Abstract
Background and Objective
Periodontitis is currently diagnosed almost entirely on gross clinical manifestations that have been in situ for more than 50 years without significant improvement. The general objective of this study was, therefore, to evaluate whether mid-infrared spectroscopy can be used to identify disease-specific molecular alterations to the overall biochemical profile of tissues and body fluids.
Material and Methods
A total of 190 gingival crevicular fluid samples were obtained from periodontitis (n = 64), gingivitis (n = 61) and normal sites (n = 65). Corresponding infrared absorption spectra of gingival crevicular fluid samples were acquired and processed, and the relative contributions of key functional groups in the infrared spectra were analysed. The qualitative assessment of clinical relevance of these gingival crevicular fluid spectra was interpreted with the multivariate statistical analysis-linear discriminant analysis.
Results
Using infrared spectroscopy, we have been able to identify four molecular signatures (representing vibrations in amide I, amide II/tyrosine rings and symmetric and asymmetric stretching vibrations of phosphodiester groups in DNA) in the gingival crevicular fluid of subjects with periodontitis or gingivitis and healthy control subjects that clearly demarcate healthy and diseased periodontal tissues. Furthermore, the diagnostic accuracy for distinction between periodontally healthy and periodontitis sites revealed by multivariate classification of gingival crevicular fluid spectra was 98.4% for a training set of samples and 93.1% for a validation set.
Conclusion
We have established that mid-infrared spectroscopy can be used to identify periodontitis-specific molecular signatures in gingival crevicular fluid and to confirm clinical diagnoses. Future longitudinal studies will assess whether mid-infrared spectroscopy represents a potential prognostic tool, recognized as key to advancement of periodontics.
Keywords: gingival crevicular fluid, inflammation, infrared spectroscopy, periodontitis
Periodontitis is an infectious disease of the tissues surrounding the teeth, occurring in about 50% of the population and resulting in significant debilitation for about half of these persons (1). A leading cause of edentulism, periodontitis has been associated with increased risk of multiple systemic illnesses, including vascular diseases and diabetes (2). Therefore, the prevention or treatment of local inflammatory disease (periodontitis) may be an important factor in the control of systemic inflammatory conditions.
Periodontitis is currently diagnosed almost entirely on gross clinical manifestations: signs of gingival inflammation such as redness and bleeding on probing, periodontal pocketing and loss of periodontal attachment or alveolar bone (3). These clinically based diagnostic parameters have been in situ for more than 50 years, but do not reliably identify susceptible individuals or distinguish between progressing lesions and lesions that represent the consequences of historical disease activity. The American Academy of Periodontology, in partnership with Centers of Disease Control, has recognized that the development of improved surveillance and prognostic tools is key to advancement of periodontics (4–7). Indeed, considerable efforts are underway to develop biochemical and microbiological diagnostic and prognostic tools (8,9).
Gingival crevicular fluid is an inflammatory exudate with constituents derived from a number of sources, including serum, the connective tissue and the epithelium through which gingival crevicular fluid passes on its way to the gingival crevice, in addition to inflammatory cells and bacteria present in the tissues and crevice (9). Over 65 biologically active components have been identified in gingival crevicular fluid. Explored diagnostic and prognostic plaque or gingival crevicular fluid markers for periodontitis include the presence of the following: (1) specific bacteria (including Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans) (10); (2) their products [e.g. volatile sulfur compounds (11) or specific proteases (12)]; (3) biomarkers involved in the disease process but produced by the host [e.g. matrix metalloproteinases (13), neutrophil elastase and alkaline phosphatase (9,14)]; (4) biomarkers of tissue damage (e.g. hydroxyproline/collagen fragments) (9,15); and (5) other markers of the inflammatory process such as prostaglandin E2 and interleukin-1 (9). However, despite the complex nature of periodontal diseases, which involves a multifaceted immune and inflammatory reaction to a polymicrobial flora, and the inter-individual variation in inflammatory responses (16,17), such potential biomarkers are generally studied individually and rarely in small numbers. This may explain why the predictive value of potential biomarkers studied to date has not been sufficient for routine clinical use (9).
Infrared spectroscopy (IRS) measures transitions in vibrational levels on infrared (IR) absorption and reveals disease-specific alterations to multiple biologically significant molecules. Infrared spectroscopy has recently emerged as a powerful modality for the diagnosis of disease (18–21). Infrared spectroscopy analysis of gingival crevicular fluid, unlike prior biochemical analyses, measures the total contents of gingival crevicular fluid and should prove a powerful diagnostic and prognostic tool for periodontal diseases. Therefore, we employed IRS to characterize gingival crevicular fluid from healthy, gingivitis and periodontitis sites and determined specific spectral signatures that clearly demarcate healthy and diseased tissues. The long-term goal is to develop a simple, reagent-free, user-friendly, rapid and minimally invasive prognostic tool for inflammatory periodontal diseases.
