Abstract
The concept of homeostasis has been inextricably linked to the function of the kidneys for more than a century when it was recognized that the kidneys had the ability to maintain the “internal milieu” and allow organisms the “physiologic freedom” to move into varying environments and take in varying diets and fluids. Early ingenious, albeit rudimentary, experiments unlocked a wealth of secrets on the mechanisms involved in the formation of urine and renal handling of the gamut of electrolytes, as well as that of water, acid, and protein. Recent scientific advances have confirmed these prescient postulates such that the modern clinician is the beneficiary of a rich understanding of the nephron and the kidney’s critical role in homeostasis down to the molecular level. This review summarizes those early achievements and provides a framework and introduction for the new CJASN series on renal physiology.
Keywords: renal physiology, kidney, nephron
Introduction
Critical advances in our understanding of renal physiology are unfolding at a rapid pace. Yet, remarkably, the lessons learned from early crude measurements and careful study still hold true; indeed, classic articles still serve as the basis for introductory textbooks on renal physiology and provide a solid working knowledge to clinicians. Drawings with just a handful of transporters at each nephron segment, known for more than half a century, are sufficient to understand basic mechanisms of autoregulation, clearance, and the effects of diuretics—the tools needed to care for patients. Yet we clinicians also benefit from a treasure trove of subsequent scientific advances, which have given us a detailed and comprehensive understanding of how the kidney maintains stable body chemistries and volume balance.
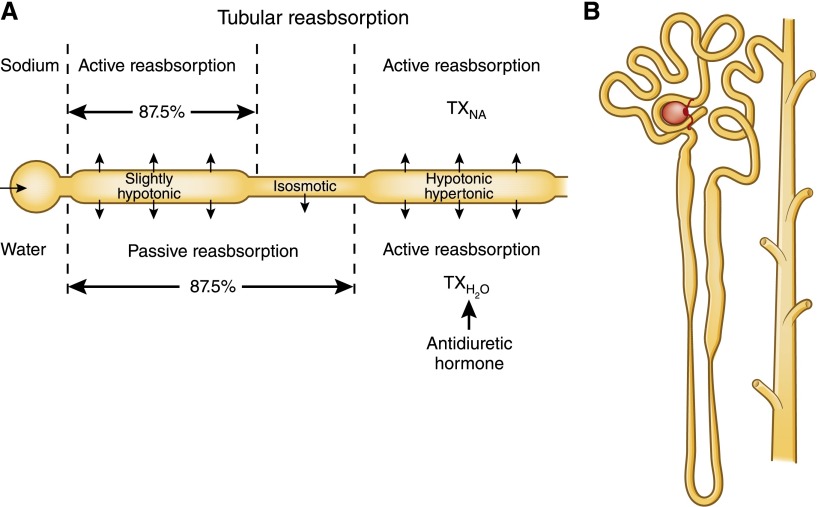
The layers of complexity and the mysteries that continue to unravel make it difficult to stay abreast of current research. Still, the modern nephrologist is in good company. In 1959, a medical student wrote to Homer Smith, the uncontested patriarch of modern nephrology at the time, to inquire about his rectilinear depiction of the nephron (Figure 1) and why he failed to mention the counter current theory in his famous 1956 textbook, the Principles of Renal Physiology (1,2). Indeed, the structure of the loop of Henle had been well known since the mid-1800s, but the importance of that eponymous structure, the gradient that it generated, and its role in the final product urine was only just elucidated at the time of the student’s correspondence. Before this, Homer Smith felt that the hairpin turn was just a vestige of embryology. This student’s missive was a curiosity, rather than a criticism. Carefully framed questions have always served to advance our understanding. In this overview, we will describe, all too briefly, the ingenious methods used by early investigators and the secrets they unlocked to help create the in-depth understanding of renal physiology and pathophysiology that we enjoy today. Additional details will follow in the new CJASN series of review articles on the physiology of the kidney.
Figure 1.
Study of the kidney requires consideration of both the role of each segment and the three-dimensional architecture. (A) Homer Smith’s rectilinear nephron mimics the straight trajectory of the fish nephron. (B) The human nephron is more intricate; the distal nephron greets its own glomerulus before it returns to the environs established by the loop of Henle. TX, treatment. A is modified from reference 1, with permission.
The Milieu Intérieur and the Kidney’s Essential Role
In the early 1800s, Darwin’s Theory of Evolution combined with the recognition that the body chemistries of many disparate species were remarkably similar led Claude Bernard to develop his theory of the Milieu Intérieur: “The constancy of the internal environment is the condition of a free and independent existence” (3). By this, he meant that the ability of our ancestor organisms to leave the oceans required that they develop the ability to “carry the ocean with them” in the form of an internal ocean, bathing their cells constantly in fluids that resemble the very seas from which they evolved. This concept, although reminiscent of the notion of bodily humors (4,5), marked an enormous advance because Bernard described both the features of bodily fluids and the need to maintain that internal milieu. Maintenance of the internal milieu was first called the “wisdom of the body” by Starling (6), who recognized that organisms must maintain the constancy of this internal ocean despite great fluctuations in diet, fluid intake, and other environmental conditions. The term homeostasis was later coined by behaviorist Walter Cannon to describe the physiologic processes that, in aggregate, maintain the constancy of the internal chemistries, as well as BP, body temperature, and energy balance (7).
The earliest insights into renal physiology came from the assiduous study of anatomy because, to a large degree, renal function follows structure. Meticulous drawings and histologic study of the animal and human kidney from William Bowman (8,9), Jacob Henle (10), and others, complete with capsule, capillaries, and convoluted tubules were available in Bernard’s time, yet the mechanisms for the formation of urine and the kidney’s role in homeostasis were not embraced until the next century. Until the 1920s, a debate raged on the mechanism of urine formation. Some researchers championed a filtration doctrine and others ascribed secretory power to both the glomerulus and tubules (11,12). The secretory theory was more popular, however, because the sheer volume of blood that would first need to be filtered and then reabsorbed by the kidney was enormous. Homer Smith noted that the filter-reabsorption strategy “seemed extravagant and physiologically complicated” (2).
