Abstract
Mice adapted to drink a flavored saccharin solution (CS−) paired with intragastric (IG) self-infusions of water rapidly increase their intake of a new flavored solution (CS+) that is paired with IG glucose self-infusions. The present study extends this method to examine post-oral glucose appetition in rats. Food-restricted rats were trained to consume a CS− flavor (e.g., grape saccharin) paired with IG water in 5 daily 1-h tests. In the next 3 tests, they drank a CS+ (e.g., cherry saccharin) paired with IG glucose. Rats infused with 8% glucose increased intake significantly on CS+ test 1, but those infused with 16% glucose showed only a small increase in intake, which may reflect a counteracting satiating effect. Both groups further increased CS+ intakes in tests 2 and 3, and preferred (81%) the CS+ to the CS− in a two-bottle test without infusions. A second experiment investigated rats’ responses to IG alpha-methyl-D-glucopyranoside (MDG), a non-metabolizable sugar analog which stimulates CS+ intake and preference in mice. The rats reduced their intake of the MDG-paired CS+ flavor over sessions, and preferred the CS− to the CS+ in the choice test. The glucose data show that rats, like mice, rapidly detect the sugar’s positive post-oral effects that can stimulate intake within the first hour of exposure. The MDG avoidance may indicate a greater sensitivity to its post-oral inhibitory effects in rats than in mice, or perhaps slower clearance of MDG in rats. The test protocol described here can be used to investigate the peripheral and central processes involved in stimulation of intake by post-oral nutrients in rats.
Keywords: Post-oral nutrient conditioning, Glucose, Intragastric, Appetition
1. Introduction
Recent mouse studies demonstrate that, in addition to well documented satiation effects, the post-oral actions of sugar and fat can rapidly stimulate intake and condition a flavor preference through a process we refer to as appetition [32,33,42–44]. In the case of glucose, this intake stimulating effect can occur within minutes, suggesting that the glucose appetition signal is generated in the gut [42–44]. This is consistent with studies showing that the positive signal that serves as an unconditioned stimulus (US) in flavor preference learning is generated in the intestine [3,43], although post-absorptive glucose has been reported to condition food and place preferences in rats [19,35]. Our recent mouse studies involved concurrent intragastric (IG) self-infusion of glucose solution when the animals consumed a flavored conditioned stimulus (CS+), which ensured that the effects of the glucose were strictly post-oral in nature.
Flavor preferences conditioned by IG glucose and glucose-containing saccharides (sucrose, maltose, Polycose) have been extensively documented in rats [3–6,9–12,14,21,24,25,36–38,40,41]. Typically these studies involve multiple one-bottle, alternating training sessions with a CS+ flavor paired with IG glucose and a CS− flavor paired with IG water before the flavor preference is assessed in a two-bottle CS+ vs. CS− test. Two experiments focused on speed of acquisition, using only a single exposure to each CS prior to the first two-bottle test, which was sufficient for the development of CS+ preference [2,15]. Rapid stimulation of CS+ intake by glucose self-infusions was not observed in these or other studies that involved short-term daily sessions [10,25,36–39], but the training methods were designed to produce strong preferences rather than to probe for glucose stimulation of intake.
In the present study we sought to determine if IG glucose self-infusions can rapidly stimulate CS+ intake in the rat using the training procedure that we developed to reveal post-oral appetition in the mouse [42,44]. In brief, food-restricted C57BL/6J (B6) mice were adapted to drink a CS− flavor paired with IG water self-infusions over several consecutive 1 h/day sessions. They were then given a novel CS+ flavor paired with IG glucose self-infusions for three consecutive 1 h/day sessions and their licking responses were continuously recorded. Mice that self-infused 8% or 16% glucose increased their rate of licking within 6–9 min in the first CS+ session and further increased their drinking rates in subsequent CS+ sessions. Mice that self-infused 2% or 4% glucose showed little or no stimulation of licking, indicating that these concentrations, which were diluted to 1% and 2% in the gut by the ingested CS+ solution, were below threshold. In contrast, mice tested with 32% glucose showed early increases in CS+ licking but not in total 1-h licks or intakes, which was attributed to the satiating action of the concentrated sugar solution.
Studying the stimulation of ongoing intake produced by post-oral detection of nutrients can help identify the gut-based appetition signals that are associated with the flavor to influence concurrent and future intake and preference [31]. The first purpose of the present work was to expand the use of this technique in rats to continue studying the nature of the glucose appetition signal.
We have made some progress in understanding the nutrient sensors involved in post-oral appetition in mice. Studies of knockout mice missing components of the sweet taste signaling system (T1R3, gustducin, Trpm5) indicate that gut sweet receptors are not essential for post-oral sugar conditioning [29,31,34]. Instead, data obtained with glucose and other sugars and sugar analogs implicate intestinal sodium glucose co-transporters/sensors (SGLT1 and SGLT3) and perhaps the GLUT2 transporter in post-oral sugar conditioning [43]. Like glucose, the non-metabolizable sugar analog alpha-methyl-D-glucopyranoside (MDG) is a ligand for the SGLTs and can mimic some post-ingestive responses to glucose [8,13,18,23]. Most relevant here, we found that like glucose, MDG was effective in stimulating CS+ intake and flavor preference in B6 mice [43]. A second purpose of the study was to extend the use of this sugar analog to rats, to ask whether the mediation of post-oral glucose detection follows the same pattern as in mice.
2. Experiment 1: Post-oral glucose appetition
Flavor preferences conditioned by IG glucose infusions are extensively documented in the rat, but as noted above, these studies did not reveal rapid stimulation of intake [2,15,25,36–39]. Myers et al. [17] recently reported, however, that food and water restricted rats trained to drink flavored saccharin solutions paired with a fixed 5-ml IG infusion of water rapidly increased their intake of a novel flavored solution that was paired with a fixed IG infusion of 16% glucose. In Experiment 1 we sought further evidence for rapid glucose appetition in rats by using the IG self-infusion protocol that is effective in mice [42–44]. That is, rats were adapted to drink a CS− flavored saccharin solution paired with matched IG self-infusions of water and were then switched to a CS+ saccharin solution paired with IG self-infusions of 8% or 16% glucose.
