Abstract
Objective
To measure levels of 8-isoprostane and Lipoxin A4 in the exhaled breath condensate of children (7–17 yrs old) recovering from status asthmaticus in a pediatric intensive care unit and to compare their respective levels in the exhaled breath condensate collected from age-matched “healthy” children enrolled from an ambulatory pediatric clinic during well-child visits.
Design
Prospective case-controlled study.
Setting
Teaching hospitals and a research laboratory.
Patients
Children recovering from status asthmaticus and age-matched controls.
Interventions
Collection of exhaled breath condensate from patients recovering from status asthmaticus and controls for purpose of measurement of 8-isoprostane and Lipoxin A4.
Measurements and Main Results
There was no difference in age (11.9 ± 3.0 vs. 12.0 ± 3.3 yrs, p = .9) between patients and control subjects. All participants completed the exhaled breath condensate collection without complications. There was no difference in the pulmonary index (3.3 ± 2.2 vs. 3.1 ± 1.9, p = 1.0) after collection of exhaled breath condensate compared with baseline values in patients with status asthmaticus. The level of 8-isoprostane was significantly higher (63 ± 9 vs. 41 ± 13 pg/mL, p < .001), whereas the level of Lipoxin A4 was significantly lower (5.6 ± 2.9 vs. 10.5 ± 3.1 ng/mL, p < .001) in the exhaled breath condensate from children recovering from status asthmaticus compared with control subjects.
Conclusions
8-Isoprostane was elevated and Lipoxin A4 is decreased in the exhaled breath condensate of children recovering from status asthmaticus in a pediatric intensive care unit. These data may provide new insight into the pathophysiology of asthma in children in this clinical setting.
Keywords: lipoxins, child, asthma
Resolution of inflammation in asthma is an active process, which includes the formation of the 15-lipoxygenase-derived lipid mediators such as the lipoxins (LXs) (1–3). Evidence continues to accumulate that lipoxins could be potential modulators of inflammation within the lungs (1, 2, 4–6). The concept that patients with severe asthma may have defective lipoxin biosynthesis was initially introduced in a study by Sanak et al (7). Subsequently, it was demonstrated in adults that LX generation and LX receptor expression were decreased in severe asthma compared with mild asthma (8, 9). Limited studies have demonstrated that LX A4 levels are decreased in the blood from children with asthma compared with control subjects (10, 11).
A number of markers implicated in the inflammatory processes in the lungs have been detected in the exhaled breath condensate (EBC), including leukotrienes, cytokines, and isoprostanes (12–16). There are limited studies in adults on levels of counterregulatory lipid-derived mediators in the EBC (1, 9). However, there are no published data on levels of LX A4 in the EBC of children recovering from status asthmaticus (SA) in a pediatric intensive care unit.
The aim of this pilot study was to measure the level of LX A4 in the EBC collected within 24 hrs of hospitalization in children recovering from SA in a pediatric intensive care unit. Because inflammation is a dynamic process, we also aimed to measure the level of 8-isoprostane, a marker of oxidative stress (15, 16), in the EBC of these children.
Our hypothesis was that the level of 8-isoprostane is increased and the level of LX A4 is decreased in the EBC of children collected within 24 hrs of hospitalization for SA compared with their respective levels in the EBC from control subjects.
MATERIALS AND METHODS
Informed consent was obtained from each child’s legal guardian and the study was approved by our institutional review board. Children 7–17 yrs of age hospitalized for SA were eligible for this study. The diagnosis of asthma and SA, classification of asthma, and the treatment of SA were based on the Global Initiative for Asthma guidelines (17).
The severity of SA on admission to the hospital was determined using the Wood clinical asthma score (Wood score) and the pulmonary index (PI) as previously described (18, 19). The Wood clinical asthma score is a scoring system developed by Wood et al (18) and involves assessing five parameters, each of which is assigned a score of 0, 1, or 2 depending on the severity of the parameter in an individual patient with SA. These parameters include: presence and degree of cyanosis, breath sounds, use of accessory muscles, the degree of expiratory wheezing, and cerebral function (18). A score of ≥5 is used to define a population of pediatric patients with severe SA and impending respiratory failure (18). The PI is a scoring system derived from a combination of respiratory rate, presence and degree of wheezing, the ratio of inspiration to exhalation, use of accessory muscles, and oxyhemoglobin saturation. This index has been shown to have a good correlation with forced expiratory volume in 1 sec in children with SA (19). The PI was also recorded before and after EBC collection to assess the effects of EBC collection on lung function. EBC samples were collected within 24 hrs of hospitalization when patients were weaned from oxygen devices that would preclude EBC collection such as an oxygen mask. However, patients were still receiving unhumidified oxygen through a nasal cannula at 1–2 L/min at this time.