Early Investigations Form the Framework
An interest in comparative physiology and the advent of the marine biologic laboratories that studded the Atlantic coast at the turn of the century helped frame an early understanding of the kidney and its role in evolution. Remarkably, increasingly complex fish with salty interiors adapted to fresh water, whereas amphibians rose from the sea to face the challenges mandated by scarce water. In his famed opus, Smith summarized the observations to date and waxed poetic (and philosophic), declaring, “Superficially, it might be said that the function of the kidneys is to make urine; but in a more considered view, one can say that the kidneys make the stuff of philosophy itself” (13). This impression, the sense of wonder at the intricate dealings of the kidney, permeates the scientific writings from that time to this day.
Studies performed in frogs, rabbits, and dogs in the early 1900s showed that the constituents of blood and urine differed because urine contained urea, potassium, and sodium salts, whereas blood contains protein, glucose, and very little urea. Furthermore, balance studies suggested that the volume and constituents of urine changed depending on changing intake or experimental infusions. In his “The Secretion of Urine” monograph published in 1917, Cushny summarized the available literature to date and described the brisk diuresis that followed sodium chloride infusions. He also reported findings that showed that the kidney produced acid urine in humans and the carnivora, whereas the herbivora had alkaline urine unless fed a protein diet (14). Despite this careful review and his presentation of the “modern view” that acknowledged that both filtration and secretion could exist, Cushny struggled with the data and felt that the theories were “diametrically opposed” because secretion and reabsorption would result in opposing currents along the renal epithelium. Clearly, methods were needed that could allow direct measurement of the filtrate and its modification along the nephron.
When Wearn and Richards introduced the micropuncture technique to the study of the kidney (first in amphibia, which had large renal structures amenable to manipulation), the debate on the formation of urine was resolved (11,15). By sampling the fluid elaborated from the glomerular capsule of a frog, the team demonstrated a protein-free filtrate that was otherwise similar to blood. By contrast, the frog bladder urine had a different composition from the blood and was free of glucose. These findings, in light of earlier data, supported the notion that urine is formed by glomerular filtration, and the urine is then modified in the tubules, by a combination of reabsorption and secretion. Subsequent studies by Walker and others inserted oil “plugs” or “blocks” in various segments of the nephron and distal to the sampling pipette so that the investigators could avoid contamination but still study urine from different segments of the nephron and, therefore, characterize each segment’s function, the ions absorbed, and the osmolarity of the fluid (16). These investigators then developed the “stop flow” technique in which they placed a pipette distal to the oil droplet, infused fluid into that segment, and then sampled the fluid at the end of that segment to determine how the fluid had been altered (Figure 2A) (17).
Figure 2.
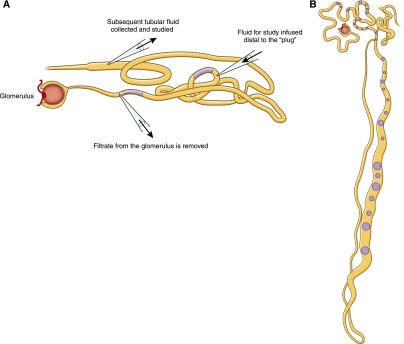
Micropuncture and “stop flow” techniques were used to help define the role of each segment of the nephron. (A) The proximal tubule from the kidney of the aquatic salamander is illustrated here. A micropipette removes the filtrate at a point just proximal to a “plug” of mineral oil. To determine the role of the tubule in handling of individual constituents (reabsorption, secretion, or diffusion), fluid was injected into the tubule at different locations and then collected distally. This “artificial” fluid could be altered to differ from the normal filtrate by one or more constituents. (B) A sketch of a camara lucida drawing of a guinea pig nephron after microdissection (these drawings were created with the aid of a light projector because photomicrographs were not readily available at the time). Oil or mercury blocks could be inserted at various points along the nephron and fluid from the lumen could be collected and studied. A is modified from reference 17, with permission; B is modified from reference 16, with permission.
