Abstract
Background
and objectives Use of bone mineral density (BMD) by dual-energy x-ray absorptiometry (DXA) is controversial for diagnosing bone loss in CKD patients on dialysis. The alternative quantitative computed tomography (QCT) is expensive and requires high radiation exposure. This study compared the two techniques and evaluated serum biochemical parameters for prediction of bone loss.
Design, setting, participants, & measurements
This prospective study enrolled patients from dialysis centers throughout Kentucky. BMD of the spine and hip was measured at baseline and after 1 year by DXA and QCT. Customary and novel serum biochemical parameters were obtained at the same times, including calcium, phosphorus, whole and intact parathyroid hormone, bone-specific alkaline phosphatase, procollagen type 1 N-terminal propeptide, tartrate-resistant acid phosphatase-5b, Dickkopf-1, fibroblast growth factor, and sclerostin. Rates of detection of osteoporosis by DXA and QCT were compared. Correlations were calculated between baseline biochemical parameters and BMD at baseline and changes over 1 year. Multivariable regression was performed to adjust for age, sex, body mass index, and race.
Results
Eighty-one patients completed the study (mean age=52.6±12.3 years, 56% men, 53% African American, and median dialysis vintage=41 months). At baseline, QCT and DXA of the spine identified similar rates of osteoporosis (13.6% and 13.6%), but at the hip, DXA identified more osteoporosis (22.2% versus 13.6%). At any site and by either method, 33.3% of the patients were osteoporotic. Baseline BMD correlated with sclerostin, intact parathyroid hormone, bone-specific alkaline phosphatase, tartrate-resistant acid phosphatase-5b, and fibroblast growth factor. At 1 year, hip QCT identified a higher number of patients experiencing bone loss (51.3%) than DXA (38.5%). After multivariable adjustment, baseline sclerostin and tartrate-resistant acid phosphatase-5b predicted bone loss measured by QCT of the hip; procollagen type 1 N-terminal propeptide predicted cortical spine bone gain by QCT.
Conclusions
QCT identified prospectively more bone loss at the hip than DXA. The baseline serum biochemical parameters sclerostin and tartrate-resistant acid phosphatase-5b were noninvasive independent predictors of bone loss in CKD patients on dialysis.
Keywords: renal osteodystrophy, CKD, dialysis
Introduction
Low bone mass is frequently seen in patients with CKD on dialysis (CKD-5D), and abnormalities in bone volume are an integral part of renal osteodystrophy (1,2). However, measurements of bone mineral density (BMD) reflecting bone mass are not common practice in the management of CKD-5D patients. Kidney Disease Outcomes Quality Initiative (KDOQI) recommends BMD measurements by dual-energy x-ray absorptiometry (DXA) only in patients in which fractures have occurred, and Kidney Disease Improving Global Outcomes (KDIGO) recommends explicitly against BMD measurements in CKD-3 to CKD-5D patients (3,4). This recommendation is explained by the lack of predictive value of BMD for fractures. However, Yenchek et al. (5) have shown in 2754 elderly patients (including 587 patients with GFR less than 60 ml/min) that lower BMD is a risk factor for fracture. It is undisputed that fractures occur at a 4.4 times higher rate in patients with CKD-5D compared with the general population (6), and bone loss is a generally accepted clinical risk factor for fracture. This study was conducted to compare DXA with quantitative computed tomography (QCT) in detection of osteoporosis and evaluate whether there are serum biochemical predictors of bone loss in CKD-5D patients.
Materials and Methods
Patients and Protocol
Patients with CKD-5D were screened, consented, and enrolled into this prospective institutional review board-approved study. Enrollment occurred from August of 2009 to April of 2012 at dialysis centers throughout Kentucky. The investigators adhered to the Declaration of Helsinki in the conduct of the study and registered the study with ClinicalTrial.gov (NCT00859612). Inclusion criteria were age 18 years or older, chronic maintenance dialysis of at least 3 months duration, mental competence, willingness to participate in the study, and calcidiol levels within the normal range. Exclusion criteria included pregnancy, systemic illnesses or organ diseases that may affect bone (except diabetes mellitus), clinical conditions that may limit study participation (e.g., respiratory distress and infections), chronic alcoholism and/or drug addiction, participation in a study of an investigational drug during the past 90 days, planning to move out of the area within 1 year, on active transplant list, and treatment within the last 6 months with drugs that may affect bone metabolism (except for treatment with calcitriol, vitamin D analogs, and/or calcimimetics). At baseline and after 1 year, patients underwent BMD determinations and blood draws for measurements of serum biochemical parameters. Demographic and clinical parameters were also recorded at the same times. The treating nephrologist determined all treatments based on standardized practice following KDOQI and KDIGO recommendations. There were no interventions by the investigators.