2.1 Method
2.1.1 Subjects
Adult male Sprague-Dawley rats (n=36) were born in the laboratory from stock purchased from Charles River Laboratories (Wilmington, MA). The rats were 10–12 weeks old at the start of training. They were individually housed in stainless steel hanging cages with ad libitum access to water in rooms maintained on a 12:12 h light:dark cycle (lights on 0800 h) at 21 degrees C. The maintenance diet was pelleted chow (No. 5001, PMI Nutrition International, Brentwood, MO; 3.3 kcal/g). Prior to the experiment, chow and tap water were available ad libitum in the home cage. The experimental protocols were approved by the Brooklyn College Institutional Animal Care and Use Committee, certifying that all subjects and procedures were in compliance with the National Institute of Health Guide for Care and Use of Laboratory Animals.
2.1.2 Surgery
Following pretraining to drink in the test cages, the rats were anesthetized with isoflurane and fitted with an IG catheter according to a technique adapted from Davis and Campbell [7]. Briefly, a silastic tube (0.040-in. i.d., 0.085-in. o.d.) was inserted into the fundic region of the stomach and secured with a pursestring suture and surgical mesh. The tube was routed through an incision in the abdominal muscle wall and subcutaneously to the back of the neck, and was connected to a luer-lock assembly fixed to the skull with dental cement and stainless steel screws. The abdominal musculature and skin were closed with sutures.
2.1.3 Apparatus
The rats were trained and tested in plastic infusion cages (23 × 24 × 31.5 cm) with stainless steel mesh flooring. Above the cage, plastic tubing from a syringe pump (A-99, Razel Scientific, Stamford, CT) was connected to the input port of a swivel on a counterbalanced lever. Plastic tubing, protected by a stainless-steel spring, connected the swivel’s output port to a luer connector attached to the rat’s luer-lock assembly for the IG catheter. Stainless steel drinking spouts were available through holes in the front wall of the cage, centered 32 mm apart. The spouts were attached to drinking tubes mounted on motorized holders (ENV-252M, Med Associates, Georgia, VT) that positioned the spouts at the front of the cage at the start of the session and retracted them at the end of the session. Fluid spillage, which was minimal, was collected in trays below the spouts; spillage amounts were recorded and used to correct the intake data. Licking behavior was monitored by an electronic lickometer (ENV-250B, Med Associates) and a microcomputer. On training trials, the rat’s licking responses activated the syringe pump. The nominal infusion rate was 1.3 ml/min but the pump was on only as long as the rat was licking; the oral intake/infusion ratio was maintained at approximately 1:1 by the computer software.
2.1.4 Test solutions
All solutions were prepared with tap water. Pretraining solutions contained 0.2%, 0.1%, or 0.05% sodium saccharin (Sigma Chemical Co., St. Louis, MO). The CS solutions contained 0.05% sodium saccharin flavored with 0.05% cherry or grape Kool-Aid (unsweetened mix; Kraft Foods, White Plains, NY). The CS− solution was paired with IG infusion of water while the CS+ solution was paired with IG infusion of glucose solution (Honeyville Food Products, Rancho Cucamonga, CA). Separate groups of rats were infused with 16% (n=20) or 8% (n=16) glucose. The flavors were counterbalanced so that the CS+ was cherry for half the rats and was grape for the remaining rats.
2.1.5 Procedure
The rats were familiarized with 0.2% saccharin for 3 days in their home cages. Then they were placed in test cages for a day of adaptation to the bottle retractors: 30-min access to 0.2% saccharin alternated with 30-min periods without fluid for 1 day, with chow ad lib. Next they were placed on restricted chow rations and given daily 30-min sessions in the test cages with saccharin, first at 0.2% and reduced to 0.1% and 0.05% over 6 days. The rats were returned to ad libitum chow prior to IG catheterization. The rats had 10 days of recovery following surgery.
Pretraining continued with the rats returned to restricted chow rations. They were given 2 days of 1-h sessions with 0.05% saccharin in the test cages, while attached to the infusion system with the pumps inactive. Then the rats self-infused water when they drank 0.05% saccharin on the next 5 adaptation days. Throughout the experiment, the rats were given chow rations 1.5 h after the session to maintain them at ~85% of free-feeding weight.
The rats were then trained with flavored saccharin solutions in daily 1-h test sessions. In the first five sessions they drank a CS− saccharin solution paired with IG water self-infusions. This was followed by three sessions with the CS+ saccharin solution paired with IG self-infusions of 16% or 8% glucose. The rats were then given four alternating sessions with the CS−, CS+, CS− and CS+, in that order, with each solution paired with its respective infusion (IG water or IG glucose). Throughout the one-bottle sessions, bottle position alternated between left and right sides over days to prevent the development of side preferences. In the final CS− and CS+ sessions the rats were given a second sipper tube containing water, not paired with IG infusions, to familiarize them with the presence of two sipper tubes in the subsequent two-bottle test. The two-bottle test, with the CS+ and CS− solutions no longer paired with IG infusions, was conducted over two 1 h/day sessions. The left-right positions of the CS+ and CS− bottles alternated daily during two-bottle testing.
2.1.6 Data analysis
CS− licks and total intakes (oral + IG infusate) during the last two of the initial five 1 h/day sessions were averaged. The data from these two sessions, referred to as Test 0, and the licks and intakes during the three CS+ sessions (Tests 1–3) were analyzed using a mixed model analysis of variance (ANOVA) with a group factor (IG glucose Concentration) and repeated measures factor (Tests 0–3). The mean licks of CS− and CS+ during the alternating sessions were compared in a separate ANOVA. Additional analyses are described in the results.
Mean cumulative lick curves were generated for Tests 0–3, and licking rates were also expressed as licks per 10-min bin for each test. The lick bin data were analyzed separately for each group with repeated measure ANOVA (Test × Bin) with each CS+ test compared to Test 0. If there was a Test × Bin interaction, simple main effects tests compared each Test 0 bin with each Test 1–3 bin.