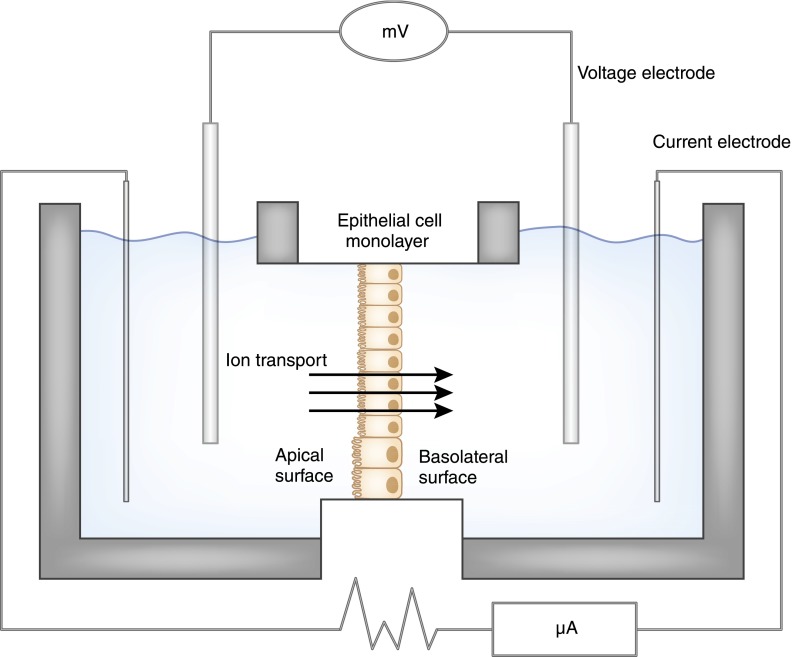
This meticulous work was confirmed in mammals by extension of the micropuncture technique to rat and guinea pig kidneys (Figure 2B). However, despite the ingenious use of oil blocks to prevent upstream tubular fluid from reaching downstream segments and then substituting artificial perfusates, micropuncture studies did not permit control of the composition of fluids on both sides of the tubular epithelium. This limitation was remedied after World War II, when Hans Ussing developed his famed chamber methods (Figure 3). With this strategy, transport across isolated epithelia could be studied quantitatively by systematically altering the ionic composition and voltages of the solutions on either side of the epithelium (18). Careful transport studies using model epithelia from nonmammals, including the toad bladder, the turtle bladder, and the flounder bladder, which anatomically and functionally model collecting duct principal cells, collecting duct intercalated cells, and distal tubule cells, respectively, gave important insights into transport mechanisms in these segments. In the late 1960s, Burg and colleagues developed methods for isolating and perfusing individual mammalian nephron segments, first from rabbits and then from mice. These preparations, along with the ability to measure minute quantities of ions and volumes from these tubules with ion-specific electrodes, including the picapnotherm (which measures minute quantities of carbon dioxide), permitted investigators to examine in detail the mechanisms, driving forces, and regulation of transport across individual nephron segments (19). With painstaking effort, investigators dissected tubules, perfused the segment with fluid of specific ion concentrations, and collected the “waste” fluid from the other end of the segment (Figure 4). This arrangement allowed investigation of individual segments of the nephron to better characterize the features of transport, electrochemical gradients, coupling with other ions, active versus passive transport, the threshold for reabsorption, and the permeability to water (1). The resulting flurry of studies, spanning nearly 2 decades, defined the phenomenology and regulation of transport, and identified, at least functionally, the transporter proteins responsible for homeostasis (20).
Figure 3.
The Ussing Chamber can be used to measure ion transport between the two sides of an epithelial cell membrane by polarized cells. Here, a monolayer of epithelial cells separates two compartments. Fluid in the two compartments is identical to eliminate the contribution of passive paracellular diffusion driven by differences in concentration, osmotic pressure, or hydrostatic pressure. Voltage electrodes placed near the epithelial membrane maintain the potential difference at zero so that the current measured by the current electrodes reflects the movement of ions by active transport through the epithelial cells.
Figure 4.
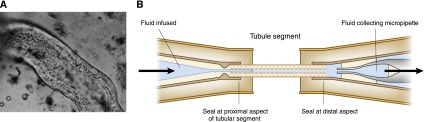
Study of isolated perfused tubular segments allowed study of each of the different nephron segments independently. (A) A photomicrograph of a portion of a rabbit proximal convoluted tubule during perfusion. (B) A schematic diagram of the technique. One end of the dissected tubule was connected to a micropipette, which was used to perfuse the lumen, and the other end was connected to a collection micropipette. Both the luminal fluid and the peritubular fluid could be controlled to assess tubular transport characteristics. A is reprinted with permission from Burg MB: Perfusion of isolated renal tubules. Yale J Biol Med 45: 321–326, 1972.
Meanwhile, in the clinical realm, the flame photometer became available in the late 1940s and this innovation made it possible to measure more than a dozen samples of blood for both sodium and potassium in under an hour. Before this time, electrolyte measurements were onerous and involved both chemical extractions and precipitations (21). Studies of electrolytes and the metabolic derangements were now possible; when this process was linked to an autoanalyzer that also provided chloride and total CO2, interest in acid-base disorders soared and the concept of “Gamblegrams” flourished (22). Dr. Gamble, a disciple of Henderson, studied a range of different insults from gastrointestinal losses to advanced CKD and their effect on electrolytes, and described the kidney as the “remarkable organ of regulation, the kidney sustains the chemical structure of extracellular fluid” (23).
Later, availability of the automatic analyzers also spurred a large literature using metabolic balance studies to characterize everything from bed rest or water immersion to the effects of pharmacologic agents like chlorothiazide (24,25). In these detailed studies, investigators characterized vital signs, weight, intake, excretion, electrolytes, clearance, plasma volume, and hormonal levels. These data helped solidify the concepts of the steady state in homeostasis (26).
The stage was now set to identify specific renal transporters, describe how they function, and characterize how they are regulated. The remarkable reabsorptive task of the nephron tubules requires energy and active transport. It was not long after Nobel laureate Jens Skou’s 1957 discovery of the Na-K-ATPase in crab nerve microsomes (27,28) that this critical transporter was identified in the kidney. Because of its abundance, the enzyme was identified in crude homogenates of the renal cortex and medulla and Na-K-ATPase activity was later measured in individual segments of the nephron. The highest activity was in the thick ascending limb and distal convoluted tubule (DCT) and considerable activity was also observed in the proximal tubule. Further study helped identify the polarity of the renal epithelial cells with the Na-K-ATPase at the basolateral membrane. This finding helped solidify the concept that energy generated from this housekeeping enzyme, which maintains the normal cellular ion concentration, is harnessed by the kidney to reabsorb the bulk of the filtered sodium along with a host of other substances (29).
With a map in place for the role of each segment of the nephron and a solid understanding of factors that influence the actions in those segments, research efforts by a multitude of investigators have set out to characterize the wide array of transporters in the nephron, their molecular structure, their distribution in the nephron, and their role in normal physiology and disease. This effort has been aided by sophisticated and rapidly evolving techniques with PCR, cloning and amplification of cDNAs, and expression in Xenopus oocytes, knockout mice, genome-wide association studies, and other models.