Measurements of BMD
For assessment of BMD, we used DXA because it is the most widely used tool for assessment of bone mass and fracture risk in the general population (7). We also used QCT because of its stronger correlation with histologically determined bone volume (8). BMD of the spine and total hip was assessed using both methods by the same operator using the same machine for the duration of the study. iDXA (GE Medical Systems Lunar, Madison, WI) was used for DXA, and the coefficients of variation for DXA measurements were spine=1.35% and hip=0.52%. SOMATOM Sensation 16 was used for QCT using the QCT PRO software (Mindways Software Inc., Austin, TX). The coefficients of variation for QCT measurements were spine=0.80% and hip=0.82%. Spine BMD includes mainly trabecular bone and a thin layer of cortex, whereas hip BMD includes mainly cortical bone. Changes in BMD were stratified into losing or gaining greater than 2% versus no change. A decrease in BMD by DXA and/or QCT of 2% was used as the threshold for bone loss (9,10). This 2% value is outside our error of DXA and QCT and the threshold defined by the International Society for Clinical Densitometry (11).
Determinations of Blood Parameters
The following biochemical parameters were measured because of their novelty or correlation with bone parameters in cross-sectional prior studies: serum parathyroid hormone (PTH; commonly used to assess bone turnover abnormalities, which have been shown to be associated with changes in bone volume) (12), bone-specific alkaline phosphatase (BSAP) and procollagen type 1 N-terminal propeptide (P1NP; which are markers of osteoblastic activity) (13,14), tartrate-resistant acid phosphatase-5b (TRAP-5b; a marker of osteoclastic activity) (15), sclerostin (a protein produced by osteocytes [16,17] and expressed at bone formation sites [18,19]), Dickkopf-1 (DKK-1; found in bone and other tissues [20], and like sclerostin, it leads to increased bone formation and bone volume when knocked out) (21,22), and fibroblast growth factor 23 (FGF23; involved in mineral metabolism with a role in bone mineralization/remodeling) (23–25). In addition, serum calcium and phosphorus were measured.
Plasma intact PTH levels were measured by a radioimmunometric assay (Scantibodies, Santee, CA). The intra- and interassay coefficients of variation for intact PTH were <5% and <7%, respectively. BSAP levels were measured using an enzyme immunoassay (EIA; Quidel, San Diego, CA). The intra- and interassay coefficients of variation were <6% and <8%, respectively. Total P1NP levels were measured using an ELISA (USCNK, Wuhan, China); the intra- and interassay coefficients of variation were <9% and <10%, respectively. TRAP-5b levels were determined using an EIA (Quidel, Santa Clara, CA).The intra- and interassay coefficients of variation were <2.2% and <3%, respectively. Serum sclerostin levels were measured using an EIA (Tecomedical Group, Sissach, Switzerland). The intra- and interassay coefficients of variation were <3.1% and <3.5%, respectively. DKK-1 was determined by an ELISA (Biomedica, Vienna, Austria). The intra- and interassay coefficients of variation were <8% and <12%, respectively.
Statistical Analyses
Results are given as mean±SD. Univariate analyses of quantitative variables were performed using Mann–Whitney U or t tests, depending on distribution and equality of variance. Bivariate correlations were calculated using Spearman’s Rho. Multivariable linear regression was used to assess predictive relationships with BMD at baseline and with BMD changes at 1 year. Serum biochemical parameters that significantly correlated with BMD or BMD changes were regressed in the presence of age group (binary variables), race (black versus other), body mass index (BMI), and sex (known confounders for BMD). Significance for analyses was set at P<0.05, except for multiple correlations, where significance was elevated to P<0.01. SPSS statistical software (version 21; IBM Corporation, Chicago, IL) was used for all calculations.
Results
Baseline Patient Characteristics
In total, 81 patients completed the study. The mean age was 52.6±12.3 years, with 56% men and 44% women. Patients were 53% African American and 46% Caucasian (including 1% Hispanic). The clinical and biochemical characteristics of the patients at baseline are shown in Table 1. There were no significant sex differences in the biochemical characteristics. Significant correlations between the biochemical parameters are shown in Table 2. Baseline BMD by QCT and DXA at the hip and spine are shown in Table 3. QCT and DXA of the spine identified similar rates of osteoporosis (13.6% and 13.6%), but at the hip, DXA identified more osteoporosis (22.2% versus 13.6%). At any site and by either method, 33.3% of the patients were osteoporotic.
Table 1.
