Abstract
Rationale
The amphetamine derivative 3,4-methylenedioxymethamphetamine(MDMA; ecstasy) is a widely abused drug, particularly in adolescent and young-adult populations. Although it was shown that MDMA-associated cues reinstate extinguished MDMA seeking in an animal relapse model, there is little information regarding the neural mechanisms underlying this behavior.
Objectives
Because the medial prefrontal cortex (mPFC) plays an important role in relapse to cocaine and methamphetamine seeking, we tested the effects of lidocaine inactivation of prelimbic(PL) and infralimbic (IL)subregions of mPFC on cue-induced relapse to MDMA seeking.
Methods
Rats were trained to respond for MDMA infusions (0.50 mg/kg/infusion, i.v.) paired with a discrete cue in daily 2-hr sessions. Responding was reinforced contingent on a modified fixed-ratio 5 schedule of reinforcement. Cue-induced reinstatement tests were conducted after responding was extinguished in the absence of MDMA and the conditioned cues. Prior to reinstatement tests, rats received bilateral microinjections of either lidocaine (100 µg/0.5 µl/side) or physiological saline (0.5 µl/side) delivered to either PL or IL mPFC.
Results
Microinjections of lidocaine into PL completely blocked reinstatement of MDMA-seeking behavior compared with saline microinjections into the same region. Lidocaine microinjections did not, however, have an effect on food-maintained responding, ruling out a nonspecific disruption of motor performance. Conversely, lidocaine inactivation of IL had no effect on reinstatement of MDMA seeking or food-maintained responding.
Conclusions
Our results provide direct support for PL activation in reinstatement of MDMA-seeking behavior. Moreover, akin to cocaine seeking, there appears to be differential involvement of PL and IL subregions in this behavior.
Keywords: cue, drug seeking, infralimbic, medial prefrontal cortex, mdma, prelimbic, reinstatement, relapse, self-administration
INTRODUCTION
Addiction to psychomotor stimulants is a chronic relapsing disorder. Relapse prevention, therefore, is a primary goal in the treatment of addiction (Mendelson and Mello 1996; O'Brien and McLellan 1996). Successful treatment is difficult, however, because addicts are hypersensitive to the incentive motivational properties of stimuli associated previously with drug taking, such as persons, places, or paraphernalia (Robinson and Berridge 1993; Stewart et al. 1984). Exposure to these stimuli, therefore, often precipitates craving and subsequent relapse (Carter and Tiffany 1999; O'Brien et al. 1992). This so-called cue-induced relapse has been studied in animals through the use of the reinstatement model (Stewart and de Wit 1987). In this model, drug-associated cues or other stimuli are used to reinstate extinguished drug-seeking responses in animals with a history of drug self-administration (Lệ and Shaham 2002; Shalev et al. 2002). Much information regarding the neural circuitry of relapse has been gleaned through the use of this model. For example, cue-induced reinstatement of cocaine seeking is attenuated by inactivation of any one of several interconnected structures of the so-called ‘motive circuit,’ including prefrontal cortex, dorsal striatum, nucleus accumbens, amygdala, and hippocampus (Fuchs et al. 2006; Fuchs et al. 2005; Fuchs et al. 2004a; b; McLaughlin and See 2003; Sun and Rebec 2003).
Although a large body of work has accumulated regarding the mechanisms underlying reinstatement of psychostimulant seeking, relatively few studies have investigated reinstatement following administration of the stimulant 3,4-methylenedioxymethamphetamine (MDMA; ecstasy). MDMA is a widely abused amphetamine derivative, particularly in adolescent and young-adult populations (Pope et al. 2001; Strote et al. 2002; Winstock et al. 2001). Similar to other amphetamine-like drugs, MDMA causes increases in extracellular catecholamine levels (Green et al. 1995), but MDMA is unique in that it potently increases serotonin (5-HT) levels as well (Gough et al. 1991; Kankaanpaa et al. 1998). Accordingly, both dopamine and 5-HT receptor mechanisms are involved in the acute neurobehavioral effects of MDMA (Ball et al. 2003; Ball and Rebec 2005; Bankson and Cunningham 2001; Bubar et al. 2004). Although it has been shown that MDMA-associated cues reinstate extinguished MDMA seeking in rats (Ball et al. 2007) and mice (Orejarena et al. 2010), there is very little information regarding the neural mechanisms underlying this behavior. Given recent evidence of maladaptive use of MDMA in a proportion of users (Cottler et al. 2001; Gouzoulis-Mayfrank et al. 2005; McCann et al. 2005; Parrott 2005; Topp et al. 1999) along with increasing preclinical evidence of the drug’s abuse potential and long-lasting neurobehavioral effects (Ball et al. 2006; Ball et al. 2011b; Ball et al. 2009; Ball et al. 2010; Schenk 2011), it is important to investigate the neural substrates underlying relapse to MDMA seeking and their overlap with the circuits driving relapse to other drugs of abuse. To begin this investigation, we focused on medial prefrontal cortex (mPFC), a region that appears to be a common anatomical substrate within the motive circuit through which a variety of stimuli converge to drive drug-seeking behaviors (Kalivas and Nakamura 1999). Because MDMA induces subregion specific structural adaptations in mPFC (Ball et al. 2009), and because dorsal and ventral subregions of mPFC play differential roles in some drug-seeking behaviors (Lasseter et al. 2010; Peters et al. 2009; Van den Oever et al. 2010) we assessed the roles of dorsal and ventral mPFC separately. Specifically, we focused on prelimbic (PL) and infralimbic (IL) subregions because these regions were targeted previously in studies of mPFC involvement in cue-induced reinstatement of other drug seeking (McLaughlin and See 2003; Rocha and Kalivas 2010; Rogers et al. 2008).