The Ultrastructure of the Glomerulus, the Concept of Clearance, and Autoregulation
Once the debate on the formation of urine was settled and it was clear that urine was formed by filtration, reabsorption, and secretion, mysteries of the elegant filtration design were explored and the concept of clearance was developed. Use of scanning electron microscopy allowed researchers to appreciate the three-dimensional structure of the cells and the ingenious design of the glomerulus. Podocytes were visualized extending their primary, secondary, and tertiary projections to interdigitate with neighboring “feet” and form the filtration slit diaphragm. Meanwhile, within the capillary bed, the delicate fenestrated endothelium drapes the basement membrane. A series of investigations into the dynamics of glomerular ultrafiltration, first in a unique strain of Wistar rats with surface glomeruli and later in primates, helped define factors that create the net driving force for filtration and provided the mathematical framework for our current understanding of these forces (30). In addition, studies on the permselectivity of the glomerular capillary wall using ferritin molecules of various sizes that were neutral, anionic, or cationic revealed that particles were restricted based on both charge and size; this explained the limited clearance of albumin at 39 A°, which is smaller than the 42 A° pores observed in the glomerular endothelial cell (31).
More recently, new techniques have helped further characterize these observations. For example, models of nephron development, such as the zebrafish, transparent and rapidly growing fish with a single pair of nephrons, have been indispensable to determining the effects of single defects on kidney function and development (32). Myriad proteins that form the filtration slit diaphragm are characterized and defects in several of these proteins can predictably cause the nephrotic syndrome. Details of the complex meshwork of the basement membrane, a joint effort by the endothelial and epithelial cells, can result in thin basement membrane disease or hereditary syndromes, such as Alport syndrome. The elixir, vascular endothelial growth factor, appears to support the endothelium; when it is compromised, endothelial cells cannot sustain the regular challenges required to support normal structure and function of this unique capillary bed and, thus, thrombosis and endothelial injury can occur. In addition, direct micropuncture of glomeruli allowed a detailed description of normal glomerular hemodynamics and identified the derangements in glomerular function that occur with disease. Application of glomerular micropuncture in animals with glomerular damage caused by hypertension, diabetes, or loss of renal mass led directly to current therapies, including dietary protein restriction and use of angiotensin-converting enzyme inhibition.
The role of the glomerulus in “clearance” has provoked significant inquiry as well. Early clinical investigators explored the clearance of urea and creatinine after ingestion or determined the clearance with the infusion equilibrium methods, first with inulin and later with a host of radioactive markers, such as 125I-iothalamate. When it became possible to measure the low concentrations of creatinine in serum, use of endogenous creatinine to calculate and later estimate clearance became possible. The pitfalls of this strategy (e.g., contribution from secretion, variability based on muscle mass, and the changes in serum levels related to diet and volume status, all of which make it less precise than the measured GFR) are more than balanced by the convenience (33).
It was soon evident that massive daily glomerular filtration could translate into life-threatening losses if there were no mechanisms in place to limit them in the event of low perfusion. Some researchers heralded these strategies as “acute renal success” (34); however, further study of the integrated response that maintains the GFR over a wide range of perfusion pressures provided an understanding of the roles of the juxtaglomerular apparatus in autoregulation and tubuloglomerular feedback (35).
Sodium and Water Homeostasis
Sodium, the major extracellular cation, plays a pivotal role in the maintenance of extracellular fluid volume and perfusion of vital organs and capillary beds. The kidney has an elaborate array of sodium transporters throughout the nephron (36). In the proximal tubule, it is linked to an elegant mechanism to reabsorb the filtered bicarbonate load by excreting H+ ions with the electroneutral antiporters or Na+/H+ exchangers. Reabsorption of the ample filtered sodium also plays an important role in the reabsorption of glucose, sulfate, phosphate, and several amino acids. The remaining fraction of filtered sodium is reabsorbed with unique transporters in each of the subsequent nephron segments in which apical reabsorption of sodium is rate limiting. These transporters include the furosemide-sensitive channel in the loop of Henle, the thiazide-sensitive sodium chloride cotransporter that is primarily in the DCT, and the epithelial sodium channel transporter that is located primarily in the collecting tubules (Figure 5).
Figure 5.
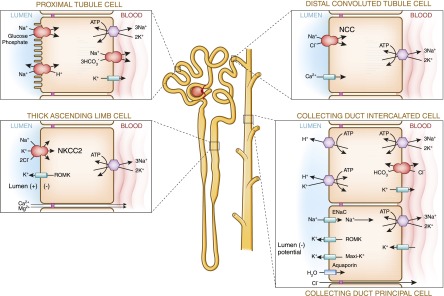
The unique transporters and cell structure of each segment of the nephron work in concert to maintain homeostasis. ENaC, epithelial sodium channel; NKCC2, Na+-K+-2Cl cotransporter; ROMK, renal outer medullary potassium.
Our understanding of the mechanisms of sodium transport along the nephron comes from disparate sources. The advent of sulfonamides, investigated initially as much-needed antibiotics after World War II, were promptly recognized for their saluretic effects and soon revealed a wealth of secrets regarding the transport of sodium throughout the nephron (37). Genetic disorders also provided important clues. Endocrinologist Frederick Bartter and others described a set of youths afflicted with growth and mental retardation, muscle cramps, salt craving, polyuria, and polydipsia. Bartter initially attributed his eponymous syndrome to a state of aldosterone excess with angiotensin resistance but when three-fourths adrenalectomy did not resolve the defect, he focused on the loop of Henle. Subsequent contributions from physiologists helped distinguish this disorder from Gitelman’s syndrome and helped define the interplay of transporters in the loop of Henle and the DCT. However, it was the stunning characterizations by geneticists that helped identify mutations in several genes; these discoveries explained the subtle differences in the phenotype of these disorders and will be carefully considered within this series (38,39)
An endocrinopathy was also the initial theory that Dr. Liddle invoked to describe a family with early onset severe hypertension and hypokalemia that was notable for suppressed renin and aldosterone. As soon as the epithelial sodium channel was characterized, investigators demonstrated complete linkage in affected individuals with a defect in this transporter that resulted in a constitutively active sodium channel (40). Insights into the molecular biology, structure, function, and regulation of each of these sodium transporters has clearly enriched our grasp of renal physiology and complemented earlier predictions.