2.2 Results
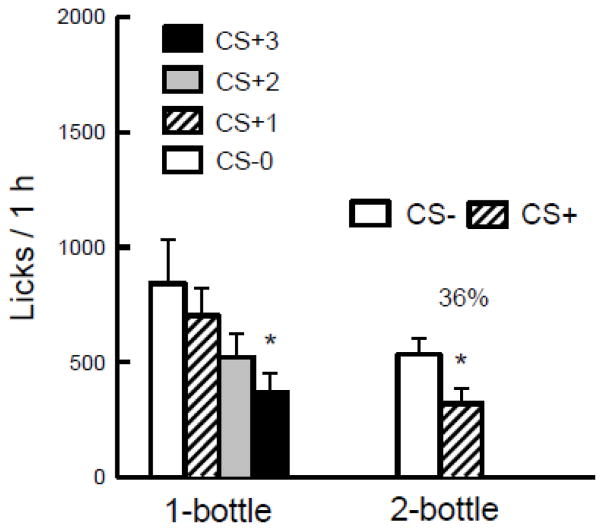
The rats increased their 1-h licks when shifted from the CS− to the CS+ [F(3,102) = 34.7, p < 0.0001] (Figure 1A). The groups did not differ significantly in the overall analysis. Within-group analyses revealed that the 8% group licked more in CS+ Test 1 than in CS− Test 0, and more in CS+ Tests 2 and 3 than in Tests 0 and 1. The 16% group licked more in CS+ Tests 2 and 3 than in Tests 0 and 1 and more in Test 3 than Test 2. Thus the 16% group, unlike the 8% group, did not show a significant increase in licks from CS− Test 0 to CS+ Test 1.
Figure 1.
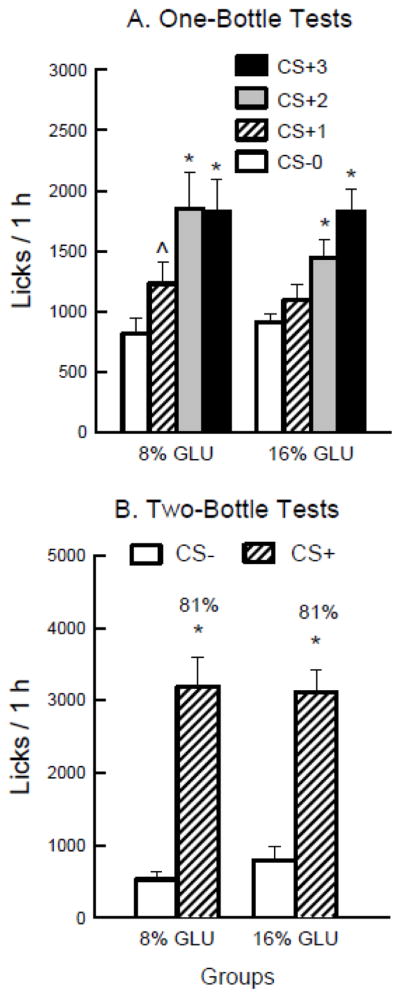
Experiment 1. A. Mean (+sem) 1-h total licks are plotted for one-bottle Tests 0–3. The rats drank (1 h/day) a CS− flavored saccharin solution paired with IG water self-infusions in Test 0 before being switched to a CS+ flavored saccharin solution paired with IG glucose self-infusions in Tests 1–3. The two IG groups were infused with 8% and 16% glucose. Significant differences (P < 0.05) between Test 0 vs. Test 1 licks are indicated by a carat, and between Tests 0–1 and Tests 2–3 licks by an asterisk. B. Mean (+sem) 1-h total licks are plotted for CS+ and CS− flavored saccharin solutions during the two-bottle preference test for the 8% and 16% IG glucose groups. CS+ and CS− intakes were not paired with IG infusions during the test. Number atop bar represents mean percent preference for the CS+ solution. Significant differences (P < 0.05) between CS+ and CS− licks are indicated by an asterisk.
The 1-h total intake data (CS solution + IG infusion g/h) revealed a similar pattern of results from Tests 0 to 3 [F(3,102) = 40.9, p < 0.0001] and there were no group differences. Within-group analyses revealed the same pattern of results as the 1-h licks. Mean intakes in Tests 0–3 were 8.3, 11.9, 16.9 and 17.5 g/h for the 8% group and 9.7, 10.8, 14.8, and 17.6 g/h for the 16% group. Compared to their CS− intake in Test 0, the 8% group increased their CS+ intakes in Tests 1–3 by 55%, 114% and 122%, respectively, and the 16% group increased their CS+ intakes by 13%, 69% and 112%, respectively. During the three CS+ sessions the 16% group self-infused almost twice the total glucose energy as the 8% group (13.7 vs. 7.2 kcal).
In the alternating CS sessions, the 8% group licked more (P < 0.05) for the CS+ than CS− (2531.0 vs. 1489.9 licks/h) whereas the 16% group did not differ in CS+ vs. CS− licks (2044.6 vs. 1971.8 licks/h) [Group × CS interaction, F(1,34) = 23.1, P = 0.0001]. The same effects were found in the analysis of intakes in the alternating sessions.
The licks for the CS+ exceeded those of the CS− in the two-bottle preference tests conducted without infusions (Figure 1B) [F(1,34) = 75.4, p < 0.0001] and CS+ intake was greater than CS− intake [F(1,34) = 86.7, p < 0.0001]. The groups did not differ significantly in the overall analyses. Percent CS+ preferences were the same (81%) for both groups.
The one-bottle lick data for CS− Test 0 and CS+ Tests 1–3 were analyzed further to identify when licking was elevated within the CS+ sessions. Figure 2 presents the CS− and CS+ licks from Tests 0 to 3 expressed as licks/10-min bin and as cumulative lick curves. Statistical analysis was performed on the 10-min data and the cumulative lick curves are included to show the evolution of the licking response during the 1-h sessions. The 8% group licked more per 10-min bin in CS+ Tests 1–3 than in CS− Test 0 [F(3,45) = 15.6, P < 0.0001]. In the comparison of Tests 0 and 1, there was a main effect of Test [F(1,15) = 15.8, P = 0.001]; the Test × Bin interaction was nonsignificant [F(5,75) = 1.90, P = 0.10] but individual tests indicated that bins 1 and 2 were greater (p < 0.01) in Test 1 than Test 0. Compared to Test 0, the 8% group licked more (p < 0.001) in bins 1 and 2 of Test 2 and bins 1, 2 and 4 of Test 3 [Test × Bin interaction, F(15,225) = 2.34, P < 0.01]. The 16% group licked more per 10-min bin in CS+ Tests 2–3 than in CS− Test 0 [F(3,57) = 21.2, P < 0.0001]. Compared to Test 0, the 16% group licked more (p < 0.0001) in bins 1 and 2 of Test 2 and bins 1–3 of Test 3 [Test × Bin interaction, F(15,285) = 5.21, P < 0.0001].