METHODS
Animals
Adult male, Sprague-Dawley rats weighing 350–400 g at the commencement of experiments were obtained from Harlan Sprague-Dawley (Indianapolis, IN). Rats were housed individually in hanging wire cages under standard laboratory conditions (12-hr light cycle from 7:00AM to 7:00 PM) with ad libitum access to water. Approximately 2 weeks prior to the commencement of experiments all rats were injected with MDMA (5.0 mg/kg; i.p.) once daily for 5 days in order to collect pilot behavioral data for a separate study. Starting 1 week before operant training rats were placed on a restricted diet and maintained at ~85% of free-feeding body weight throughout experiments to aid in the acquisition of self-administration and to facilitate greater responding during reinstatement tests (Comer et al. 1995) with the exception of a period before and after surgery during which animals were given free access to food. All procedures were conducted between 9:00 AM and 5:00 PM, were in compliance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (Committee on Guidelines for the Use of Animals in Neuroscience and Behavioral Research 2003), and were approved by the Bloomsburg University Institutional Animal Care and Use Committee.
Drugs
(±)-MDMA was provided by the National Institute on Drug Abuse (Research Triangle Park, NC) and lidocaine hydrochloride was purchased from Sigma (St. Louis, MO). Both drugs were dissolved in 0.9% sterile saline. The self-administration dose of MDMA was chosen based on published work demonstrating that similar doses support the highest levels of operant responding (Cornish et al. 2003; Ratzenboeck et al. 2001; Schenk et al. 2003) and also because this dose supported self-administration in our previous study (Ball et al. 2007). The dose of lidocaine was chosen because it was shown to decrease cue-induced reinstatement of cocaine seeking when microinjected into amygdala or ventral subiculum (Kantak et al. 2002; Sun and Rebec 2003). All doses refer to weight of salt.
Apparatus
Details on operant conditioning chambers are described elsewhere (Ball et al. 2011a). Briefly, operant conditioning chambers (Coulbourn Instruments, Whitehall, PA) were housed in sound-attenuating, ventilated cubicles and connected to a PC with the Graphic State software interface system (Coulbourn Instruments). Each chamber was equipped with an active and an inactive response lever. Each chamber was outfitted with a fluid swivel and spring leash assembly connected to a counterbalanced arm assembly. Chambers also included a house light, a row of multicolored LED cue lamps, and a tone generator. A motor-driven syringe pump (Coulbourn Instruments) for drug delivery was located outside of each cubicle.
Training
Rats first experienced 2 days of shaping sessions during which responses on the active lever were reinforced with 45-mg grain-based food pellets (Bio Serv, Frenchtown, NJ)contingent upon a fixed ratio (FR)-1 schedule of reinforcement. During shaping sessions, if 15 min elapsed without an active lever response a reinforcer was delivered non-contingently. Shaping sessions ended after 80 reinforcers were earned. Over the course of the next 7–8 days the reinforcement schedule was gradually increased to FR-5 in daily 30-min sessions. Training on the FR-5 schedule continued for 2 more days or until at least 60 reinforcers were obtained within a session.
Surgery
Following an injection of atropine sulfate (0.05 mg/kg; s.c.), animals were anesthetized with ketamine HCl (90 mg/kg; i.m.) and xylazine HCl (10 mg/kg; i.m.), with supplemental injections as needed. A catheter constructed from polyethylene tubing (PE10 and PE50; Fisher Scientific, Pittsburgh, PA) and a 22 gauge cannula-guide connector assembly (Plastics One, Roanoke, VA) was inserted into the right jugular vein. The catheter was routed subcutaneously and exited at the scapula of the skull. Following catheterization, rats were fixed in a stereotaxic apparatus and two guide cannulas (22 gauge; Plastics One) were bilaterally implanted 1.0 mm above either the PL mPFC (coordinates: AP:+3.2, ML: ±1.3, and DV –3.0 relative to bregma, midline, and skull surface, respectively, with a 10° angle away from midline) or IL mPFC (coordinates: AP:+3.2, ML: ±1.5, and DV –4.0 relative to bregma, midline, and skull surface, respectively, with a 10° angle away from midline). Four stainless steel screws were implanted in the skull for support, and the cannulas and screws were held in place with dental cement. A dummy cannula was inserted into each guide cannula to prevent blockage. During ~1 week of recovery catheters were flushed with heparinized physiological saline (50 U/ml heparin) twice daily, and 0.1 ml (10 mg/ml; i.v.) of gentamycin (Lonza, Walkersville, MD) was administered once daily. During the period of MDMA self-administration, catheter patency was evaluated by injecting 0.1 ml Brevital (1%) as necessary. Loss of muscle tone within 5 s after injection indicates a patent catheter. Two animals that began self-administration training were subsequently removed from the experiment due to catheter-related problems.
MDMA self-administration training
Rats were trained to press the lever for MDMA in daily 2-hr sessions. During these sessions rats responded on a modified FR5 schedule under which the first response on the active lever resulted in an intravenous infusion of 0.50 mg/kg of MDMA accompanied by conditioned stimuli (CS), which consisted of a tone+ flashing cue light compound stimulus presented for 5 s. Drug concentration (5.0 mg/ml) and pump delivery rate (10 µl/s) were kept constant, and dose of drug (0.50 mg/kg/infusion) was controlled by varying the duration of pump action (i.e., volume of injected solution) based on body weight. Delivery of the drug and CS was followed by a 15 s time-out period signaled by illumination of the house light. During MDMA infusions and time-outs responses were recorded but had no programmed consequences. After the first infusion, MDMA infusions and presentations of the CS were contingent upon an FR5 schedule. Rats self-administered MDMA for 14 sessions if the number of MDMA infusions varied by < 20% on at least two of the last three sessions; otherwise, sessions continued until this criterion was met.
Extinction sessions
On the day following the last self-administration session, daily 60-min extinction sessions began. During extinction sessions responses were recorded but had no programmed consequences. Extinction sessions continued for at least 5 days or until the total presses for a session were ≤ 20% of the mean number of total presses during the last three self-administration sessions.