Hormonal and sympathetic nervous input can greatly augment sodium reabsorption, particularly by angiotensin II in the proximal tubule and aldosterone in the distal nephron, whereas the effect of atrial natriuretic peptide in the medullary collecting duct was found to be the opposite (41). Knowledge of these transporters is critical to the understanding of the clinical use of diuretics and the care of patients with a wide variety of issues, from the patient with essential hypertension to the complex patient with cirrhosis.
In the 1960s, Guyton proposed that all hypertension, ultimately, is a result of the failure of the kidney to excrete the excess of total body sodium with a normal pressure natriuresis (42). Although this theory has been disputed over the years, it is notable that monogenic defects that lead to hypertension or hypotension are found exclusively in genes that encode either renal transporter proteins or proteins that regulate the function of renal transporter proteins and ultimately renal sodium handling.
Despite mounting evidence on the importance of sodium in the kidney’s role in homeostasis, investigators in the 1950s soon recognized that the measured serum sodium correlated poorly with the total body sodium by comparing these values in heterogeneous patients with a variety of chronic conditions. Instead, the serum sodium correlated well with the serum osmolality (particularly when corrections were made for the osmotic contributions of glucose and nonprotein nitrogen) (43). (Of note, the same investigators also recognized that the Na2+-K+/total body water ratio correlated closely with “corrected” serum sodium and explained the importance for accounting of potassium repletion during the treatment of hyponatremia.) Maintenance of the plasma osmolality was noted to be tightly regulated by both the release of vasopressin and the kidneys’ response (44). This interplay between the two is essential for water homeostasis, a critical factor in the maintenance of cell volume. Although cells have developed strategies to deal with excess or insufficient water, these volume regulatory changes require extrusion or inclusion of electrolytes, which alters the cellular interior milieu and wreaks havoc on normal cellular function. Later adaptations allow cells to return toward normalcy but only within a small range. Water reabsorption requires the ability to both establish an osmotic gradient in the kidney and to reabsorb water from the urinary filtrate. The kidney has an elegant strategy to concentrate or dilute the urine by its response to vasopressin and the ability to deploy aquaporins to the luminal membrane (45). At least seven aquaporin isoforms are expressed in the kidney and play important roles at different sites. In the proximal tubule and thin descending limb, aquaporin 1 appears to serve as the dominant gateway for water reabsorption, whereas trafficking of aquaporin 2 along cytoskeletal elements in the collecting duct cells allows reabsorption of water and urine concentration in the principal cells of the collecting ducts (46). Detailed study of the molecular structure and cell physiology of these transporters has allowed insight into the rare genetic diseases that affect aquaporins, such as congenital nephrogenic diabetes insipidus and the common acquired defects related to lithium, calcium, and even urinary obstruction. Similarly, study of the vasopressin receptor has resulted in new strategies and pharmacologic agents for the treatment of states of excess antidiuretic hormone and polycystic kidney disease.
Acid-Base Homeostasis
Maintenance of pH is a critical activity of the kidney and is essential for normal cellular function because the pH dictates the charged state of proteins that affects conformational shape, enzymatic activity, binding, and cellular transport and, thus, allows proteins to perform essential metabolic functions. Although some acid-base enthusiasts enjoy consideration of the “strong ion difference” to reconcile data, the normal kidney’s remarkable response to subtle differences in pH or, more likely, intracellular CO2 does not take these differences into account. Although the exact mechanisms used to sense pH are still not yet understood, it is well known that the kidney plays a dominant role in the regulation of the acid-base balance (47). Indeed, with acidosis, a complex intracellular cascade ensues, including activation of the electroneutral sodium-coupled amino acid transporter for glutamine, an increase in glutamine metabolism and ammoniagenesis, as well as an increase in the expression of the sodium hydrogen antiporter (48,49). Additional orchestrated responses in the distal nephron with the help of the machinery in the intercalated cells and neighboring principal cells contribute to the kidneys’ response to the challenges of acidemia (50).
By contrast, metabolic alkalosis can be easily and rapidly handled by the kidney by excretion of excess filtered bicarbonate or may be perpetuated by the kidney because of a response to volume depletion with secondary activation of the renin-angiotensin and aldosterone axis or from primary hyperaldosteronism. These processes are intimately linked to the regulation of potassium as well. Finally, the kidney responds to the challenges of primary respiratory disorders to offset the effects of these disturbances in a predictable fashion (51,52). In chronic respiratory alkalosis, the kidney can decrease acid excretion by decreasing ammonia production and bicarbonate retention; in respiratory acidosis, activity of the Na-H antiporter is augmented so that more bicarbonate is reabsorbed (53). Understanding of these complex processes helps in the care of actual patients who develop acid-base disorders from a wide variety of insults.
Potassium Homeostasis
Excretion of potassium in excess of the amount filtered is another triumph of the kidney in electrolyte homeostasis and its mechanism, another important milestone in the understanding of renal pathophysiology (54–56). Potassium, the major cation in the body, must be maintained in high concentrations in the intracellular space and a low concentration in the extracellular fluid to allow both normal cellular function and the considerable gradient required for excitation of nerves and contractions of muscles. The kidney plays its role by reabsorption of nearly all of the filtered load proximally and variable secretion in the distal nephron. Along the way, potassium is secreted into the lumen with the help of the renal outer medullary potassium transporter, which provides sufficient substrate to the NaKC2 transporter, and by the “big potassium,” “maxi,” or high conductance transporter (57). Both appear to play an important role in the secretion of potassium in the distal nephron dictated by the influence of aldosterone and magnitude of distal flow (58).
