Abstract
Background and objectives
Cardiovascular disease is the leading cause of death in patients with CKD. IL-10 is considered an antiatherosclerotic cytokine. However, previous studies have failed to observe an association between IL-10 and cardiovascular disease in CKD. This study aimed to evaluate whether serum IL-10 levels were associated with the risk of cardiovascular events in CKD patients.
Design, setting, participants, & measurements
Four hundred three patients with stages 1–5 CKD were followed for a mean of 38 (range=2–42) months for fatal and nonfatal cardiovascular events. IL-10 and IL-6 were measured at baseline together with surrogates of endothelial function (flow-mediated dilatation) and proinflammatory markers (high-sensitivity C-reactive protein and pentraxin-3). The association between IL-10 and flow-mediated dilatation through linear regression analyses was evaluated. The association between IL-10 and the risk of cardiovascular events was assessed with Cox regression analysis.
Results
IL-10, IL-6, high-sensitivity C-reactive protein, and pentraxin-3 levels were higher among participants with lower eGFR. Both fatal (25 of 200 versus 6 of 203 patients) and combined fatal and nonfatal (106 of 200 versus 23 of 203 patients) cardiovascular events were more common in patients with IL-10 concentration above the median. Flow-mediated dilatation was significantly lower in patients with higher serum IL-10 levels, but IL-10 was not associated with flow-mediated dilatation in multivariate analysis. Kaplan–Meier survival curves showed that patients with IL-10 below the median value (<21.5 pg/ml) had higher cumulative survival compared with patients who had IL-10 levels above the median value (log-rank test, P<0.001).
Conclusions
IL-10 levels increase along with the reduction of kidney function. Higher serum IL-10 levels were associated with the risk of cardiovascular events during follow-up. We speculate that higher IL-10 levels in this context signify an overall proinflammatory milieu.
Keywords: CKD, cardiovascular disease, kidney disease, IL-10
Introduction
Cardiovascular (CV) disease is the leading cause of death in patients with CKD (1). Disproportionately increased oxidative stress and inflammation are considered among the main culprits of this heightened risk (2).
IL-10 acts as an immunoregulatory cytokine that balances increases of proinflammatory cytokines and shuts off the system (3). Several experimental studies have confirmed the anti-inflammatory effect of IL-10 (4). In addition, IL-10 has also been shown to have antithrombotic and antiatherosclerotic properties (5).
Several clinical studies have been conducted to answer the question of whether serum IL-10 levels are associated with the development of adverse CV events. In contrast to animal experiments, contradictory results have been reported in the clinical setting. Although Heeschen et al. (6) found that higher serum IL-10 levels were related to better clinical outcomes in terms of CV morbidity and mortality among patients with acute coronary syndrome (ACS), Mälarstig et al. (7) showed the opposite. Several potential explanations for this discordance have been suggested (8,9). Furthermore, it has been suggested that IL-10 kinetics are different in CKD patients (4). An earlier study showed large interindividual differences in serum IL-10 levels in patients undergoing hemodialysis caused by promoter gene polymorphism (8).
With this background in mind, our main goal was to evaluate whether serum IL-10 is associated with the development of CV events in CKD patients. Such a plausible association needs to be analyzed in the context of the proinflammatory counterbalance, and for that reason, we also assessed high-sensitivity C-reactive protein (hsCRP), IL-6, and pentraxin-3 (PTX-3) levels. Finally, because endothelial dysfunction is recognized by many as one of the earliest discernible steps of atherosclerotic process (10), we studied—as a secondary aim—the possible association between IL-10 and flow-mediated dilatation (FMD) as an endothelial dysfunction surrogate.
Materials and Methods
Patients and Study Design
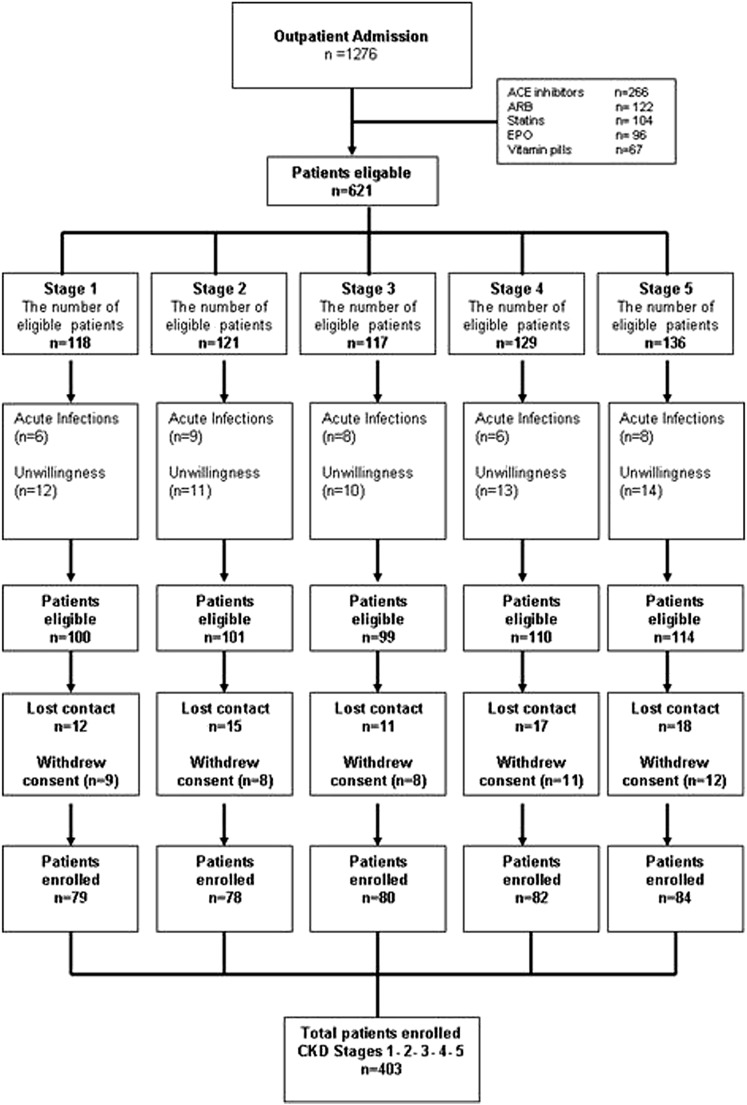
This study is an ancillary study performed in an observational prospective cohort study already collected (11). The initial objective of the cohort study was to identify risk factors for endothelial dysfunction in nondialyzed CKD patients, and for that reason, a number of exclusions was done a priori. Between March 2006 and February 2011, 1276 patients were referred to the Renal Unit of the Gulhane School of Medicine Medical Center, Ankara, Turkey for the first time because of suspected or manifest CKD. All patients included in the study were diagnosed as having CKD according to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative Guidelines (12). By protocol and to minimize any confounding effects of conditions that may influence endothelial dysfunction measurements, 873 patients who were already taking medications that may influence endothelial function, including angiotensin converting enzyme inhibitors (n=266), angiotensin receptor blockers (n=222), statins (n=104), erythropoiesis-stimulating agents (n=96), and supplemental vitamin pills (n=67), were excluded. Other exclusion criteria included acute infections and unwillingness to participate in the study (n=65); 153 eligible patients dropped out for the following reasons: lost to follow-up (n=80) or withdrew consent (n=73). A flow chart of the patients is shown in Figure 1. Stages of CKD were determined using eGFR, which was calculated using the Modification of Diet in Renal Disease equation (13). Four hundred three patients were included in the final analysis. None of the patients with stage 5 CKD were on dialysis at the time of investigation.