Experiment 1: effect of lidocaine inactivation of PL mPFC on CS-induced reinstatement of MDMA-seeking behavior
Following extinction, animals underwent 1-hr CS-induced reinstatement sessions. Before these sessions, two separate groups of rats (n = 5/group) received bilateral microinjections of either lidocaine (100 µg/0.5 µl/side) or physiological saline (0.5 µl/side) delivered to PL mPFC through 28 gauge internal cannulas that extended 1 mm below the guide cannula (Plastics One). Microinjections were delivered by an infusion pump (Fisher Scientific, Newark, DE) with 10 µl syringes (Hamilton Company, Reno, NV) over a 1 min period. Syringes were connected to internal cannulas with polyethylene tubing. Internal cannulas were left in place for 1 min after injections to allow for drug diffusion. Following microinjections, rats were immediately placed into the chambers and the reinstatement session began with a noncontingent presentation of the CS over a 5-s period followed by a 15-s time-out. Thereafter, responding was reinforced by the CS alone, contingent upon an FR-5 schedule except that the first response was reinforced by the CS.
Experiment 2: effect of lidocaine inactivation of PL mPFC on food self-administration behavior
After reinstatement testing, a subset of rats from Experiment 1 (n = 6; three from each treatment group) resumed daily 30-min food self-administration sessions under an FR-5 schedule. When responding was stable (≤ 20% difference in response rate across 3 sessions) rats received microinjections of either saline or lidocaine according to the procedure described in Experiment 1 immediately before commencement of the next daily session. Rats that received lidocaine in Experiment 1 received saline and rats that received saline in Experiment 1 received lidocaine. Following a daily session in which no treatment was administered, testing was repeated in a counterbalanced design in which rats treated 2 days previously with saline microinjections received lidocaine, and vice versa, prior to the food self-administration session.
Experiment 3: effect of lidocaine inactivation of IL mPFC on CS-induced reinstatement of MDMA-seeking behavior
Following extinction, two separate groups of rats (n = 8 and 5 for saline and lidocaine groups, respectively) underwent the exact procedures described in Experiment 1, except that microinjections targeted IL mPFC.
Experiment 4: effect of lidocaine inactivation of IL mPFC on food self-administration behavior
After reinstatement testing, a subset of rats from Experiment 3 (n = 6; three from each treatment group) underwent the exact procedures described in Experiment 2, except that microinjections targeted IL mPFC.
Histology
Upon completion of experiments, rats were deeply anesthetized with pentobarbital (200 mg/kg, i.p.) and then perfused transcardially with 0.9% saline followed by 10% formaline. Brains were removed and stored in formalin solution for at least 1 week. After storage in formalin, brains were stored in a 30% sucrose/10% formalin solution for at least 1week, then frozen, cut (60 µm sections), mounted on slides, and stained with cresylecht violet to verify microinjection sites.
Data analysis
For Experiments 1 and 3, response rates were calculated as responses per hour and analyzed by means of two-way, mixed ANOVAs with treatment (saline or lidocaine microinjections before reinstatement test) as the between factor and session [self-adminstration (calculated as mean number of presses per hour during the last three self-administration sessions), last extinction session, and reinstatement session] as the within factor. Additionally, for time-course analyses during reinstatement sessions, responses were calculated in 20-min blocks and analyzed by means of two-way, mixed ANOVAs with treatment (saline or lidocaine microinjections) as the between factor and time (in 20-min blocks) as the within factor. All ANOVAs were followed by Bonferroni post-tests. For Experiments 2 and 4, response rates during food self-administration following saline and lidocaine treatment were calculated as responses per 30 min and analyzed by means of paired t-tests. In all cases, total responses per session, including responses during time outs, were used for analyses.
RESULTS
Histology
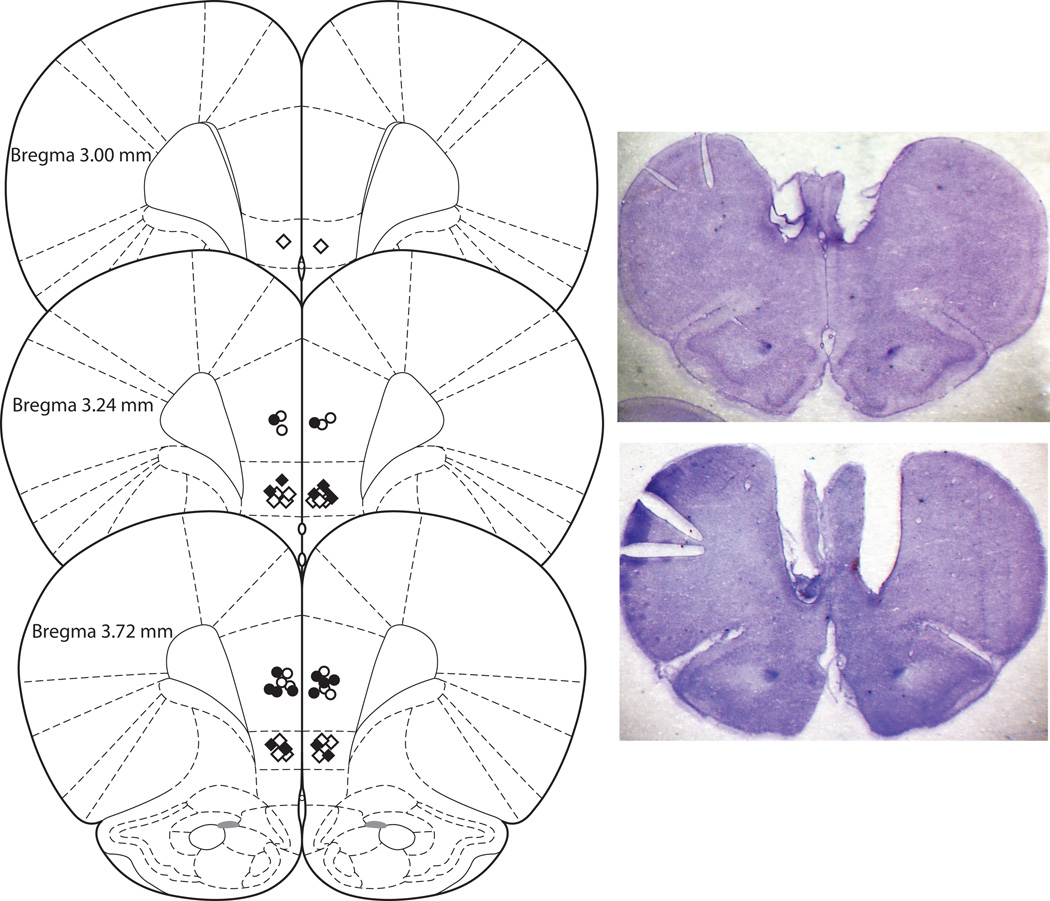
Fig. 1 shows the positions of all microinjection sites in PL and IL mPFC. Proper cannula placement was verified in all 23 animals that completed the experiments.