Material and methods
Subjects
Thirty patients [mean age (± SD), 54.4 ± 16.6 years; 14 females] with chronic moderate to severe periodontitis, attending the Graduate Periodontics Clinics at the University of Manitoba, participated in the study. The research protocol was approved by each of the Research Ethics Boards of the University of Manitoba and by the Institute for Biodiagnostics, National Research Council of Canada. Informed, written consent was obtained from each individual prior to collection of samples. Periodontitis sites were defined as those with periodontal probing depth ≥ 5 mm and clinical attachment loss ≥ 3 mm, with or without bleeding on probing. Gingivitis sites were defined as those with periodontal probing depths < 3 mm and bleeding on probing. Healthy sites were defined as those with periodontal probing depths < 3 mm and no bleeding on probing and positioned contralateral, or nearest to contralateral, to the diseased site. Both healthy and diseased sites were chosen from the same patients. All gingival crevicular fluid samples were collected prior to clinical measurements from 64 periodontitis sites, 61 gingivitis sites and 65 normal sites. All clinical parameters were assessed and gingival crevicular fluid samples obtained by the same examiner (X.M.X.).
Collection of gingival crevicular fluid
Gingival crevicular fluid samples were collected from healthy, gingivitis and/or periodontitis sites within an individual essentially as we have previously described (22). Briefly, sites were isolated with cotton wool in order to minimize the risk of salivary contamination. Sites to be sampled were gently air-dried, following the removal of supragingival plaque with a curette. A strip of perio-paper was then inserted into the gingival crevice until it met with light resistance. The strip was removed after 30 s, and the volume of gingival crevicular fluid measured using a precalibrated Periotron 6000 (IDE Interstate, Amityville, NY, USA). The perio-paper strip was then placed into a sterile 2 mL conical tube containing 80 μL ice-cold saline. The tube was then vortexed for 30 s and centrifuged (10 min, 800 g) with the strip placed slightly above the fluid meniscus. Any samples obviously contaminated by saliva or blood were rejected.
Acquisition of infrared spectra
Samples were prepared for spectroscopic measurements by drying gingival crevicular fluid onto a silicon wafer demarcated into 96 circular wells (5 mm diameter) by an adhesive plastic mask (23). Infrared spectra of such films were recorded with a Bruker Vector 22 spectrometer (Bruker Optics, Billerica, MA, USA) equipped with a high-throughput sampling accessory and a home-built wafer mount. The high-throughput sampling accessory allows for the automated, sequential acquisition of spectra for 95 films and a blank well used for the background measurement distributed on the silicon wafer with the same spacing as a 96-well microtiter plate. Spectra were acquired at a nominal resolution of 4 cm−1, with 256 scans co-added per spectrum and for background measurement. Absorbance spectra were converted into second derivative spectra using the Savitzky– Golay algorithm with a nine-point window for the multivariate statistical analysis. The band integrations of the relative components of each spectrum were calculated using GRAMS/32 AI software (Thermo Scientific, Waltham, MA, USA). This software integrates and computes the area of selected peaks in a user-interactive mode by defining two end-points on the bottom trace of the spectrum. To make it consistent and comparable, the end-points for each band area integration were predefined to avoid artificial errors.
Multivariate statistical analysis of infrared spectra
Linear discriminant analysis (LDA) is known as a supervised method because the class identity of each sample is supplied to the classification algorithm and used in establishing boundaries to distinguish among groups of spectra in different classes (23,24). Taking the spectra and the corresponding diagnoses (class designations) as input, it explicitly chooses the boundaries that best separate the classes. Class assignment of any given spectrum comprises computing its distance from all class centroids (representative class average spectra) and allotting it to the class whose centroid is nearest. To properly assess the predictive value of this classification procedure, the spectra were split into a training set, which was used as the basis to identify discriminatory patterns, and an independent test set to assess the accuracy of the trained algorithm in classifying samples of unknown origin. Approximately two-thirds of the samples were designated the training set and the remaining one-third the test set, as we have previously described (25). The training set for periodontitis and control groups comprised 27 periodontitis and 35 control samples, and the test set comprised 16 periodontitis and 12 control samples. Similarly, the training set for gingivitis and control groups consists of 25 gingivitis and 33 control gingival crevicular fluid samples, while 15 gingivitis and 14 control gingival crevicular fluid samples were used for the test set.
Results
Clinical characteristics
The clinical characteristics of the sites from recruited subjects are presented in Table 1.
Table 1. Periodontal health among subject groups.
| Clinical parameters | ||
|---|---|---|
|
|
||
| Group | Probing depth | Clinical attachment loss |
| Healthy sites (n = 65) | 2.06 ± 0.74 | 2.64 ± 1.06 |
| Gingivitis sites (n = 61) | 2.60 ± 0.54* | 3.49 ± 1.22* |
| Periodontitis sites (n = 64) | 6.00 ± 1.41*† | 6.46 ± 1.42*† |
p < 0.01 compared with healthy sites;
p < 0.01 compared with gingivitis sites. The clinical data are expressed as means ± SD.