Figure 1.
Flow chart of the study population. ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; EPO, erythropoietin.
All included patients were followed for time-to-event analysis until occurrence of fatal or nonfatal CV events. Fatal and nonfatal CV events, including death, stroke, and myocardial infarction, were recorded by telephone contacts and routine outpatient clinic visits. The Gulhane School of Medicine local ethical committee approved the study protocol, and all patients were included in the study after signing informed consent forms.
Laboratory Measurements
All blood samples were obtained from patients in the morning between 7:00 and 11:00 A.M. after 12 hours of fasting for measurement of fasting plasma glucose, serum basal insulin, serum albumin, total serum cholesterol, triglyceride, HDL and LDL cholesterol, and intact parathyroid hormone. Samples were kept frozen at −80°C. hsCRP, IL-10, IL-6, and PTX-3 parameters were studied at the same time. An insulin resistance score (Homeostasis Model Assessment–Insulin Resistance [HOMA-IR]) was computed using the following equation (14): HOMA-IR=fasting plasma glucose (milligrams per deciliter)×immunoreactive insulin (microinternational units per milliliter)/405. Proteinuria was quantified using a 24-hour timed urine collection.
Serum hsCRP, Human IL-10, IL-6, and PTX-3 Measurements
hsCRP was measured by a photometric method. Serum levels of IL-10 were determined using a commercially available sandwich enzyme immunoassay kit (Human IL-10 Platinum ELISA Kit; eBioscience, Bender MedSystems, Vienna, Austria). The calculated overall intra-assay coefficient of variation was 3.2%, and the calculated overall interassay coefficient of variation was 5.6%. After spiking of human IL-10 into normal human serum, average spike recoveries ranged from 81% to 106%, and overall mean recovery of 97% was found.
Serum levels of IL-6 were analyzed using human IL-6 ELISA kits (Quantikine HS Human IL-6 Immunoassay; R&D Systems, Minneapolis, MN) with a sensitivity of 0.16 pg/ml and an interassay coefficient of variation of <7.8%. Plasma PTX-3 concentration was measured by using a commercially available ELISA kit (Perseus Proteomics Inc., Japan). A description of these results has been published elsewhere (14).
Assessment of Endothelial Function
Endothelium-dependent vasodilatation (FMD) and endothelium-independent vasodilatation (nitroglycerine-mediated dilatation) of the brachial artery were assessed noninvasively using high-resolution ultrasound as described by Celermajer et al. (15). The method for the vascular assessment met the criteria imposed by the International Brachial Artery Reactivity Task Force (16). Measurements were made by a single observer using an ATL 5000 ultrasound system (Advanced Technology Laboratories Inc., Bothell, WA) with a 12-MHz probe. A detailed description of the endothelial measurements is provided elsewhere (15,16).
Statistical Analyses
All statistical analyses were performed using an SPSS 11.0 (SPSS Inc., Chicago, IL) statistical package. Non-normally distributed variables were expressed as median (range), and normally distributed variables were expressed as mean±SD as appropriate. A P value<0.05 was considered to be statistically significant. Between-group comparisons were assessed for categorical variables with the chi-squared test, and the Kruskal–Wallis test (ANOVA) was used for the rest of the variables. Spearman rank correlation was used to determine correlations between paired variables. Stepwise multivariate regression analysis was used to assess the independent associates of FMD as a mediator of CV disease. Kaplan–Meier curves were drawn to present differences between two different IL-6/IL-10 ratio groups. Survival and time-to-event analysis of CV outcomes were done using Cox proportional hazards models, including adjustment for potential confounding factors. Data are presented in the form of hazard ratios and 95% confidence intervals. The sample size was calculated by using Power and Sample Size Calculations V.3.0 (Vanderbilt University). We assumed that the number of the patients who have IL-6/IL-10 levels smaller and greater than 0.028 is almost equal. For a 1.5-fold hazard ratio, a 5% α-error, and an 80% β-error, the sample size was calculated at 192 for each group. Recruitment time was 36 months, with a 6-month additional follow-up period.