Divalent Cations and Phosphate Homeostasis
The kidney plays a critical role in maintaining both normal extracellular calcium ion levels and the vast repositories of calcium needed for normal intracellular function and maintenance of the skeleton. Integrated control by parathyroid hormone and 1,25-dihydroxyvitamin D helps achieve this end (59). Calcium is reabsorbed through a paracellular route in the proximal tubule and the thick ascending limb, whereas there are unique, well characterized transient receptor potential ion channels in the DCT. Each of these regions is controlled by local effects prescribed by the calcium sensing receptor and modulated by the pH (60). There is also an interesting pas de deux between sodium and calcium handling. Volume depletion or decreased sodium delivery decreases urinary calcium either by increasing proximal reabsorption or by promoting reabsorption in the DCT with changes in the activity of the basolateral Na+/Ca+ exchanger or hyperpolarization of the luminal plasma membrane (61,62). The fate of magnesium homeostasis is intimately linked to that of other cations. In parallel to calcium, the majority of filtered magnesium is reabsorbed in the proximal tubule and thick ascending limb by paracellular movement mediated, in part, by the claudin proteins, which govern ion movement through the otherwise tight junctions in those regions (63). In addition, the action of the renal outer medullary potassium transporter to supply more potassium in the lumen for the NaKC2 transporter is thought to create a lumen-positive transepithelial potential difference that can favor cation reabsorption in this segment (64). By contrast, in the DCT, movement of Mg2+ is an active transcellular process. At this segment, cation channels from the melastin transient receptor potential subfamily play a major role in this endeavor, evidenced by Mg2+ wasting seen in the rare genetic disorders with defects in this channel (65).
Emerging details on phosphate metabolism have identified a family of sodium phosphate transporters that help reabsorb the bulk of the filtered phosphate in the proximal tubule (66). These transporters appear to be affected by a series of factors, including the recently characterized fibroblast growth factor 23 and its obligate coreceptor Klotho, which together, via the fibroblast growth factor receptor, inhibit the reabsorption of sodium-dependent phosphate reabsorption and lower vitamin D levels by downregulating the gene for 1α-hydroxylase (67). Phosphate that escapes proximal reabsorption and is delivered distally is available to bind H+ as an important source of “titratable acid” (68).
Protein Metabolism
Even Richard Bright (69) in the 1820s recognized that in “dropsy” (or edema) of renal origin, urea was increased in the blood and decreased in the urine such that urea could serve as a marker of kidney failure. One hundred years later, Thomas Addis tried to assess renal function using urea clearance and “rest” the kidneys from the “work” of clearing proteins by prescribing a low-protein diet (70). Landmark studies by Brenner’s group suggested that this strategy was correct because high-protein diets fed to laboratory animals can be shown to increase renal blood flow and glomerular filtration and subsequently contribute to the progression of CKD (71). Nevertheless, the specific mediators that lead to hyperfiltration and contribute to the changes seen with a high-protein diet are yet to be determined.
Renal Physiology Has Significant Clinical Relevance
One of the considerable gifts to the field of nephrology is that there is a deep and growing understanding of the intricacies of kidney function and the ingenious methods that the kidney uses to govern homeostasis. These discoveries complement observations made with careful consideration and primitive measurements in the past. Predictions on the movement of ions have been translated by detailed characterization, on the molecular level, of ion transporters and provide insight into the integrated responses of the kidney to the maintenance of the internal milieu. Those of us who are privileged enough to care for patients with disorders of the kidney can utilize knowledge gleaned in the laboratory to understand real clinical concerns.
Because it is difficult for any practicing nephrologist to stay abreast of the rapidly unfolding revelations, this new renal physiology series will serve as an update to the current understanding of the nephron. CJASN will provide careful reviews of the nephron, sequentially, from the glomerulus to the collecting duct, followed by a review on the control of urinary drainage and bladder function. Next, there will be a series of cohesive reviews that will address how the kidney factors in the integrated response to sodium and water homeostasis, potassium handling, acid-base homeostasis, excretion of organic cations and anions, and divalent cations and phosphate homeostasis. Protein metabolism and control of renal nitrogen excretion as well as sensory functions of the kidney will follow. Finally, the role of the interstitium and hormonal function of the kidney will be considered.
In jingoistic banter that many nephrologists can echo with sincerity, Homer Smith asserted, “The responsibility for maintaining the composition of [the internal milieu] … devolves to the kidneys. It is no exaggeration to say that the composition of the body fluids is determined not by what the mouth takes in but by what the kidneys keep; they are the master chemists of our internal environment” (13).With this series in hand, the CJASN reader will be privy to the cutting-edge science of nephrology; knowledge of the dramatic advances in our understanding will likely turn any nephrologist into a philosopher.