Infrared spectral features of gingival crevicular fluid samples
Several major differentiating IR bands were visualized in the spectral range of 900-1800 cm−1 (Fig. 1). The two prominent absorptions at 1652 and 1542 cm−1 arise from C=O stretching (amide I band) and N–H bending (amide II band) vibrations of peptide groups (20,21). The bands at 1087 and 1240 cm−1 are stretching vibrations of phosphodiester groups in DNA, while the band at 1740 cm−1 originates from lipid ester C=O groups (20,26). The lipid ester intensity in the gingival crevicular fluid from periodontitis sites was much lower than that in healthy gingival crevicular fluid. A cluster of CH2 and CH3 stretching vibrations, mainly attributing to gingival crevicular fluid lipids, were found in the spectral range of 2800-3100 cm-1. Other distinguishing spectral features included the proteinaceous amide B band at 3050 cm−1 and the amide A band at 3290 cm−1 (20,27). Thus, disease-specific cellular and molecular alterations to the composition of gingival crevicular fluid are clear, most obviously the increased intensity of the 1652 cm−1 amide I band at inflammatory sites (gingivitis and periodontitis) compared with healthy crevices.
Fig. 1.
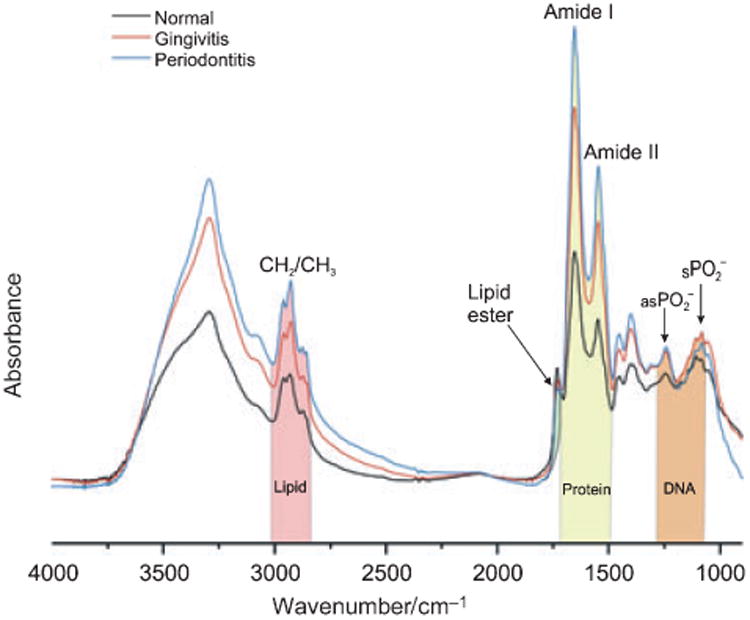
Mean mid-infrared spectra of gingival crevicular fluid samples from healthy, gingivitis and periodontitis sites. The shaded bands represent the infrared bands originating from molecular signatures from lipid, protein and nucleic acid in gingival crevicular fluid samples. , Symmetric ; as , asymmetric .
While much of the mean gingival crevicular fluid spectra of the inflammatory and control groups appears superficially similar, we used Fourier self-deconvolution to narrow effective bandwidths, enhance resolution and increase available discriminatory data (26; Fig. 2), which revealed important discriminatory features, such as the DNA band at 1713 cm−1 characteristic of base-paired DNA strands (28). By integrating the three major DNA sensitive bands (the bands at 1087 and 1240 cm−1 arising from symmetric and asymmetric stretching vibrations of phosphodiester groups in DNA and the 1713 cm−1 band) we can see that gingival crevicular fluid DNA concentrations in diseased sites are increased compared with healthy sites. The increased DNA content in the inflammatory gingival crevicular fluid samples might suggest a provocative condition during the inflammatory condition, with active enrolled leukocytes, bacteria and shedding epithelial cells in the gingival crevicular fluid samples.
Fig. 2.
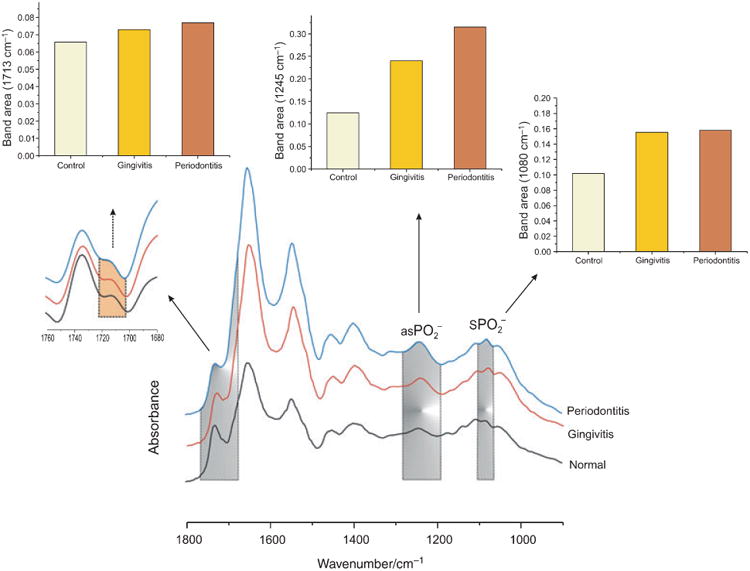
Relative DNA contributions are increased in diseased gingival crevicular fluid groups. The shaded areas highlight DNA-specific signals in gingival crevicular fluid. The inset traces are an enlarged area of another important DNA band, 1713 cm−1, arising from DNA pair base vibration after Fourier self-deconvolution. Also shown are histograms representing the integrated area (relative DNA content) in the mean spectra from the three groups. , symmetric ; as , asymmetric .