Figure 2.

Experiment 1. Licks per 10-min bin are plotted for Test 0 with CS− flavored saccharin solution paired with IG water self-infusions, and for Tests 1–3 with CS+ flavored saccharin solution paired with IG glucose self-infusions. Graph insets plot cumulative lick curves for Tests 0–3. A. 8% IG glucose group. The rats licked more (P < 0.001) for CS+ in bins 1–2 of Test 2 and bins 1, 2 and 4 of Test 3 than in Test 0. B. 16% IG glucose group. The rats licked more (P < 0.0001) for CS+ in bins 1–2 of Test 2 and bins 1–3 of Test 3 than in Test 0.
2.3 Discussion
Both groups increased licking and intakes when infused with glucose, though only the 8% group licked more in the first session. Within-session analyses indicated that the enhanced licking was largely in the first 20 or 30 minutes of the hour. The 8% group was already drinking maximally in CS+ Test 2, with no further increase in CS+ Test 3. The more gradual increase in licking over tests in the 16% group hints at a greater satiating effect with more concentrated glucose. This possibility is bolstered by the similar CS+ and CS− intake by the 16% group in the alternating sessions; in contrast, CS+ intake of the 8% group increased further during this period, so that they displayed greater CS+ than CS− intake. Despite these differences during one-bottle access, the groups were very similar in the two-bottle preference tests conducted without infusions, and both showed an 81% CS+ preference. Thus the lower concentration of glucose was just as effective at conditioning a preference in this procedure, and permitted an earlier appearance of lick stimulation than did 16% glucose.
By comparison, B6 mice responded more rapidly to a shift from CS− Test 0 to CS+ Tests 1–3 with IG glucose self-infusions [44]. Licking was maximal on CS+ Test 1 for the 8% group, and was clearly enhanced in CS+ Test 1 for the 16% group, with further increase by Test 3. The 8% mice licked more for the CS+ than they had for CS− in the first half of the CS+ sessions, whereas the 16% mice reduced their licking earlier in the sessions, suggesting greater satiation than the 8% group. Like the rats, the 8% group drank more CS+ than CS− in alternating sessions, though the difference was smaller in the mice. Another difference was a numerically smaller CS+ preference for CS+8% than for CS+16% in mice (70% vs. 80%). These were the two concentrations that elicited the greatest licking by mice in Tests 1–3; licks and intakes were reduced with 32% glucose infusion, but the preference was highest at 91%, showing that greater satiety did not interfere with the development of CS+ preference. Lower concentrations of 2% and 4% glucose were largely ineffective in stimulating licking or CS+ preferences.
The rapid stimulation of CS+ licking and intake produced by IG glucose self-infusions in this experiment contrasts with the similar CS+ and CS− intakes observed in prior studies with our standard training procedures [25,36–39]. In these earlier studies, rats were trained with sweeter (0.2% saccharin) CS solutions with the CS+ and CS− presented in alternating sessions, and the CS+ paired with IG self-infusions of 8% or 16% glucose. The method used here, in which the animal first becomes familiar with the CS− solution for several sessions and is trained with less sweet (0.05% saccharin) CS solutions provides for lower CS− baseline intakes, which enhances the ability of the IG glucose self-infusions to stimulate licking and intake.
3. Experiment 2: Post-oral MDG appetition
Our study of B6 mice showed that glucose and other sugars that are ligands of SGLT1 and SGLT3 could serve as post-oral reward for flavor preference conditioning [43]. One such sugar was the non-metabolizable glucose analog MDG. Intragastric 8% MDG stimulated CS+ licking and preference similar to that produced by IG 8% glucose self-infusions. In Experiment 2, we determined if IG self-infusions of MDG would have post-oral appetition effects similar to those of glucose in rats.
3.1 Method
New rats (n=15) of the same description were studied as in Experiment 1. They were trained as in the first experiment except that the CS+ was paired with self-infusions of 8.6% MDG, which is isomolar to 8% glucose.
3.2 Results
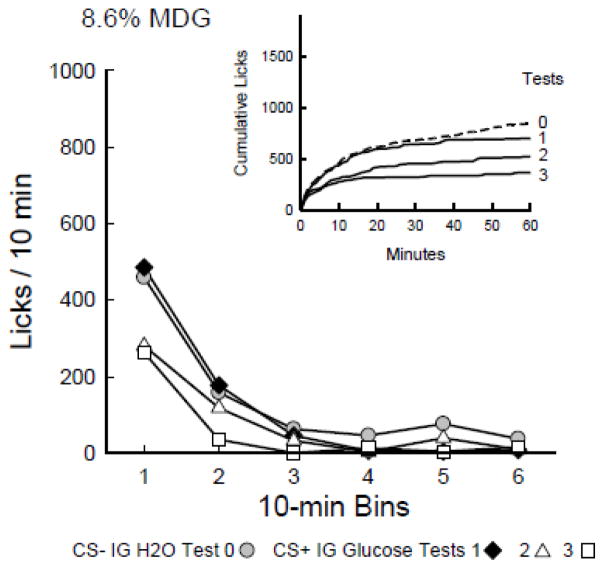
The rats reduced their 1-h licks and total intakes when shifted from the CS− in Test 0 to the CS+ in Tests 1–3 (Figure 3). In particular, they licked less (P < 0.05) in CS+ Test 3 than in CS− Test 0 [F(3,42) = 4.1, p < 0.05]. The 1-h total intake data (CS solution + IG infusion g/h) also reflected reductions [F(3,42) = 5.4, p < 0.01], with lower intake in Test 3 than in Tests 0 and 1. The rats consumed 8.0, 7.0, 5.8, and 3.9 g/h in Tests 0–3.
Figure 3.
