Abstract
Background
In cohort studies, elevated plasma levels of non-esterified free fatty acids (P-NEFA) have been associated with increased risk of sudden cardiac death (SCD) in men, but blood samples were drawn several years prior to SCD.
Objective
We sought to confirm this relationship by evaluating P-NEFA at the time of the SCD event in a group of both men and women.
Methods
From the ongoing Oregon Sudden Unexpected Death Study, we compared P-NEFA in 149 SCD cases presenting with ventricular fibrillation (mean age 64±12 yrs, 73% male) and 149 age and sex-matched controls with coronary artery disease. Plasma was processed from blood drawn at the time of arrest (cases) and at a routine visit (controls). P-NEFA levels were compared after categorizing into quartiles based on control values. Conditional logistic regression was used to predict adjusted odds of SCD associated with P-NEFA levels per increased quartile.
Results
P-NEFA was significantly higher in SCD cases compared to controls (median 0.39; interquartile range 0.28–0.60 vs. 0.32 mmol/L; 0.20–0.49, P=0.002). There were no significant differences in body mass index, smoking, or diabetes. The odds of SCD were 1.42 (95% CI 1.14–1.78) per quartile increase in P-NEFA level (P=0.002). Individuals with P-NEFA levels above the pre-specified cut-off point of 0.32 mmol/L were at increased risk of SCD [OR 2.00 (1.20–3.34) P=0.008].
Conclusions
These findings strengthen the role of P-NEFA as a potential biomarker for assessment of SCD risk.
Keywords: fatty acids, sudden cardiac death, cardiac arrest, biomarker, risk prediction
Introduction
Sudden cardiac death (SCD) has been estimated to account for as much as 50% of all cardiovascular mortality in the United States (1). Even though the majority of cases are found to have significant coronary artery disease (CAD), most will present with sudden cardiac arrest as the first manifestation of cardiac disease (2). This latter observation highlights the need for, and importance of improved SCD risk prediction models that incorporate the cumulative effects of multiple biomarkers (3,4). In order to be useful for risk stratification in the general population, such tools need to be easy to deploy, and affordable (5,6). Therefore, the analysis of a marker must be robust, readily available on a larger scale, and easy to perform. Some, but not all, plasma biomarkers fit that description and there has been an increased interest in this field of research in recent years.
One biomarker with robust analysis methodology that has been evaluated in several cohort studies is the level of circulating non-esterified free fatty acids (NEFAs), compounds released from triglycerides stored in adipose tissue. Elevated levels of NEFAs have in experimental settings been shown to be proarrhythmic (7,8), associated with risk of SCD in larger cohort studies (9–11) and not associated in other reports (12). However, definitions of SCD have not been consistent, the cohorts may not always be reflective of the general population, and the numbers of events have been relatively low. Most importantly, the samples have been drawn several years and even decades prior to the SCD event, which is the inherent weakness of such analyses (13).
Therefore, we sought to validate the utility of elevated plasma NEFA as a risk predictor of SCD by analysing samples drawn at the time of the SCD event from an ongoing study with well-characterized SCD cases and control subjects.
Methods
The Oregon Sudden Unexpected Death Study (Oregon SUDS) is an ongoing, prospective, community-wide study of out-of-hospital SCD that has been described in detail previously (13).
In brief, the study is geographically located in the Portland, OR, metropolitan area with a population of ~1,000,000. Out-of-hospital SCD cases were identified through multiple sources; the Medical Examiner’s office, Emergency Medical Services, and all local hospitals. All available medical records (including pre-arrest medical records as well as Medical Examiner and Emergency Medical Services records) were obtained for each subject and reviewed in detail. A diagnosis of SCD was assigned by majority consensus after in-house adjudication by 3 physicians who closely evaluated the circumstances of arrest in combination with all available clinical information. SCD was defined as a sudden and unexpected pulseless condition of likely cardiac etiology (3). An exhaustive review was performed of all available clinical and autopsy information in order to exclude all SCD cases with non-cardiac cause, and those with trauma/violent death, presence of a terminal illness or death due to drug overdose.