Increased protein (amide I at 1652 cm−1) and lipid signals (symmetric CH2 stretching vibration at 2853 cm-1 from the fatty acyl chains) are also evident at diseased sites (Fig. 3). The integrated area of the =CH band at 3012 cm−1 is used as an index of the relative concentration of double bonds in lipid structures from unsaturated fatty acyl chains (linolenic, arachidonic, etc.) arising from lipid peroxidation (27,29). Interestingly, lipid oxidation is increased in the inflammatory groups, as evidenced by the olefinic =CH band at 3012 cm−1, which provides further evidence of the importance of lipid peroxidation in periodontal disease pathogenesis (30,31). The lipid ester band arising from C=O group at 1740 cm−1, however, decreased in the periodontitis group.
Fig. 3.
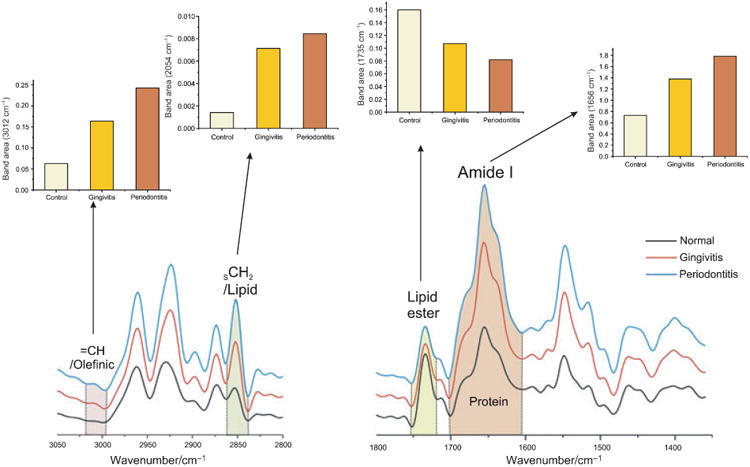
Relative concentration of protein and lipid components derived from gingival crevicular fluid IR mean spectra after Fourier self-deconvolution procedure. The histograms represent the integrated area (relative protein, lipid and lipid peroxidation content) in the spectra from the three groups. The differences in protein and lipid content of gingival crevicular fluid from diseased and healthy sites are apparent.
Linear discriminant analysis
Qualitative analyses were used to correlate observed spectral differences of gingival crevicular fluid from inflammatory groups (gingivitis and periodontitis) and normal control samples. Linear discriminant analysis was the classifier of choice because of its robustness and speed. Four proteinand DNA-specific spectral regions selected by the optimal regional selection algorithm for best differentiation of diseased and control groups by LDA are presented in Fig. 4. In the first step, 62 gingival crevicular fluid spectra from two groups (control and periodontitis) were used as a training set, whereby the LDA algorithm was trained to recognize and predict the spectral features characteristic of control and periodontitis samples. Training and prediction was optimized using the leave-one-out method of cross-validation (23,24). Sixty-one of the 62 spectra were split into control and periodontitis samples and used to train the algorithm to recognize features characteristic of each group. The trained algorithm was then used to classify the 62nd spectrum (not used in the training) based upon the presence or absence of these characteristic features. This process was then repeated with a different spectrum omitted from the training set until the LDA was performed 62 times. The validation set (29 control and periodontitis gingival crevicular fluid spectra) was then presented to the model blindly to probe the predictive accuracy. The ability of the LDA procedure to adequately predict periodontitis was impressive, as shown in Table 2.
Fig. 4.
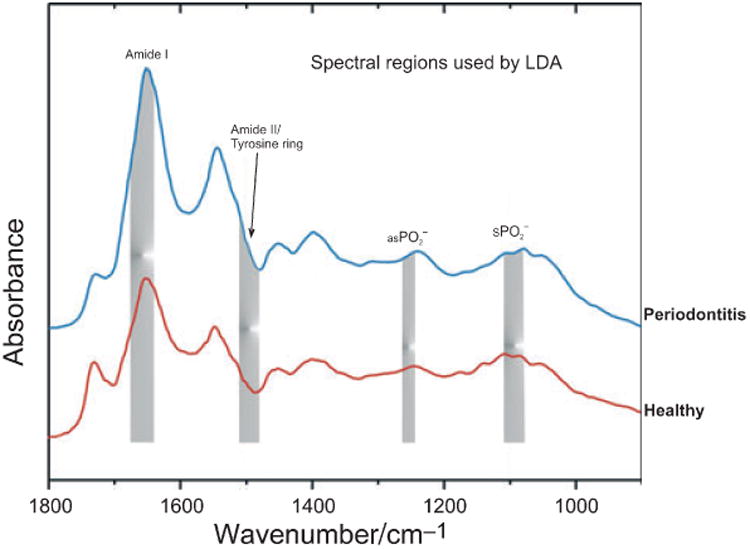
Linear discriminant analysis of the normal and periodontitis groups. The shaded areas identify four signature spectral regions selected by the optimal regional selection algorithm as contributing most significantly to the differentiation of the normal and periodontitis groups by linear discriminant analysis. , symmetric ; as , asymmetric .