Experiment 2. Mean (+sem) 1-h total licks are plotted for one-bottle Tests 0–3 and the two-bottle preference test. The rats drank (1 h/day) a CS− flavored saccharin solution paired with IG water self-infusions in Test 0 before being switched to a CS+ flavored saccharin solution paired with IG 8.6% MDG self-infusions in Tests 1–3. CS+ and CS− intakes were not paired with IG infusions during the two-bottle test. Significant differences (P < 0.05) between Test 0 vs. Test 3 licks and between CS+ and CS− licks are indicated by an asterisk. Number atop bar represents mean percent preference for the CS+ solution.
In the alternating CS sessions, the rats licked less for CS+ than for CS− (341.4 vs. 539.4 licks/h, t(14) = 2.62, p < 0.05) and consumed less CS+ than CS− (3.4 vs. 5.7 g/h, t(14) = 2.94, p = 0.01). CS− licks and intakes did not differ significantly from those of CS− Test 0, and CS+ licks and intakes did not differ from those of CS+ Test 3.
In the two-bottle preference tests (Figure 3), the rats licked less for CS+ than CS− (321.9 vs. 535.1 licks/h, t(14) = 3.30, p < 0.01) and consumed less CS+ than CS− (1.8 vs. 2.8 g/h, t(14) = 3.21, p = 0.01). Percent CS+ preferences were 36% in licks and 37% in intakes.
Figure 4 presents the CS− and CS+ licks from Tests 0 to 3 expressed as licks/10-min bin and as cumulative lick curves. The rats licked less per 10-min bin in CS+ Test 3 than in CS− Test 0 [F(3,42) = 4.1, P = 0.01]. In the comparisons of Tests 0 and 1, and Tests 0 and 2, there were main effects of Bin only. In CS+ Test 3, the MDG rats licked less (p < 0.05) in bins 1 and 2 than CS− in Test 0 [Test × Bin interaction, F(5,70) = 4.09, P < 0.01].
Figure 4.
Experiment 2. Licks per 10-min bin are plotted for Test 0 with CS− flavored saccharin solution paired with IG water self-infusions, and for Tests 1–3 with CS+ flavored saccharin solution paired with IG 8.6% MDG self-infusions. Graph insets plot cumulative lick curves for Tests 0–3. The rats licked less (P < 0.05) for CS+ in bins 1–2 of Test 3 than in Test 0.
3.4 Discussion
Both the declining CS+ intakes in 1-bottle sessions and the avoidance of the CS+ in the 2-bottle tests indicated that infusion of 8.6% MDG did not provide post-oral rewarding stimulation to rats. This stands in sharp contrast to the findings obtained with B6 mice, in which 8% MDG infusions stimulated CS+ intake and conditioned preferences similar to that of 8% glucose [43]. The small difference in MDG concentration (8.6% vs. 8%) used in the two experiments does not account for the differential response of rats and mice to IG MDG infusions. IG self-infusions of 12% and 16% MDG also stimulated CS+ licking in mice, although less so than 8% MDG and much less than 12% and 16% glucose. This was interpreted as a possible effect of accumulating inhibitory signals generated by the non-metabolizable MDG which, unlike glucose, is not actively transported out of intestinal cells. It appears that rats are more sensitive to the post-oral inhibitory actions of MDG than mice, or it may be that rats clear the MDG more slowly and thus accumulate more in the gut than do mice. The latter possibility is suggested by the gradual reduction in CS+ intake over days.
4. General Discussion
The present study showed that rats, like mice, rapidly detect the positive post-oral effects of glucose and increase their ingestion rate within 10 min in the first session with a CS+ paired with IG self-infusions of 8% glucose. The animals also prefer the glucose-paired CS+ flavor in two-bottle tests versus the water-paired CS− flavor. Thus the appetition effects that have been established in mice can also be observed in rats trained with this procedure. These data complement those of the established alternating-stimulus training method for flavor preference conditioning, which was not designed to evaluate such rapid behavioral signs of nutrient reward detection. A second purpose of this study, the evaluation of rats’ responses to the nonmetabolizable glucose analog MDG, found that rats, unlike mice, do not derive positive rewarding effects from this agent. This disparity may reflect a species difference in sensitivity to the inhibitory effects of MDG, or in the clearance of MDG from the gut, rather than a difference in the system for detecting post-oral glucose reward.
The control of intake by post-oral nutrient actions has been viewed as largely inhibitory, with accumulating nutrient in the gut terminating ingestion, yet the acquisition of flavor preferences suggests positive feedback that stimulates appetite [31,32]. Rats learn preferences when trained in alternating sessions with a flavor paired with glucose infusion and another flavor paired with water infusion. Typically, multiple training sessions are conducted before preferences are assessed, but two recent studies reported glucose-conditioned CS+ preferences in rats, following single brief training sessions with CS+ and CS− flavors paired with IG glucose and water infusions, respectively [2,15]. These studies, however, did not reveal how rapidly the infused glucose generated the appetition signal that conditioned the flavor preference because the critical CS+ vs. CS− choice test was not conducted until a day or more after the last training session. Another conditioning procedure, however, suggested that a rapid within-session association can form between a CS+ flavor and post-oral glucose effects [16]. Rats were trained in short daily sessions with two successive flavors, the early flavor in the first 8 min and the late flavor in the second 8 min. On alternate training days, early and late CS+ flavors were paired with IG infusions of 12% glucose, and on other days early and late CS− flavors were paired with IG water infusions. In subsequent choice tests, the rats preferred the early and late CS+ flavors over their respective CS− flavors. The preference for the early CS+ over the early CS− was taken as evidence that the rats experienced post-oral glucose reward within the 8-min early CS+ session.
In apparent conflict with the idea that glucose generates a rapid post-oral appetition signal, Puerto et al. [22] reported no CS+ preference in rats trained 10 min/day with CS+ and CS− flavors paired with IG infusions of 18% glucose and water, respectively. However, their rats were given concurrent access to the two CS solutions, which makes it very difficult for the animal to associate an individual flavor with the post-oral actions of glucose within the short training session. We also failed to observe glucose-conditioned preferences in rats given concurrent access to CS+ and CS− flavors during short (30 min) daily sessions but obtained significant CS+ preferences and profound stimulation of CS+ intakes in rats trained 20 h/day ([1]; Sclafani & Ackroff, unpublished observation). With long sessions, rats have the opportunity to consume discrete CS+ and CS− bouts and experience their differential post-oral consequences [1,9].