During the same period, controls with CAD without a history of SCD were enrolled from the same geographic area. The rationale for using a CAD population as a control group is based on prior studies showing that the vast majority (>80–95%) of SCD victims older than 50 have significant post-mortem findings of CAD (1,14,15). This study design facilitates the identification of factors associated with SCD in the presence of CAD. Control subjects across a broad spectrum of CAD were identified from patients undergoing coronary angiography at one of the region’s major participating health systems, patients transported by the region’s Emergency Medical Service system with complaints suggestive of coronary ischemia, and patients with existing CAD enrolled in a regional HMO. Medical records for each potential control were reviewed after obtaining consent; those with documented CAD (defined as below) were enrolled during a single visit.
This study was approved by the Institutional Review Board of Cedars-Sinai Medical Center, Oregon Health and Science University, as well as all participating hospitals and health systems.
Study Population
For this analysis, cases identified from February 1, 2002 to January 31, 2011 were eligible if they were aged 35 – 84, white race, with a first monitored rhythm of ventricular fibrillation (VF) and a sufficient blood sample drawn at the time of cardiac arrest. Cases (n=150) were randomly selected from the entire pool of eligible cases. Controls (n=150), also white, were age and sex-matched to cases.
Definitions
Details of clinical history such as hypertension, hyperlipidemia, heart failure and diabetes mellitus were obtained from the clinical records. Any subject with a stenosis occluding more than 50% of the coronary artery lumen on coronary angiogram (prior to SCD) or autopsy, or a history of myocardial infarction or coronary vascularization, was considered as having CAD. Body mass index (BMI) was calculated as mass (kg) divided by the height squared (m2). A subset of patients had undergone quantitative assessment of left ventricular systolic function with echocardiogram prior to but not related to the SCD event or enrollment visit. Left ventricular ejection fraction (LVEF), a variable associated with SCD (16) was assessed and treated as a categorical variable and dichotomized as >35% or ≤35% in order to make it comparable to clinically relevant cut-offs (2).
Plasma analysis
For cases, blood samples were drawn during resuscitation but before time of death. For controls, blood was drawn during a routine out-patient visit. The collection containers were non-heparinized and no subjects were on heparin therapy. Plasma was separated and stored at −80 C° until thawed for assays. Total levels of NEFA were measured using ATAC 8000 (Elan Diagnostics, Smithfield, RI) with a NEFA kit (Wako Diagnostics, Richmond, VA) for enzymatic colorimetric assays. Intra- and interassay coefficients of variation were <5%. The sensitivity of the method was 0.0014 mmol/L.
Canine model to assess effects of VF arrest on NEFA levels
In order to evaluate the potential influence of the VF arrest event on the circulating levels of NEFA, 17 female hound dogs (age 9–19 months) were included in a canine ancillary study. Blood was drawn 1 week prior to inducing VF, immediately prior to cardiac arrest, 1 minute post VF, and 5 minutes post VF. Dogs were intubated and placed on a ventilator. Pre-anesthesia, anesthesia and paralysis were peformed using acepromazine subcutaneously, propofol intravenously, and isoflurane and oxygen as gas anesthesia. Ventricular fibrillation was induced by rapid ventricular pacing. Blood was collected in EDTA coated tubes. Plasma was processed and stored at −80°C. NEFA levels were measured using a Free Fatty Acid Quantification Kit according to the manufacturer’s protocol. This part of the study was approved by the Institutional Animal Care and Use Committee.
Statistical Analysis
Continuous variables were expressed as mean±standard deviation (SD) or median and interquartile range. Categorical variables were expressed as proportions. Comparisons were made using t-tests, Wilcoxon rank sum tests or Chi Square tests. Subjects were categorized into quartiles based on the plasma NEFA distribution of control values. Categorical values of plasma NEFA levels (quartiles or above/below median) were used for logistic regression analyses. Due to the lack of clinically established NEFA cut-off values in the context of SCD, the present study also used the median NEFA value in controls as a sensitivity analysis. The association between SCD and NEFA levels alone, as well as in combination with different clinical variables was assessed using conditional logistic regression. The median value of each quartile was used in a separate conditional logistic regression model to test for a linear trend across NEFA quartiles. For all analyses, a value of P<0.05 was considered statistically significant.