Results
Patient Characteristics
In total, 79 patients with stage 1 CKD, 78 patients with stage 2 CKD, 80 patients with stage 3 CKD, 82 patients with stage 4 CKD, and 84 patients with stage 5 CKD were included in the study. Demographic and clinical characteristics of the entire study cohort are depicted in Table 1. There was no difference among the groups in terms of age, sex distribution, and body mass index. Laboratory values, including inflammatory cytokines and vascular measurements, are shown in Table 1. As expected, serum calcium and albumin decreased, whereas serum phosphorus and intact parathyroid hormone levels increased across CKD stages 1–5. There was a significant declining trend for serum LDL cholesterol from stage 1 to stage 5 (P=0.04). There were significant increases in serum levels of hsCRP, PTX-3, and IL-6 across increasing CKD stages. In a similar way, IL-10 levels were significantly increased as eGFR decreased. Despite significant increases in both IL-6 and IL-10, the IL-6/IL-10 ratio significantly decreased from stage 1 to stage 5 CKD. Both endothelium-dependent (expressed as FMD) and endothelium-independent vasodilation (expressed as nitroglycerine-mediated dilatation) were decreased from stage 1 CKD to stage 5 CKD.
Table 1.
Clinicodemographic characteristics, biochemical parameters, and vascular assessments according to CKD stages
| Parameters | Stage 1 (≥90 ml/min; n=79) | Stage 2 (60–89 ml/min; n=78) | Stage 3 (30–59 ml/min; n=80) | Stage 4 (15–29 ml/min; n=82) | Stage 5 (<15 ml/min; n=84) | P Valuea |
|---|---|---|---|---|---|---|
| Age, yr | 50 (28–71) | 56 (30–71) | 52 (29–71) | 55 (31–73) | 53 (28–71) | 0.31 |
| Sex (men/women) | 45/34 | 46/32 | 46/34 | 40/42 | 51/33 | 0.59 |
| Body mass index, kg/m2 | 26.6±2.2 | 26.3±3.1 | 25.9±2.4 | 26.2±2.9 | 25.4±2.8 | 0.06 |
| History of CV disease, n | ||||||
| CV episode | 9 (11%) | 3 (4%) | 10 (13%) | 12 (15%) | 20 (24%) | 0.02 |
| Stroke | 3 (4%) | 2 (3%) | 1 (1%) | — | 3 (4%) | 0.08 |
| Peripheral vascular disease | 4 (5%) | 1 (1%) | — | 2 (3%) | 3 (4%) | 0.07 |
| Aortic aneurysm | 3 (4%) | 1 (1%) | — | — | — | 0.11 |
| Etiology of CKD, n | ||||||
| Diabetes | 12 (15%) | 22 (28%) | 17 (21%) | 20 (24%) | 20 (24%) | 0.38 |
| GN | 11 (14%) | 9 (12%) | 13 (16%) | 11 (13%) | 13 (15%) | 0.67 |
| Hypertension | 9 (12%) | 19 (24%) | 16 (20%) | 10 (12%) | 10 (12%) | 0.08 |
| Polycystic kidney disease | 5 (6%) | 2 (3%) | 3 (4%) | 4 (5%) | 2 (2%) | 0.23 |
| Reflux nephropathy | 3 (4%) | 3 (4%) | 1 (1%) | 4 (5%) | 2 (2%) | 0.13 |
| Amyloidosis | 4 (5%) | 6 (8%) | 4 (5%) | 5 (6%) | 5 (6%) | 0.68 |
| Unknown | 35 (44%) | 17 (22%) | 26 (33%) | 28 (34) | 32 (38%) | 0.72 |
| Smoking (current), n | 37 (47%) | 37 | 36 (45%) | 32 (39%) | 41 (49%) | 0.15 |
| Total cholesterol, mg/dl | 200 (160–239) | 202 (170–243) | 200 (171–245) | 199 (159–244) | 195 (157–243) | 0.10 |
| Triglycerides, mg/dl | 142±15 | 144±12 | 147±16 | 145±14 | 142±20 | 0.20 |
| LDL cholesterol, mg/dl | 128±17 | 131±16 | 126±15 | 129±14 | 123±21 | 0.04 |
| Systolic BP, mmHg | 135±9 | 138±12 | 135±13 | 136±15 | 134±10 | 0.10 |
| Diastolic BP, mmHg | 83±4 | 84±3 | 85±4 | 84±6 | 84±5 | 0.07 |
| Serum calcium, mg/dl | 8.9±0.5 | 8.7±0.5 | 8.3±0.5 | 8.1±0.4 | 8.1±0.4 | <0.001 |
| Serum phosphate, mg/dl | 4.1±0.4 | 4.3±0.9 | 4.6±0.8 | 5.7±1.3 | 6.6±1.6 | <0.001 |
| iPTH, pg/ml | 51±12 | 70±28 | 152±42 | 167±33 | 257±41 | <0.001 |
| Serum albumin, g/dl | 4.0 (3.6–4.8) | 4.0 (3.5–4.6) | 4.3 (3.5–4.8) | 4.0 (3.4–4.6) | 3.9 (3.2–4.6) | <0.001 |
| HOMA-IR index | 1.64±0.72 | 1.78±0.61 | 1.89±1.08 | 1.96±1.00 | 1.86±0.95 | 0.20 |
| 24-h proteinuria, g/d | 1.39 (0.38–2.45) | 1.68 (0.37–3.92) | 1.71 (0.57–5.15) | 1.57 (0.48–4.39) | 1.7 (0.8–5.45) | <0.001 |
| hsCRP, mg/L | 8.0 (3.2–13.6) | 11.2 (5.0–16.0) | 17.0 (5.0–22.0) | 23.0 (6.7–35.0) | 25.5 (4.0–44.0) | <0.001 |
| PTX-3, ng/ml | 3.6 (1.3–45.2) | 6.9 (1.4–42.8) | 8.41 (0.5–60.5) | 9.15 (0.6–48.7) | 15.2 (0.8–67.3) | <0.001 |
| IL-6, pg/ml | 5.2 (1.0–6.6) | 5.5 (1.0–7.3) | 6.1 (1.0–12.6) | 7.4 (1.0–15.4) | 12.0 (1.3–27.4) | <0.001 |
| IL-10, pg/ml | 9.7 (2.1–47.2) | 12.0 (2.2–59.0) | 16.8 (3.6–58.7) | 32.5 (3.2–62.6) | 41.5 (5.6–66.0) | <0.001 |
| IL-6/IL-10 ratio | 0.47 (0.03–2.18) | 0.33 (0.03–2.54) | 0.30 (0.02–2.52) | 0.20 (0.02–2.27) | 0.23 (0.02–2.18) | <0.001 |
| NMD, % | 13.0 (11.0–13.8) | 13.1 (12.4–13.8) | 12.9 (12.0–13.9) | 13.0 (11.6–13.8) | 12.2 (10.0–13.1) | <0.001 |
| FMD, % | 8.4±0.7 | 7.3±0.6 | 7.0±0.7 | 6.4±0.8 | 5.7±1.0 | <0.001 |
Units for CKD stages are presented as ml/min per 1.73 m2. CV, cardiovascular; iPTH, intact parathyroid hormone; HOMA-IR, Homeostasis Model Assessment–Insulin Resistance; hsCRP, high-sensitivity C-reactive protein; PTX-3, pentraxin-3; NMD, nitroglycerine-mediated dilatation; FMD, flow-mediated dilatation.