Patients with CKD on dialysis clinical and serum biochemical characteristics at baseline and 1 year
| Variables and Serum Biochemical Values | At Baselinea | Normal Range | At 1 Yeara | Differencea | P Value |
|---|---|---|---|---|---|
| Variables | |||||
| No. of patients | 81 | ||||
| BMI, mean±SD | 31.3±7.8 | ||||
| Dialysis vintage, median mo (range) | 41 (3–241) | ||||
| Patients with diabetes, % | 46.1 | ||||
| Smoked within 2 yr, % | 28.6 | ||||
| Tx with active 1,25 OH D, % | 56.8 | 64.2 | 7.4 | 0.42 | |
| Tx with cinacalcet, % | 23.8 | 29.5 | +5.7 | 0.28 | |
| Tx with phosphate binders, % | 91.3 | 85.1 | −6.2 | 0.20 | |
| Calcium containing, % | 40.7 | 37.0 | −10.0 | 0.75 | |
| Serum biochemical values | |||||
| Calcium, mg/dl | 8.8±0.8 | 8.4–10.2 | 9.0±0.9 | 0.2±0.9 | 0.09 |
| Phosphorus, mg/dl | 5.8±1.6 | 2.6–4.5 | 5.5±1.5 | −0.2±1.8 | 0.50 |
| Intact PTH, pg/ml | 342 (176–522) | 14–66 | 315 (174–491) | 14 (−162–166) | 0.94 |
| BSAP, unit/L | 63.0±37.8 | 15–75 | 58.6±44.0 | −2.5±51.4 | 0.54 |
| FGF23, RU/ml | 5056 (1659–12,998) | <180 | 9701 (2270–15,945) | 1454 (−246–7905) | <0.001b |
| TRAP-5b, unit/L | 7.50±4.34 | 1.2–6.7 | 7.64±3.74 | 0.60±3.09 | 0.11 |
| P1NP, ng/ml | 47.3±25.0 | 32.8–57.4 | 19.6±19.5 | −28.1±31.0 | <0.001b |
| Sclerostin, pg/ml | 1282±696 | 130–1160 | 1376±640 | 182±384 | <0.001b |
| DKK-1, pmol/L | 30.5±28.4 | 28–256 | 41.9±23.6 | 14.9±28.8 | <0.001b |
BMI, body mass index; Tx, treatment; 1,25 OH D, 1,25-dihydroxyvitamin D; PTH, parathyroid hormone; BSAP, bone-specific alkaline phosphatase; FGF23, fibroblast growth factor 23; TRAP-5b, tartrate-resistant acid phosphatase-5b; P1NP, procollagen type 1 N-terminal propeptide; DKK-1, Dickkopf-1.
Mean±SD or median (interquartile range) or percentage.
P<0.001.
Table 2.
Spearman’s Rho correlations between biochemical parameters at baseline
| Biochemical Parameters | Intact PTH | FGF23 | BSAP | TRAP-5b | Sclerostin |
|---|---|---|---|---|---|
| Phosphorus | 0.25a | 0.46b | 0.01 | 0.06 | −0.36b |
| Intact PTH | 0.21 | 0.55b | 0.50b | −0.49b | |
| FGF23 | −0.17 | −0.02 | −0.21 | ||
| BSAP | 0.60b | −0.32b |
P<0.05.
P<0.01.
Table 3.
Bone mineral density at baseline and changes after 1 year
| Method of Measurement | Baseline |
Change at 1 Year | ||
|---|---|---|---|---|
| BMD, Mean±SD | Percent Losers≤−2% | Percent No Change=−1.9%–+1.9% | Percent Gainers≥+2% | |
| QCT total hip, g/cm3 | 291±72 | 51.3 | 27.6 | 21.1 |
| Cortical | 942±212 | 19.7 | 43.4 | 36.8 |
| Trabecular | 147±38 | 36.8 | 32.9 | 30.3 |
| QCT spine, mg/cm3 | ||||
| Trabecular | 168±58 | 39.2 | 15.2 | 45.6 |
| Cortical | 393±40 | 27.8 | 31.6 | 40.5 |
| DXA total hip, g/cm2 | 0.959±0.198 | 38.5 | 38.4 | 23.1 |
| DXA spine, g/cm2 | 1.22±0.206 | 38.3 | 30.9 | 30.8 |
BMD, bone mineral density; QCT, quantitative computed tomography; DXA, dual-energy x-ray absorptiometry.
Univariate and Multivariable Correlates with Baseline BMD
Age correlated negatively with QCT BMD of total hip and cortical spine (Rho=−0.53 and −0.50, respectively, P<0.001) and DXA BMD of total hip (Rho=−0.35, P<0.001) but not the spine. BMI correlated positively with DXA BMD of the hip (Rho=0.25, P=0.03). Men had higher DXA BMD than women at both the hip and spine (t test P values=0.003 and 0.004, respectively) and higher cortical BMD of the spine by QCT (P<0.01). Patients treated with vitamin D had lower QCT BMD of the hip, lower trabecular BMD of the spine, and lower DXA BMD of the hip (t test P values=0.04, 0.02, and 0.03, respectively) than patients not treated. Patients of African-American race had higher trabecular BMD of the spine (t test P<0.001) and higher BMD by QCT and DXA at the hip (P<0.01 and 0.05, respectively). Dialysis vintage, diabetes, and recent smoking were not associated with baseline BMD.
At baseline, intact PTH correlated inversely with QCT cortical spine and DXA spine; BSAP and TRAP-5b correlated inversely with QCT cortical spine, and sclerostin correlated positively with DXA of both hip and spine and QCT hip (Table 4). No significant correlations were found between baseline BMD and serum calcium, phosphorus, P1NP, or DKK-1. No correlations were found with differentiated cortex and trabecular total hip BMD.
Table 4.