Fig. 1.
Schematic illustration [modified from Paxinos and Watson (2005)] of coronal sections from the rat brain showing approximate injection sites in mPFC. Open and filled circles represent the PL injection sites in saline and lidocaine groups, respectively, and open and filled diamonds represent the IL injection sites in saline and lidocaine groups, respectively. Inset: Photomicrographs of coronal sections illustrating cannula tracts from animals with PL (top) and IL (bottom) placements. Notches indicate right hemisphere
Experiment 1: effect of lidocaine inactivation of PL mPFC on CS-induced reinstatement of MDMA-seeking behavior
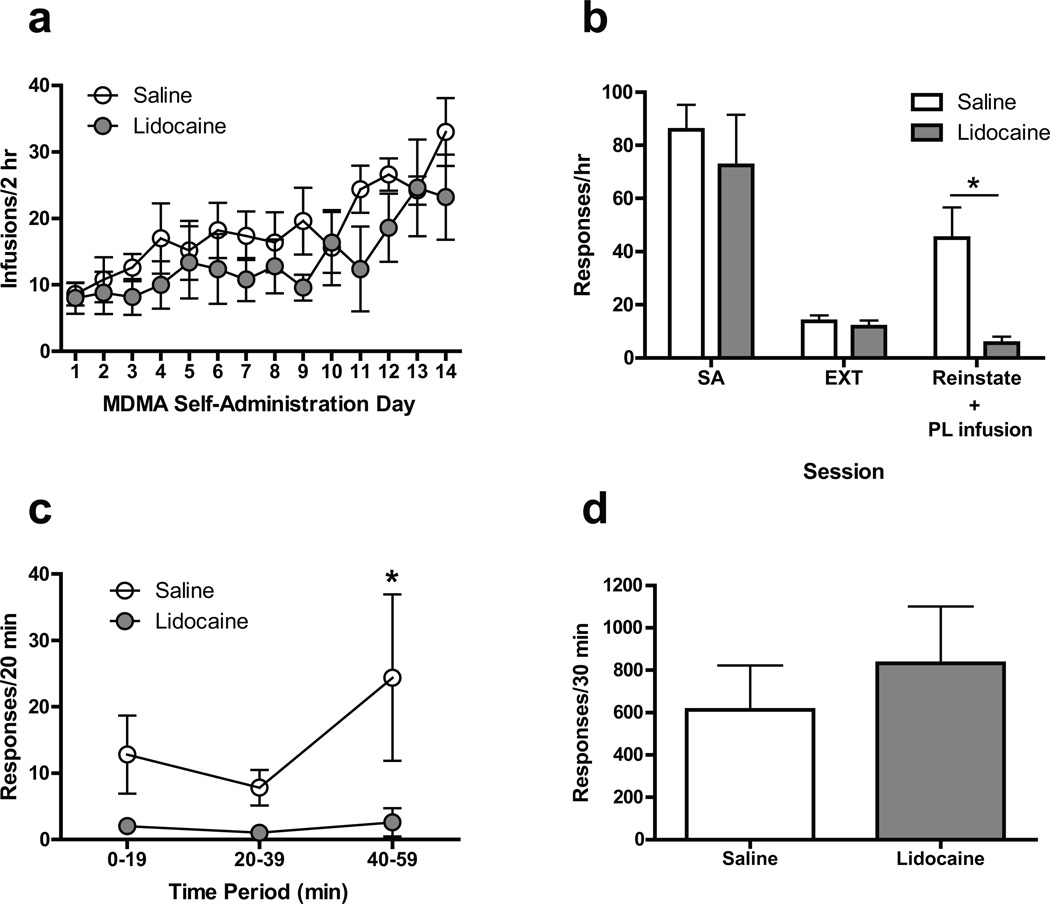
On the day following the last extinction session, CS-induced reinstatement tests were conducted in rats pretreated with either saline or lidocaine microinjections delivered to PL mPFC. Mean response rates for individual animals over the last 3 days of MDMA self-administration ranged from 59.33 to 110.33 and 35.17 to 144.00 responses/hr for the saline and lidocaine groups, respectively. As shown in Figs. 2a and 2b, mean response rates during self-administration and on the last day of extinction training for the saline and lidocaine groups were similar. During CS-induced reinstatement sessions, however, responding was significantly lower following pretreatment with lidocaine compared to saline [F(2, 16) = 23.70, p < .001 and F(1, 8) = 5.41, p < .05 for main effect of session and main effect of treatment, respectively]. Note that in lidocaine-treated rats responding during reinstatement was reduced to levels comparable to extinction. The time-course data in Fig. 2c show that responding was higher in the saline group compared with the lidocaine group across the entire reinstatement session [main effect of treatment, F(1, 8) = 10.97, p < .05]
Fig. 2.