Sixteen age-matched children without a history of asthma or atopic disease or a history of a respiratory illness in the previous 6 wks were recruited from the ambulatory well-child clinic as control subjects. Ten subjects were black, five were white, and one subject was Hispanic. None of the control subjects had received systemic corticosteroids in the preceding 3 months. In these individuals, the EBC was collected on random days. We enrolled only 16 subjects in the control group because of limitation of resources.
Collection of EBC
Exhaled breath condensate was collected using a commercial device (R tube; Respiratory Research, Inc, Charlottesville, VA) as previously described (20, 21) and following the guidelines of the American Thoracic Society/European Respiratory Society (21). Children did not eat or drink for 3 hrs before EBC collection. They were asked to rinse their mouth with distilled water before the procedure and 7 mins into the procedure to minimize nasal contamination. Subjects were instructed to breathe tidally for 15 mins through the mouthpiece of the R tube with a one-way valve, which was connected to a condenser. In children 7–17 yrs of age, 15 mins of tidal breathing through an R-tube yields approximately 1 mL of EBC (20, 21). Because most patients were still receiving unhumidified oxygen through a nasal cannula (at 1–2 L/min) for the underlying SA, it was not practical to use a nose clip.
Subjects were also instructed to temporarily discontinue EBC collection if they needed to swallow saliva or had an urge to cough. The EBC samples were transferred into sterile containers and immediately stored at −70°C until analysis.
Measurement of 8-Isoprostane and LX A4
8-Isoprostane and LX A4 were extracted from EBC using a procedure for eicosanoid extraction as previously described (22). Briefly, 1-mL EBC samples were added to a mixture of methanol and 0.1 M sodium phosphate buffer, pH 7.4, to yield a final methanol concentration of 20%. This mixture was passed through C18 Sep-Pak cartridges (Waters Associates, Milford, MA). After washing the Sep-Pak cartridges with a mixture containing 20% methanol in 0.1 M sodium phosphate buffer followed by water, 8-isoprostane and LX A4 were eluted with 80% methanol in water. The extracts were evaporated to dryness under a gentle stream of nitrogen to prevent oxidation of the lipids and stored at −80°C. Extracts were resuspended in immunoassay buffer and assayed for 8-isoprostane (Cayman Chemical, Ann Arbor, MI) and LX A4 (Neogen, Lexington, KY) by enzyme-linked immunosorbent assay according to the manufacturer’s instructions. The detection limits of the assays were 2.7 pg/mL (8-isoprostane) and 30 pg/mL (LX A4). The assays were validated using gas chromatography/mass spectroscopy, which showed a high correlation (r = .95) between a known amount of 8-isoprostane and LX A4 and the concentration measured by enzyme immunoassay (3, 8, 9).
Statistical Analyses
Data are presented as mean ± SD or frequency (%). Age and 8-isoprostane values were compared between groups using two-tailed Student’s t test; Mann-Whitney U test was used to compare LX A4 values between groups; chi-square test examined group differences with respect to sex; and the signed-rank test was used to test for changes in PI before and after EBC collection in the patients with SA. Correlations were analyzed by Spearman correlation coefficients. p values < .05 were considered statistically significant. Statistical analyses were performed by using SPSS version 15.0 (SPSS Inc, Chicago, IL) and SAS version 8 (SAS Institute Inc, Cary, NC).
