Abstract
Background and objectives
Cystatin C is a 13.3 kD middle molecule of similar size to β2-microglobulin and a marker of GFR in CKD. This study aimed to determine cystatin C kinetics in hemodialysis to understand whether blood concentrations may predict residual renal function and middle-molecule clearance.
Design, setting, participants, & measurements
Cystatin C removal and rebound kinetics were studied in 24 patients on high-flux hemodialysis or hemodiafiltration. To determine whether cystatin C concentrations are predictable, an iterative two-pool mathematical model was applied.
Results
Cystatin C was cleared effectively, although less than β2-microglobulin (reduction ratios±SD are 39%±11 and 51%±11). Cystatin C rebounded to 95%±5% of predialysis concentration by 12 hours postdialysis. The two-pool kinetic model showed excellent goodness of fit. Modeled extracellular cystatin C pool volume is smaller than that predicted, comprising 25.5%±9.2 of total body water. Iterated parameters, including nonrenal clearance, showed wide interindividual variation. Modeled nonrenal clearance was substantially higher than renal clearance in this population at 25.1±6.6 ml/min per 1.73 m2 body surface area.
Conclusions
Plasma cystatin C levels may be used to measure middle-molecule clearance. Levels rebound substantially postdialysis and plateau in the interdialytic period. At low GFR, nonrenal clearance predominates over renal clearance, and its interindividual variation will limit use of cystatin C to predict residual renal function in advanced kidney disease.
Keywords: GFR, cystatin C, kinetics, hemodialysis
Introduction
Cystatin C is a lysosomal protease and cysteine proteinase inhibitor produced by all nucleated cells (1,2). It is freely filtered by glomeruli and metabolized by proximal tubules (3,4). Considerable interest has developed in use of cystatin C as a marker of GFR (5). Cystatin C–based GFR estimates do not require correction for muscle bulk, sex, and race (6–8). Cystatin C predicts mortality in CKD independent of GFR (9). There has been interest in use of cystatin C as a marker of residual renal function (RRF) in both acute renal failure and ESRD (10–12).
Previous data suggest that, at low GFR, the close relationship of cystatin C with GFR weakens (11). However, if cystatin C kinetics are predictable, then it may be possible to use parameters, such as predialysis cystatin C, to predict RRF. This use would have wide-ranging benefits and allow estimation of RRF without the need for interdialytic urine collection (13).
There is increasing interest in techniques that maximize middle-molecule clearance, such as hemodiafiltration (HDF) (14,15). Clearance of these molecules may be an important marker of adequate treatment in both peritoneal dialysis (10,16) and hemodialysis (HD) (11,17). Cystatin C has been proposed as a candidate for such a marker (18–20), but there are little data describing its removal and rebound kinetics in the maintenance HD population.
We aimed to determine plasma cystatin C kinetics in high-flux HD and HDF during dialysis and the interdialytic period to establish the relationship of cystatin C levels to dialyzer and renal clearance. Cystatin C generation rate (G) and nonrenal clearance (KNR) have been previously estimated (21). Because the sieving coefficient of cystatin C is close to unity (4), the renal clearance (KR) approximates GFR. Intercompartmental transfer rates are unknown, but limited information suggests that the major pool is extracellular.
To determine cystatin C kinetics in high-flux HD and HDF and whether plasma concentrations are predictable, we developed a two-pool mass transfer iterative computer model. This model used previously available data on G, KNR, measured GFR, and plasma cystatin C levels sampled throughout the HD cycle.
Materials and Methods
Study Design and Setting
After approval by a local research ethics committee and in accordance with the Declaration of Helsinki, 24 patients on either chronic high-flux HD or HDF at the Lister Hospital in Stevenage, United Kingdom gave informed written consent to be studied. Because of the extended blood sampling required, only hospital inpatients were included. All patients were hemodynamically stable. Patients with fluid overload or anemia (hemoglobin<9 g/dl) were excluded.
HD and HDF Procedures
Patients were dialyzed using an incremental HD algorithm, where the target total Kt/V was 1.2 (22). GFR was estimated as the mean of urea and creatinine clearance calculated from a midweek 48-hour interdialytic urine collection performed during the study period±7 days. Patients with a urine output<100 ml/d were classified as having 0 GFR. Urea and creatinine clearance were calculated using the formula
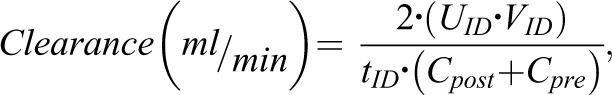 |
where UID is the urinary concentration of the relevant solute (millimoles per liter or micromoles per liter), VID is the urine collection volume (milliliters), tID is duration of interdialytic period (minutes), and Cpost and Cpre are the post- and predialysis plasma concentrations of the relevant solute (millimoles per liter or micromoles per liter).
Dialysis data recorded included blood flow (QB), dialysis fluid flow (QD), ultrafiltration volume (UF), and body size parameters.
Sampling of Plasma for Clearance Molecules
Samples were obtained during dialysis from the arterial needle immediately before the first dialysis session (baseline) and at four intervals equal to 20% of the dialysis time. The end of dialysis sample was taken 30 seconds after slowing the blood pump to 50 ml/min (23). Postdialysis samples were obtained at 1, 2, 4, 8, 12, and 24 hours and immediately before the subsequent dialysis session. For the first five (pilot) patients, additional postdialysis samples were taken at 2, 15, and 30 minutes (the washback was after the 2-minute sample).