As an alternative method of detecting how rapidly rodents detect the positive post-oral actions of nutrients, we developed the procedure of adapting animals to drink a CS− flavor paired with IG water infusions for several 1-h sessions and then switching them to a CS+ flavor paired with an IG nutrient, while monitoring their within-session licking behavior [42–44]. Experiment 1 revealed that rats tested with a CS+ paired with IG 8% glucose self-infusions increased their rate of licking within 10 min in the first session and even more so in subsequent sessions. With a 16% glucose infusion, the rats showed a smaller, nonsignificant stimulation of licking in the first session, although they did increase their CS+ intakes in subsequent sessions. Using a different test procedure, Myers et al. [17] recently reported rapid stimulation of licking with IG 16% glucose in female rats. In their study, food and water-restricted rats were first adapted to drink several novel flavors paired with a fixed 5-ml IG water infusion during the first 15 min of daily 2-h sessions. The use of multiple “CS−” flavors was intended to reduce neophobia to new flavors. On the critical test day, the rats were given another novel flavor (the CS+) paired with a 5-ml IG infusion of 16% glucose, and they increased their 2-h intake by 29% compared to the preceding CS− tests. Lick curves indicated that glucose increased the rate of drinking 4 – 18 min into the 2-h session. Thus, it is possible to obtain rapid stimulation of drinking with a 16% glucose infusion in rats. Given the many differences in method (fixed vs. lick-driven infusion, multiple vs. two flavors, food plus water restriction vs. food only, female vs. male rats), we cannot identify the reason(s) why 16% glucose stimulated drinking more rapidly in the Myers et al. study than in the present study.
The glucose-induced appetition observed in the present study with rats is generally similar to that we previously observed in B6 mice [42–44]. One exception is that 16% glucose stimulated intake in CS+ Test 1 in the mice but not in the rats. Yet, by CS+ Test 3 the rats and mice showed similar increases in CS+ intake, relative to the CS− baseline (~110% vs. 90% increases). Note that the rat and mouse studies used different CS solutions; the rats were tested with 0.05% saccharin solutions flavored with grape and cherry Kool-Aid while the mice were tested with 0.025% saccharin solutions flavored with ethyl acetate and propyl acetate. We used 0.05% saccharin in the present study because it is difficult to get reliable intakes of more dilute saccharin solutions in Sprague-Dawley rats compared to inbred B6 mice (see [17]). The isopreferred Kool-Aid flavors were used here, as in prior rat studies, because preliminary findings indicated that rats show an unconditioned preference for ethyl acetate over propyl acetate.
The stimulation of licking observed in rats and mice in the first CS+ test may represent an unconditioned response to the appetition signal generated by glucose in the gut as well as, perhaps, a rapidly learned association of the glucose with the CS+ flavor. The greatly elevated licking rates at the start of CS+ Tests 2 and 3, which occurred with both glucose concentrations, represents a conditioned increase in the reward value of the CS+ flavor. During the first CS+ test session, and perhaps continuing after the session has ended, the animals associate the CS+ flavor with post-oral effects, so that in subsequent sessions, they immediately drink more vigorously. This enhancement in early drinking is also revealed in non-reinforced tests when the CS+ is paired with IG water (unpublished findings; [26]). In prior studies rats trained with a CS+ paired with IG glucose or glucose-containing saccharides often did not drink more CS+ than CS− in later training sessions [25,36–39]. One reason that enhanced CS+ drinking was not always detected in these studies is that the rats were trained with sweeter CS flavors (containing 0.2% rather than 0.05% saccharin), which encouraged maximal drinking in the short sessions. In long sessions (20–24 h) rats and mice trained with CS solutions sweetened with 0.2% saccharin invariably consumed more of a glucose-paired CS+ than of the water-paired CS− [1,4,27,28,30].
Whereas rats and mice are similar in their appetition response to IG glucose, they displayed opposite responses to IG MDG infusions. In Exp. 2 the MDG infusions suppressed CS+ intake during training and conditioned a CS+ avoidance, whereas MDG infusions stimulated CS+ intake and preference in B6 mice [43]. Mice and rats also differ in their oral preference for MDG solutions. In 24-h two-bottle tests (Supplementary Fig. 1), B6 mice strongly preferred 4% and 8% MDG to water (92%, 97%), whereas Sprague-Dawley rats avoided both 4% and 8% MDG (29%, 15%). The rat’s oral avoidance did not appear to reflect an aversive taste, as food-restricted rats preferred 8.6% MDG to water (82%) in a 3-min two-bottle test that minimizes post-oral effects (Supplementary Fig. 2). The taste of MDG is not strongly positive to the rats, however, as 8.6% MDG was less preferred to 8% and 4% glucose but isopreferred to 2% glucose in 3-min tests. In marked contrast, B6 mice significantly preferred isomolar 8.6% MDG to 8% glucose in 3-min choice tests. These findings indicate that mice and rats differ in their taste response as well as their post-oral conditioning response to MDG. On the other hand, MDG can induce similar physiological responses, such as stimulating the gut hormone GLP-1, in the two species [13,20,23].
The nature of the sensors and signaling process responsible for post-oral glucose appetition is not completely understood, but some aspects are known. Rat studies implicate the upper intestine as a primary site of action [3], and mouse studies implicate the intestinal sugar sensors SGLT1 and SGLT3 rather than sweet receptor components T1R2/T1R3 in the process [29,43]. The evidence against a critical role for intestinal T1R2/T1R3 receptors is that IG infusions of fructose or sucralose, potent T1R2/T1R3 ligands, do not condition flavor preferences and that IG infusions of glucose or sucrose condition strong preferences in T1R3 knockout mice [29,30]. The evidence implicating the SGLT sensors in the appetition process includes the findings that IG infusions of SGLT ligands glucose, galactose, MDG, and 3-O-methyl-D-glucopyranoside stimulated CS+ intake in mice, with the SGLT3 selective sugars (glucose, MDG) being the most effective [43]. In addition, the SGLT inhibitor phloridzin blocked MDG-induced appetition, although both phloridzin and the GLUT2 inhibitor phloretin were needed to block glucose-induced appetition in mice [43]. The present finding that IG MDG suppressed rather than stimulated CS+ intake in rats obviously questions the role of SGLT sensors in sugar appetition in this species, as does our earlier finding that IG galactose conditioned a CS+ avoidance in rats [25]. Note that galactose is a weak appetition stimulus in B6 mice and does not condition a CS+ preference with 24-h training sessions, which may be due to inhibitory actions related to incomplete galactose metabolism in adult animals [30,43]. It may that rats are more sensitive to the inhibitory actions of MDG and galactose than mice, which might account for the ineffectiveness of these sugars to stimulate CS+ intake and preferences. Clearly rodents show a range of responses to sugars and their analogs, demonstrating that post-oral sugar reward is not a simple function of energy provision. Further studies with this and related methods should continue to identify the stimuli and sensors for post-oral sugar reward with greater precision.