(a) Acquisition of MDMA self-administrationin rats assigned to receive intra-PL lidocaine or saline during subsequent reinstatement tests (number of 0.50 mg/kg infusions per 2-hr session).(b) Effect of bilateral microinjections of lidocaine (100 µg/0.5 µl/side) or saline (0.5 µl/side) into PL mPFC on CS-induced reinstatement of MDMA seeking. Open and closed bars indicate data obtained from rats assigned to receive saline and lidocaine injections, respectively, before the reinstatement test. SA indicates the mean number of presses per hour during the last three self-administration sessions. EXT indicates the extinction session before reinstatement. Reinstate indicates the reinstatement session, which was preceded by a saline or lidocaine microinjection into PL mPFC. * Indicates Bonferroni post-test, p < .05. (c) Time course of the effect of intra-PL lidocaine or saline on reinstatement responding. * Indicates difference from lidocaine group, Bonferroni post-test, p < .05. (d) Effect of intra-PL lidocaine or saline on food-reinforced operant responding. All data in figure are represented as mean ± SEM
Experiment 2: effect of lidocaine inactivation of PL mPFC on food self-administration behavior
After reinstatement testing, a subset of rats from Experiment 1 was used to assess the effects of PL mPFC microinjections of saline and lidocaine on food-reinforced operant responding. As shown in Fig. 2d, lidocaine treatment had no significant effect on responding when compared with saline treatment.
Experiment 3: effect of lidocaine inactivation of IL mPFC on CS-induced reinstatement of MDMA-seeking behavior
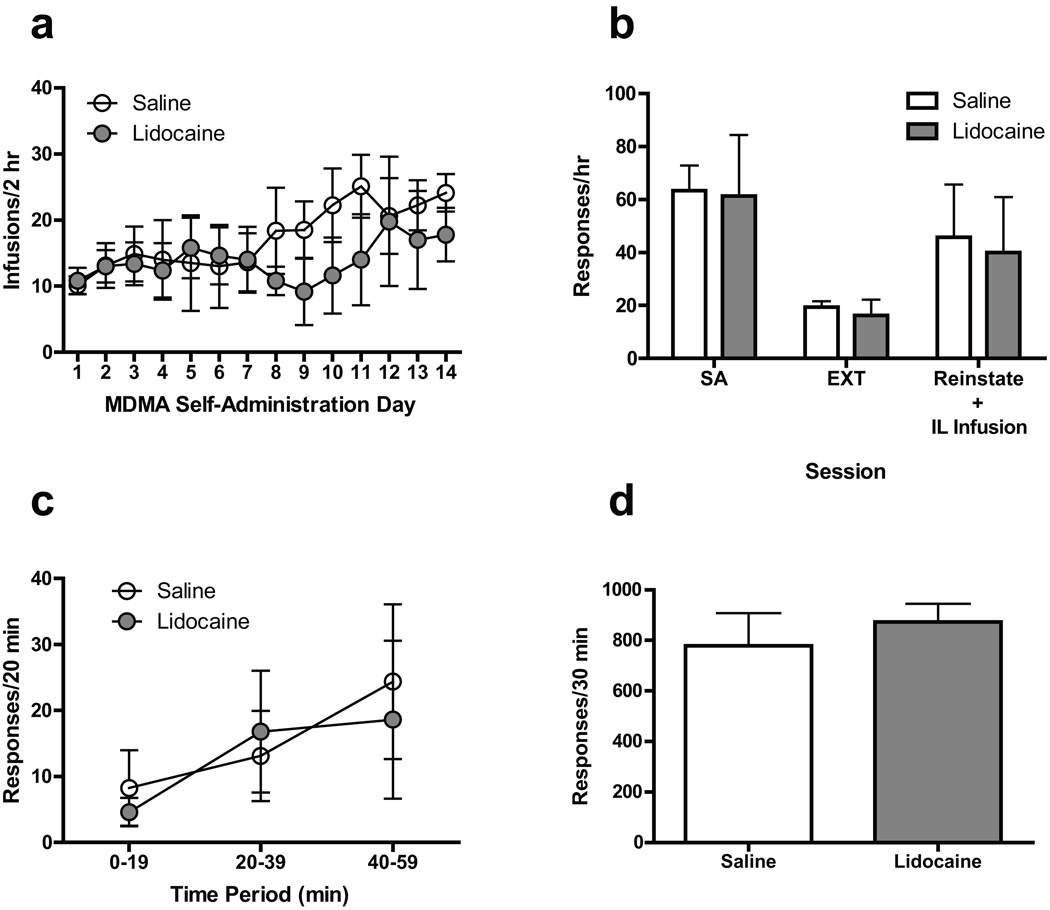
Using the same procedures as in Experiment 1, separate groups of rats with IL mPFC cannula placements underwent CS-induced reinstatement testing. Mean response rates for individual animals over the last 3 days of MDMA self-administration ranged from 20.34 to 115.00 and 17.50 to 145.34 responses/hr for the saline and lidocaine groups, respectively. As shown in Figs. 3a and 3b, mean response rates during self-administration, extinction, and CS-induced reinstatement sessions for the saline and lidocaine groups were similar. Although there was greater within-group variability in responding for both saline and lidocaine groups during reinstatement tests compared with Experiment 1, the mean (±SEM) reinstatement response rates [45.75 (±19.91) and 40.00 (±20.97)responses per hour for saline and lidocaine groups, respectively] were similar to that of saline-treated rats in Experiment 1 [45.00 (±11.64) responses per hour]. As Fig. 3c shows, reinstatement responding was similar in the saline and lidocaine groups across the entire session.
Fig. 3.