Differences assessed by chi-squared test for categorical variables and Kruskal–Wallis test. Statistically significant if P<0.05.
Phenotypical Characteristics Associated with High IL-10 Concentrations
We stratified the entire study cohort into two groups according to the median serum IL-10 level (Table 2). All of the studied inflammatory cytokines were significantly higher in the group with IL-10 concentrations above the median value compared with the group with IL-10 concentrations below the median value. Diabetes mellitus was more frequent in patients with high serum IL-10, and FMD was significantly lower. The number of both fatal and nonfatal CV events was significantly higher in patients with high IL-10 levels.
Table 2.
Biochemical parameters, vascular assessment results, and composite CV events in patient groups, which were stratified by median IL-10 levels
| Parameters | IL-10 (<20.02 pg/ml; n=203) | IL-10 (≥20.02 pg/ml; n=200) | P Valuea |
|---|---|---|---|
| Total cholesterol, mg/dl | 200 (159–245) | 200 (157–244) | 0.30 |
| Triglycerides, mg/dl | 144±14 | 144±17 | 0.80 |
| LDL cholesterol, mg/dl | 128±16 | 126±17 | 0.20 |
| Systolic BP, mmHg | 133 (110–180) | 135 (110–190) | 0.40 |
| Diastolic BP, mmHg | 84±5 | 84±5 | 0.80 |
| Serum calcium, mg/dl | 8.62±0.58 | 8.22±0.50 | <0.001 |
| Serum phosphate, mg/dl | 4.54±0.96 | 5.70±1.56 | <0.001 |
| iPTH, pg/ml | 96±56 | 187±77 | <0.001 |
| Serum albumin, g/dl | 4.0 (3.5–4.8) | 4.0 (3.2–4.8) | 0.08 |
| HOMA-IR index | 1.73±0.77 | 1.93±1.00 | 0.03 |
| 24-h proteinuria, g/d | 1.62 (0.37–5.45) | 1.65 (0.48–5.45) | 0.09 |
| hsCRP, mg/L | 11.5 (3.2–33.0) | 20.0 (4.0–44.0) | <0.001 |
| PTX-3, ng/ml | 4.9 (0.8–42.7) | 8.8 (0.5–67.3) | <0.001 |
| IL-6, pg/ml | 5.67±3.39 | 7.80±5.47 | <0.001 |
| IL-10, pg/ml | 9.5 (2.1–21.5) | 41.1 (21.6–66.0) | <0.001 |
| IL-6/IL-10 ratio | 0.58 (0.06–2.54) | 0.17 (0.02–1.24) | <0.001 |
| NMD, % | 13.0 (11.6–13.8) | 12.9 (10.0–13.8) | <0.001 |
| FMD, % | 7.4±0.9 | 6.5±1.2 | <0.001 |
| Diabetes, n | 31 (15%) | 60 (30%) | 0.001 |
| Hypertension, n | 31 (15%) | 33 (17%) | 0.80 |
| Smoking, n | 87 (43%) | 96 (48%) | 0.30 |
| History of CV disease, n | 33 (16%) | 44 (22%) | 0.06 |
| CV events, n | 23 (11%) | 106 (53%) | <0.001 |
| Mortality, n | 6 (3%) | 25 (13%) | <0.001 |
Differences assessed by chi-squared test for categorical variables and Kruskal–Wallis test. Statistically significant if P<0.05. iPTH, intact parathyroid hormone; NMD, nitroglycerine-mediated dilatation.
Multivariate Determinants of FMD
Although all proinflammatory/anti-inflammatory markers (PTX-3, IL-6, IL-10, and the IL-6/IL-10 ratio) were significantly related to FMD in univariate analysis, the multivariate analysis showed that only the IL-6/IL-10 ratio and serum PTX-3 levels were associated with FMD (Table 3).
Table 3.