Three logistic regression models were performed. The first (Model A) adjusted for NEFA quartiles, age, and gender. The second model (Model B) adjusted for those variables plus hyperlipidemia and hypertension. A third model (Model C) further adjusted for LVEF (≤35% or >35%). A final model (Model D) included clinical history of heart failure as a covariate, instead of LVEF.
Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC) and Stata Version 12.0 (StataCorp, College Station, TX).
Results
Study population characteristics
The study population was comprised of 149 SCD cases (mean age 64±12 years, 73% male) and 149 controls (age 64±12, 73% male). The biochemical analysis of one control subject did not provide data for analysis and in one case we were not able to confirm presentation with VF. None of the plasma samples measured had NEFA levels below the sensitivity of the assay. Baseline clinical characteristics of the two groups are shown in Table 1. The SCD and control groups were well-matched by age and gender, and there were no significant differences between the groups by BMI, smoking or prevalence of diabetes mellitus (all P>0.05). Hypertension and dyslipidemia were more prevalent in the control group, consistent with the fact that the control population had clinically-established CAD. Heart failure and LVEF≤35% were more prevalent among SCD cases.
Table 1.
Baseline characteristics of the two study groups.
| SCD cases (n=149) |
Controls (n=149) |
P-value | |
|---|---|---|---|
| Age (years) | 64±12 | 64±12 | Matching variable |
| Male (%) | 73 | 73 | Matching variable |
| NEFA (mmol/L) | 0.39 (0.28–0.60) | 0.32 (0.20–0.49) | 0.015 |
| BMI (kg/m2)* | 29.6±6.4 | 29.8±6.2 | 0.79 |
| Diabetes (%) | 31 | 33 | 0.71 |
| Hyperlipidemia (%) | 48 | 85 | <0.0001 |
| Hypertension (%) | 50 | 72 | <0.0001 |
| Smoking (%)† | 45 | 39 | 0.43 |
| History of heart failure (%) | 22 | 13 | 0.03 |
| LVEF‡ | |||
| ≥55% | 29 | 56 | |
| 36–54% | 32 | 31 | |
| ≤35% | 39 | 14 |
Continuous variables are presented as mean ± SD (age, body mass index) with p-values from independent samples t-test or median and interquartile range (NEFA) with p-value from Wilcoxon ; categorical variables are presented as proportions (%) with p-values from the chi-squared test.
Data on body mass index available for 85 cases and 149 controls.
Data on smoking history available for 83 cases and 93 controls.
Data on LVEF available for 38 cases and 117 controls.
Plasma NEFA levels
Plasma NEFA levels were significantly higher in SCD cases compared to controls (median 0.39; interquartile range 0.28–0.60 vs. 0.32 mmol/L; 0.20–0.49, P=0.002). There were no significant differences between NEFA levels in men and women (0.35; 0.24–0.53 mmol/L vs. 0.36; 0.22–0.63, P=0.39).