Table 2. Diagnostic accuracy of periodontitis based on IR spectra of gingival crevicular fluid.
| Accuracy (%) | Specificity (%) | Positive predictive value (%) | |||
|---|---|---|---|---|---|
| Training set | |||||
| Control | 26 | 1 | 96.3 | 100 | 100 |
| Periodontitis | 0 | 35 | 100 | 96.3 | 97.2 |
| Validation set | |||||
| Control | 11 | 2 | 84.6 | 100 | 100 |
| Periodontitis | 0 | 16 | 100 | 84.6 | 88.9 |
Overall accuracy was 98.4% on the training set and 93.l% on the test set. Diagnosis of periodontitis was determined by linear discriminant analysis of the infrared spectra. Bold numbers indicate accurate classifications. Underlined numbers indicate inaccurate classifications.
A similar approach was employed to classify gingival crevicular fluid from gingivitis and healthy control sites, as shown in Table 3. The overall accuracy for the classification of gingival crevicular fluid samples as control or gingivitis was 91.4% for the training set and 72.4% for the validation set. This suggests that the gingivitis-specific molecular alterations to gingival crevicular fluid are less profound than in periodontitis, as would be expected.
Table 3. Diagnostic accuracy of gingivitis based on IR spectra of gingival crevicular fluid.
| Accuracy (%) | Specificity (%) | Positive predictive value (%) | |||
|---|---|---|---|---|---|
| Training set | |||||
| Control | 32 | 1 | 97.0 | 84.0 | 88.9 |
| Gingivitis | 4 | 21 | 84.0 | 97.4 | 95.5 |
| Validation set | |||||
| Control | 12 | 2 | 85.7 | 60.0 | 66.7 |
| Gingivitis | 6 | 9 | 60.0 | 85.7 | 81.8 |
Overall accuracy was 91.4% on the training set and 72.4% on the test set. Diagnosis of periodontitis was determined by linear discriminant analysis of the infrared spectra. Bold numbers indicate accurate classifications. Underlined numbers indicate inaccurate classifications.
Discussion
Gingival crevicular fluid is composed of serum and locally generated materials, including tissue breakdown products, bacterial components and products, and inflammatory and immune mediators. Thus, gingival crevicular fluid contains a wealth of information on periodontal disease processes. A major attraction of gingival crevicular fluid as a diagnostic marker is the site-specific nature of the sample. This allows sophisticated analyses of gingival crevicular fluid components to be linked to clinical assessment at the site of sample collection. Owing to the complexity of the pathogenic tissue alterations associated with periodontitis, which are reflected in gingival crevicular fluid composition, we had hypothesized that IR spectroscopy could reliably differentiate diseased and healthy periodontal sites. We have been able to show that even in unprocressed spectral data (Fig. 1), subtle differences in spectral band intensity and positions arising from the three major components, i.e. lipid, protein and DNA, can be seen in gingival crevicular fluid from healthy, gingivitis and periodontitis groups.
A more sophisticated means with which to extract the relevant biological information hidden in raw infrared spectra is to assess the relative contributions of key functional groups by integrating the band areas, which usually provide more information about concentrations than band intensities. Indeed, as is clearly demonstrated in Figs 2 and 3, we are able to reliably analyze and compare specific gingival crevicular fluid components among disease groups. For example, the protein concentration in both disease groups is higher than in control samples, in agreement with prior reports of increased total protein levels in periodontitis gingival crevicular fluid (32), and a significant correlation between total gingival crevicular fluid protein concentration and disease severity (33). Many gingival crevicular fluid proteins have been extensively explored as potential diagnostic markers that define periodontal inflammation. They include inflammatory mediators, particularly cytokines and matrix metalloproteinases, and tissue breakdown products, such as fibronectin, collagen fragments and hydroxyproline, which should reflect the extent of underlying tissue destruction. Our results suggest that the total protein signal is elevated in periodontal disease, which is consistent with our spectral observations.
Infrared spectroscopy is increasingly recognized as an alternative modality for accurate determination of lipid peroxidation in various biological samples (29,34,35). Increased oxidative stress is the consequence of enhanced production of reactive oxygen species and/or attenuated reactive oxygen species scavenging capacity, resulting in tissue damage reflected in increased lipid peroxidation. Loss of unsaturation during lipid peroxidation reactions was compensated by the presence of double bonds in the lipid peroxidation products such as malondialdehyde, lipid aldehydes and alkyl radicals. It is well recognized that lipid peroxidation markedly increases during periodontal inflammation (36–38). For instance, the malondialdehyde contents of chronic apical periodontitis tissues are higher than in healthy tissue of the same individuals (39). Tsai et al. (31) recently reported that the total amount of gingival crevicular fluid lipid peroxidation correlated positively with several clinical disease parameters, indicating that the more severe the inflammation of the periodontal tissue the higher the level of lipid peroxidation. Furthermore, periodontal oxidative stress may even have systemic consequences, with higher blood lipid peroxidation concentrations noted in a rat model of periodontitis compared with periodontally healthy control animals (40). Several studies have also demonstrated that IR spectroscopic analysis of the integrated area in the 3030–2990 cm−1 region, representing the olefinic band arising from the unsaturated lipids, can be employed to assess lipid peroxidation in various biological samples (29,35,41). In keeping with such prior reports, our spectral data show that significant disease-related changes occur in the lipid content of gingival crevicular fluid, indicative of increased oxidative stress (see Fig. 3).