All samples were analyzed for urea, creatinine, β2-microglobulin, and cystatin C as described in Supplemental Material.
Design of Two-Pool Mass Transfer Model of Cystatin C Kinetics
Model Design.
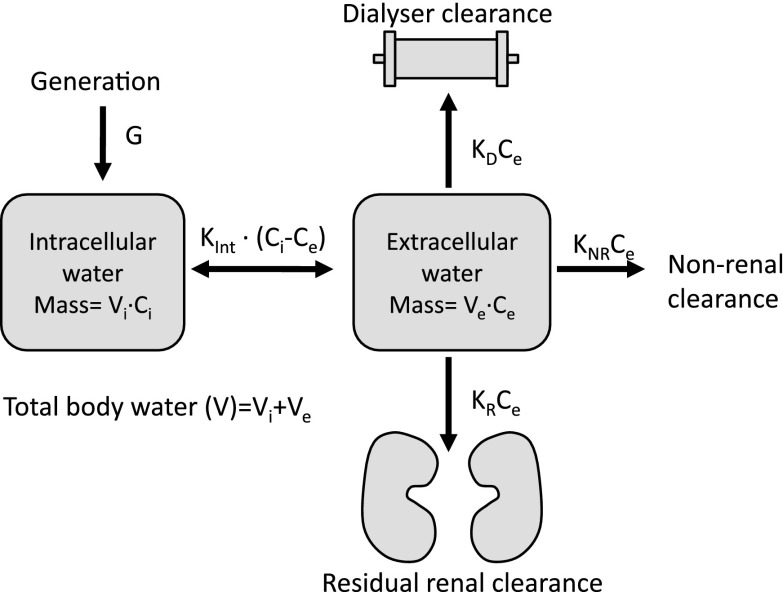
Model design is shown in Figure 1. Cystatin C was assumed to be distributed in intracellular and extracellular water with concentrations Ci and Ce, respectively. Mass transfer rate between pools was assumed to equal to the mass transfer coefficient for the intracellular/extracellular barrier (Kint) multiplied by the difference between Ci and Ce. G was assumed to be into intracellular space at a constant rate. Cystatin C removal from extracellular space was assumed to occur by three routes: KR, KNR, and dialyzer clearance (KD). Each of these rates was equal to the product of the clearance constant (KR, KNR, or KD) and Ce. The model used a variable volume, with ultrafiltration volume being total fluid removed over the dialysis period and assumed to be at a constant rate during HD from the extracellular compartment.
Figure 1.
Two-pool mathematical mass transfer model of cystatin C kinetics showing distribution of the molecule which was assumed to between the extracellular and intracellular water spaces only. Removal from the extracellular space was by renal (KR), nonrenal (KNR), and dialyzer clearance (KD). Kint represents a mass transfer coefficient between pools. Ce, extracellular concentration; Ci, intracellular concentration; G, cystatin C mass generation rate; Ve, extracellular volume; Vi, intracellular volume.
Cystatin C concentration in the intracellular and extracellular pools was calculated from the mass of cystatin C in the respective pool divided by the pool volume (Vi or Ve).
The Watson volume (VWatson) (24) was assumed to be equal to postdialysis body water (TBWpost) and calculated using the clinically assessed dry weight. Predialysis total body water (TBWpre) was considered to be VWatson plus the UF volume (TBWpre=VWatson+UF). TBWpre was considered to consist of predialysis extracellular and intracellular water volumes (Ve_pre and Vi_pre, respectively). A constant (ECWFraction) defined the proportion of TBWpre occupied by extracellular water (Ve_pre/TBWpre). The fraction of TBWpre occupied by intracellular water was, therefore, 1−ECWFraction. Equations defining the initial (predialysis) pool volumes were, therefore, Ve_pre=ECWFraction×TBWpre and Vi_pre=(1−ECWFraction)×TBWpre. Mass transfer in or out of pools in the model was calculated using the integral of the mass transfer rates.
Modeling was performed using Vissim Optimize-Pro v8.0 (Westford, MA), permitting an iterative model to be designed for mass flow between compartments using integration. This environment permits importing of observed data and comparison with modeled plasma cystatin C concentration, and it has previously been used for dialysis kinetic modeling (25).
Iterative Procedure.
Certain parameters were taken as constants—either measured or assumed from previously reported data. Unknown parameters were iterated. Constants were cystatin C KD, KR, and G. Iterated parameters were KNR, ECWFraction, and Kint. Published cystatin C clearance data for high-flux dialyzer membranes are limited, but data indicate that it is approximately 10% lower than in vivo β2-microglobulin clearance (26–28). We, therefore, estimated cystatin C KD to be 90% of β2-microglobulin clearance based on extensively studied β2-microglobulin clearance data, which took account of modality (HD/HDF), blood flow, and dialyzer size (29). KR was assumed to be equal to measured GFR that was unadjusted for body surface area, which assumed a sieving coefficient of one for cystatin C (4). G was taken as 0.124 mg/min per 1.73 m2 body surface area in accordance with previous data (21).