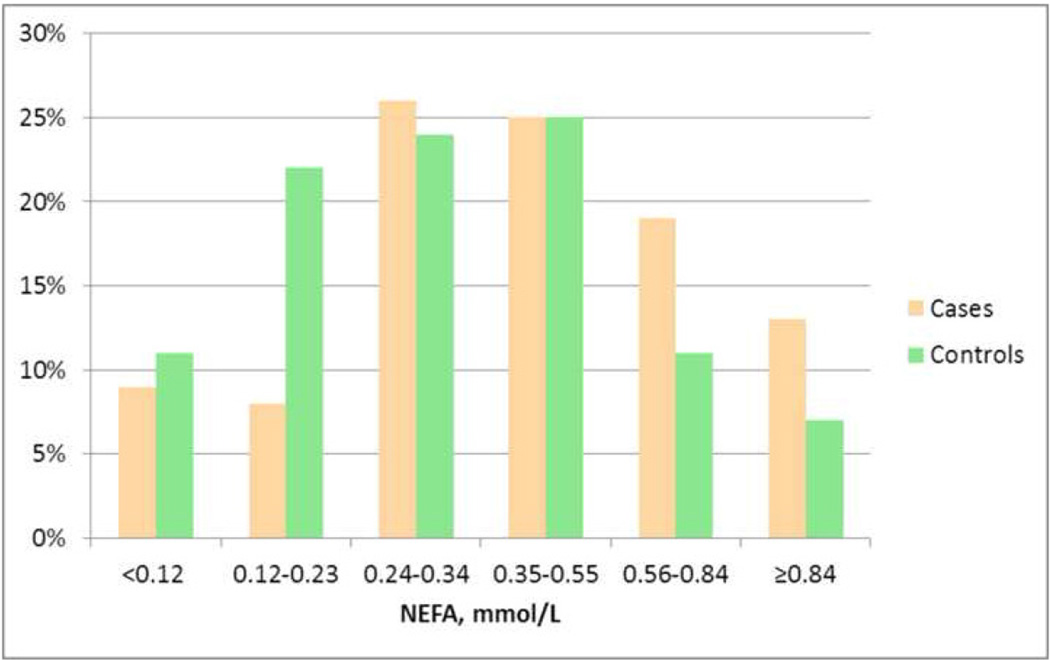
Levels of NEFA were higher among SCD cases across the spectrum of NEFA, as illustrated in the Figure with cases predominating over the 75th percentile (P=0.006).
Figure.
Distribution (%) of SCD cases (tan colored columns) and controls (green columns) in the plasma free fatty acid (NEFA) categories corresponding to the 10th, 25th, 50th, 75th, and 90th percentiles among pooled subjects (<0.12, 0.12–0.23, 0.24–0.34, 0.35–0.55, 0.56–0.84, and ≥0.84 mmol/L). SCD cases were more likely than controls to have plasma NEFA levels in the higher categories (P=0.006).
Risk of SCD
Using a conditional logistic regression model, the odds ratio for SCD was 1.42 (95% CI 1.14–1.78) per quartile increase in NEFA levels (P=0.002). Similar point estimates were observed when adjusting for hyperlipidemia and hypertension as well as history of heart failure (Table 2a). However, the model adjusting for LVEF≤35% was non-significant. The same models were evaluated using the pre-specified cut-off of the median NEFA in controls (0.32 mmol/L). The odds ratio for elevated NEFA alone (above the cut-off) increased to 2.00 (95% CI 1.20–3.34, P=0.008). Again, similar point estimates were observed when adjusting for hyperlipidemia and hypertension as well as history of heart failure but not when using LVEF≤35% as a covariate. We also performed an analysis using the original age- and sex-matched design in which we included the propensity score as a covariate in the conditional logistic regression model. The results of this analysis were consistent with the original analysis, with an OR of 1.51 per NEFA quartile for risk of SCD, 95% CI 1.18–1.93, p=0.001).
Table 2.
| a. Risk of SCD with each quartile increase of NEFA level | ||
|---|---|---|
| Conditional logistic regression |
SCD case OR (95% CI) |
P-value |
| Model A | 1.42 (1.14–1.78) | 0.002 |
| Model B | 1.43 (1.09–1.87) | 0.01 |
| Model C | 1.45 (0.72–2.90) | 0.30 |
| Model D | 1.43 (1.14–1.80) | 0.002 |
| b. Risk of SCD with significant NEFA levels (≥median) | ||
|---|---|---|
| Conditional logistic regression |
SCD case OR (95% CI) |
P-value |
| Model A | 2.00 (1.20 – 3.34) | 0.008 |
| Model B | 1.88 (1.02 – 3.47) | 0.04 |
| Model C | 1.19 (0.26 – 5.41) | 0.83 |
| Model D | 2.02 (1.20 – 3.40) | 0.008 |
All models n=298 unless otherwise noted.