RESULTS
Twenty patients with SA (ten with moderate persistent asthma, eight with mild persistent asthma, and two with mild intermittent asthma) and 16 control subjects were enrolled in the study. Thirteen patients were black, six were white, and one patient was Hispanic. All patients with SA were treated with continuous nebulized albuterol (0.45 mg/kg/hr up to a maximum hourly dose of 20 mg) for at least 4 hrs. Subsequently, they received intermittent albuterol nebulization (0.45 mg/kg up to a maximum of dose of 5 mg) every 2–6 hrs as needed. All patients also received intravenous methylprednisolone (2 mg/kg body weight, divided into four doses, every 6 hrs). All patients had received at least two doses of intravenous methylprednisolone before EBC collection. None of the patients with SA enrolled in this study required positive pressure ventilation and they were not treated with intravenous magnesium or a helium–oxygen mixture.
All participants completed EBC collection without adverse effects. In patients recovering from SA, the PI after EBC collection was not significantly different compared with the index before EBC collection (3.3 ± 2.2 vs. 3.1 ± 1.9; average change 0.2 ± 0.7, median change 0, p = 1.0) suggesting that EBC collection did not have any deleterious effects on patients with SA.
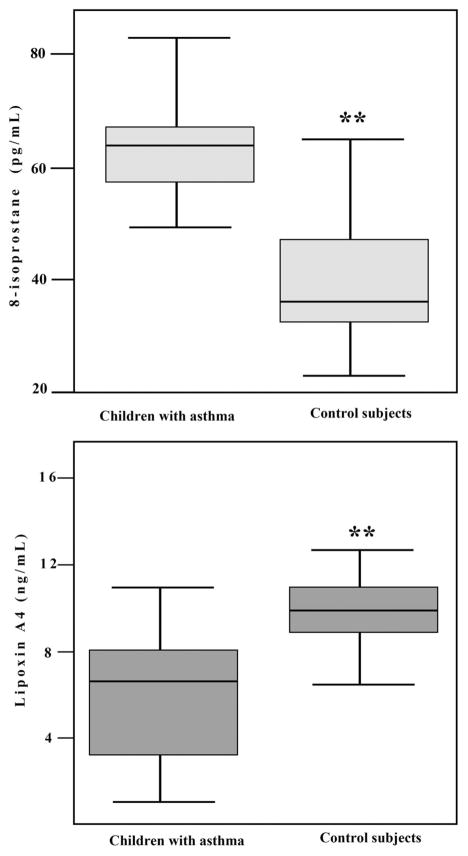
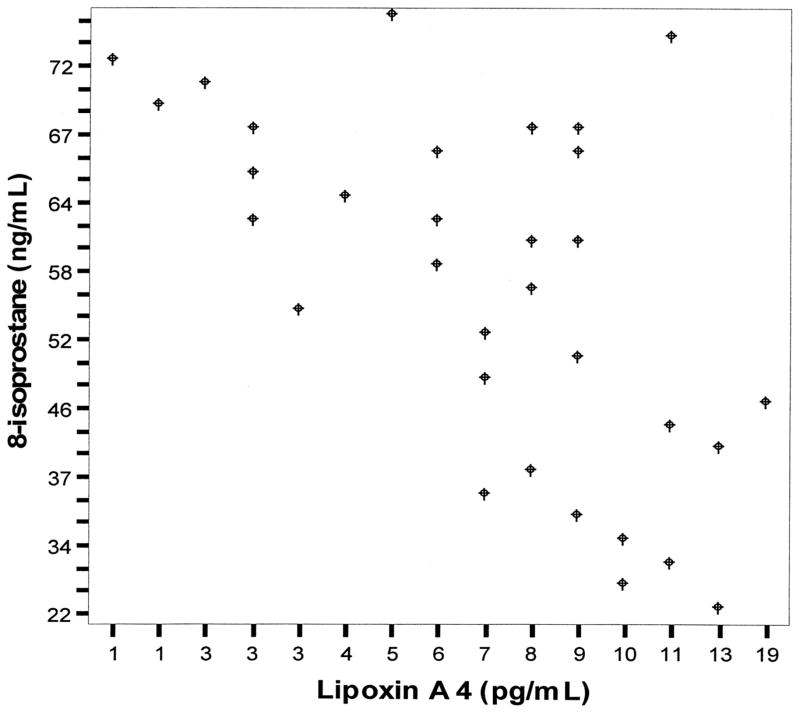
Clinical data for control subjects and patients with SA at the time of admission to the hospital are summarized in Table 1. In children with SA, 8-isoprostane level in the EBC was significantly higher (Fig. 1), whereas the level of LX A4 was significantly lower (Fig. 1) compared with control subjects. There was a statistically significant negative correlation (Fig. 2) between levels of 8-isoprostane and levels of LX A4 in the EBC (ρ = −0.65, p < .01). There was no correlation between LX A4 levels and PI before EBC collection in children with SA (ρ = −0.08, p = .8).