Authors’ Note
The landmark works described in this article are freely accessible on the Internet for those who would like to indulge in the primary sources. These texts and manuscripts have been made available as part of Google Scholar and the Internet Archive, nonprofit digital libraries with the mission to allow universal access to all knowledge. These archival texts include Homer Smith’s The Principals of Renal Physiology and From Fish to Philosopher, Bowman’s treatise On the Structure and Use of the Malpighian Bodies of the Kidney, and texts by Starling, Cushny, Cannon, and Bernard.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Smith HW: Principles of Renal Physiology, New York, Oxford University Press, 1956 [Google Scholar]
- 2.Smith HW: The fate of sodium and water in the renal tubules. Bull N Y Acad Med 35: 293–316, 1959 [PMC free article] [PubMed] [Google Scholar]
- 3.Bernard C: Leçons sur les phénomènes de la vie communs aux animaux et aux vegetaux, Paris, Bailliere JB, 1878 [Google Scholar]
- 4.Bernard C: An Introduction to the Study of Experimental Medicine 1865, London, Macmillan & Co Ltd, 1927 [Google Scholar]
- 5.Gross CG: Claude Bernard and the constancy of the internal environment. Neuroscientist 4: 380–385, 1998 [Google Scholar]
- 6.Starling EH: The Harvreian Oration, delivered before The Royal College of Physicians of London on St. Luke’s Day, 1923. BMJ 2: 685–690, 1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon WB: Wisdom of the Body, New York, WW Norton, 1939 [Google Scholar]
- 8.Bowman W: On the structure and use of the malphighian bodies of the kidney, with observations on the circulation through that gland. Philos Trans R Soc Lond 132: 57–80, 1942 [Google Scholar]
- 9.Eknoyan G: Sir William Bowman: His contributions to physiology and nephrology. Kidney Int 50: 2120–2128, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Kinne-Saffran E, Kinne RK: Jacob Henle: The kidney and beyond. Am J Nephrol 14: 355–360, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Wearn HT, Richards AN: Observations on the composition of glomerular urine, with particular reference to the problem of reabsorption in the renal tubules. Am J Phys 71: 209–227, 1924 [Google Scholar]
- 12.Gottschalk CW: A history of renal physiology to 1950. in: The Kidney: Physiology and Pathophysiology, edited by Seldin DW, Giebisch G , 2nd Ed., New York, Raven Press, Ltd, 1992 [Google Scholar]
- 13.Smith HW: From Fish to Philosopher, Garden City, NY, Doubleday and Company, Inc, 1961 [Google Scholar]
- 14.Cushny AR: The secretion of urine. In: Monographs on Physiology, edited by Starling EH, London, Longmans, Green and Co., 1917 [Google Scholar]
- 15.Sands JM: Micropuncture: Unlocking the secrets of renal function. Am J Physiol Renal Physiol 287: F866–F867, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Walker AM, Bott PA, Oliver J, MacDowell MD: The collection and analysis of fluid from single nephrons of the mammalian kidney. Am J Physiol 134: 580–595, 1941 [Google Scholar]
- 17.Richards AN, Walker AM: Methods of collecting fluid from known regions of the renal tubules of amphibia and of perfusing the lumen of a single tubule. Am J Physiol 118: 111–120, 1937 [Google Scholar]
- 18.Ussing HH, Zerahn K: Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand 23: 110–127, 1951 [DOI] [PubMed] [Google Scholar]
- 19.Burg MB, Grantham J, Abramow M, Orloff J, Schafer JA: Preparation and study of fragments of single rabbit nephrons. J Am Soc Nephrol 8: 675–683, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Burg MB, Knepper MA: Single tubule perfusion techniques. Kidney Int 30: 166–170, 1986 [DOI] [PubMed] [Google Scholar]
- 21.Peitzman SJ: The flame photometer as engine of nephrology: A biography. Am J Kidney Dis 56: 379–386, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Gamble JL: Chemical Anatomy, Physiology and Pathology of Extracellular Fluid, A Lecture Syllabus, Cambridge, Harvard University Press, 1942 [Google Scholar]
- 23.Harvey AM: Classics in clinical science: James L. Gamble and “Gamblegrams”. Am J Med 66: 904–906, 1979 [DOI] [PubMed] [Google Scholar]
- 24.Hollander W, Chobanian AV, Wilkins RW: Relationship between diuretic and antihypertensive effects of chlorothiazide and mercurial diuretics. Circulation 19: 827–838, 1959 [DOI] [PubMed] [Google Scholar]
- 25.Chobanian AV, Lille RD, Tercyak A, Blevins P: The metabolic and hemodynamic effects of prolonged bed rest in normal subjects. Circulation 49: 551–559, 1974 [DOI] [PubMed] [Google Scholar]
- 26.Bonventre JV, Leaf A: Sodium homeostasis: Steady states without a set point. Kidney Int 21: 880–883, 1982 [DOI] [PubMed] [Google Scholar]
- 27.Skou JC: The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta 23: 394–401, 1957 [DOI] [PubMed] [Google Scholar]
- 28.Skou JC: Nobel Lecture. The identification of the sodium pump. Biosci Rep 18: 155–169, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Katz AI: Renal Na-K-ATPase: Its role in tubular sodium and potassium transport. Am J Physiol 242: F207–F219, 1982 [DOI] [PubMed] [Google Scholar]
- 30.Maddox DA, Deen WM, Brenner BM: Dynamics of glomerular ultrafiltration. VI. Studies in the primate. Kidney Int 5: 271–278, 1974 [DOI] [PubMed] [Google Scholar]
- 31.Bohrer MP, Baylis C, Humes HD, Glassock RJ, Robertson CR, Brenner BM: Permselectivity of the glomerular capillary wall. Facilitated filtration of circulating polycations. J Clin Invest 61: 72–78, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drummond IA: Kidney development and disease in the zebrafish. J Am Soc Nephrol 16: 299–304, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Narayanan S, Appleton HD: Creatinine: A review. Clin Chem 26: 1119–1126, 1980 [PubMed] [Google Scholar]
- 34.Thurau K, Boylan JW: Acute renal success. The unexpected logic of oliguria in acute renal failure. Am J Med 61: 308–315, 1976 [DOI] [PubMed] [Google Scholar]
- 35.Peti-Peterdi J, Harris RC: Macula densa sensing and signaling mechanisms of renin release. J Am Soc Nephrol 21: 1093–1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Féraille E, Doucet A: Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: Hormonal control. Physiol Rev 81: 345–418, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Suki W, Rector FC, Jr, Seldin DW: The site of action of furosemide and other sulfonamide diuretics in the dog. J Clin Invest 44: 1458–1469, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartter FC, Pronove P, Gill JR, Jr, MacCardle RC: Hyperplasia of the juxtaglomerular complex with hyperaldosteronism and hypokalemic alkalosis. A new syndrome. Am J Med 33: 811–828, 1962 [DOI] [PubMed] [Google Scholar]