A: NEFA Quartiles (<0.20, 0.20–0.32, 0.32–0.49, 0.49+ mmol/L) in age and sex-matched pairs
B: Model A plus history of hyperlipidemia and hypertension
C: Model A plus left ventricular systolic impairment (EF≤35% vs. >35%) (n=155 with LVEF assessed; 38 cases, 117 controls)
D: Model A plus clinical history of heart failure
Canine model
Median plasma NEFA was similar immediately before and 1 minute after VF and attempted resuscitation (0.21 mmol/L, IQR 0.11–0.41 vs. 0.23 mmol/L, IQR 0.13–0.42, P=0.20). The median change per dog from before to after VF was an increase of 0.02 mmol/L, which was non-significant (P=0.17, Wilcoxon signed rank sum test). Similar values were seen when comparing immediately before and 5 minutes after VF (0.03 mmol/L, P=0.43). No significant increase was observed between NEFA 1 minute after and 5 minutes after VF (0.01 mmol/L, P=1.0).
Discussion
The main finding of this prospective, community-based case-control study is that elevated levels of plasma NEFA measured at the time of the resuscitation process during cardiac arrest are also associated with risk of SCD. Taken together with previous observations in longitudinal cohort studies that have identified this association in samples drawn several years remote from the SCD event, these data underscore the potential of plasma NEFA as a biomarker for SCD risk prediction.
There is growing evidence supporting the association between elevated levels of NEFAs and SCD (7,17,18). The mechanisms of proarrhythmia pertaining to NEFAs are likely to be multi-factorial and complex. In experimental models, ventricular arrhythmias following NEFA injections have been reported in rats (19,20) as well as in dogs (21). It has also been shown that plasma lipolysis induced by heparin administration will result in ventricular fibrillation in canines (22). This also points to the importance of using non-heparinized sampling containers for NEFA analysis as in the present study. Other experimental studies have indicated a link between elevated fatty acid levels and heart failure (23,24), an association which was subsequently confirmed in the Cardiovascular Health Study (25). In the present study NEFA was associated with SCD, even after adjustment for history of heart failure. However, when adjusting for LVEF, p-values were non-significant possibly due to the small numbers with LVEF available for analysis.
Nonetheless, these findings suggest that elevated levels of NEFAs are not just a consequence of an adrenal surge associated with a proarrhythmic state, but may also contribute to ventricular arrhythmogenesis. Other proarrhythmic properties attributed to NEFA include the ability to inhibit the Na+, K+, ATPase pump (26), activate Ca2+ channels (27), as well as influence the regulation of K+ channels (28). The experimental links between NEFA and ventricular arrhythmias are supported by data from large prospective cohort studies.
The first larger cohort study that specifically investigated the relationship between NEFA levels and SCD was the Paris Prospective Study I (9). This study by Jouven et al followed almost 8,000 middle-aged men free of known cardiovascular disease for >20 years. Elevated levels of circulating NEFAs at baseline examination were found to be an independent risk factor for sudden death (adjusted relative risk 2.05; 95% CI, 1.34 to 3.15). Interestingly, no association was observed with non-sudden fatal myocardial infarction. Pilz and co-workers later confirmed these findings in a German cohort of intermediate to high-risk individuals (10). They measured circulating NEFAs in 3,315 subjects referred for coronary angiography and found elevated plasma NEFAs to be an independent risk predictor of SCD (adjusted hazard ratio 1.76, 95% CI 1.03–3.00; P=0.038) over a median follow-up time of ~7 years. The study followed on a previous report from the same cohort reporting that NEFA levels were associated with all-cause and cardiovascular mortality (29). In contrast, Djousse and co-workers did not observe any association between P-NEFA drawn at baseline and subsequent SCD over a 10-year follow-up period in the Cardiovascular Health Study. The apparent conflicting results might be explained by differences of the study populations. For example, the baseline samples of the Cardiovascular Health Study were drawn at the average age of 75 years in a predominantly female group (58%). This contrasts with the population of the present study (average age 64 years at the time of event and only 27% females) (12). To what extent such major demographic differences may influence biometabolism of P-NEFA and its role as a biomarker remains to be elucidated.
While these findings are of particular interest, the present study has addressed an important unanswered question: whether or not this association between increased NEFA levels and SCD actually contributed to the pathophysiological processes, given the long periods of time between baseline examinations and the sudden death event (13). Therefore, the congruence of the findings of the present study, conducted from blood drawn at the time of SCD, with those of previous cohort studies (9,10,29) strengthens the case for NEFAs as a biomarker associated with SCD and possibly useful in risk prediction for SCD.