Unknown parameters (KNR, ECWFraction, and Kint) were iterated by optimal fitting of the model to the observed patient data. Estimates of anticipated parameter values were used as initial values. The initial iteration value of KNR was 22.3 ml/min per 1.73 m2 body surface area from previous data (21). Simultaneous iteration of both G and KNR was not possible because of their opposite overall effects on the model, which would result in multiple possible solutions. In view of the potential for multiple pool effects for cystatin C, we iterated ECWFraction. The initial iteration value of ECWFraction (ECWFraction_initial_value) was calculated as the predicted predialysis proportion of TBW occupied by extracellular water according to
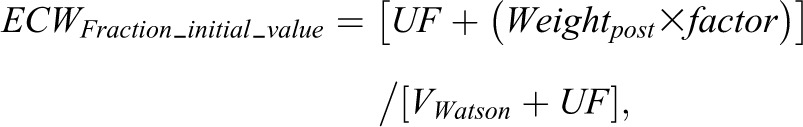 |
where Weightpost is postdialysis weight and factor represents the predicted ratio of extracellular water to body weight, which was taken as 0.239 and 0.214 L/kg in men and women, respectively (30). In one patient with a unilateral below knee amputation, VWatson was corrected (31), and initial value of ECWFraction was assumed to be 0.4.
The initial value of Kint for each patient was calculated by running a test model for each subject, in which KNR and ECWFraction were considered constants (not iterated) at their initial values (see above).
Ce was measured. It can be seen (Figure 1) that, at steady state (predialysis), G=Kint (Ci − Ce). Ci was, therefore, calculated as G/Kint+Ce. From initial values of Ci and Ce, it is possible to calculate initial cystatin C mass in the intracellular and extracellular compartments given the initial pool volumes Ve_pre and Vi_pre calculated above.
The iterative procedure aimed to optimize the degree of fit by minimizing a cost function equal to the SD of the difference between the data and the model. The expression used to calculate this function was
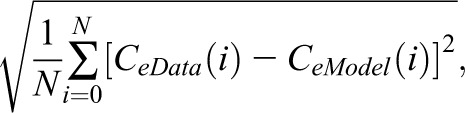 |
where N is number of cystatin C samples obtained for the subject, CeData is the measured cystatin C concentration, and CeModel is the modeled cystatin C. Although not used for iteration, model goodness of fit was assessed using the coefficient of determination (r2) calculated as the sum of squares of the differences between observed data and modeled data normalized to the sum of squares of the differences between observed data and the mean of observed data.
Results
Study Population
Patient demographics, comorbidities, and dialysis parameters are detailed in Table 1; 24 patients were recruited (13 patients on high-flux HD and 11 patients on online HDF). Hospitalized patients were studied before planned discharge after they were considered hemodynamically and clinically stable. Hospitalization had been related to vascular access (21%), infections (25%), cardiovascular problems (38%), and other reasons (16%).
Table 1.
Baseline characteristics, comorbidities, and dialysis parameters in the study population
| Descriptive | Parameter |
|---|---|
| Demographics | |
| Age, yr | 62.0±14.6 |
| Men/women, % | 46/54 |
| Weight, kg | 67.2±22.1a |
| Watson volume, L | 34±8.74a |
| Body surface area, m2 | 1.69±0.28a |
| Comorbidities | |
| Diabetes mellitus, % | 67 |
| Cardiovascular disease, % | 50 |
| Active malignancy, % | 8.3 |
| C reactive protein, mg/L | 22 (62)b |
| Dialysis parameters | |
| Dialysis time, min | 188±33a |
| Blood flow (QB), ml/min | 283±43a |
| Dialysis fluid flow (QD), ml/min | 587±139a |
| High-flux hemodialysis/hemodiafiltration, % | 54/46 |
| Ultrafiltration, L | 1.49±1.05a |
| Estimated in vivo cystatin C dialyzer clearance (KD), ml/min | 70.3±20.1a |
| GFR, ml/min per 1.73 m2 body surface area | 1.43 (0.22–2.33)b |
| Single pool Kt/Vurea | 1.19±0.52 |
| Total Kt/Vurea (renal+dialyzer) | 1.24±0.49 |
| Vascular access | |
| Arteriovenous fistula, % | 12.5 |
| Vascular catheter, % | 87.5 |
Total Kt/Vurea represents the sum of residual renal and dialyzer clearance, which was used for incremental dialysis calculations (Materials and Methods).
Mean±SD.
Median (interquartile range).