We have previously shown that by using the DNA-specific signals embedded in the IR spectra of the leukocytes, one can precisely diagnose leukemia and predict leukocytic apoptosis (20,24,29). We can now apply this newly developed technology to the study of periodontal diseases. Gingival crevicular fluid contains a diverse population of cells, which include bacteria, desquamated epithelia and transmigrating leukocytes (42,43). The increased DNA component in gingival crevicular fluid from gingivitis and periodontitis sites, relative to healthy control sites, is likely to be due to a combination of an inflammation-driven increase in leukocyte migration into the gingival crevicular fluid, particularly neutrophils, an increase in epithelial turnover, reflecting ongoing tissue remodelling, and the inflammatory stimulus itself, i.e. plaque bacteria.
Periodontal disease is clearly multi-factorial, yet previous attempts to use gingival crevicular fluid analysis as a diagnostic tool have generally relied on the measurement of only one or two specific gingival crevicular fluid components. Since IR spectroscopy measures the composite molecular content of gingival crevicular fluid, then assuming that molecular alterations do occur during the disease process, the chance for IR spectroscopy to distinguish various stages of periodontitis should be promising, in contrast to previous one-dimensional biochemical approaches. This hypothesis is supported by our LDA studies, which consider multiple components in the gingival crevicular fluid as the basis to designate individual spectra as healthy or diseased. The accuracy of this LDA approach in diagnosing periodontitis was 98.4% for the training set and 93.1% for the validation set. However, the overall accuracy for the gingival crevicular fluid spectral classification between control sampless and gingivitis was 91.4%, and for the training set 72.4%. This discrepancy might reflect fundamental pathological differences between periodontitis and gingivitis, with irreversible tissue damage the hallmark of periodontitis. Also, the changes in three major components (protein, lipid and DNA; Figs 2 and 3) in the gingival crevicular fluid from the periodontitis group were much higher than in the gingivitis group. Such molecular data were readily picked up by LDA, leading to optimal diagnostic accuracy for the periodontitis group (93.1%) but only an acceptable level for the gingivitis group (72.4%).
In addition to high accuracy, there are several other advantages to IR spectroscopic screening and diagnosis of periodontitis. Infrared spectroscopy is reagent free and requires only small sample volumes. The gingival crevicular fluid samples are essentially unprocessed and the process is readily automated. Infrared spectroscopy is straightforward, and minimal training is required of operators following automation. Gingival crevicular fluid samples are easily collected by clinicians, with sample collection targeted to specific sites or to a representative set of teeth.
In summary, these studies show that we have established the appropriate IRS technology and the proof of principle that IRS can be used to identify disease-specific molecular signatures in gingival crevicular fluid cross-sectionally. Longitudinal studies are required to ascertain whether IRS is a suitable prognostic tool, long-sought by the periodontal community (4–7).
Acknowledgments
This study was generously supported by Natural Sciences and Engineering Research Council and Canadian Institute of Health Research, Canada (CPG 75128), and National Institute of Dental and Craniofacial Research, USA (R21DE017160).