Table 1.
Characteristics of patients with status asthmaticus and control subjects
| Patients With Asthma | Control Subjects | p | |
|---|---|---|---|
| Number | 20 | 16 | |
| Age, yrs | 11.9 ± 2.9 | 12 ± 3.3 | NS |
| Sex, male/female | 12/8 | 10/6 | |
| Initial clinical asthma score of Wood | 3.1 ± 2.1 | N/A | |
| Initial pulmonary index | 7.1 ± 3.2 | N/A | |
| Initial WBCa | 13 ± 2.7 | N/A | |
| Percent eosinophils on WBC | 1.8 ± 1.5 | N/A | |
| Percent polys on WBC | 88 ± 12 | N/A | |
| EBC volume, mL | 1.4 ± 0.3 | 1.5 ± 0.2 | NS |
WBC, white blood cell count; NS, not significant; NA, not applicable; Polys, polymorphonuclear leukocytes; EBC, exhaled breath condensate.
WBC in numbers/mm3. Data are expressed as mean ± SD.
Figure 1.
Box–whisker plots comparing levels of 8-isoprostane (pg/mL) and lipoxin A 4 (ng/mL) in the exhaled breath condensate in children with status asthmaticus and control subjects. **p < .01.
Figure 2.
The correlation between 8-isoprostane (ng/mL) and lipoxin A4 (pg/mL) levels in the exhaled breath condensate (Spearman correlation coefficient [ρ] = −0.65, p < .01).
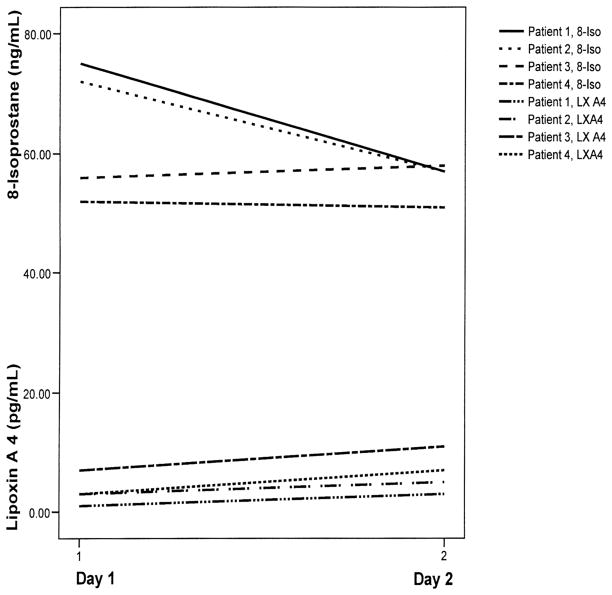
We were able to collect EBC between 24 and 48 hrs of hospitalization in four patients. The trend in the level of 8-isoprostane in the EBC collected >24 hrs after hospitalization was mixed (Fig. 3). In two of the patients, the trend was downward, whereas in the other two patients, it did not change substantially. Figure 3 also shows the trend in the levels of LX A4 in the same four patients. There was a trend toward slightly higher levels of LX A4 in the second samples.
Figure 3.
Changes in the levels of 8-isoprostane (8-Iso) (ng/mL) and lipoxin A4 (LX A4) (pg/mL) in the exhaled breath condensate at >24 hrs compared with <24 hrs after hospitalization in four children with status asthmaticus.
DISCUSSION
In this pilot study, LX A4 levels were lower and 8-isoprostane levels were higher in the EBC of children recovering from SA in a pediatric intensive care unit compared with control subjects.
The success rate for EBC collection in children with SA was 100% without significant adverse effects, which is consistent with previous observations (15, 20).
We did not document forced expiratory volume in 1 sec in any of the patients, because they were not well enough to cooperate with spirometry. However, we did use the PI, which has been shown to correlate well with forced expiratory volume in 1 sec in children with SA (19).