Univariate and multivariate associates of FMD in CKD patients
| Parameters | FMD (%) | 95% Confidence Interval for the Coefficients | |
|---|---|---|---|
| Univariatea Correlation Coefficient (P Value) | Multivariate Adjusted Difference (P Value) | ||
| IL-10, pg/ml | −0.39 (<0.001)a | −0.06 (0.26) | −0.01 to 0.01 |
| IL-6, pg/ml | −0.35 (<0.001)a | 0.09 (0.89) | 0.01 to 0.04 |
| IL-6/IL-10 ratio | 0.12 (0.02)a | −0.12 (<0.001)a | −0.64 to −0.22 |
| hsCRP, mg/L | −0.57 (<0.001)a | −0.01 (0.88) | −0.01 to 0.01 |
| PTX-3, ng/ml | −0.27 (<0.001)a | −0.11 (0.001)a | −0.01 to <0.01 |
| NMD, % | 0.37 (<0.001)a | 0.19 (<0.001)a | 0.21 to 0.44 |
| Age, yr | −0.03 (0.60) | −0.05 (0.32) | −0.01 to <0.01 |
| Sex (men/women) | −0.02 (0.70) | −0.08 (0.13) | −0.28 to 0.02 |
| Smoking | −0.06 (0.21) | −0.10 (0.06) | −0.27 to 0.01 |
| Systolic BP, mmHg | −0.10 (0.04)a | −0.12 (<0.001)a | −0.02 to −0.01 |
| LDL cholesterol, mg/dl | 0.06 (0.22) | −0.01 to 0.01 | |
| Diabetes | −0.15 (0.002)a | −0.07 (0.14) | −0.30 to 0.08 |
| Previous CV disease | −0.09 (0.08) | 0.02 (0.73) | −0.07 to 0.10 |
| HOMA-IR | −0.19 (<0.001)a | −0.09 (<0.01)a | −0.17 to 0.01 |
| Serum albumin, g/dl | 0.18 (<0.001)a | 0.11 (<0.001)a | 0.17 to 0.61 |
| 24-h proteinuria, mg/d | −0.15 (0.002)a | −0.01 (0.86) | 0.01 to 0.01 |
| Serum calcium, mg/dl | 0.43 (<0.001)a | 0.04 (0.40) | −0.13 to 0.20 |
| Serum phosphate, mg/dl | −0.54 (<0.001)a | −0.06 (0.13) | −0.11 to 0.03 |
| iPTH, pg/ml | −0.68 (<0.001)a | −0.04 (0.44) | −0.02 to 0.02 |
| eGFR, ml/min per 1.73 m2 | 0.74 (<0.001)a | 0.64 (<0.001)a | 0.02 to 0.03 |
NMD, nitroglycerine-mediated dilatation; iPTH, intact parathyroid hormone.
Statistically significant (P<0.05). n values as assessed by Spearman rank test as well as estimates and P values from multivariate regression models. The r2 value of the multivariate model was 0.61. Variables known to influence FMD levels (diabetes, history of CV disease, age, sex, IL-10, IL-6, IL-6/IL-10 ratio, PTX-3, hsCRP, NMD, HOMA-IR, systolic BP, albumin, 24-hour proteinuria, calcium, phosphate, iPTH, and eGFR) were initially included in the multivariate analyses.
CV Outcomes
CV outcomes were determined from patient inclusion onward, with a mean follow-up period of 38 (range=2–42) months; 36 patients died, and 31 of those deaths were presumably because of CV causes. CV mortality was defined as death caused by coronary heart disease (n=16), sudden death (n=4), stroke (n=10), or complicated peripheral vascular disease (n=1). In total, 98 additional nonfatal major adverse CV events took place during the follow-up period. These events included stroke (n=27), myocardial infarction (n=64), and peripheral vascular disease (n=7). The predictors for time-to-CV event (fatal and nonfatal CV events=129) were studied by univariate and multivariate Cox regression analyses.
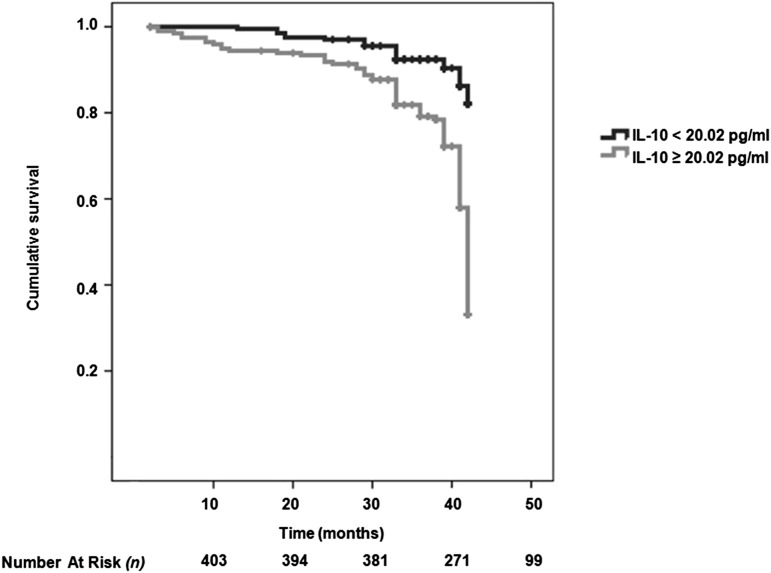
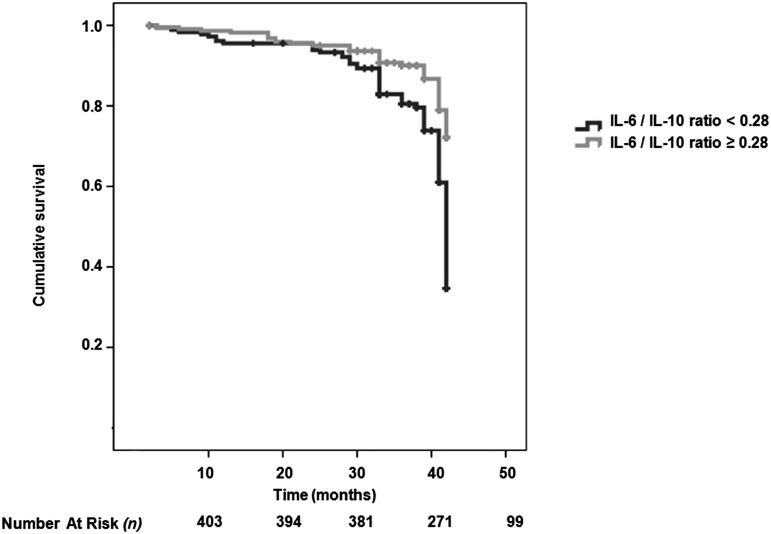
We included all significant parameters derived from the univariate analysis and well known risk factors for CV disease (such as age and sex) into the multivariate Cox model. The multivariate Cox analysis showed that FMD, PTX-3, tertiles of IL-10 (<11.57, 11.57–33.8, and >33.8 pg/ml) and IL-6 (<5.25, 5.25–6.73, and >6.73 pg/ml), the IL-6/IL-10 ratio, and the presence of diabetes mellitus were associated with the risk of CV events (Table 4). In addition, Kaplan–Meier survival curves showed that patients with IL-10 below the median value (<20.02 pg/ml) had higher cumulative survival compared with patients who had IL-10 levels above the median value (log-rank test, P<0.001) (Figure 2). The median for the IL-6/IL-10 ratio in the whole cohort was 0.28, and Kaplan–Meier curves showed a survival advantage associated with patients with an IL-6/IL-10 ratio above this value; furthermore, time to event was shorter in patients with the IL-6/IL-10 ratio above the median value (Figure 3).