Lemaitre and co-workers also performed a case-control study in which they recruited out-of-hospital cardiac arrests and compared them to age and gender matched controls (30). In contrast to the previously mentioned cohort studies and the present one, they analysed red blood cell membrane levels of trans-fatty acids. They reported an association of the relative distribution of the trans-18:2 isomer with risk of SCD (adjusted odds ratio 3.1, 95% CI, 1.7 to 5.4 when comparing the 80th to the 20th percentile) (30). It is possible that red blood cell membrane levels of trans-fatty acids better reflect the dietary intake and exposure of a person. However, membrane lysis during processing could affect precision of measurement (31). The more automated enzymatic methods for analysing plasma NEFA may facilitate the future utilization of this biomarker as a risk predictor of SCD on a larger scale. Risk prediction of a complex condition such as SCD is likely to require multiple-variable models (6) and it is generally recognized that current risk prediction strategies are inadequate (3).
In summary, our study is the first to show an association between elevated plasma NEFA levels at the time of arrest and SCD and is consistent with most findings of previous studies of NEFA and SCD where the blood was drawn several years before the SCD event.
Limitations of the Study
This study has several limitations that warrant discussion. Even though our results are similar to those from studies where blood was drawn many years prior to the cardiac arrest, it cannot be ruled out that part of the elevation of NEFA in cases can be explained by circumstances related to the event itself or the dietary status of the subjects as well as factors we were unable to control for such as electrolyte levels. However, the ancillary canine study results did not identify any effects of the cardiac arrest itself on NEFA levels, suggesting that this would be unlikely. Moreover, it has previously been shown that inhibition of adipose tissue lipolysis and subsequent lower NEFA levels reduces ventricular arrhythmias, a finding not explained by adrenergic tone as measured by cathecolamine levels (32). Experimental animal models might be one way of further elucidating this process. Indeed, Siscovick et al have reported that changes to red blood cell membrane fatty acid levels from the cardiac arrest itself were only slight in primates (33) and we did not find any significant difference in our canine model in which we tried to replicate the circumstances of cardiac arrest. However, it cannot be ruled out that the canine ancillary study was underpowered to verify an existing difference related to the arrest given the low number of dogs (n=17) and a borderline P-value at 0.17. Larger experimental animal models of different variety might be useful to further explore the effects of the arrest itself on P-NEFA and other biomarkers. Interestingly, in humans, different fatty acid markers have been shown to be stable during the acute phase after myocardial infarction as well as after sudden cardiac arrest (34,35).
Finally, the majority of residents in the community are of white European descent. Since the sample size of non-whites was relatively small and any subgroup analyses would have been underpowered, we limited our analysis to whites. Our findings may not readily be generalized to other communities or races.
Conclusion
These findings confirm the association between elevated NEFA levels and SCD risk, when drawn during the acute SCD event. NEFA levels hold promise as a biomarker that may enhance methodology for risk assessment of SCD.
Acknowledgements
The authors would like to acknowledge the significant contribution of American Medical Response, Portland/Gresham fire departments and the Oregon State Medical Examiner’s office. They are grateful to Katherine Jerger, Jo Ayala and Vicki Yeheng Liu for technical assistance; and to Rohan Dharmakumar, Ivan Cokic and colleagues for their assistance with the canine ancillary study.
Financial support: RH is supported by a grant from the Swedish Research Council (#2011-1071). SSC is the Pauline and Harold Price Endowed Professor of Cardiac Electrophysiology at the Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA. There are no other disclosures.