(a) Acquisition of MDMA self-administration in rats assigned to receive intra-IL lidocaine or saline during subsequent reinstatement tests.(b) Effect of bilateral microinjections of lidocaine (100 µg/0.5 µl/side) or saline (0.5 µl/side) into IL mPFC on CS-induced reinstatement of MDMA seeking. (c) Time course of the effect of intra-IL lidocaine or saline on reinstatement responding. (d) Effect of intra-IL lidocaine or saline on food-reinforced operant responding. See Fig. 2 for other details
Experiment 4: effect of lidocaine inactivation of IL mPFC on food self-administration behavior
Using the same procedures as in Experiment 2, a subset of rats from Experiment 3 was used to assess the effects of IL mPFC microinjections of saline and lidocaine on food-reinforced operant responding. As shown in Fig. 3d, lidocaine treatment had no significant effect on responding when compared with saline treatment.
DISCUSSION
We used the reinstatement model of drug seeking to investigate the neural substrates of cue-induced relapse to MDMA-seeking behavior. Given the involvement of mPFC in cue-induced reinstatement of cocaine and methamphetamine seeking (McLaughlin and See 2003; Rocha and Kalivas 2010), we assessed the effects of temporary lidocaine inactivation of dorsal (PL) and ventral (IL) mPFC on the resumption of lever pressing that was previously reinforced by i.v. MDMA. We found that inactivation of PL, but not IL, mPFC blocked discrete cue-induced reinstatement of MDMA seeking. Moreover, the reduction in reinstatement responding following PL inactivation could not be attributed to a nonspecific disruption of motor performance because inactivation of this region did not reduce high rates of responding for food on an FR-5 schedule. These results not only support our previous study showing that discrete MDMA-paired cues can reinstate extinguished MDMA-seeking behavior in rats (Ball et al. 2007), but also provide direct support for PL mPFC activation in this behavior.
Animals with IL cannula placements displayed greater within-group variability in responding during reinstatement tests (Experiment 3) compared to animals with PL placements (Experiment 1). Given our data from Experiment 1 showing that PL activation is necessary for reinstatement, it is possible that the variability was due to damage to PL as a result of IL cannula placements. Thus, although cannulas were lowered at an angle to reduce damage to overlying cortical areas, cannulas targeting IL nonetheless passed through the lateral portions of PL. Consistent with this reasoning, the relatively large within-group variability observed during reinstatement tests in Experiment 3 was not observed in the subset of rats tested for food-reinforced operant responding (Experiment 4), a behavior that is not affected by PL inactivation (see results of Experiment 2). It is not likely that the cause of variability was diffusion of injections from IL to PL regions because similar amounts of variability were observed in both the saline and the lidocaine groups. In fact, approximately the same proportions of animals in the saline and lidocaine groups showed robust reinstatement responding [3 of 8 (38%) and 2 of 5 (40%), respectively; mean increase in responding compared to prior extinction session for those animals was 358% and 314%, respectively]. Conversely, every subject with PL cannula placements and prior lidocaine injections displayed reinstatement responding similar to extinction, indicating a specific role for this region indiscrete cue-induced MDMA seeking.
Our findings are in agreement with the cocaine literature, pointing to a dorsal/ventral dichotomy with regard to the role of mPFC in relapse to drug seeking [for reviews see(Lasseter et al. 2010; Peters et al. 2009; Van den Oever et al. 2010)]. Regarding discrete cue-induced reinstatement specifically, our results indicating PL, but not IL, involvement are consistent with findings in animals extinguished from cocaine self-administration(McLaughlin and See 2003), but in contrast to findings with other drugs. Thus, inactivation of either PL or IL attenuated cue-induced reinstatement of methamphetamine and heroin seeking (Rocha and Kalivas 2010; Rogers et al. 2008). It is noteworthy that in contrast to methamphetamine and heroin, both MDMA and cocaine induce relatively large increases in extracellular levels of 5-HT (Bradberry et al. 1993; Gough et al. 1991; Kankaanpaa et al. 1998; Teneud et al. 1996). Whether serotonergic mechanisms might account for the differential findings regarding IL involvement in cue-induced reinstatement of methamphetamine and heroin vs. cocaine and MDMA seeking is a question worthy of further investigation.
Exposure to the psychomotor stimulants amphetamine, cocaine, and nicotine is associated with enduring morphological adaptations in mPFC, including increases in spine density and dendritic branching [for review see (Robinson and Kolb 2004)]. Moreover, we recently reported that repeated exposure to MDMA results in long-lasting and subregion-specific structural alterations in mPFC (Ball et al. 2009). For example, the PL, but not anterior cingulate, subregion showed increased spine density on distal dendrites of layer V pyramidal neurons. The anterior cingulate, on the other hand, displayed decreased spine density on proximal dendrites. Thus, the regional differences in function related to MDMA seeking in the present study may be the result of regional differences in MDMA-associated structural adaptations. Although we did not assess MDMA-induced structural changes in IL in our previous morphological study (Ball et al. 2009), anatomical and functional connections between mPFC and other components of the motive circuit support this hypothesis. Thus, compared with other subregions of mPFC, PL projects preferentially to the core subregion of nucleus accumbens (Berendse et al. 1992; Sesack et al. 1989) and also sends approximately three times as many efferents to the ventral tegmental area (Geisler et al. 2007). Importantly, glutamate transmission in both nucleus accumbens core and ventral tegmental area is critical to reinstatement of cocaine-seeking behavior (McFarland et al. 2003; Park et al. 2002; Sun et al. 2005). It is noteworthy, however, that several studies support the involvement of IL mPFC and nucleus accumbens shell in certain types of psychostimulant reinstatement (e.g., (Famous et al. 2008; Rocha and Kalivas 2010; Vassoler et al. 2008)), and also that psychostimulants, including MDMA, induce structural adaptations in multiple cortico-striato-limbic regions, including nucleus accumbens shell and dorsal striatum (Ball et al. 2009; Ball et al. 2010; Robinson and Kolb 2004). Taken together, these data suggest that while engagement of PL mPFC is a common feature of psychostimulant relapse induced by a variety of stimuli, the degree of involvement of other motive circuit structures is dependent on the specific circumstances of relapse, as well as the particular drug in question.