Table 4.
Univariate and multivariate Cox analysis predicting fatal and nonfatal CV events (a composite of 129 fatal and nonfatal events)
| Parameters | Univariate Cox | Multivariate Cox | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | P Value | Hazard Ratio | 95% Confidence Interval | P Value | |
| FMD (%) | 0.61 | 0.53 to 0.71 | <0.001a | |||
| IL-10 tertiles, pg/ml | 1.04 | 1.03 to 1.05 | <0.001a | 1.62 | 1.09 to 2.40 | 0.02a |
| IL-6 tertiles, pg/ml | 1.29 | 1.04 to 1.60 | 0.02a | 1.35 | 1.04 to 1.74 | 0.02a |
| IL-6/IL-10 ratio | 0.09 | 0.04 to 0.22 | <0.001a | 0.25 | 0.09 to 0.71 | <0.01a |
| PTX-3, ng/ml | 1.03 | 1.02 to 1.04 | <0.001a | 1.02 | 1.01 to 1.03 | 0.004a |
| hsCRP, mg/L | 1.04 | 1.02 to 1.06 | 0.001a | |||
| Serum albumin, g/dl | 0.56 | 0.33 to 0.97 | 0.04a | |||
| eGFR, ml/min | 0.99 | 0.98 to 0.99 | <0.001a | |||
| Diabetes (yes/no) | 5.11 | 3.62 to 7.23 | <0.001a | 4.41 | 3.08 to 6.33 | <0.001a |
| Hypertension (yes/no) | 2.15 | 1.45 to 3.19 | <0.001a | |||
| Smoking (yes/no) | 1.82 | 1.28 to 2.59 | 0.001a | |||
| History of CV disease | 1.01 | 0.84 to 1.23 | 0.90 | |||
| 24-h proteinuria, mg/d | 1.00 | 1.00 to 1.01 | 0.63 | |||
| Systolic BP, mmHg | 1.03 | 1.01 to 1.04 | <0.001a | |||
| LDL cholesterol, mg/dl | 1.00 | 0.99 to 1.01 | 0.79 | |||
| HOMA-IR | 1.36 | 1.19 to 1.55 | <0.001a | |||
| Calcium, mg/dl | 0.86 | 0.63 to 1.17 | 0.35 | |||
| Phosphate, mg/dl | 1.14 | 1.03 to 1.28 | 0.02a | |||
| iPTH, pg/ml | 1.01 | 1.00 to 1.01 | <0.001a | |||
| Age, yr | 1.00 | 0.99 to 1.01 | 0.90 | |||
| Sex (men/women) | 1.31 | 0.91 to 1.87 | 0.14 | |||
iPTH, intact parathyroid hormone.
Statistically significant (P<0.05).
Figure 2.
Survival curve showing survival advantage of participants with serum IL-10 level below median value. Kaplan–Meier survival curves (a composite of 129 fatal and nonfatal events) according to IL-10 levels<20.02 or ≥20.02 pg/ml.
Figure 3.
Survival curve showing survival advantage of participants with IL-6/IL-10 ratio above 0.28. Kaplan–Meier survival curves (a composite of 129 fatal and nonfatal events) according to IL-6/IL-10 ratio<0.28 or ≥0.28.
We did additional sensitivity analyses to assess the association of IL-10 and the development of new CV events in patients without a previous history of CV disease (n=326). The number of fatal and nonfatal events was 103. The multivariate Cox analysis showed that PTX-3, tertiles of IL-10, and the presence of diabetes mellitus were independently associated with the risk of CV events (Supplemental Table 1). Kaplan–Meier survival curves showed that patients with IL-10 below the median value (<20.02 pg/ml) had higher cumulative survival compared with patients who had IL-10 levels above the median value (log-rank test, P<0.001) (Supplemental Figure 1). Similarly, the median for the IL-6/IL-10 ratio in the whole cohort was 0.284, and Kaplan–Meier curves showed a survival advantage associated with patients with an IL-6/IL-10 ratio above this value (Supplemental Figure 2).
Discussion
The main findings of this study were as follows. First, IL-10 levels increase across worsening CKD stages. Second, serum IL-10 levels showed a negative association with FMD, but only the IL-6/IL-10 ratio remained significantly associated in multivariate models. Third, IL-10 was directly associated with the risk of CV events during follow-up together with IL-6 and PTX-3.
IL-10 is an immunomodulatory cytokine secreted mainly by activated monocytes, lymphocytes, and macrophages (3). IL-10 downregulates the inflammatory activation of monocyte–macrophage cells by transcriptional and post-transcriptional inhibition of the entire range of proinflammatory cytokines (17). In addition to its broad range of anti-inflammatory activity, IL-10 also has putative antiatherosclerotic properties. IL-10 is found in the atheromatous plaque, possibly as a result of local production by tissue macrophages (18). IL-10 interferes with the initial steps in the atherogenetic process by downregulating adhesion molecules, such as CD18, CD60L, and intercellular adhesion molecule 1 (5,14). IL-10 also reduces production of lytic enzymes, such as matrix metalloproteinases, suppresses superoxide anion production, and consequently, helps stability of atheromatous plaques (19).