Spearman’s Rho correlations and multivariable regression coefficients between biochemical measures and BMD at baseline
| Baseline Variable | QCT BMD Total Hip, mg/cm3 | QCT BMD Trabecular Spine, mg/cm3 | QCT BMD Cortical Spine, mg/cm3 | DXA Total Hip, g/cm2 | DXA Spine, g/cm2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rhoa | Coefficientb | Rhoa | Coefficientb | Rhoa | Coefficientb | Rhoa | Coefficientb | Rhoa | Coefficientb | |
| Intact PTH | −0.20 | — | −0.15 | — | −0.39c | −0.35c | −0.28d | −0.42e | −0.32c | −0.38d |
| BSAP | −0.19 | — | 0.09 | — | −0.34c | −0.29d | −0.26d | −0.28d | −0.23 | — |
| TRAP-5b | −0.15 | — | 0.06 | — | −0.33c | −0.33d | −0.18 | — | −0.14 | — |
| FGF23 | −0.19 | — | −0.22 | — | −0.25d | −0.27d | −0.22 | — | −0.27d | −0.25d |
| Sclerostin | 0.35c | 0.45e | 0.18 | — | 0.23 | — | 0.39e | 0.40e | 0.32c | 0.36c |
Regression coefficients are standardized and adjusted for age group, sex, race, and BMI (n=65–78). Significance for multiple correlations was set at P<0.01 and P<0.05 for multivariable regressions.
Bivariate correlation Rho.
Multivariable regression coefficient.
P<0.01.
P<0.05.
P<0.001.
With adjustment for age group, sex, race, and BMI, intact PTH correlated with baseline QCT cortical spine and DXA hip and spine, BSAP correlated with QCT cortical spine and DXA hip, TRAP-5b correlated with QCT cortical spine, FGF23 correlated with QCT cortical spine, and sclerostin correlated with QCT hip and DXA hip and spine (Table 4).
Biochemical Changes at 1 Year
Four biochemical parameters changed significantly over the 1 year of the study. Mean FGF23, sclerostin, and DKK-1 increased, whereas mean P1NP decreased (Table 1). Treatment modalities were not associated with changes in these parameters, except that DKK-1 increased more in patients not treated with active vitamin D or cinacalcet. Mean intact PTH did not change significantly over 1 year.
Correlations with and Predictors of Bone Loss at 1 Year
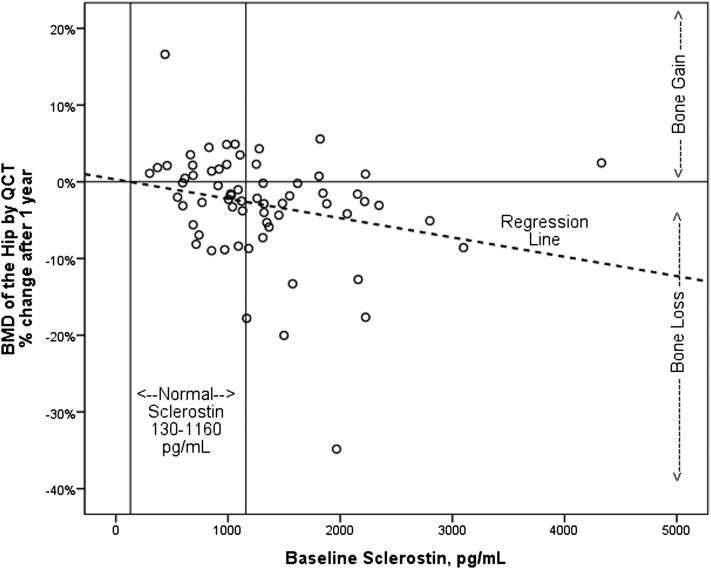
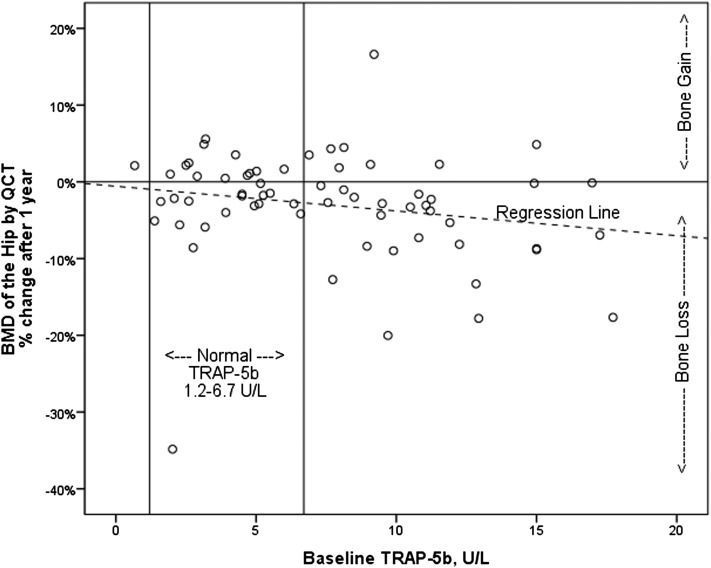
The percent of patients losing or gaining greater than 2% BMD over 1 year is shown in Table 3. QCT at the hip measured a greater frequency of bone loss than QCT of the spine or DXA of the hip or spine (Table 2). Demographic and clinical variables, including therapeutic modalities, were not associated with 1-year changes in BMD, and baseline BMD measures did not correlate with changes in BMD. Baseline serum biochemical measures that correlated with 1-year changes in BMD included sclerostin with change in QCT hip (Figure 1) and FGF23 negatively with change in DXA spine (Table 5). Baseline TRAP-5b, BSAP, P1NP, DKK-1, and intact PTH did not correlate with changes in BMD. With adjustment for age, sex, race, and BMI, TRAP-5b and sclerostin were independent predictors of bone loss in QCT total hip, P1NP was an independent predictor of changes in QCT cortical spine, and FGF23 was an independent predictor of bone loss in the spine by DXA (Figure 2, Table 5). Seventy-three percent of patients with both elevated sclerostin and TRAP-5b lost BMD by QCT of the hip.