Clearance of Cystatin C Compared with Small and Middle Molecules
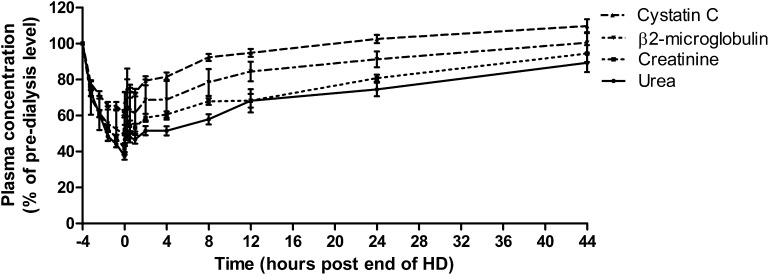
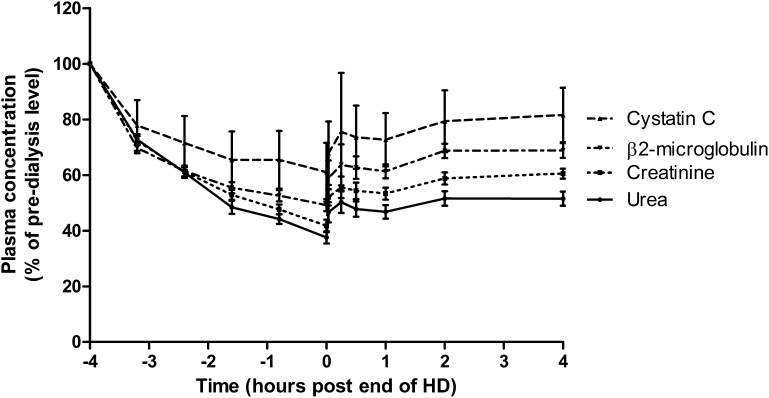
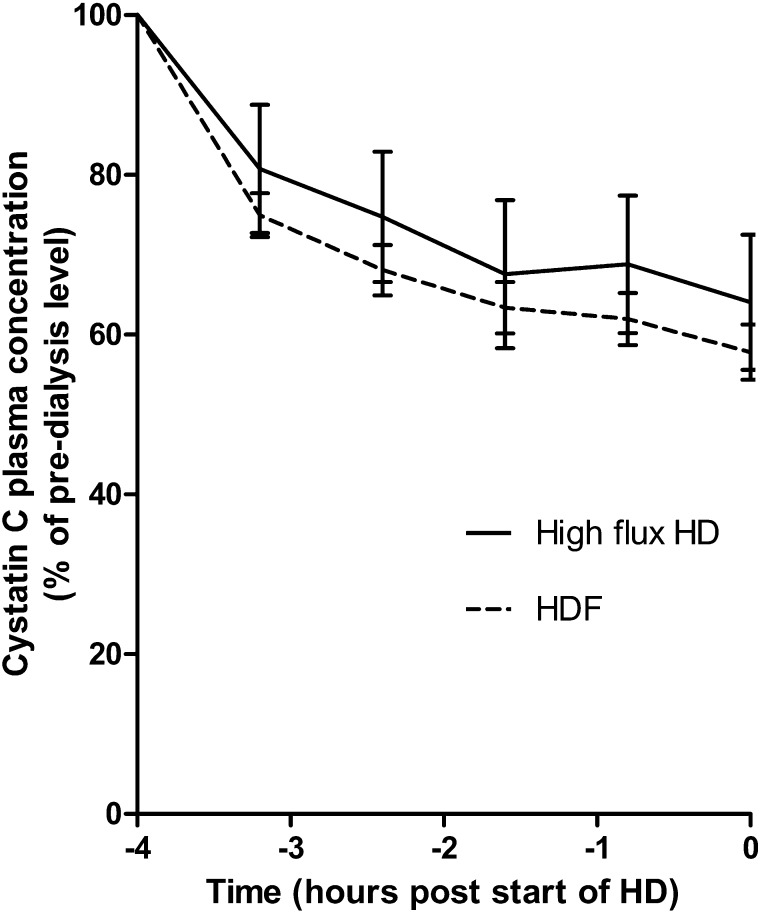
Figures 2 and 3 show observed clearance and rebound kinetics for urea, creatinine, β2-microglobulin, and cystatin C; reduction ratios for each molecule (±SD) were 63%±11, 58%±12, 51%±11, and 39%±11, respectively. For all molecules, a rapid rebound occurred in the first 1 hour after termination of dialysis followed by a shallower rebound. For each molecule, there was no significant difference in mean concentration between +2- and +4-hours postdialysis time points. Concentrations of urea, creatinine, and β2-microglobulin did not rebound to their predialysis levels during the interdialytic period, whereas for cystatin C, the mean 12-hour postdialysis concentration was 95%±5 of the predialysis concentration, and by 24 hours, it had reached 103%±7 (Figure 2). For patients treated by high-flux HD, the mean cystatin C reduction ratio was 34%±9%, and for those patients on HDF, it was 44%±11% (P=0.02) (Figure 4). Likewise, for β2-microglobulin, the mean reduction ratio on high-flux HD was 46%±11% compared with 56%±10% on HDF (P=0.02). There was no difference in predialysis cystatin C concentrations in HDF and high-flux HD groups (P=0.58).
Figure 2.
Removal and rebound kinetics of small and middle molecules compared with cystatin C showing mean±SD level calculated as the percentage of predialysis concentration. All dialysis session lengths have been normalized to a 4-hour duration for the purpose of graphical representation. Data for the intradialytic and immediate postdialytic period are shown in more detail in Figure 3.
Figure 3.
Removal and rebound kinetics of cystatin C during dialysis and the immediate postdialytic period. Data points show the mean±SD concentration calculated as percentage of the predialysis concentration. All session lengths are normalized to a 4-hour duration for diagrammatic representation.
Figure 4.
Difference in removal of cystatin C during high-flux hemodialysis (HD) and hemodiafiltration (HDF) groups. Mean±SD plasma cystatin C concentration is shown as a percentage of predialysis concentration. HD session lengths are normalized to a 4-hour duration for comparison.
Cystatin C reduction ratio correlated significantly with weekly standard Kt/V (r=0.49, P=0.01) but not single pool Kt/V (r=0.35, P=0.08). Predialysis plasma cystatin C correlated negatively with residual renal urea clearance and GFR (r=−0.51, P=0.01 in both cases).