Consistent with the aforementioned anti-inflammatory and antiatherosclerotic actions of IL-10, animal studies showed that both systemic and local IL-10 gene transfers attenuate atherogenesis (17,20). In contrast to these consistent experimental biologic actions, clinical studies reported controversial results. Heeschen et al. (6) showed that patients with ACS who had elevated serum IL-10 levels at presentation had favorable clinical outcomes during a 6-month follow-up. However, this clinical benefit was confined only to patients who had concurrently higher serum CRP values at baseline evaluation. In contrast, Mälarstig et al. (7) evaluated data from the Fragmin and Fast Revascularization during Instability in Coronary Artery Disease II trial and found that higher baseline IL-10 levels in acute myocardial infarction patients were predictive of poor CV outcomes during a 1-year follow-up. Trying to settle this controversy, Cavusoglu et al. (21) recently conducted a prospective observational study, in which they followed ACS patients for 5 years. In accordance with study by Mälarstig et al. (7), Cavusoglu et al. (21) found that elevated baseline plasma levels of IL-10 are a strong and independent predictor of adverse CV outcomes.
Some hypotheses have been put forward to explain the discrepancies observed in these clinical studies. First, because levels of inflammatory cytokines including IL-10 are subject to considerable change with time, sampling time may have affected the results. In almost all studies, only one blood sample had been studied. Second, IL-10 may have some currently unknown harmful effects, and increased levels of IL-10 may reflect a compensatory or counter-regulatory mechanism in response to a heightened level of proinflammatory cytokines. The results by Heeschen et al. (6) support such a notion, because a predictive ability for favorable outcomes related to serum IL-10 levels was only evident in patients with higher serum CRP values. Third, the survival benefit of IL-10 may require a longer follow-up time to be evident. Indeed, this benefit was only apparent after 1 year in the study of Cavusoglu et al. (21). Thus, variable follow-up durations may have influenced observed results in these studies. Fourth, one could also consider the fact that circulating level of IL-10 does not accurately reflect tissue levels of IL-10 (particularly in atheromatous plaques) (5).
Genetic variations in the promoter region of the IL-10 gene are associated with different levels of IL-10 production and resultant circulating levels (22). Girndt et al. (8) showed that carriers of the 1082A allele (low producers of IL-10) experienced higher rates of CV morbidity in hemodialysis patients. These patients had lower serum levels of IL-10 and higher CRP than patients with the 1082G allele. Notably, this association between IL-10 polymorphism and CV outcomes has not been verified in the general population (23).
In our study, CKD patients did not have recent ACS, and IL-10 values should reflect baseline and not response to stress levels. It is recognized that release of IL-10 always follows elevation of proinflammatory cytokines. Thus, timing of blood sampling may not be as critical compared with previous studies in patients with acute events. Elevation of serum IL-10 level was continuous across CKD stages and positively correlated with other inflammatory markers. The IL-6/IL-10 ratio was associated with worse outcomes rather than better outcomes, which might be expected based on the antiatherogenic properties of IL-10 and the associations with gene polymorphisms. A higher ratio is partially a marker for lower GFR. The elevated levels of serum IL-10 in CKD (11,24) are a joint product of impaired renal clearance of IL-10 by glomerular filtration and tubular metabolism (4) and the ability of uremic monocytes to secrete more IL-10 compared with healthy monocytes (25). An alternative possibility is that, with a decreasing GFR, the proinflammatory status is not sufficiently compensated by the anti-inflammatory/antiatherogenetic properties of IL-10.
One of the few studies addressing IL-10 and CV risk in CKD patients comes from Weber et al. (24), and this study failed to show an association between IL-10 and vascular disease assessed by pulse wave velocity. In addition, Weber et al. (24) found that increased serum IL-10 levels were associated with mortality. Our findings are in line with these findings, because IL-10 was not associated with FMD in multivariate analyses, but using a wider spectrum of renal dysfunction, we confirmed the potential impact on mortality. One could speculate that IL-10 is produced in response to heightened inflammation as a result of the toxic internal uremic milieu. Indeed, our results showed significant increases in serum levels of IL-6, CRP, and PTX-3 together with IL-10 elevations. The patient with a favorable genetic polymorphism can oppose the harmful results of the inflammatory state, which is not the case for low producers of serum IL-10. Therefore, the exact role of serum IL-10 for predicting adverse CV outcomes should ideally be evaluated together with genetic polymorphism studies and a comprehensive evaluation of serum proinflammatory markers as renal function declines. Additional studies should also focus on tissue cytokine levels to elucidate the pathogenesis of such an association.
It has been suggested that the IL-6/IL-10 ratio is a better reflection of the change in the inflammation status in patients with systemic inflammatory response syndrome (26). Thus, we calculated this ratio and found that, despite continuous and significant increases in both IL-10 and IL-6 across increasing CKD stages, the ratio of IL-6/IL-10 decreased when renal function declined. This result implies that, whereas both cytokines increase with reduced GFR, the change in IL-10 is proportionally greater than that of IL-6. Our observation lends support to the hypothesis that IL-10 may increase as a compensatory mechanism secondary to proinflammatory cytokines. This ratio seemed to be an independent predictor of composite end points in addition to serum IL-10 levels.
There are some limitations of the present study that deserve mention. The first limitation is the strict exclusion criteria, which were applied to study more unbiased factors associated with endothelial function. Exclusion of patients taking angiotensin converting enzyme inhibitors/angiotensin receptor blockers, statins, erythropoiesis-stimulating agents, and vitamin supplements provided a very selective patient population that is difficult to extrapolate into the general nondialyzed CKD population. However, this exclusion would also decrease any unwanted background pathophysiological noise that would most likely interfere with the IL-10/IL-6 impact on outcomes. Our study, therefore, needs to be taken as a necessary preliminary academic approach in search of determinants and associations of IL-10 rather than clinical application. A second limitation is the existence of only one patient visit/blood sampling. We must also acknowledge the lack of adjudication of CV events. Our hospital system and records unfortunately do not allow this adjudication, and events were identified by telephone calls and patient visits.