References
- 1.Brown LJ, Loe H. Prevalence, extent, severity and progression of periodontal disease. Periodontol 2000. 1993;2:57–71. doi: 10.1111/j.1600-0757.1993.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 2.Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13(suppl 4):3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 3.Armitage GC. Diagnosis of periodontal diseases. J Periodontol. 2003;74:1237–1247. doi: 10.1902/jop.2003.74.8.1237. [DOI] [PubMed] [Google Scholar]
- 4.Giannobile WV. Periodontal surveillance – prospects for the future. J Periodontol. 2007;78:1365. doi: 10.1902/jop.2007.077002. [DOI] [PubMed] [Google Scholar]
- 5.Kwok V, Caton JG. Commentary: prognosis revisited: a system for assigning periodontal prognosis. J Periodontol. 2007;78:2063–2071. doi: 10.1902/jop.2007.070210. [DOI] [PubMed] [Google Scholar]
- 6.Eke PI, Genco RJ. CDC Periodontal Disease Surveillance Project: background, objectives, and progress report. J Periodontol. 2007;78:1366–1371. doi: 10.1902/jop.2007.070134. [DOI] [PubMed] [Google Scholar]
- 7.Tomar SL. Public health perspectives on surveillance for periodontal diseases. J Periodontol. 2007;78:1380–1386. doi: 10.1902/jop.2007.060340. [DOI] [PubMed] [Google Scholar]
- 8.Kinney JS, Ramseier CA, Giannobile WV. Oral fluid-based biomarkers of alveolar bone loss in periodontitis. Ann N Y Acad Sci. 2007;1098:230–251. doi: 10.1196/annals.1384.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamster IB, Ahlo JK. Analysis of gingival crevicular fluid as applied to the diagnosis of oral and systemic diseases. Ann N Y Acad Sci. 2007;1098:216–229. doi: 10.1196/annals.1384.027. [DOI] [PubMed] [Google Scholar]
- 10.D'Ercole S, Catamo G, Piccolomini R. Diagnosis in periodontology: a further aid through microbiological tests. Crit Rev Microbiol. 2008;34:33–41. doi: 10.1080/10408410701693317. [DOI] [PubMed] [Google Scholar]
- 11.Morita M, Wang HL. Relationship of sulcular sulfide level to severity of periodontal disease and BANA test. J Periodontol. 2001;72:74–78. doi: 10.1902/jop.2001.72.1.74. [DOI] [PubMed] [Google Scholar]
- 12.Loesche WJ, Bretz WA, Kerschensteiner D, et al. Development of a diagnostic test for anaerobic periodontal infections based on plaque hydrolysis of benzoyl-DL-arginine-naphthylamide. J Clin Microbiol. 1990;28:1551–1559. doi: 10.1128/jcm.28.7.1551-1559.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munjal SK, Prescher N, Struck F, Sorsa T, Maier K, Netuschil L. Evaluation of immunoassay-based MMP-8 detection in gingival crevicular fluid on a point-of-care platform. Ann N Y Acad Sci. 2007;1098:490–492. doi: 10.1196/annals.1384.018. [DOI] [PubMed] [Google Scholar]
- 14.Bowers MR, Fisher LW, Termine JD, Somerman MJ. Connective tissue-associated proteins in crevicular fluid: potential markers for periodontal diseases. J Periodontol. 1989;60:448–451. doi: 10.1902/jop.1989.60.8.448. [DOI] [PubMed] [Google Scholar]
- 15.Huynh QN, Wang S, Tafolla E, et al. Specific fibronectin fragments as markers of periodontal disease status. J Periodontol. 2002;73:1101–1110. doi: 10.1902/jop.2002.73.10.1101. [DOI] [PubMed] [Google Scholar]
- 16.Kinane DF, Hart TC. Genes and gene polymorphisms associated with periodontal disease. Crit Rev Oral Biol Med. 2003;14:430–449. doi: 10.1177/154411130301400605. [DOI] [PubMed] [Google Scholar]
- 17.Kinane DF, Mark Bartold P. Clinical relevance of the host responses of periodontitis. Periodontol 2000. 2007;43:278–293. doi: 10.1111/j.1600-0757.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 18.Sahu R, Mordechai S. Fourier transform infrared spectroscopy in cancer detection. Future Oncol. 2005;1:635–647. doi: 10.2217/14796694.1.5.635. [DOI] [PubMed] [Google Scholar]
- 19.Hynes A, Scott DA, Man A, Singer DL, Sowa MG, Liu KZ. Molecular mapping of periodontal tissues using infrared microspectroscopy. BMC Med Imaging. 2005;5:2. doi: 10.1186/1471-2342-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu KZ, Xu M, Scott DA. Biomolecular characterisation of leucocytes by infrared spectroscopy. Br J Haematol. 2007;136:713–722. doi: 10.1111/j.1365-2141.2006.06474.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu KZ, Tsang KS, Li CK, Shaw RA, Mantsch HH. Infrared spectroscopic identification of beta-thalassemia. Clin Chem. 2003;49:1125–1132. doi: 10.1373/49.7.1125. [DOI] [PubMed] [Google Scholar]
- 22.Fraser HS, Palmer RM, Wilson RF, Coward PY, Scott DA. Elevated systemic concentrations of soluble ICAM-1 (sICAM) are not reflected in the gingival crevicular fluid of smokers with periodontitis. J Dent Res. 2001;80:1643–1647. doi: 10.1177/00220345010800070901. [DOI] [PubMed] [Google Scholar]
- 23.Somorjai RL, Nikulin AE, Pizzi N, et al. Computerized consensus diagnosis: a classification strategy for the robust analysis of MR spectra. I. Application to 1H spectra of thyroid neoplasms. Magn Reson Med. 1995;33:257–263. doi: 10.1002/mrm.1910330217. [DOI] [PubMed] [Google Scholar]