In summary, we showed that functional inactivation of the PL, but not IL, region of mPFC completely blocked CS-induced reinstatement of MDMA-seeking behavior without affecting food-reinforced operant responding. The differential involvement of PL and IL in MDMA seeking is consistent with the cocaine literature, but in contrast to findings with methamphetamine and heroin. Collectively, these findings underscore the importance of comparative studies, and highlight that, even within the same class of drugs, there may be differences in the circuitry underlying relapse that could have important implications for addiction treatment. Finally, given the popularity of MDMA and our results showing overlap in the neural circuits underlying relapse to MDMA seeking and the circuits driving relapse to other highly addictive drugs, further research on the mechanisms of MDMA seeking is warranted.
Acknowledgements
This work was supported by National Institutes of Health grant DA027960 (K.T.B.). MDMA was generously provided by the National Institute on Drug Abuse. The authors wish to thank Dr. Keith Fargo for his generous assistance in photomicrograph preparation.
Footnotes
Conflict of Interest: The authors declare no financial conflicts of interest.
REFERENCES
- Ball KT, Budreau D, Rebec GV. Acute effects of 3,4-methylenedioxymethamphetamine on striatal single-unit activity and behavior in freely moving rats: differential involvement of dopamine D(1) and D(2) receptors. Brain Research. 2003;994:203–215. doi: 10.1016/j.brainres.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Ball KT, Budreau D, Rebec GV. Context-dependent behavioural and neuronal sensitization in striatum to MDMA (ecstasy) administration in rats. European Journal of Neuroscience. 2006;24:217–228. doi: 10.1111/j.1460-9568.2006.04885.x. [DOI] [PubMed] [Google Scholar]
- Ball KT, Combs T, Beyer D. Opposing roles for dopamine D1- and D2-like receptors in discrete cue-induced reinstatement of food seeking. Behavioural Brain Research. 2011a;222:390–393. doi: 10.1016/j.bbr.2011.03.064. [DOI] [PubMed] [Google Scholar]
- Ball KT, Klein JE, Plocinski JA, Slack R. Behavioral sensitization to 3,4-methylenedioxymethamphetamine is long-lasting and modulated by the context of drug administration. Behavioural Pharmacology. 2011b;22:847–850. doi: 10.1097/FBP.0b013e32834d13b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KT, Rebec GV. Role of 5-HT2A and 5-HT2C/B receptors in the acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on striatal single-unit activity and locomotion in freely moving rats. Psychopharmacology (Berlin) 2005;181:676–687. doi: 10.1007/s00213-005-0038-z. [DOI] [PubMed] [Google Scholar]
- Ball KT, Walsh KM, Rebec GV. Reinstatement of MDMA (ecstasy) seeking by exposure to discrete drug-conditioned cues. Pharmacology, Biochemistry, and Behavior. 2007;87:420–425. doi: 10.1016/j.pbb.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KT, Wellman CL, Fortenberry E, Rebec GV. Sensitizing regimens of (±)3,4-methylenedioxymethamphetamine (ecstasy) elicit enduring and differential structural alterations in the brain motive circuit of the rat. Neuroscience. 2009;160:264–274. doi: 10.1016/j.neuroscience.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KT, Wellman CL, Miller BR, Rebec GV. Electrophysiological and structural alterations in striatum associated with behavioral sensitization to (+/−)3,4-methylenedioxymethamphetamine (ecstasy) in rats: role of drug context. Neuroscience. 2010;171:794–811. doi: 10.1016/j.neuroscience.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. 3,4-methylenedioxymethamphetamine (MDMA) as a unique model of serotonin receptor function and serotonin-dopamine interactions. Journal of Pharmacology and Experimental Therapeutics. 2001;297:846–852. [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. Journal of Computational Neurology. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Nobiletti JB, Elsworth JD, Murphy B, Jatlow P, Roth RH. Cocaine and cocaethylene: microdialysis comparison of brain drug levels and effects on dopamine and serotonin. Journal of Neurochemistry. 1993;60:1429–1435. doi: 10.1111/j.1471-4159.1993.tb03305.x. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Pack KM, Frankel PS, Cunningham KA. Effects of dopamine D1- or D2-like receptor antagonists on the hypermotive and discriminative stimulus effects of (+)-MDMA. Psychopharmacology. 2004;173:326–336. doi: 10.1007/s00213-004-1790-1. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Comer SD, Lac ST, Wyvell CL, Curtis LK, Carroll ME. Food deprivation affects extinction and reinstatement of responding in rats. Psychopharmacology (Berl) 1995;121:150–157. doi: 10.1007/BF02245624. [DOI] [PubMed] [Google Scholar]
- Committee on Guidelines for the Use of Animals in Neuroscience and Behavioral Research NRC. Guidelines for the care and use of mammals in neuroscience and behavioral research. The National Academies Press; 2003. The National Academies Press. [PubMed] [Google Scholar]
- Cornish JL, Shahnawaz Z, Thompson MR, Wong S, Morley KC, Hunt GE, McGregor IS. Heat increases 3,4-methylenedioxymethamphetamine self-administration and social effects in rats. European Journal of Pharmacology. 2003;482:339–341. doi: 10.1016/j.ejphar.2003.09.060. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Womack SB, Compton WM, Ben-Abdallah A. Ecstasy abuse and dependence among adolescents and young adults: applicability and reliability of DSM-IV criteria. Human Psychopharmacology: Clinical and Experimental. 2001;16:599–606. doi: 10.1002/hup.343. [DOI] [PubMed] [Google Scholar]
- Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha J-HJ, Pierce RC. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. Journal of Neuroscience. 2008;28:11061–11070. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. Journal of Neuroscience. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropscyhopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2004a;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. Journal of Neuroscience. 2004b;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. Journal of Neuroscience. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough B, Ali SF, Slikker W, Jr, Holson RR. Acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on monoamines in rat caudate. Pharmacology Biochemistry & Behavior. 1991;39:619–623. doi: 10.1016/0091-3057(91)90137-q. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Fischermann T, Rezk M, Thimm B, Hensen G, Daumann J. Memory performance in polyvalent MDMA (ecstasy) users who continue or discontinue MDMA use. Drug and Alcohol Dependence. 2005;78:317–323. doi: 10.1016/j.drugalcdep.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Green AR, Cross AJ, Goodwin GM. Review of the pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA or "Ecstasy") Psychopharmacology (Berlin) 1995;119:247–260. doi: 10.1007/BF02246288. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Current Opinion in Neurobiology. 1999;9:223–227. doi: 10.1016/s0959-4388(99)80031-2. [DOI] [PubMed] [Google Scholar]
- Kankaanpaa A, Meririnne E, Lillsunde P, Seppala T. The acute effects of amphetamine derivatives on extracellular serotonin and dopamine levels in rat nucleus accumbens. Pharmacology Biochemistry & Behavior. 1998;59:1003–1009. doi: 10.1016/s0091-3057(97)00527-3. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. Journal of Neuroscience. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Curr Top Behav Neurosci. 2010;3:101–117. doi: 10.1007/7854_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lệ AD, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacology and Therapeutics. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Seckin E, Rosenblatt P, Mathews WB, Ravert HT, al e. Quantitative PET studies of the serotonin transporter in MDMA uses and controls using [(11)C]McN5652 and [(11)C]DASB. Neuropsychopharmacology. 2005;30:1741–1750. doi: 10.1038/sj.npp.1300736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. Journal of Neuroscience. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Management of cocaine abuse and dependence. New England Journal of Medicine. 1996;334:965–972. doi: 10.1056/NEJM199604113341507. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Annals of the New York Academy of Sciences. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, McLellan AT. Myths about the treatment of addiction. Lancet. 1996;347:237–240. doi: 10.1016/s0140-6736(96)90409-2. [DOI] [PubMed] [Google Scholar]
- Orejarena MJ, Lanfumey L, Maldonado R, Robledo P. Involvement of 5-HT2A receptors in MDMA reinforcement and cue-induced reinstatement of MDMA-seeking behaviour. International Journal of Neuropsychopharmacology. 2010;14:1–14. doi: 10.1017/S1461145710001215. [DOI] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. Journal of Neuroscience. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC. Chronic tolerance to recreational MDMA (3,4-methylenedioxymethamphetamine) or ecstasy. Journal of Psychopharmacology. 2005;19:71–83. doi: 10.1177/0269881105048900. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5 edn. Academic Press; 2005. Academic Press. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Jr, Ionescu-Pioggia M, Pope KW. Drug use and life style among college undergraduates: a 30-year longitudinal study. American Journal of Psychiatry. 2001;158:1519–1521. doi: 10.1176/appi.ajp.158.9.1519. [DOI] [PubMed] [Google Scholar]
- Ratzenboeck E, Saria A, Kriechbaum N, Zernig G. Reinforcing effects of MDMA ("ecstasy") in drug-naive and cocaine-trained rats. Pharmacology. 2001;62:138–144. doi: 10.1159/000056086. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. European Journal of Neuroscience. 2010;31:903–909. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. Epub 2007 Oct 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S. MDMA ("ecstasy") abuse as an example of dopamine neuroplasticity. Neuroscience and Biobehavioral Reviews. 2011;35:1203–1218. doi: 10.1016/j.neubiorev.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Schenk S, Gittings D, Johnstone M, Daniela E. Development, maintenance and temporal pattern of self-administration maintained by ecstasy (MDMA) in rats. Psychopharmacology (Berlin) 2003;169:21–27. doi: 10.1007/s00213-003-1407-0. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. Journal of Computational Neurology. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacological Reviews. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H. Reinstatement of drug-taking behavior as a method of assessing incentive motivational properties of drugs. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. New York: Springer-Verlag; 1987. pp. 211–227. [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychological Review. 1984;91:251–268. [PubMed] [Google Scholar]
- Strote J, Lee JE, Wechsler H. Increasing MDMA use among college students: results of a national survey. Journal of Adolescent Health. 2002;30:64–72. doi: 10.1016/s1054-139x(01)00315-9. [DOI] [PubMed] [Google Scholar]
- Sun W, Akins CK, Mattingly AE, Rebec GV. Ionotropic glutamate receptors in the ventral tegmental area regulate cocaine-seeking behavior in rats. Neuropsychopharmacology. 2005;30:2073–2081. doi: 10.1038/sj.npp.1300744. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. Journal of Neuroscience. 2003;23:10258–10264. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teneud LM, Baptista T, Murzi E, Hoebel BG, Hernandez L. Systemic and local cocaine increase extracellular serotonin in the nucleus accumbens. Pharmacology Biochemistry & Behavior. 1996;53:747–752. doi: 10.1016/0091-3057(95)02087-x. [DOI] [PubMed] [Google Scholar]
- Topp L, Hando J, Dillon P, Roche A, Solowij N. Ecstasy use in Australia: patterns of use and associated harm. Drug and Alcohol Dependence. 1999;55:105–115. doi: 10.1016/s0376-8716(99)00002-2. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, Spijker S, Smit AB, De Vries TJ. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neuroscience and Biobehavioral Reviews. 2010;35:276–284. doi: 10.1016/j.neubiorev.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, Schmidt HD, Gerard ME, Famous KR, Ciraulo DA, Kornetsky C, Knapp CM, Pierce RC. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. Journal of Neuroscience. 2008;28:8735–8739. doi: 10.1523/JNEUROSCI.5277-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock AR, Griffiths P, Stewart D. Drugs and the dance music scene: a survey of current drug use patterns among a sample of dance music enthusiasts in the UK. Drug and Alcohol Dependence. 2001;64:9–17. doi: 10.1016/s0376-8716(00)00215-5. [DOI] [PubMed] [Google Scholar]