List of abbreviations
- BMI
body mass index
- CAD
coronary artery disease
- CI
confidence interval
- IQR
interquartile range
- LVEF
left ventricular ejection fraction
- NEFA
non-esterified free fatty acids
- OR
odds ratio
- SCD
sudden cardiac death
- SD
standard deviation
- VF
ventricular fibrillation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
References
- 1.Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death: epidemiology, transient risk, and intervention assessment. Ann Intern Med. 1993;119:1187–1197. doi: 10.7326/0003-4819-119-12-199312150-00006. [DOI] [PubMed] [Google Scholar]
- 2.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace. 2006;8:746–837. doi: 10.1093/europace/eul108. [DOI] [PubMed] [Google Scholar]
- 3.Fishman GI, Chugh SS, Dimarco JP, et al. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 5.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 6.Havmoller R, Chugh SS. Plasma biomarkers for prediction of sudden cardiac death: another piece of the risk stratification puzzle? Circulation Arrhythmia and electrophysiology. 2012;5:237–243. doi: 10.1161/CIRCEP.111.968057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliver MF, Opie LH. Effects of glucose and fatty acids on myocardial ischaemia and arrhythmias. Lancet. 1994;343:155–158. doi: 10.1016/s0140-6736(94)90939-3. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui RA, Harvey KA, Ruzmetov N, Miller SJ, Zaloga GP. n-3 fatty acids prevent whereas trans-fatty acids induce vascular inflammation and sudden cardiac death. Br J Nutr. 2009;102:1811–1819. doi: 10.1017/S0007114509992030. [DOI] [PubMed] [Google Scholar]
- 9.Jouven X, Charles MA, Desnos M, Ducimetiere P. Circulating nonesterified fatty acid level as a predictive risk factor for sudden death in the population. Circulation. 2001;104:756–761. doi: 10.1161/hc3201.094151. [DOI] [PubMed] [Google Scholar]
- 10.Pilz S, Scharnagl H, Tiran B, et al. Elevated plasma free fatty acids predict sudden cardiac death: a 6.85-year follow-up of 3315 patients after coronary angiography. Eur Heart J. 2007;28:2763–2769. doi: 10.1093/eurheartj/ehm343. [DOI] [PubMed] [Google Scholar]
- 11.Lemaitre RN, King IB, Mozaffarian D, et al. Plasma phospholipid trans fatty acids, fatal ischemic heart disease, and sudden cardiac death in older adults: the cardiovascular health study. Circulation. 2006;114:209–215. doi: 10.1161/CIRCULATIONAHA.106.620336. [DOI] [PubMed] [Google Scholar]
- 12.Djoussé L, Biggs ML, Ix JH, et al. Nonesterified fatty acids and risk of sudden cardiac death in older adults. Circ Arrhythm Electrophysiol. 2012;5:273–278. doi: 10.1161/CIRCEP.111.967661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chugh SS, Reinier K. Predicting sudden death in the general population: another step, N terminal B-type natriuretic factor levels. Circulation. 2009;119:2863–2864. doi: 10.1161/CIRCULATIONAHA.109.865436. [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB, Gagnon DR, Cupples LA. Epidemiology of sudden coronary death: population at risk. Can J Cardiol. 1990;6:439–444. [PubMed] [Google Scholar]
- 15.Kannel WB, Schatzkin A. Sudden death: lessons from subsets in population studies. J Am Coll Cardiol. 1985;5:141B–149B. doi: 10.1016/s0735-1097(85)80545-3. [DOI] [PubMed] [Google Scholar]
- 16.Reinier K, Dervan C, Singh T, et al. Increased left ventricular mass and decreased left ventricular systolic function have independent pathways to ventricular arrhythmogenesis in coronary artery disease. Heart Rhythm. 2011;8:1177–1182. doi: 10.1016/j.hrthm.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliver MF. Sudden cardiac death: the lost fatty acid hypothesis. QJM. 2006;99:701–709. doi: 10.1093/qjmed/hcl084. [DOI] [PubMed] [Google Scholar]