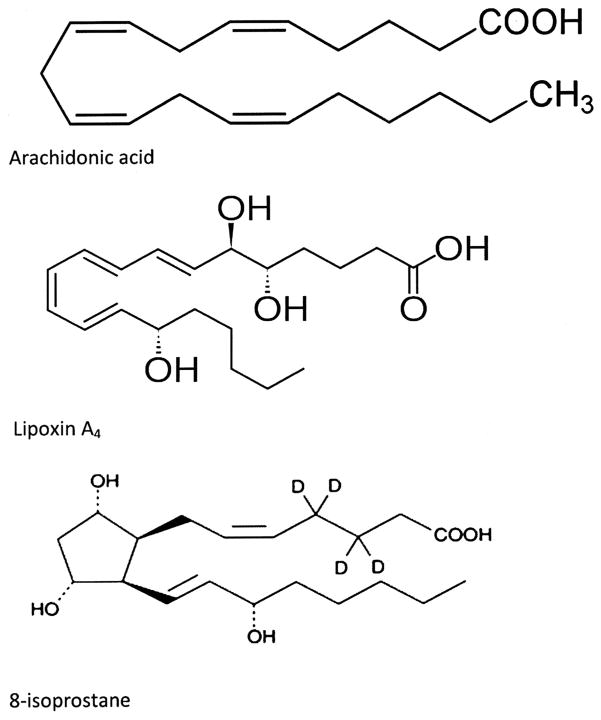
Persistent airway inflammation in asthma may result from excess proinflammatory mediators in the respiratory tissue or ongoing lipid peroxidation (16, 23, 24). However, it could also result from inadequate production of counter-regulatory mediators such as LXs, which serve to dampen inflammation (2–4, 7). LXs including LX A4 (Fig. 4) are a unique class of eicosanoids derived from 15-lipoxigenase oxidation of arachidonic acid that are distinguished from most other eicosanoids because of their counterregulatory signals that dampen airway inflammation (8, 9). In this study, the level of LX A4 was lower in the EBC collected from patients with SA compared with control subjects. This may create an imbalance favoring persistent airway inflammation and air flow limitation, which are typical of acute asthma.
Figure 4.
Chemical structures of arachidonic acid, 8-isoprostane, and lipoxin A4.
LXs promote resolution of cytokine-driven acute inflammation in the lungs through a number of mechanisms (5, 25). It has been demonstrated that in experimental allergic lung inflammation, signaling of LX A4 receptors decreases the generation of interleukin-5, interleukin-13, eotaxin, and cysteinyl leukotrienes as well as eosinophils trafficking and IgE formation (4, 5). LXs also promote resolution of inflammation by enhancing phagocytosis of apoptotic neutrophils by macrophages and by inhibition of nuclear factor κB activation (6, 25, 26).
A number of biosynthetic routes can lead to formation of lipoxins (2, 4, 8, 9). One pathway involves initial lipoxygenation of arachidonic acid by 15-lipoxygenase to from 15-HETE, which is then converted by 5-lipoxygenase and epoxide hydrase to LX A4 (4, 8, 9). Transgenic rabbits with increased 15-lipoxygenase expression in monocytes and macrophages display the ability to produce more lipoxins at sites of inflammation and are protected from chronic inflammatory diseases (27). In contrast, the lungs of animals deficient in 15-lipoxygenase display a more intense inflammatory response to antigen-dependent allergic airway inflammation (28). Collectively, these properties are consistent with the biological actions of LXs as regulators of airway inflammation in asthma. Insufficient production of LX A4 may facilitate influx of inflammatory cells into the respiratory tissue (3, 6, 8). This would perpetuate the inflammatory process with resultant bronchospasm and airway edema (3, 6, 8).
The level of 8-isoprostane (Fig. 4) was still significantly elevated in the EBC samples collected from children recovering from SA within 24 hrs of hospitalization but after at least two doses of intravenous systemic corticosteroids, suggesting ongoing lipid peroxidation. The trend in the level of 8-isoprostane in the EBC collected >24 hrs after hospitalization was mixed (Fig. 3). In two of the patients, the trend was downward, whereas in the other two patients, it did not change substantially. It is difficult to draw a conclusion from these limited data; however, a study by Baraldi et al (15) documented that the level of 8-isoporstane was still significantly elevated in the EBC of children with asthma exacerbation after a 5-day course of systemic corticosteroids in an ambulatory setting suggesting that the process of lipid peroxidation may not be sensitive to the effects of systemic cortico-steroids.