To conclude, the results of this study showed that increased levels of serum IL-10 and the IL-6/IL-10 ratio might independently associate with CV outcomes. Serum IL-10 levels were higher among patients with lower eGFR in parallel to other inflammatory markers. This seemingly paradoxical increase of an antiatherosclerotic cytokine and its independent association with adverse CV clinical outcomes might be considered as a compensatory increase in patients with heightened inflammatory status. Although limited by our cross-sectional study design and inability to establish causality in the observations reported, we hope our work will entice the initiation of prospective studies to better delineate the role of serum IL-10 in the development of the atherogenic uremic phenotype.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the patients and personnel involved in the creation of this patient material. We also thank Familial Mediterranean Fever Arthritis Vasculitis and Orphan Diseases Research (FAVOR; www.favor.org.tr) web registries at Gulhane Military Medical Academy, Institute of Health Sciences for their support in epidemiological and statistical advisory and invaluable guidance for the preparation of the manuscript.
We acknowledge support from the Gulhane School of Medicine and the Swedish Research Council.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08660813/-/DCSupplemental.
References
- 1.McCullough PA, Steigerwalt S, Tolia K, Chen SC, Li S, Norris KC, Whaley-Connell A, KEEP Investigators : Cardiovascular disease in chronic kidney disease: Data from the Kidney Early Evaluation Program (KEEP). Curr Diab Rep 11: 47–55, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massy ZA, Stenvinkel P, Drueke TB: The role of oxidative stress in chronic kidney disease. Semin Dial 22: 405–408, 2009 [DOI] [PubMed] [Google Scholar]
- 3.de Vries JE: Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann Med 27: 537–541, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Morita Y, Yamamura M, Kashihara N, Makino H: Increased production of interleukin-10 and inflammatory cytokines in blood monocytes of hemodialysis patients. Res Commun Mol Pathol Pharmacol 98: 19–33, 1997 [PubMed] [Google Scholar]
- 5.Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, Heimbürger O, Cederholm T, Girndt M: IL-10, IL-6, and TNF-alpha: Central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int 67: 1216–1233, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Heeschen C, Dimmeler S, Hamm CW, Fichtlscherer S, Boersma E, Simoons ML, Zeiher AM, CAPTURE Study Investigators : Serum level of the antiinflammatory cytokine interleukin-10 is an important prognostic determinant in patients with acute coronary syndromes. Circulation 107: 2109–2114, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Mälarstig A, Eriksson P, Hamsten A, Lindahl B, Wallentin L, Siegbahn A: Raised interleukin-10 is an indicator of poor outcome and enhanced systemic inflammation in patients with acute coronary syndrome. Heart 94: 724–729, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girndt M, Kaul H, Sester U, Ulrich C, Sester M, Georg T, Köhler H: Anti-inflammatory interleukin-10 genotype protects dialysis patients from cardiovascular events. Kidney Int 62: 949–955, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Straczkowski M, Kowalska I, Nikolajuk A, Krukowska A, Gorska M: Plasma interleukin-10 concentration is positively related to insulin sensitivity in young healthy individuals. Diabetes Care 28: 2036–2037, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Davignon J, Ganz P: Role of endothelial dysfunction in atherosclerosis. Circulation 109[Suppl 1]: III27–III32, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972 [PubMed] [Google Scholar]
- 12.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[4 Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 13.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Song S, Ling-Hu H, Roebuck KA, Rabbi MF, Donnelly RP, Finnegan A: Interleukin-10 inhibits interferon-gamma-induced intercellular adhesion molecule-1 gene transcription in human monocytes. Blood 89: 4461–4469, 1997 [PubMed] [Google Scholar]
- 15.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE: Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R, International Brachial Artery Reactivity Task Force : Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Von Der Thüsen JH, Kuiper J, Fekkes ML, De Vos P, Van Berkel TJ, Biessen EA: Attenuation of atherogenesis by systemic and local adenovirus-mediated gene transfer of interleukin-10 in LDLr-/- mice. FASEB J 15: 2730–2732, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Mallat Z, Heymes C, Ohan J, Faggin E, Lesèche G, Tedgui A: Expression of interleukin-10 in advanced human atherosclerotic plaques: Relation to inducible nitric oxide synthase expression and cell death. Arterioscler Thromb Vasc Biol 19: 611–616, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Lacraz S, Nicod LP, Chicheportiche R, Welgus HG, Dayer JM: IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J Clin Invest 96: 2304–2310, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinderski Oslund LJ, Hedrick CC, Olvera T, Hagenbaugh A, Territo M, Berliner JA, Fyfe AI: Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol 19: 2847–2853, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Cavusoglu E, Marmur JD, Hojjati MR, Chopra V, Butala M, Subnani R, Huda MS, Yanamadala S, Ruwende C, Eng C, Pinsky DJ: Plasma interleukin-10 levels and adverse outcomes in acute coronary syndrome. Am J Med 124: 724–730, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Eskdale J, Gallagher G: A polymorphic dinucleotide repeat in the human IL-10 promoter. Immunogenetics 42: 444–445, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Koch W, Kastrati A, Böttiger C, Mehilli J, von Beckerath N, Schömig A: Interleukin-10 and tumor necrosis factor gene polymorphisms and risk of coronary artery disease and myocardial infarction. Atherosclerosis 159: 137–144, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Weber C, Sigrist M, Romann A, Chiarelli G, Levin A: Novel biomarkers do not correlate with severity of vascular stiffness in CKD patients with severe co-morbid disease. Nephron Clin Pract 119: c261–c268, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Brunet P, Capo C, Dellacasagrande J, Thirion X, Mege JL, Berland Y: IL-10 synthesis and secretion by peripheral blood mononuclear cells in haemodialysis patients. Nephrol Dial Transplant 13: 1745–1751, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Taniguchi T, Koido Y, Aiboshi J, Yamashita T, Suzaki S, Kurokawa A: Change in the ratio of interleukin-6 to interleukin-10 predicts a poor outcome in patients with systemic inflammatory response syndrome. Crit Care Med 27: 1262–1264, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.