- 18.Paolisso G, Gualdiero P, Manzella D, et al. Association of fasting plasma free fatty acid concentration and frequency of ventricular premature complexes in nonischemic non-insulin-dependent diabetic patients. Am J Cardiol. 1997;80:932–937. doi: 10.1016/s0002-9149(97)00548-1. [DOI] [PubMed] [Google Scholar]
- 19.Willerbrands AF, ter Welle HF, Tasseron SJ. The effect of a high molar FFA-albumin ratio in the perfusion medium on rhythm and contractility of the isolated rat heart. J Mol Cell Cardiol. 1973;5:259–273. doi: 10.1016/0022-2828(73)90066-7. [DOI] [PubMed] [Google Scholar]
- 20.Makiguchi M, Kawaguchi H, Tamura M, Yasuda H. Effect of palmitic acid and fatty acid binding protein on ventricular fibrillation threshold in the perfused rat heart. Cardiovasc Drugs Ther. 1991;5:753–761. doi: 10.1007/BF03029751. [DOI] [PubMed] [Google Scholar]
- 21.Soloff LA. Arrhythmias following infusions of fatty acids. Am Heart J. 1970;80:671–674. doi: 10.1016/0002-8703(70)90012-8. [DOI] [PubMed] [Google Scholar]
- 22.Kurien VA, Yates PA, Oliver MF. Free fatty acids, heparin, and arrhythmias during experimental myocardial infarction. Lancet. 1969;2:185–187. doi: 10.1016/s0140-6736(69)91424-x. [DOI] [PubMed] [Google Scholar]
- 23.Chiu HC, Kovacs A, Blanton RM, et al. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96:225–233. doi: 10.1161/01.RES.0000154079.20681.B9. [DOI] [PubMed] [Google Scholar]
- 24.Yagyu H, Chen G, Yokoyama M, et al. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111:419–426. doi: 10.1172/JCI16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Djoussé L, Benkeser D, Arnold A, et al. Plasma free fatty acids and risk of heart failure: the Cardiovascular Health Study. Circ Heart Fail. 2013;6:964–969. doi: 10.1161/CIRCHEARTFAILURE.113.000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly RA, O'Hara DS, Mitch WE, Smith TW. Identification of NaK-ATPase inhibitors in human plasma as nonesterified fatty acids and lysophospholipids. J Biol Chem. 1986;261:11704–11711. [PubMed] [Google Scholar]
- 27.Huang JM, Xian H, Bacaner M. Long-chain fatty acids activate calcium channels in ventricular myocytes. Proc Natl Acad Sci U S A. 1992;89:6452–6456. doi: 10.1073/pnas.89.14.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim D, Duff RA. Regulation of K+ channels in cardiac myocytes by free fatty acids. Circ Res. 1990;67:1040–1046. doi: 10.1161/01.res.67.4.1040. [DOI] [PubMed] [Google Scholar]
- 29.Pilz S, Scharnagl H, Tiran B, et al. Free fatty acids are independently associated with all-cause and cardiovascular mortality in subjects with coronary artery disease. J Clin Endocrinol Metab. 2006;91:2542–2547. doi: 10.1210/jc.2006-0195. [DOI] [PubMed] [Google Scholar]
- 30.Lemaitre RN, King IB, Raghunathan TE, et al. Cell membrane trans-fatty acids and the risk of primary cardiac arrest. Circulation. 2002;105:697–701. doi: 10.1161/hc0602.103583. [DOI] [PubMed] [Google Scholar]
- 31.Arab L. Biomarkers of fat and fatty acid intake. J Nutr. 2003;133(Suppl 3):925S–932S. doi: 10.1093/jn/133.3.925S. [DOI] [PubMed] [Google Scholar]
- 32.Rowe MJ, Neilson JM, Oliver MF. Control of ventricular arrhythmias during myocardial infarction by antilipolytic treatment using a nicotinic-acid analogue. Lancet. 1975;1:295–300. doi: 10.1016/s0140-6736(75)91206-4. [DOI] [PubMed] [Google Scholar]
- 33.Siscovick DS, Raghunathan TE, King I, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 34.Aarsetoey H, Aarsetoey R, Lindner T, Staines H, Harris WS, Nilsen DW. Low levels of the omega-3 index are associated with sudden cardiac arrest and remain stable in survivors in the subacute phase. Lipids. 2011;46:151–161. doi: 10.1007/s11745-010-3511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kark JD, Manor O, Goldman S, Berry EM. Stability of red blood cell membrane fatty acid composition after acute myocardial infarction. J Clin Epidemiol. 1995;48:889–895. doi: 10.1016/0895-4356(94)00218-f. [DOI] [PubMed] [Google Scholar]