5. Conclusions
Sugar appetite is initiated by stimulation of sweet taste receptors in the mouth and is further enhanced by post-oral sugar sensors through appetition, as revealed in rodents by the increased intake and preference for a flavored non-nutritive solution paired with intragastric sugar infusions. The test protocol described here can be used to investigate the peripheral and central processes involved in unconditioned and conditioned stimulation of intake by post-oral nutrients. If we can determine the nature of the signals and their receptors in the gut, this information could lead to new methods to enhance preferences for nutritious foods with complex flavors that are not unconditionally preferred.
Supplementary Material
Highlights.
Intragastric infusions of glucose rapidly stimulated intake and preference in rats.
These appetition effects resembled those previously seen in mice.
Non-metabolizable alpha-methyl-D-glucose (MDG) reduced intake and preference in rats.
In contrast, moderate MDG concentrations are post-orally rewarding in mice.
The method can be used to study peripheral and central post-oral food reward.
Acknowledgments
This research was supported by grant DK-31135 from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors thank Kwame McCartney and Martin Zartarian for their expert technical assistance.
Appendix A. Supplementary data
Supplementary data to this article can be found online.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ackroff K, Touzani K, Peets TK, Sclafani A. Flavor preferences conditioned by intragastric fructose and glucose: differences in reinforcement potency. Physiol Behav. 2001;72:691–703. doi: 10.1016/s0031-9384(01)00442-5. [DOI] [PubMed] [Google Scholar]
- 2.Ackroff K, Dym C, Yiin Y-M, Sclafani A. Rapid acquisition of conditioned flavor preferences in rats. Physiol Behav. 2009;97:406–13. doi: 10.1016/j.physbeh.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackroff K, Yiin Y-M, Sclafani A. Post-oral infusion sites that support glucose-conditioned flavor preferences in rats. Physiol Behav. 2010;99:402–11. doi: 10.1016/j.physbeh.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzara AV, Sclafani A. Flavor preferences conditioned by intragastric sugar infusions in rats: Maltose is more reinforcing than sucrose. Physiol Behav. 1998;64:535–41. doi: 10.1016/s0031-9384(98)00113-9. [DOI] [PubMed] [Google Scholar]
- 5.Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. Naltrexone fails to block the acquisition or expression of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacol Biochem Behav. 2000;67:545–57. doi: 10.1016/s0091-3057(00)00395-6. [DOI] [PubMed] [Google Scholar]
- 6.Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. D1 but not D2 dopamine receptor antagonism blocks the acquisition of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacol Biochem Behav. 2001;68:709–20. doi: 10.1016/s0091-3057(01)00484-1. [DOI] [PubMed] [Google Scholar]
- 7.Davis JD, Campbell CS. Chronic intrajugular, intraportal, gastric, and duodenal cannulae for the rat. In: Singh D, Avery DD, editors. Physiological techniques in behavioral research. Monterey: Brooks Cole; 1975. pp. 163–77. [Google Scholar]
- 8.Delaere F, Duchampt A, Mounien L, Seyer P, Duraffourd C, Zitoun C, et al. The role of sodium-coupled glucose co-transporter 3 in the satiety effect of portal glucose sensing. Molec Metab. 2013;2:47–53. doi: 10.1016/j.molmet.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drucker DB, Ackroff K, Sclafani A. Flavor preference produced by intragastric Polycose infusions in rats using a concurrent conditioning procedure. Physiol Behav. 1993;54:351–5. doi: 10.1016/0031-9384(93)90122-v. [DOI] [PubMed] [Google Scholar]
- 10.Drucker DB, Sclafani A. The role of gastric and post-gastric sites in glucose-conditioned flavor preferences in rats. Physiol Behav. 1997;61:351–8. doi: 10.1016/s0031-9384(96)00414-3. [DOI] [PubMed] [Google Scholar]
- 11.Elizalde G, Sclafani A. Flavor preferences conditioned by intragastric Polycose: A detailed analysis using an electronic esophagus preparation. Physiol Behav. 1990;47:63–77. doi: 10.1016/0031-9384(90)90043-4. [DOI] [PubMed] [Google Scholar]
- 12.Lucas F, Azzara AV, Sclafani A. Flavor preferences conditioned by intragastric Polycose in rats: More concentrated Polycose is not always more reinforcing. Physiol Behav. 1998;63:7–14. doi: 10.1016/s0031-9384(97)00364-8. [DOI] [PubMed] [Google Scholar]
- 13.Moriya R, Shirakura T, Ito J, Mashiko S, Seo T. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol. 2009;297:E1358–65. doi: 10.1152/ajpendo.00412.2009. [DOI] [PubMed] [Google Scholar]
- 14.Myers KP, Sclafani A. Conditioned acceptance and preference but not altered taste reactivity responses to bitter and sour flavors paired with intragastric glucose infusion. Physiol Behav. 2003;78:173–83. doi: 10.1016/s0031-9384(02)00890-9. [DOI] [PubMed] [Google Scholar]
- 15.Myers KP. Robust preference for a flavor paired with intragastric glucose acquired in a single trial. Appetite. 2007;48:123–7. doi: 10.1016/j.appet.2006.07.077. [DOI] [PubMed] [Google Scholar]
- 16.Myers KP, Whitney MC. Rats’ learned preferences for flavors encountered early or late in a meal paired with the postingestive effects of glucose. Physiol Behav. 2011;102:466–74. doi: 10.1016/j.physbeh.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Myers KP, Taddeo MS, Richards EK. Sensory-specific appetition: postingestive detection of glucose rapidly promotes continued consumption of a recently encountered flavor. Physiol Behav. 2013;121:125–33. doi: 10.1016/j.physbeh.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 18.O’Malley D, Reimann F, Simpson AK, Gribble FM. Sodium-coupled glucose cotransporters contribute to hypothalamic glucose sensing. Diabetes. 2006;55:3381–6. doi: 10.2337/db06-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliveira-Maia AJ, Roberts CD, Walker QD, Luo B, Kuhn C, Simon SA, et al. Intravascular food reward. PLoS One. 2011;6:e24992. doi: 10.1371/journal.pone.0024992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia. 2009;52:289–98. doi: 10.1007/s00125-008-1202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pérez C, Lucas F, Sclafani A. Increased flavor acceptance and preference conditioned by the postingestive actions of glucose. Physiol Behav. 1998;64:483–92. doi: 10.1016/s0031-9384(98)00104-8. [DOI] [PubMed] [Google Scholar]