Predialysis cystatin C concentration correlated significantly with GFR (P=0.01, r=0.51).
Two-Pool Modeling of Cystatin C Clearance
Overall Model Results.
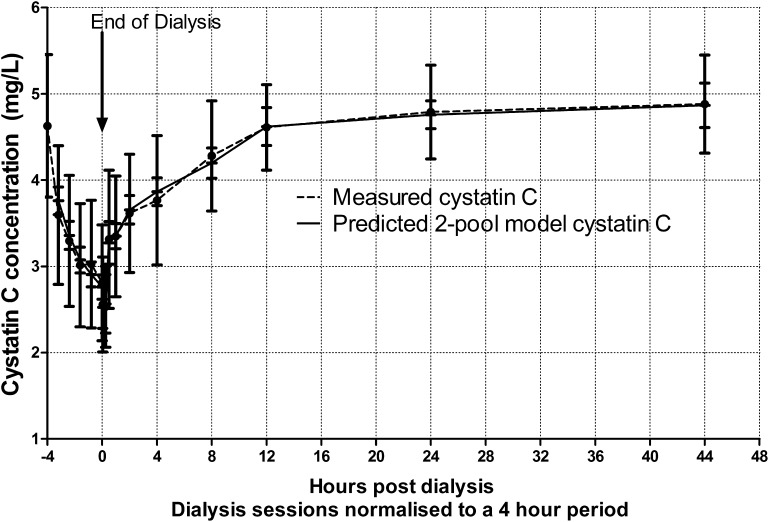
Parameter estimates for KNR, Kint, and ECWFraction were obtained from iterative modeling for each individual subject. Figure 5 shows combined data from all patients comparing measured and modeled cystatin C levels at each time point throughout the dialysis cycle. Goodness of fit, measured as SD of the difference between the measured and modeled data, had a mean of 0.19 mg/L (SD=0.10), with a range of 0.03–0.42 mg/L. Using the coefficient of determination (r2) to assess goodness of fit, mean r2 was 0.89 (SD=0.09), with a range of 0.67–0.99. In the postdialysis period, there was no significant difference between the modeled and observed data using paired t tests (P>0.05). However, in the interdialytic period, the model underestimated cystatin C at 80% and 100% of dialysis duration time points (P<0.001 and P=0.04, respectively), although by only a small magnitude, with mean differences between the data and the model of 0.21 and 0.14 mg/L, respectively.
Figure 5.
Comparison of measured cystatin C clearance and rebound data to two-pool modelled plasma cystatin C. Errors bars shown represent the mean±SD.
Iterated Parameter Estimates from Model.
Mean iterated KNR was 25.1 ml/min per 1.73 m2 body surface area (SD=6.6). The transfer coefficient for the intracellular–extracellular pool barrier (Kint) showed a wide variation between patients, with a median of 63.7 ml/min per 1.73 m2 body surface area (interquartile range=29.4—157.1). These clearance parameters are corrected to body surface area to allow comparison with KR, which is usually body surface area–adjusted. The mean modeled ratio of the extracellular water to total body water (ECWFraction) was 0.255 (SD=0.092).
Prediction of Postrebound Cystatin C Concentration
There was no significant correlation between dialysis duration and the magnitude of postdialysis rebound measured by the rise in plasma cystatin C from the end of dialysis to 2 hours postdialysis. The magnitude of the modeled rebound at 2 hours postdialysis was 50.0%±10.9% of the intradialytic fall in cystatin C.
Discussion
In the normal population, cystatin C may be superior to creatinine as a single unadjusted marker for predicting GFR (5,32–34), although the finding is not universal (35). Cystatin C–based equations perform as least as well as, if not better than, the Modification of Diet in Renal Disease equation in individuals without substantial renal impairment (3,36–40), although perhaps less well in stage 3 CKD (8). However, in end stage renal failure, its usefulness as a predictor of RRF has not been established. We set out to define the kinetics of cystatin C and study whether it is possible to accurately model cystatin C concentration, which might allow RRF to be estimated from plasma levels.
We have shown that the kinetics of cystatin C are more complex than the kinetics of small molecules, such as urea. Although we were able to accurately model cystatin C concentration using a two-pool iterative model, important model parameters (ECWFraction, Kint, and KNR) showed large interindividual variance. Mean modeled KNR of 25.1 ml/min per 1.73 m2 body surface area was similar to that reported by Sjöström et al. (21), perhaps unsurprisingly, because we used the estimated G from this publication. Levels, however, showed wide variation.
Because predialysis (plateau) concentration of cystatin C is defined by the balance of generation and clearance (renal and nonrenal), the wide variation in the ratio of generation rate to nonrenal clearance limits the usefulness of predialysis levels as a marker of RRF in this setting. Assuming that generation rate is relatively stable, then at high levels of GFR, predialysis cystatin C concentration will be largely determined by GFR, whereas at low levels, nonrenal clearance will predominate. In line with this finding, in peritoneal dialysis patients, GFR predicted by a cystatin C–based equation (10) correlated with EDTA clearance but only explained 23% of the variance (41).