- 39.Proesmans W: Threading through the mizmaze of Bartter syndrome. Pediatr Nephrol 21: 896–902, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR, Jr, Ulick S, Milora RV, Findling JW, Canessa CM, Rossier BC, Lifton RP: Liddle’s syndrome: Heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79: 407–414, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Zeidel ML, Kikeri D, Silva P, Burrowes M, Brenner BM: Atrial natriuretic peptides inhibit conductive sodium uptake by rabbit inner medullary collecting duct cells. J Clin Invest 82: 1067–1074, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montani J, Van Vliet BN: Understanding the contribution of Guyton’s large circulatory model to long-term control of arterial blood pressure. Exp Physiol 94: 382–398, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Edelman IS, Leibman J, O’Meara MP, Birkenfeld LW: Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest 37: 1236–1256, 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verney EB: Renal excretion of water and salt. Lancet 273: 1237–1242, 1957 [DOI] [PubMed] [Google Scholar]
- 45.Knepper MA: Molecular physiology of urinary concentrating mechanism: Regulation of aquaporins water channels by vasopressin. Am J Physiol Renal Physiol 272: F3–F12, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA: Aquaporins in the kidney: From molecules to medicine. Physiol Rev 82: 205–244, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Pitts RF, Lotspeich WD, Schiess WA, Ayer JL, Miner P: The renal regulation of acid-base balance in man; the nature of the mechanism for acidifying the urine. J Clin Invest 27: 48–56, 1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boron WF: Acid-base transport by the renal proximal tubule. J Am Soc Nephrol 17: 2368–2382, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Curthoys NP, Gstraunthaler G: Mechanism of increased renal gene expression during metabolic acidosis. Am J Physiol Renal Physiol 281: F381–F390, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Koeppen BM: The kidney and acid-base regulation. Adv Physiol Educ 33: 275–281, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Arbus GS, Herbert LA, Levesque PR, Etsten BE, Schwartz WB: Characterization and clinical application of the “significance band” for acute respiratory alkalosis. N Engl J Med 280: 117–123, 1969 [DOI] [PubMed] [Google Scholar]
- 52.Brackett NC, Jr, Wingo CF, Muren O, Solano JT: Acid-base response to chronic hypercapnia in man. N Engl J Med 280: 124–130, 1969 [DOI] [PubMed] [Google Scholar]
- 53.Krapf R, Pearce D, Lynch C, Xi XP, Reudelhuber TL, Pouysségur J, Rector FC, Jr: Expression of rat renal Na/H antiporter mRNA levels in response to respiratory and metabolic acidosis. J Clin Invest 87: 747–751, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berliner RW, Kennedy TJ, Jr: Renal tubular secretion of potassium in the normal dog. J Am Soc Nephrol 9: 1341–1344, discussion 1344–1345, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Malnic G, Klose RM, Giebisch G: Micropuncture study of renal potassium excretion in the rat. Am J Physiol 206: 674–686, 1964 [DOI] [PubMed] [Google Scholar]
- 56.Giebisch G: Renal potassium transport: Mechanisms and regulation. Am J Physiol 274: F817–F833, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Greger R, Schlatter E, Hebert SC: Milestones in nephrology: Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle’s loop of rabbit kidney. J Am Soc Nephrol 12: 1788–1793, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Sansom SC, Welling PA: Two channels for one job. Kidney Int 72: 529–530, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Peacock M: Calcium metabolism in health and disease. Clin J Am Soc Nephrol 5[Suppl 1]: S23–S30, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Felsenfeld AJ, Levine BS: Milk alkali syndrome and the dynamics of calcium homeostasis. Clin J Am Soc Nephrol 1: 641–654, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ: Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 115: 1651–1658, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gagnon KB, Delpire E: Physiology of SLC12 transporters: Lessons from inherited human genetic mutations and genetically engineered mouse knockouts. Am J Physiol Cell Physiol 304: C693–C714, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hou J, Rajagopal M, Yu ASL: Claudins and the kidney. Annu Rev Physiol 75: 479–501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferrè S, Hoenderop JG, Bindels RJM: Sensing mechanisms involved in Ca2+ and Mg2+ homeostasis. Kidney Int 82: 1157–1166, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Dimke H, Monnens L, Hoenderop JG, Bindels RJ: Evaluation of hypomagnesemia: Lessons from disorders of tubular transport. Am J Kidney Dis 62: 377–383, 2013 [DOI] [PubMed] [Google Scholar]
- 66.Lederer E, Miyamoto K: Clinical consequences of mutations in sodium phosphate cotransporters. Clin J Am Soc Nephrol 7: 1179–1187, 2012 [DOI] [PubMed] [Google Scholar]
- 67.Hu MC, Shiizaki K, Kuro-o M, Moe OW: Fibroblast growth factor 23 and Klotho: Physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 75: 503–533, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scheiss WA, Ayer JL, Lotspeich WD, Pitts RF: The renal regulation of acid-base balance in man. Factors affecting the excretion of titratable acid by the normal human subject. J Clin Invest 27: 57–64, 1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jay V: Richard Bright—physician extraordinaire. Arch Pathol Lab Med 124: 1262–1263, 2000 [DOI] [PubMed] [Google Scholar]
- 70.Lemley KV, Pauling LS: Thomas Addis: 1881-1949, Washington, DC, National Academy of Sciences, 1994 [PubMed] [Google Scholar]
- 71.Brenner BM, Meyer TW, Hostetter TH: Dietary protein intake and the progressive nature of kidney disease: The role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med 307: 652–659, 1982 [DOI] [PubMed] [Google Scholar]