- 24.Liu KZ, Schultz CP, Johnston JB, Lee K, Mantsch HH. Comparison of infrared spectra of CLL cells with their ex vivo sensitivity (MTT assay) to chlorambucil and cladribine. Leuk Res. 1997;21:1125–1133. doi: 10.1016/s0145-2126(97)00060-x. [DOI] [PubMed] [Google Scholar]
- 25.Borden JT, Man A, Scott DA, Liu KZ. Tobacco-induced alterations to the Fourier-transform infrared spectrum of serum. J Mol Med. 2003;81:788–794. doi: 10.1007/s00109-003-0490-3. [DOI] [PubMed] [Google Scholar]
- 26.Surewicz WK, Mantsch HH. New insight into protein secondary structure from resolution-enhanced infrared spectra. Biochim Biophys Acta. 1988;952:115–130. doi: 10.1016/0167-4838(88)90107-0. [DOI] [PubMed] [Google Scholar]
- 27.Liu KZ, Jia L, Kelsey SM, Newland AC, Mantsch HH. Quantitative determination of apoptosis on leukemia cells by infrared spectroscopy. Apoptosis. 2001;6:269–278. doi: 10.1023/a:1011383408381. [DOI] [PubMed] [Google Scholar]
- 28.Taillandier E, Liquier J. Infrared spectroscopy of DNA. Methods Enzymol. 1992;211:307–335. doi: 10.1016/0076-6879(92)11018-e. [DOI] [PubMed] [Google Scholar]
- 29.Severcan F, Gorgulu G, Gorgulu ST, Guray T. Rapid monitoring of diabetes-induced lipid peroxidation by Fourier transform infrared spectroscopy: evidence from rat liver microsomal membranes. Anal Biochem. 2005;339:36–40. doi: 10.1016/j.ab.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Sheikhi M, Bouhafs RK, Hammarstrom KJ, Jarstrand C. Lipid peroxidation caused by oxygen radicals from Fusobacterium-stimulated neutrophils as a possible model for the emergence of periodontitis. Oral Dis. 2001;7:41–46. [PubMed] [Google Scholar]
- 31.Tsai CC, Chen HS, Chen SL, et al. Lipid peroxidation: a possible role in the induction and progression of chronic periodontitis. J Periodontal Res. 2005;40:378–384. doi: 10.1111/j.1600-0765.2005.00818.x. [DOI] [PubMed] [Google Scholar]
- 32.Akalin FA, Sengun D, Eratalay K, Renda N, Caglayan G. Hydroxyproline and total protein levels in gingiva and gingival crevicular fluid in patients with juvenile, rapidly progressive, and adult periodontitis. J Periodontol. 1993;64:323–329. doi: 10.1902/jop.1993.64.5.323. [DOI] [PubMed] [Google Scholar]
- 33.Baltacioglu E, Akalin FA, Alver A, Deger O, Karabulut E. Protein carbonyl levels in serum and gingival crevicular fluid in patients with chronic periodontitis. Arch Oral Biol. 2008;53:716–722. doi: 10.1016/j.archoralbio.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Sills RH, Moore DJ, Mendelsohn R. Erythrocyte peroxidation: quantitation by Fourier transform infrared spectroscopy. Anal Biochem. 1994;218:118–123. doi: 10.1006/abio.1994.1149. [DOI] [PubMed] [Google Scholar]
- 35.Liu KZ, Bose R, Mantsch HH. Infrared spectroscopic study of diabetic platelets. Vib Spectrosc. 2002;28:131–136. [Google Scholar]
- 36.Battino M, Ferreiro MS, Gallardo I, Newman HN, Bullon P. The antioxidant capacity of saliva. J Clin Periodontol. 2002;29:189–194. doi: 10.1034/j.1600-051x.2002.290301x.x. [DOI] [PubMed] [Google Scholar]
- 37.Panjamurthy K, Manoharan S, Ramachandran CR. Lipid peroxidation and antioxidant status in patients with periodontitis. Cell Mol Biol Lett. 2005;10:255–264. [PubMed] [Google Scholar]
- 38.Buduneli N, Kardesler L, Isik H, et al. Effects of smoking and gingival inflammation on salivary antioxidant capacity. J Clin Periodontol. 2006;33:159–164. doi: 10.1111/j.1600-051X.2006.00892.x. [DOI] [PubMed] [Google Scholar]
- 39.Marton IJ, Balla G, Hegedus C, et al. The role of reactive oxygen intermediates in the pathogenesis of chronic apical periodontitis. Oral Microbiol Immunol. 1993;8:254–257. doi: 10.1111/j.1399-302x.1993.tb00570.x. [DOI] [PubMed] [Google Scholar]
- 40.Sobaniec H, Sobaniec-Lotowska ME. Morphological examinations of hard tissues of periodontium and evaluation of selected processes of lipid peroxidation in blood serum of rats in the course of experimental periodontitis. Med Sci Monit. 2000;6:875–881. [PubMed] [Google Scholar]
- 41.Toyran N, Zorlu F, Donmez G, Oge K, Severcan F. Chronic hypoperfusion alters the content and structure of proteins and lipids of rat brain homogenates: a Fourier transform infrared spectroscopy study. Eur Biophys J. 2004;33:549–554. doi: 10.1007/s00249-004-0396-1. [DOI] [PubMed] [Google Scholar]
- 42.Delima AJ, Van Dyke TE. Origin and function of the cellular components in gingival crevice fluid. Periodontol 2000. 2003;31:55–76. doi: 10.1034/j.1600-0757.2003.03105.x. [DOI] [PubMed] [Google Scholar]
- 43.Palmer RM, Wilson RF, Hasan AS, Scott DA. Mechanisms of action of environmental factors – tobacco smoking. J Clin Periodontol. 2005;32(suppl 6):180–195. doi: 10.1111/j.1600-051X.2005.00786.x. [DOI] [PubMed] [Google Scholar]


