cytokines released from adipose tissue are associated with an increased risk for the presence and increasing severity of venous disease, independent of the degree of obesity. Additional analyses also revealed that the adipokines studied do not likely mediate the association between obesity and venous disease suggesting distinct mechanisms by which higher adiposity results in an increased risk for chronic venous disease. Future studies, especially those that are longitudinal in nature, are warranted to further refine the current knowledge of the association between obesity and chronic venous disease.
ACKNOWLEDGMENTS
The authors are grateful to the participants and staff of the San Diego Population Study. Funding for this project was provided by R01 HL083926 (Dr. Cushman) and R01 HL53487 (Dr. Criqui).
REFERENCES
- 1.Immelman EJ, Jeffrey PC. The post-phlebitic syndrome: pathophysiology, prevention and management. Clin Chest Med. 1984;5:537–550. [PubMed] [Google Scholar]
- 2.Iannuzzi A, Panico S, Ciardullo AV, Bellati C, Cioffi V, Iannuzzo G, Celentano E, Berrino F, Rubba P. Varicose veins of the lower limbs and venous capacitance in postmenopausal women: Relationship with obesity. J Vasc Surg. 2002;36:965–968. doi: 10.1067/mva.2002.128315. [DOI] [PubMed] [Google Scholar]
- 3.Padberg F, Jr., Cerveira JJ, Lal BK, Pappas PJ, Varma S, Hobson RW., 2nd Does severe venous insufficiency have a different etiology in the morbidly obese? Is it venous? J Vasc Surg. 2003;37:79–85. doi: 10.1067/mva.2003.61. [DOI] [PubMed] [Google Scholar]
- 4.van Rij AM, De Alwis CS, Jiang P, Christie RA, Hill GB, Dutton SJ, Thomson IA. Obesity and Impaired Venous Function. Eur J Vasc Endovasc Surg. 2008;35:739–744. doi: 10.1016/j.ejvs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Danielsson G, Eklof B, Grandinetti A, L Kistner R. The Influence of Obesity on Chronic Venous Disease. Vasc Endovascular Surg. 2002;36:271–276. doi: 10.1177/153857440203600404. [DOI] [PubMed] [Google Scholar]
- 6.Khaodhiar L, Ling PR, Blackburn GL, Bistrian BR. Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. JPEN J Parenter Enteral Nutr. 2004;28:410–415. doi: 10.1177/0148607104028006410. [DOI] [PubMed] [Google Scholar]
- 7.Lemieux I, Pascot A, Prud’homme D, Almeras N, Bogaty P, Nadeau A, Bergeron J, Despres JP. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol. 2001;21:961–967. doi: 10.1161/01.atv.21.6.961. [DOI] [PubMed] [Google Scholar]
- 8.Park KG, Park KS, Kim MJ, Kim HS, Suh YS, Ahn JD, Park KK, Chang YC, Lee IK. Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Res Clin Pract. 2004;63:135–142. doi: 10.1016/j.diabres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Ahima RS. Central actions of adipocyte hormones. Trends Endocrinol Metab. 2005;16:307–313. doi: 10.1016/j.tem.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Bouloumie A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 11.Bullo M, Salas-Salvado J, Garcia-Lorda P. Adiponectin expression and adipose tissue lipolytic activity in lean and obese women. Obes Surg. 2005;15:382–386. doi: 10.1381/0960892053576776. [DOI] [PubMed] [Google Scholar]
- 12.Chen CC, Li TC, Li CI, Liu CS, Wang HJ, Lin CC. Serum resistin level among healthy subjects: relationship to anthropometric and metabolic parameters. Metabolism. 2005;54:471–475. doi: 10.1016/j.metabol.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Qasim A, Mehta NN, Tadesse MG, Wolfe ML, Rhodes T, Girman C, Reilly MP. Adipokines, Insulin Resistance, and Coronary Artery Calcification. J Am Coll Cardiol. 2008;52:231–236. doi: 10.1016/j.jacc.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Criqui MH, Jamosmos M, Fronek A, Denenberg JO, Langer RD, Bergan J, Golomb BA. Chronic venous disease in an ethnically diverse population: the San Diego Population Study. Am J Epidemiol. 2003;158:448–456. doi: 10.1093/aje/kwg166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langer RD, Ho E, Denenberg JO, Fronek A, Allison M, Criqui MH. Relationships between symptoms and venous disease: the San Diego population study. Arch Intern Med. 2005;165:1420–1424. doi: 10.1001/archinte.165.12.1420. [DOI] [PubMed] [Google Scholar]