Cystatin C clearance during dialysis was of less than that of urea, creatinine, and β2-microglobulin, reflecting its molecular size (13 kD). Cystatin C reduction ratio was greater with HDF than high-flux HD, which has been shown previously (42,43). The rate of cystatin C reduction fell during the HD session, suggesting a lag in transfer to the vascular compartment. Mass removal of cystatin C is greater in the initial phase of dialysis, suggesting that increments in sessional frequency may be a more effective removal strategy than increased duration. We found, as have others, that predialysis cystatin C levels correlate with weekly standard Kt/V but not single pool Kt/V (19,20). This finding probably reflects the contribution of RRF to the former parameter. The relationship has been used by the work by Huang et al. (13), which recently described a method of predicting GFR based on plasma cystatin C levels corrected for standard Kt/V.
Of the solutes studied, the magnitude of the postdialysis rebound was greatest for cystatin C. Unlike the other molecules, cystatin C levels plateaued in the interdialytic period. This finding confirms that predialysis levels are in steady state, in which generation rate is balanced by clearance (renal and nonrenal). The wide interindividual variation in KNR that we have shown implies that predialysis cystatin C concentration will not correlate closely with GFR.
In our two-pool model, the rebound kinetics of cystatin C are predominantly defined by the relative pool sizes of ECW and ICW (determined by ECWFraction) and the mass transfer rate between body compartments (Kint). Both showed wide variance. The apparent relative pool size of the dialyzed extracellular pool in relation to total body water (ECWFraction) was smaller than that predicted from anthropometric equations, which was hypothesized by Tidman et al. (44). Hence, rebound characteristics are not fully explained by re-equilibration of cystatin C from the intracellular to extracellular pool. The reduction in apparent dialyzed pool size implies the presence of one or more pools of sequestered cystatin C. It should be emphasized that ECWFraction represents an apparent pool size derived from modeling rather than a true physiologic parameter.
There is evidence that cystatin C is dimerized and monomerizes as part of the normal cellular processing pathway (45). There are no data on the relative pool size of dimerized cystatin C. It is likely that the assay that we used measured predominantly monomeric cystatin C, although there are no published data. Cystatin C may also be bound to the C4 component of complement (46) and other proteins, which may contribute to the reduction in apparent pool size. In our model, we iterated the relative sizes of the apparent intracellular and extracellular spaces, which will have taken into account the effect of any additional pools. Similar multiple pool effects have been shown for other molecules, such as serum phosphate (25).
Our model assumed also that transfer across the intracellular–extracellular space was a concentration-driven process akin to diffusion, but the process may be more complex and involve active secretion, which is known to occur for cystatin C in the endoplasmic reticulum–lysosome pathway (45,47). Wallin et al. (47) have recently identified a hydrophobic cystatin C region in the cysteine cathepsin binding region of the molecule that seems to be a crucial determinant of cellular uptake. We iterated the mass transfer coefficient (Kint), and, therefore, the parameter is likely to represent the combined effects of diffusion, active transport, and perhaps, other cellular processes that might affect the rebound in cystatin C.
We chose to fix G at 0.124 mg/min per 1.73 m2 as previously reported, because it seems to vary little between individuals (11). In the model, it was necessary to constrain either G or KNR to prevent multiple solutions to the model being possible (multiple global minima). By constraining G, we may have overestimated the interindividual variation in nonrenal clearance. The model also assumed that cystatin C generation occurred into the intracellular space and that concentrations were uniform throughout both pools. However, there is evidence that, although cystatin C functions as a housekeeping protein, concentration varies in different body fluid spaces. The dominant type of cysteine protease inhibitors may also vary between tissues (48). Body water was not iterated, because it would have resulted in models with multiple solutions. Similarly, dialyzer clearance was not iterated but included as a constant estimated from in vivo clearance data for β2-microglobulin, a similar-sized middle molecule. Limited published data suggest that cystatin C clearance is approximately 90% of β2-microglobulin clearance for high-flux dialyzers in HD and HDF (26–28). There are extensive in vivo clearance data available for β2-microglobulin (29) that are specific to dialysis modality (high-flux HD or HDF), blood flow, and dialyzer size. However, we acknowledge that more extensive in vivo cystatin C clearance studies are required to confirm this result.
Despite these limitations, this two-pool model is able to accurately model the rebound in cystatin C, with the modeled concentration not differing significantly from the measured concentration at any time point. In the interdialytic period, fit was not quite as good, with modeled levels differing, albeit marginally, from measured levels at the two time points toward the end of the dialysis session. This result may suggest that ECWFraction decreases to the end of dialysis, reflecting release of cystatin C from sequestered pools, perhaps because of monomerization.
In conclusion, plasma cystatin C concentration may be successfully modeled using two-pool kinetics, but it is necessary to iterate the relative sizes of the intracellular and extracellular pools nonphysiologically. This finding implies that the kinetics are substantially more complex than for molecules such as urea and that the incorporation of multiple pools would be required to improve modeling further. Importantly, the rebound in plasma cystatin C is of large magnitude, and the kinetics are difficult to predict. The wide variation in interindividual modeled nonrenal clearance indicates that, at low levels of GFR, single plasma cystatin C concentrations are unlikely to be useful as an indicator of RRF. Lack of interindividual predictability of modeled parameters indicates that, although cystatin C rebound may be modeled using two pools for one individual, prediction of interdialytic concentration for another individual without formal modeling is not possible, unlike for urea. These findings show the limitations in using single point plasma measures of cystatin C to accurately predict GFR in patients on HD. Cystatin C is cleared by high-flux membranes, and its usefulness as a marker of middle-molecule clearance should be further explored.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was funded by a grant from the British Renal Society and supported by a Kidney Research United Kingdom Fellowship. We acknowledge the support of the National Institutes for Health Research through the Comprehensive Local Research Network.