- 22.Puerto A, Deutsch JA, Molina F, Roll P. Rapid discrimination of rewarding nutrient by the upper gastrointestinal tract. Science. 1976;192:485–7. doi: 10.1126/science.1257784. [DOI] [PubMed] [Google Scholar]
- 23.Ritzel U, Fromme A, Ottleben M, Leonhardt U, Ramadori G. Release of glucagon-like peptide-1 (GLP-1) by carbohydrates in the perfused rat ileum. Acta Diabetol. 1997;34:18–21. doi: 10.1007/s005920050059. [DOI] [PubMed] [Google Scholar]
- 24.Sclafani A, Cardieri C, Tucker K, Blusk D, Ackroff K. Intragastric glucose but not fructose conditions robust flavor preferences in rats. Am J Physiol. 1993;265:R320–R5. doi: 10.1152/ajpregu.1993.265.2.R320. [DOI] [PubMed] [Google Scholar]
- 25.Sclafani A, Fanizza LJ, Azzara AV. Conditioned flavor avoidance, preference and indifference produced by intragastric infusion of galactose, glucose, and fructose in rats. Physiol Behav. 1999;67:227–34. doi: 10.1016/s0031-9384(99)00053-0. [DOI] [PubMed] [Google Scholar]
- 26.Sclafani A. Post-ingestive positive controls of ingestive behavior. Appetite. 2001;36:79–83. doi: 10.1006/appe.2000.0370. [DOI] [PubMed] [Google Scholar]
- 27.Sclafani A, Glendinning JI. Flavor preferences conditioned in C57BL/6 mice by intragastric carbohydrate self-infusion. Physiol Behav. 2003;79:783–8. doi: 10.1016/s0031-9384(03)00174-4. [DOI] [PubMed] [Google Scholar]
- 28.Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol. 2005;289:R712–20. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- 29.Sclafani A, Glass D, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol. 2010;299:R1643–R50. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sclafani A, Ackroff K. Flavor preferences conditioned by intragastric glucose but not fructose or galactose in C57BL/6J mice. Physiol Behav. 2012;106:457–61. doi: 10.1016/j.physbeh.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sclafani A, Ackroff K. The role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1119–33. doi: 10.1152/ajpregu.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sclafani A. Gut-brain nutrient signaling: appetition vs. satiation. Appetite. 2013;71:454–8. doi: 10.1016/j.appet.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sclafani A, Zukerman S, Ackroff K. GPR40 and GPR120 fatty acid sensors are critical for post-oral but not oral mediation of fat preferences in the mouse. Am J Physiol Reg Integ Physiol. 2013;305:R1490–R7. doi: 10.1152/ajpregu.00440.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sclafani A, Marambaud P, Ackroff K. Sucrose-conditioned flavor preferences in sweet ageusic T1r3 and Calhm1 knockout mice. Physiol Behav. 2014;126:25–9. doi: 10.1016/j.physbeh.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tordoff MG, Friedman MI. Hepatic portal glucose infusions decrease food intake and increase food preference. Am J Physiol. 1986;251:R192–R6. doi: 10.1152/ajpregu.1986.251.1.R192. [DOI] [PubMed] [Google Scholar]
- 36.Touzani K, Bodnar RJ, Sclafani A. Lateral hypothalamus dopamine D1-like receptors and glucose-conditioned flavor preferences in rats. Neurobiol Learn Mem. 2009;92:464–7. doi: 10.1016/j.nlm.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Touzani K, Bodnar RJ, Sclafani A. Dopamine D1-like receptor antagonism in amygdala impairs the acquisition of glucose-conditioned flavor preference in rats. Eur J Neurosci. 2009;30:289–98. doi: 10.1111/j.1460-9568.2009.06829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Touzani K, Bodnar RJ, Sclafani A. Acquisition of glucose-conditioned flavor preference requires the activation of dopamine D1-like receptors within the medial prefrontal cortex in rats. Neurobiol Learn Mem. 2010;94:214–9. doi: 10.1016/j.nlm.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Touzani K, Bodnar RJ, Sclafani A. Glucose-conditioned flavor preference learning requires co-activation of NMDA and dopamine D1-like receptors within the amygdala. Neurobiol Learn Mem. 2013;106C:95–101. doi: 10.1016/j.nlm.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yiin Y-M, Ackroff K, Sclafani A. Food deprivation enhances the expression but not acquisition of flavor acceptance conditioning in rats. Appetite. 2005;45:152–60. doi: 10.1016/j.appet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Yiin Y-M, Ackroff K, Sclafani A. Flavor preferences conditioned by intragastric nutrient infusions in food restricted and free-feeding rats. Physiol Behav. 2005;84:217–31. doi: 10.1016/j.physbeh.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Zukerman S, Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol. 2011;301:R1635–R47. doi: 10.1152/ajpregu.00425.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zukerman S, Ackroff K, Sclafani A. Post-oral appetite stimulation by sugars and non-metabolizable sugar analogs in mice: role of intestinal SGLT sugar sensors. Am J Physiol. 2013;305:R840–R53. doi: 10.1152/ajpregu.00297.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zukerman S, Ackroff K, Sclafani A. Post-oral glucose stimulation of intake and conditioned flavor preference in C57BL/6J mice: A concentration-response study. Physiol Behav. 2013;109:33–41. doi: 10.1016/j.physbeh.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.