Figure 1.
Relationship between bone mineral density (BMD) and baseline serum sclerostin. The figure shows one-year percent change in BMD of total hip by quantitative computed tomography (QCT) with corresponding baseline serum sclerostin levels. Rho=−0.37, P<0.001.
Table 5.
Spearman’s Rho correlations and multivariable regression coefficients between biochemical measures and change in BMD after 1 year
| Variable | ∆QCT BMD Total Hip, mg/cm3 | ∆QCT Trabecular Spine, mg/cm3 | ∆QCT Cortical Spine, mg/cm3 | ∆DXA BMD Total Hip, g/cm2 | ∆DXA BMD Spine, g/cm2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rhoa | Coefficientb | Rhoa | Coefficientb | Rhoa | Coefficientb | Rhoa | Coefficientb | Rhoa | Coefficientb | |
| Baseline | ||||||||||
| TRAP-5b | −0.27c | −0.28c | −0.01 | — | −0.14 | — | −0.09 | — | −0.11 | — |
| FGF23 | 0.08 | — | −0.16 | — | 0.01 | — | −0.01 | — | −0.39d | −0.33e |
| P1NP | 0.25c | 0.23 | 0.12 | — | 0.31c | 0.30c | 0.02 | — | 0.22 | — |
| Sclerostin | −0.37e | −0.31c | −0.01 | — | 0.08 | — | −0.06 | — | −0.10 | — |
| Change at 1 yr | — | |||||||||
| ∆Sclerostin | 0.12 | — | −0.36e | −0.40e | 0.13 | — | 0.02 | — | 0.32c | 0.28 |
| ∆Intact PTH | 0.02 | — | 0.35e | 0.36c | −0.03 | — | −0.16 | — | −0.07 | — |
Regression coefficients are standardized and adjusted for age group, sex, race, and BMI (n=65–78). Significance for multiple correlations was set at P<0.01 and P<0.05 for multivariable regressions.
Bivariate correlation Rho.
Multivariable regression coefficient.
P<0.05.
P<0.001.
P<0.01.
Figure 2.
Relationship between BMD and baseline serum tartrate-resistant acid phosphatase-5b (TRAP-5b). The figure shows one-year percent change in BMD of total hip by QCT with corresponding baseline serum TRAP-5b levels. Rho=−0.27, P=0.03.
Increase in intact PTH correlated with trabecular bone gain by QCT of the spine. Increase in sclerostin correlated with trabecular bone loss by QCT of the spine (Table 5).
Discussion
Our findings show, for the first time in CKD-5D patients, that bone loss is predicted by three noninvasive parameters. One parameter, TRAP-5b, is a measure of bone resorption, whereas sclerostin and P1NP are considered to reflect predominantly bone formation.
TRAP-5b is produced by osteoclasts and circulates at increased concentrations in various metabolic bone disorders with increased turnover (26), after oophorectomy (27), and in a percentage of patients with osteoporosis (28). Serum concentrations of TRAP-5b have been shown to be related to osteoclast number more than osteoclast activity (29,30). Comparing patients with CKD with bone fractures with patients without fractures, TRAP-5b was found to be 29% higher in patients with fractures (31). These findings are extended by our results, which identify elevated TRAP-5b as an independent predictor for bone loss.
Sclerostin is a soluble inhibitor of Wingless/integrated (Wnt) signaling that binds to the low-density lipoprotein receptor–related proteins 5 and 6 coreceptors and impedes the canonical Wnt/β-catenin signaling pathway to regulate bone formation in the adult skeleton (32). Knockout or loss of sclerostin leads to increased bone formation in mice and sclerosteosis in humans (33–35). Treatment with sclerostin-neutralizing mAb showed osteoanabolic effects with increases in bone formation and bone strength in rats and primates (36,37). Moreover, postmenopausal women treated with a sclerostin antibody were found to have a 5.3% increase in lumbar spine BMD (18). Sclerostin has been shown to be increased in CKD-5D patients (38). A strong inverse relationship was observed between GFR and serum sclerostin (39). Our findings show that bone loss can be predicted by elevated serum levels of sclerostin in patients with CKD-5D. This finding is in agreement with results in non-CKD animals and patients showing lower bone formation with higher sclerostin levels (16,17,32). Also, in prior studies in CKD-5D patients, we found a strong negative correlation between sclerostin blood levels and bone formation parameters (38), which could explain why, prospectively, patients with CKD-5D and higher sclerostin lose more bone than patients with lower sclerostin levels. The baseline finding of a positive relationship between sclerostin and baseline BMD in CKD-5D patients is puzzling but in keeping with at least six earlier reports (40–45).