The results in this paper have not been published previously in whole or part except in abstract format at the Renal Association Conference 2011, on June 6–9, 2011, in Birmingham, UK.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07510713/-/DCSupplemental.
References
- 1.Grubb A: Diagnostic value of analysis of cystatin C and protein HC in biological fluids. Clin Nephrol 38[Suppl 1]: S20–S27, 1992 [PubMed] [Google Scholar]
- 2.Grubb AO: Cystatin C—properties and use as diagnostic marker. Adv Clin Chem 35: 63–99, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coll E, Botey A, Alvarez L, Poch E, Quintó L, Saurina A, Vera M, Piera C, Darnell A: Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis 36: 29–34, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Tenstad O, Roald AB, Grubb A, Aukland K: Renal handling of radiolabelled human cystatin C in the rat. Scand J Clin Lab Invest 56: 409–414, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Dharnidharka VR, Kwon C, Stevens G: Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis 40: 221–226, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Vinge E, Lindergård B, Nilsson-Ehle P, Grubb A: Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest 59: 587–592, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Bökenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J: Cystatin C—a new marker of glomerular filtration rate in children independent of age and height. Pediatrics 101: 875–881, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tangri N, Inker LA, Tighiouart H, Sorensen E, Menon V, Beck G, Shlipak M, Coresh J, Levey AS, Sarnak MJ: Filtration markers may have prognostic value independent of glomerular filtration rate. J Am Soc Nephrol 23: 351–359, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoek FJ, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT: Estimation of residual glomerular filtration rate in dialysis patients from the plasma cystatin C level. Nephrol Dial Transplant 22: 1633–1638, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Sjöström PA, Jones IL, Tidman MA: Cystatin C as a filtration marker—haemodialysis patients expose its strengths and limitations. Scand J Clin Lab Invest 69: 65–72, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Mayeur N, Rostaing L, Nogier MB, Jaafar A, Cointault O, Kamar N, Conil JM, Fourcade O, Lavayssiere L: Kinetics of plasmatic cytokines and cystatin C during and after hemodialysis in septic shock-related acute renal failure. Crit Care 14: R115, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang SH, Filler G, Lindsay RM: Residual renal function calculated from serum cystatin C measurements and knowledge of the weekly standard Kt/V urea. Perit Dial Int 32: 102–104, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maduell F, Moreso F, Pons M, Ramos R, Mora-Macià J, Foraster A, Soler J, Galceran JM, Martinez-Castelao A, Online Hemodiafiltration Study Group from the Catalonian Society of Nephrology : Design and patient characteristics of ESHOL study, a Catalonian prospective randomized study. J Nephrol 24: 196–202, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Vilar E, Fry AC, Wellsted D, Tattersall JE, Greenwood RN, Farrington K: Long-term outcomes in online hemodiafiltration and high-flux hemodialysis: A comparative analysis. Clin J Am Soc Nephrol 4: 1944–1953, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Q, Li R, Zhong Z, Mao H, Fan J, Lin J, Yang X, Wang X, Li Z, Yu X: Is cystatin C a better marker than creatinine for evaluating residual renal function in patients on continuous ambulatory peritoneal dialysis? Nephrol Dial Transplant 26: 3358–3365, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Krishnamurthy N, Arumugasamy K, Anand U, Anand CV, Aruna V, Venu G, Gayathri R: Effect of hemodialysis on circulating cystatin c levels in patients with end stage renal disease. Indian J Clin Biochem 25: 43–46, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JS, Kim GH, Kang CM, Lee CH: Application of cystatin C reduction ratio to high-flux hemodialysis as an alternative indicator of the clearance of middle molecules. Korean J Intern Med 25: 77–81, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Malki N, Heidenheim PA, Filler G, Yasin A, Lindsay RM: Cystatin C levels in functionally anephric patients undergoing dialysis: The effect of different methods and intensities. Clin J Am Soc Nephrol 4: 1606–1610, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang SH, Filler G, Yasin A, Lindsay RM: Cystatin C reduction ratio depends on normalized blood liters processed and fluid removal during hemodialysis. Clin J Am Soc Nephrol 6: 319–325, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sjöström P, Tidman M, Jones I: Determination of the production rate and non-renal clearance of cystatin C and estimation of the glomerular filtration rate from the serum concentration of cystatin C in humans. Scand J Clin Lab Invest 65: 111–124, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Vilar E, Wellsted D, Chandna SM, Greenwood RN, Farrington K: Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol Dial Transplant 24: 2502–2510, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Hemodialysis Adequacy 2006 Work Group : Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis 48[Suppl 1]: S2–S90, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Watson PE, Watson ID, Batt RD: Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr 33: 27–39, 1980 [DOI] [PubMed] [Google Scholar]