Distinct from 8-isoprostane, the level of LX A4, an anti-inflammatory mediator, was decreased in the EBC, after at least two doses of systemic corticosteroids. Furthermore, in four patients in whom data were available, the trend in the levels of LX A4 was more or less flat in the EBC collected >24 hrs after hospitalization when compared with the levels in the EBC collected within 24 hrs of hospitalization. It is difficult to draw a conclusion as a result of the small sample size; however, a study by Bhavsar et al (29) recently demonstrated suppression of LX A4 generation by alveolar macrophages from patients with severe asthma when these cells were pretreated with dexamethasone. The suppression of LX A4 generation was more pronounced in patients with severe asthma compared with patients with mild asthma and control subjects, suggesting that in patients with severe asthma, the overall balance was in favor of a proinflammatory response (29).
This study has several limitations. First, it includes a relatively small number of children with SA from a single institution. Given the clinical heterogeneity of children with SA, a study that includes larger numbers of patients with characterization of the degree of asthma severity and by parallel quantification of proinflammatory and anti-inflammatory mediators would allow for more meaningful statistical comparisons. We did not establish a firm correlation between levels of 8-isoprostane or LX A4 and lung function. This may be related to the small size of the current study population but is consistent with a previous observation by Planaguma et al (9) on this relationship. Second, it was not practical to use a nose clip in these patients because most of them were still receiving oxygen supplementation through a nasal cannula. Therefore, a certain degree of nasal contamination of the EBC is possible. Finally, the impact of exposure to 100% oxygen used to drive albuterol nebulization treatment on oxidative stress was not evaluated. Because of these limitations, our study results may not be applicable to other institutions or other clinical settings.
Although there are limitations, the results of this study are novel and address an important relationship between acute asthma in children requiring hospitalization and LXs in the EBC, which may be measured noninvasively with minimal complications.
Acknowledgments
This study was supported by Blue Cross Blue Shield of Michigan Foundation.
Footnotes
The authors have not disclosed any other conflicts of interests.
For information regarding this article, rashedh48@gmail.com
References
- 1.Serhan CN, Clish CB, Brannon CJ, et al. Novel functional sets of lipid-derived mediators with anti-inflammatory actions generated from omega-3-fatty acids via cycloxygenase 2-non-steroidal anti-inflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serhan CN. Resolution phase of inflammation: Novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 3.Levy BD, Kohli P, Gotlinger K, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy BD, Lukaacs NE, Berlin AA, et al. Lipoxin A4 stable analogs reduce allergic airway response via mechanisms distinct from CysLT1 antagonism. FASEB J. 2007;21:3877–3884. doi: 10.1096/fj.07-8653com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy BD, De Sanctis GT, Devchand PR, et al. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A4. Nat Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 6.Godson C, Mitchell S, Harvey K, et al. Cutting edge: Lipoxins rapidly stimulate non-phlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 7.Sanak M, Levy BD, Clish CB, et al. Aspirin-tolerant asthmatics generate more lipoxins than aspirin-intolerant asthmatics. Eur Respir J. 2000;16:44–49. doi: 10.1034/j.1399-3003.2000.16a08.x. [DOI] [PubMed] [Google Scholar]
- 8.Levy BD, Bonnans C, Silverman ES, et al. Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172:824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Planaguma A, Kazani S, Marigowda G, et al. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am J Respir Crit Care Med. 2008;178:574–582. doi: 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tahan F, Saraymen R, Gumus H. The role of lipoxin A4 in exercise-induced bronchoconstriction in asthma. J Asthma. 2008;45:161–164. doi: 10.1080/02770900701847068. [DOI] [PubMed] [Google Scholar]