P1NP is a protein produced by osteoblasts. During the extracellular processing of type I procollagen, the amino terminal propeptide of type 1 collagen is cleaved, resulting in free trimeric P1NP, which in turn, is cleaved into monomers. These propeptides circulate in blood and are used as markers of bone formation. Our prospective findings that P1NP predicts cortical bone gain in the spine by QCT are novel and suggest a role of bone formation in maintaining bone volume in CKD-5D patients.
These findings describe a potentially valuable clinical role for measurement of sclerostin, P1NP, and TRAP-5b in CKD-5D patients. Given the known relationships of sclerostin and P1NP with bone formation and TRAP-5b with bone resorption, these biochemical measurements may provide the basis for choosing between therapeutics aimed mainly at suppression of resorption or stimulation of bone formation in the treatment of bone loss in CKD-5D patients. Obviously, additional studies quantifying relationships between bone turnover markers and bone histomorphometric parameters are required.
The current work complements the work by Nickolas et al. (46), which addresses BMD changes in CKD patients in various stages of CKD (9 of 53 patients were on dialysis). Our findings are exclusively on CKD-5D patients. In the patient cohort in the work by Nickolas et al. (46), mean PTH levels increased, whereas in our patients, mean PTH did not change. However, change in intact PTH was an independent predictor of trabecular spine bone gain by QCT.
It is intriguing to learn that higher FGF23 levels predict lower DXA BMD of the spine at baseline and more bone loss after 1 year. These findings do not confirm the results of Desjardins et al. (47) and Ureña et al. (48) that FGF23 does not correlate with DXA BMD. This finding may be because of possible differences in patient populations and more importantly, the cross-sectional nature of these two studies. This explanation is contrary to the report that FGF23 is associated with increased vascular calcifications (47) at more proximal sites of the spine. Other than site differences, there are possible differences in patient characteristics along with the known limitation of cross-sectional results. A confounding of DXA by extraosseous calcifications can explain differences between DXA and QCT in detection of osteoporosis.
Nonparametric analyses of correlations between the measured biochemical parameters confirmed the known relationships between FGF23 and phosphorus, BSAP and intact PTH, BSAP and TRAP-5b, TRAP-5b and intact PTH, and sclerostin and intact PTH (negative). Novel relationships were found between sclerostin and phosphorus (negative) as well as between sclerostin and BSAP (negative) in CKD-5D patients. These relationships can be explained by the negative correlation between sclerostin and PTH (38), resulting in lower bone turnover with higher sclerostin levels.
The finding of lower BMD at baseline in patients treated with vitamin D compared with no vitamin D treatment might be explained by patients’ characteristics. Vitamin D is customarily prescribed to patients with high PTH levels. Higher PTH levels are, in turn, associated with lower cortical bone mass (12). The presented results show that baseline BMD does not predict future changes in BMD. This finding suggests that cross-sectional BMD measurements cannot predict bone loss and that serial DXA measurements are necessary for monitoring for bone loss.
Limitations found with cross-sectional observations are resolved here by our prospective 1-year results. Our regression results are valuable for diagnosis and prediction of bone loss but do not allow us to comment on mechanisms. Additional studies over longer periods of time might identify additional predictors of bone loss. Other studies will need to examine mechanisms. The present study should help forward efforts to clinical applications. These efforts will have to overcome the lack of routine availability of assays for sclerostin and TRAP-5b for clinical purposes.
In conclusion, QCT identified prospectively more bone loss at the hip than DXA. The baseline serum biochemical parameters sclerostin and TRAP-5b were noninvasive independent predictors of bone loss in patients with CKD-5D.
Disclosures
None.
Acknowledgments
The authors thank Kimberly McLaughlin and Nedda Hughes of the University of Kentucky, Division of Nephrology, Bone and Mineral Metabolism, for their invaluable and knowledgeable work in patient enrollment and data collection.