- 25.Spalding EM, Chamney PW, Farrington K: Phosphate kinetics during hemodialysis: Evidence for biphasic regulation. Kidney Int 61: 655–667, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Schmidt JJ, Hafer C, Clajus C, Hadem J, Beutel G, Schmidt BM, Kielstein JT: New high-cutoff dialyzer allows improved middle molecule clearance without an increase in albumin loss: A clinical crossover comparison in extended dialysis. Blood Purif 34: 246–252, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Krieter D: Optimum sieving and biocompatibility profiles of the new high-flux dialysis membrane Purema H. Presented at the 23rd Annual Meeting of the International Society of Blood Purification, Rotterdam, The Netherlands, September 1, 2005 [Google Scholar]
- 28.Meert N, Eloot S, Schepers E, Lemke HD, Dhondt A, Glorieux G, Van Landschoot M, Waterloos MA, Vanholder R: Comparison of removal capacity of two consecutive generations of high-flux dialysers during different treatment modalities. Nephrol Dial Transplant 26: 2624–2630, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Ronco C, Bowry SK, Brendolan A, Crepaldi C, Soffiati G, Fortunato A, Bordoni V, Granziero A, Torsello G, La Greca G: Hemodialyzer: From macro-design to membrane nanostructure; the case of the FX-class of hemodialyzers. Kidney Int Suppl 80: 126–142, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Chamney PW, Krämer M, Rode C, Kleinekofort W, Wizemann V: A new technique for establishing dry weight in hemodialysis patients via whole body bioimpedance. Kidney Int 61: 2250–2258, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Tzamaloukas AH, Saddler MS, Murphy G, Morgan K, Goldman RS, Murata GH, Malhotra D: Volume of distribution and fractional clearance of urea in amputees on continuous ambulatory peritoneal dialysis. Perit Dial Int 14: 356–361, 1994 [PubMed] [Google Scholar]
- 32.Siersbaek-Nielsen K, Hansen JM, Kampmann J, Kristensen M: Rapid evaluation of creatinine clearance. Lancet 1: 1133–1134, 1971 [DOI] [PubMed] [Google Scholar]
- 33.Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S: Measurement of muscle mass in humans: Validity of the 24-hour urinary creatinine method. Am J Clin Nutr 37: 478–494, 1983 [DOI] [PubMed] [Google Scholar]
- 34.van Olden RW, Krediet RT, Struijk DG, Arisz L: Measurement of residual renal function in patients treated with continuous ambulatory peritoneal dialysis. J Am Soc Nephrol 7: 745–750, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Stickle D, Cole B, Hock K, Hruska KA, Scott MG: Correlation of plasma concentrations of cystatin C and creatinine to inulin clearance in a pediatric population. Clin Chem 44: 1334–1338, 1998 [PubMed] [Google Scholar]
- 36.Grubb A, Nyman U, Björk J, Lindström V, Rippe B, Sterner G, Christensson A: Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem 51: 1420–1431, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Grubb A, Simonsen O, Sturfelt G, Truedsson L, Thysell H: Serum concentration of cystatin C, factor D and beta 2-microglobulin as a measure of glomerular filtration rate. Acta Med Scand 218: 499–503, 1985 [DOI] [PubMed] [Google Scholar]
- 38.Kyhse-Andersen J, Schmidt C, Nordin G, Andersson B, Nilsson-Ehle P, Lindström V, Grubb A: Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin Chem 40: 1921–1926, 1994 [PubMed] [Google Scholar]
- 39.Simonsen O, Grubb A, Thysell H: The blood serum concentration of cystatin C (gamma-trace) as a measure of the glomerular filtration rate. Scand J Clin Lab Invest 45: 97–101, 1985 [DOI] [PubMed] [Google Scholar]
- 40.Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, Price CP: Serum cystatin C measured by automated immunoassay: A more sensitive marker of changes in GFR than serum creatinine. Kidney Int 47: 312–318, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Carter JL, Lane CE, Fan SL, Lamb EJ: Estimation of residual glomerular filtration rate in peritoneal dialysis patients using cystatin C: Comparison with 51Cr-EDTA clearance. Nephrol Dial Transplant 26: 3729–3732, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Campo A, Lanfranco G, Gramaglia L, Goia F, Cottino R, Giusto V: Could plasma cystatin C be useful as a marker of hemodialysis low molecular weight proteins removal? Nephron Clin Pract 98: c79–c82, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Lindström V, Grubb A, Alquist Hegbrant M, Christensson A: Different elimination patterns of beta-trace protein, beta2-microglobulin and cystatin C in haemodialysis, haemodiafiltration and haemofiltration. Scand J Clin Lab Invest 68: 685–691, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Tidman M, Sjöström P, Jones I: Plasma cystatin C for estimating residual GFR (rGFR) in dialysis patients. Nephrol Dial Transplant 23: 1072–1073, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Merz GS, Benedikz E, Schwenk V, Johansen TE, Vogel LK, Rushbrook JI, Wisniewski HM: Human cystatin C forms an inactive dimer during intracellular trafficking in transfected CHO cells. J Cell Physiol 173: 423–432, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Ghiso J, Saball E, Leoni J, Rostagno A, Frangione B: Binding of cystatin C to C4: The importance of sense-antisense peptides in their interaction. Proc Natl Acad Sci U S A 87: 1288–1291, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallin H, Abrahamson M, Ekström U: Cystatin C properties crucial for uptake and inhibition of intracellular target enzymes. J Biol Chem 288: 17019–17029, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abrahamson M, Barrett AJ, Salvesen G, Grubb A: Isolation of six cysteine proteinase inhibitors from human urine. Their physicochemical and enzyme kinetic properties and concentrations in biological fluids. J Biol Chem 261: 11282–11289, 1986 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.