- 16.Ix JH, Allison MA, Denenberg JO, Cushman M, Criqui MH. Novel cardiovascular risk factors do not completely explain the higher prevalence of peripheral arterial disease among African Americans. The San Diego Population Study. J Am Coll Cardiol. 2008;51:2347–2354. doi: 10.1016/j.jacc.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Fronek A, Criqui MH, Denenberg J, Langer RD. Common femoral vein dimensions and hemodynamics including Valsalva response as a function of sex, age, and ethnicity in a population study. J Vasc Surg. 2001;33:1050–1056. doi: 10.1067/mva.2001.113496. [DOI] [PubMed] [Google Scholar]
- 18.Hosmer DW, Jr., Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons, Inc.; 2000. [Google Scholar]
- 19.Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E, Prins MH. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 20.Bernardi E, Prandoni P. The post-thrombotic syndrome. Curr Opin Pulm Med. 2000;6:335–342. doi: 10.1097/00063198-200007000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Sugerman H, Windsor A, Bessos M, Wolfe L. Intra-abdominal pressure, sagittal abdominal diameter and obesity comorbidity. J Intern Med. 1997;241:71–79. doi: 10.1046/j.1365-2796.1997.89104000.x. [DOI] [PubMed] [Google Scholar]
- 22.Scott TE, LaMorte WW, Gorin DR, Menzoian JO. Risk factors for chronic venous insufficiency: A dual case-control study. J Vasc Surg. 1995;22:622–628. doi: 10.1016/s0741-5214(95)70050-1. [DOI] [PubMed] [Google Scholar]
- 23.Criqui MH, Denenberg JO, Bergan J, Langer RD, Fronek A. Risk factors for chronic venous disease: the San Diego Population Study. J Vasc Surg. 2007;46:331–337. doi: 10.1016/j.jvs.2007.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biguzzi E, Mozzi E, Alatri A, Taioli E, Moia M, Mannucci PM. The post-thrombotic syndrome in young women: retrospective evaluation of prognostic factors. Thromb Haemost. 1998;80:575–577. [PubMed] [Google Scholar]
- 25.Hivert MF, Langlois MF, Carpentier AC. The entero-insular axis and adipose tissue-related factors in the prediction of weight gain in humans. Int J Obes. 2006;31:731–742. doi: 10.1038/sj.ijo.0803500. [DOI] [PubMed] [Google Scholar]
- 26.Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C, Mantzoros CS. Serum Adiponectin Levels Are Inversely Associated with Overall and Central Fat Distribution but Are Not Directly Regulated by Acute Fasting or Leptin Administration in Humans: Cross-Sectional and Interventional Studies. J Clin Endocrinol Metab. 2003;88:4823–4831. doi: 10.1210/jc.2003-030214. [DOI] [PubMed] [Google Scholar]
- 27.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 28.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.La Cava A, Alviggi C, Matarese G. Unraveling the multiple roles of leptin in inflammation and autoimmunity. J Mol Med. 2004;82:4–11. doi: 10.1007/s00109-003-0492-1. [DOI] [PubMed] [Google Scholar]
- 30.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 31.Franchini M, Targher G, Montagnana M, Lippi G. The metabolic syndrome and the risk of arterial and venous thrombosis. Thromb Res. 2008;122:727–735. doi: 10.1016/j.thromres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Goldhaber SZ, Grodstein F, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Willett WC, CH H. A prospective study of risk factors for pulmonary embolism in women. JAMA. 1997;277:642–645. [PubMed] [Google Scholar]
- 33.Tsai A, Cushman M, Rosamond W, Heckbert S, Polak JF, Folsom AR. Cardiovascular risk factors and VTE incidence. Arch Int Med. 2002;162:1182–1189. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- 34.Gordon DOL. Common risk factors for both arterial and venous thrombosis. Br J Haematol. 2008;;140:488–495. doi: 10.1111/j.1365-2141.2007.06973.x. [DOI] [PubMed] [Google Scholar]