- 11.Wu SH, Yin PL, Zhang YM, et al. Reversed changes of lipoxin A4 and leukotrienes in children with asthma of different severity degree. Pediatr Pulmonol. 2010;45:333–340. doi: 10.1002/ppul.21186. [DOI] [PubMed] [Google Scholar]
- 12.Prieto L, Ferrer A, Palop J, et al. Differences in exhaled breath condensate pH measurement between samples obtained with two commercial devices. Respir Med. 2007;101:1715–1720. doi: 10.1016/j.rmed.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001;163:1693–1722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- 14.Hunt JF, Fong Km, Malik R, et al. Endogenous airway acidification: Implications for asthma pathophysiology. Am J Respir Crit Care Med. 2000;161:694–699. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 15.Baraldi E, Carraro S, Alinovi R, et al. Cysteinyl leukotrienes and 8-isoprostane in exhaled breath condensate of children with asthma exacerbations. Thorax. 2003;58:505–509. doi: 10.1136/thorax.58.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montuschi P, Corradi M, Ciabattoni G, et al. Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. Am J Respir Crit Care Med. 1999;160:216–220. doi: 10.1164/ajrccm.160.1.9809140. [DOI] [PubMed] [Google Scholar]
- 17.National Heart, Lung, and Blood Institute: Global Initiative for Asthma; Global Strategy. National Heart, Lung, and Blood Institute. Global Initiative for Asthma; Global Strategy for Asthma Management and Prevention; NHLBI/WHO Workshop Report. Bethesda, MD: National Institute of Health; 2006. Publication No. 02-3659. [Google Scholar]
- 18.Wood DW, Downes JJ, Leck HI. A clinical scoring system for the diagnoses of respiratory failure. Am J Dis Child. 1972;123:227–228. doi: 10.1001/archpedi.1972.02110090097011. [DOI] [PubMed] [Google Scholar]
- 19.Becker AB, Nelson NA, Simons ER. The pulmonary index: Assessment of a clinical score for asthma. Am J Dis Child. 1984;138:574–576. doi: 10.1001/archpedi.1984.02140440058015. [DOI] [PubMed] [Google Scholar]
- 20.Hasan RA, Thomas J, Davidson B, et al. 8-Isoprostane in the exhaled breath condensate of children hospitalized for status asthmaticus. Pediatr Crit Care Med. 2010;12:e25–28. doi: 10.1097/PCC.0b013e3181dbeac6. [DOI] [PubMed] [Google Scholar]
- 21.Horvath I, Hunt J, Barnes PJ. Exhaled breath condensate: Methodological recommendations and unanswered questions. Eur Respir J. 2005;26:523–548. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 22.Peters-Golden M, Shelley CJ. Inhibitory effects of exogenous arachidonic acid on alveolar macrophage 5-lipoxygenase metabolism-role of ATP depletion. J Immunol. 1988;140:1958–1966. [PubMed] [Google Scholar]
- 23.Barreto M, Villa MP, Olita C, et al. 8-Isoprostane in exhaled breath condensate and exercise-induced bronchoconstriction in asthmatic children and adolescents. Chest. 2009;135:66–73. doi: 10.1378/chest.08-0722. [DOI] [PubMed] [Google Scholar]
- 24.Levy BD, Clish CB, Schmidt B, et al. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 25.Jozsef L, Zouki C, Petasis NA, et al. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit peroxynitrite formation, NF-κB and AP-1 activation, and IL-8 gene expression in human leukocytes. Proc Nat Acad Sci U S A. 2002;99:13266–13271. doi: 10.1073/pnas.202296999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gagliardo R, Chanez P, Mathieu M, et al. Persistent activation of nuclear factor-κB signaling pathway in severe uncontrolled asthma. Am J Respir Crit Care Med. 2003;168:1190–1198. doi: 10.1164/rccm.200205-479OC. [DOI] [PubMed] [Google Scholar]
- 27.Serhan CN, Jain A, Marleau S, et al. Reduced inflammation and tissue damage in transgenic rabbits over expressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856–6865. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- 28.Conrad DJ, Dai X. Murine 12/15-lipoxygenase (12/15-LO) inhibits antigen-dependent airway inflammation and has a distinct pattern of tissue expression. Am J Resp Crit Care Med. 2002;165:B46. [Google Scholar]
- 29.Bhavsar PK, Levy BD, Hew MK, et al. Corticosteroid suppression of lipoxin A4 and leukotrienes B4 from alveolar macrophages in severe asthma. Respir Res. 2010;11:71. doi: 10.1186/1465-9921-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]