Research reported in this publication was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health Award R01080770, the Kentucky Nephrology Research Trust, and National Center for Research Resources and National Center for Advancing Translational Sciences, National Institutes of Health Grant UL1-TR000117.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank the following colleagues for their support and making their patients available for our study: Dr. Don Duff and colleagues of Nephrology Associates of Kentuckiana, Dr. Eleanor Lederer and colleagues of the University of Louisville Division of Nephrology, Dr. Thomas Ferguson and colleagues of Nephrology Associates of Lexington, Dr. Khalid Bhatti and Dr. Mahendra Patel of Nephrology Associates of Central Kentucky, Dr. Jyotin Chandarana of dialysis clinic in Hazard, KY, Dr. Rezkalla Butros of dialysis clinic in Mt. Sterling, KY, Dr. Ashutosh Lohe and Dr. Syed Hasni of dialysis clinic in Barbourville, KY, Dr. Harold Helton of Cumberland Nephrology, and Dr. Mostafa Amr of Central Kentucky Nephrology.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “The Utility of Circulating Markers to Predict Bone Loss across the CKD Spectrum,” on pages 1160–1162.
References
- 1.Malluche HH, Monier-Faugere MC: Renal osteodystrophy: What’s in a name? Presentation of a clinically useful new model to interpret bone histologic findings. Clin Nephrol 65: 235–242, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G, Kidney Disease: Improving Global Outcomes (KDIGO) : Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Yenchek RH, Ix JH, Shlipak MG, Bauer DC, Rianon NJ, Kritchevsky SB, Harris TB, Newman AB, Cauley JA, Fried LF, Health, Aging, and Body Composition Study : Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol 7: 1130–1136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, Wong C, Stehman-Breen C: Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 58: 396–399, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Erlichman M, Holohan TV: Bone densitometry: Patients with end-stage renal disease. Health Technol Assess (Rockv) 8: 1–27, 1996 [PubMed] [Google Scholar]
- 8.Malluche HH, Arnala I, Faugere MC: Values of noninvasive techniques in predicting bone histology. Ann Chir Gynaecol 77: 246–250, 1988 [PubMed] [Google Scholar]
- 9.Cauley JA, Zmuda JM, Wisniewski SR, Krishnaswami S, Palermo L, Stone KL, Black DM, Nevitt MC: Bone mineral density and prevalent vertebral fractures in men and women. Osteoporos Int 15: 32–37, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Hui SL, Slemenda CW, Johnston CC, Jr.: Age and bone mass as predictors of fracture in a prospective study. J Clin Invest 81: 1804–1809, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leib ES, Lewiecki EM, Binkley N, Hamdy RC, International Society for Clinical Densitometry : Official positions of the International Society for Clinical Densitometry. J Clin Densitom 7: 1–6, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Malluche HH, Mawad HW, Monier-Faugere MC: Renal osteodystrophy in the first decade of the new millennium: Analysis of 630 bone biopsies in black and white patients. J Bone Miner Res 26: 1368–1376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fishman WH: Alkaline phosphatase isozymes: Recent progress. Clin Biochem 23: 99–104, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Hale LV, Galvin RJ, Risteli J, Ma YL, Harvey AK, Yang X, Cain RL, Zeng Q, Frolik CA, Sato M, Schmidt AL, Geiser AG: PINP: A serum biomarker of bone formation in the rat. Bone 40: 1103–1109, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Halleen JM, Alatalo SL, Suominen H, Cheng S, Janckila AJ, Väänänen HK: Tartrate-resistant acid phosphatase 5b: A novel serum marker of bone resorption. J Bone Miner Res 15: 1337–1345, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Veverka V, Henry AJ, Slocombe PM, Ventom A, Mulloy B, Muskett FW, Muzylak M, Greenslade K, Moore A, Zhang L, Gong J, Qian X, Paszty C, Taylor RJ, Robinson MK, Carr MD: Characterization of the structural features and interactions of sclerostin: Molecular insight into a key regulator of Wnt-mediated bone formation. J Biol Chem 284: 10890–10900, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA: Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22: 6267–6276, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padhi D, Jang G, Stouch B, Fang L, Posvar E: Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 26: 19–26, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Kusu N, Laurikkala J, Imanishi M, Usui H, Konishi M, Miyake A, Thesleff I, Itoh N: Sclerostin is a novel secreted osteoclast-derived bone morphogenetic protein antagonist with unique ligand specificity. J Biol Chem 278: 24113–24117, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Pinzone JJ, Hall BM, Thudi NK, Vonau M, Qiang YW, Rosol TJ, Shaughnessy JD, Jr.: The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood 113: 517–525, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA, Shen Z, Patel N, Tai YT, Chauhan D, Mitsiades C, Prabhala R, Raje N, Anderson KC, Stover DR, Munshi NC: Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood 114: 371–379, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morvan F, Boulukos K, Clément-Lacroix P, Roman Roman S, Suc-Royer I, Vayssière B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G: Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res 21: 934–945, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE: Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38: 1310–1315, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S, Rowe PS, Vierthaler L, Zhou J, Quarles LD: Phosphorylated acidic serine-aspartate-rich MEPE-associated motif peptide from matrix extracellular phosphoglycoprotein inhibits phosphate regulating gene with homologies to endopeptidases on the X-chromosome enzyme activity. J Endocrinol 192: 261–267, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD: Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab 291: E38–E49, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Stĕpan JJ, Silinková-Málková E, Havránek T, Formánková J, Zichová M, Lachmanová J, Straková M, Broulik P, Pacovský V: Relationship of plasma tartrate resistant acid phosphatase to the bone isoenzyme of serum alkaline phosphatase in hyperparathyroidism. Clin Chim Acta 133: 189–200, 1983 [DOI] [PubMed] [Google Scholar]
- 27.Stĕpán JJ, Pospíchal J, Presl J, Pacovský V: Bone loss and biochemical indices of bone remodeling in surgically induced postmenopausal women. Bone 8: 279–284, 1987 [DOI] [PubMed] [Google Scholar]
- 28.de la Piedra C, Torres R, Rapado A, Diaz Curiel M, Castro N: Serum tartrate-resistant acid phosphatase and bone mineral content in postmenopausal osteoporosis. Calcif Tissue Int 45: 58–60, 1989 [DOI] [PubMed] [Google Scholar]
- 29.Cheung MS, Glorieux FH, Rauch F: Large osteoclasts in pediatric osteogenesis imperfecta patients receiving intravenous pamidronate. J Bone Miner Res 24: 669–674, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Christiensen C, Hassager C, Riis BJ: Biochemical markers of bone turnover. In: Metabolic Bone Disease and Clinically Related Disorders, edited by Avioli LV, Krane SM, San Diego, CA, Academic Press, 1998 [Google Scholar]
- 31.Nickolas TL, Cremers S, Zhang A, Thomas V, Stein E, Cohen A, Chauncey R, Nikkel L, Yin MT, Liu XS, Boutroy S, Staron RB, Leonard MB, McMahon DJ, Dworakowski E, Shane E: Discriminants of prevalent fractures in chronic kidney disease. J Am Soc Nephrol 22: 1560–1572, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baron R, Rawadi G: Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 148: 2635–2643, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W: Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10: 537–543, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W: Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet 39: 91–97, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C: Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23: 860–869, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, Chen Q, Winters A, Boone T, Geng Z, Niu QT, Ke HZ, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C: Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24: 578–588, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Ominsky MS, Vlasseros F, Jolette J, Smith SY, Stouch B, Doellgast G, Gong J, Gao Y, Cao J, Graham K, Tipton B, Cai J, Deshpande R, Zhou L, Hale MD, Lightwood DJ, Henry AJ, Popplewell AG, Moore AR, Robinson MK, Lacey DL, Simonet WS, Paszty C: Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res 25: 948–959, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Cejka D, Herberth J, Branscum AJ, Fardo DW, Monier-Faugere MC, Diarra D, Haas M, Malluche HH: Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol 6: 877–882, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelletier S, Dubourg L, Carlier MC, Hadj-Aissa A, Fouque D: The relation between renal function and serum sclerostin in adult patients with CKD. Clin J Am Soc Nephrol 8: 819–823, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amrein K, Amrein S, Drexler C, Dimai HP, Dobnig H, Pfeifer K, Tomaschitz A, Pieber TR, Fahrleitner-Pammer A: Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults. J Clin Endocrinol Metab 97: 148–154, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Ardawi MS, Al-Kadi HA, Rouzi AA, Qari MH: Determinants of serum sclerostin in healthy pre- and postmenopausal women. J Bone Miner Res 26: 2812–2822, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Cejka D, Jäger-Lansky A, Kieweg H, Weber M, Bieglmayer C, Haider DG, Diarra D, Patsch JM, Kainberger F, Bohle B, Haas M: Sclerostin serum levels correlate positively with bone mineral density and microarchitecture in haemodialysis patients. Nephrol Dial Transplant 27: 226–230, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Durosier C, van Lierop A, Ferrari S, Chevalley T, Papapoulos S, Rizzoli R: Association of circulating sclerostin with bone mineral mass, microstructure, and turnover biochemical markers in healthy elderly men and women. J Clin Endocrinol Metab 98: 3873–3883, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Mödder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, Melton LJ, 3rd, Khosla S: Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res 26: 373–379, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polyzos SA, Anastasilakis AD, Bratengeier C, Woloszczuk W, Papatheodorou A, Terpos E: Serum sclerostin levels positively correlate with lumbar spinal bone mineral density in postmenopausal women—the six-month effect of risedronate and teriparatide. Osteoporos Int 23: 1171–1176, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Nickolas TL, Stein EM, Dworakowski E, Nishiyama KK, Komandah-Kosseh M, Zhang CA, McMahon DJ, Liu XS, Boutroy S, Cremers S, Shane E: Rapid cortical bone loss in patients with chronic kidney disease. J Bone Miner Res 28: 1811–1820, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desjardins L, Liabeuf S, Renard C, Lenglet A, Lemke HD, Choukroun G, Drueke TB, Massy ZA, European Uremic Toxin (EUTox) Work Group : FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporos Int 23: 2017–2025, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Ureña P, Bernard-Poenaru O, Ostertag A, Baudoin C, Cohen-Solal M, Cantor T, de Vernejoul MC: Bone mineral density, biochemical markers and skeletal fractures in haemodialysis patients. Nephrol Dial Transplant 18: 2325–2331, 2003 [DOI] [PubMed] [Google Scholar]