Abstract
Background and objectives
Increased bone resorption, low bone formation, and abnormal mineralization have been described in stone formers with idiopathic hypercalciuria. It has been previously shown that the receptor activator of NF-κB ligand mediates bone resorption in idiopathic hypercalciuria (IH). The present study aimed to determine the expression of fibroblast growth factor 23 (FGF-23), vitamin D receptor (VDR), and sclerostin in bone tissue from IH stone formers.
Design, setting, participants, & measurements
Immunohistochemical analysis was performed in undecalcified bone samples previously obtained for histomorphometry from 30 transiliac bone biopsies of idiopathic hypercalciuria stone-forming patients between 1992 and 2002 and 33 healthy individuals (controls). Serum parameters were obtained from their medical records.
Results
Histomorphometry disclosed 21 IH patients with high and 9 IH patients with normal bone resorption. Importantly, eroded surfaces (ES/BS) from IH patients but not controls were significantly correlated with VDR immunostaining in osteoblasts (r=0.51; P=0.004), sclerostin immunostaining in osteocytes (r=0.41; P=0.02), and serum 1,25-dihydroxyvitamin D (r=0.55; P<0.01). Of note, both VDR and sclerostin immunostaining were significantly correlated with serum 1,25-dihydroxyvitamin D in IH patients (r=0.52; P=0.01 and r=0.53; P=0.02, respectively), although VDR and sclerostin expression did not differ between IH and controls. IH patients with high bone resorption exhibited a significantly stronger sclerostin immunostaining than IH patients with normal bone resorption. FGF-23 expression in osteocytes from IH patients did not differ from controls and was not correlated with any histomorphometric parameter.
Conclusions
These findings suggest the contribution of VDR and sclerostin, as well as 1,25-dihydroxyvitamin D, to increase bone resorption in idiopathic hypercalciuria but do not implicate FGF-23 in the bone alterations seen in these patients.
Keywords: hypercalciuria, immunohistochemistry, sclerostin, FGF-23, VDR
Introduction
Idiopathic hypercalciuria (IH) represents a generalized dysregulation of calcium homeostasis, which includes increased gut calcium absorption, decreased renal calcium reabsorption, and a tendency to lose calcium from the bone (1,2).
Abnormal bone remodeling among hypercalciuric stone formers has been disclosed by histomorphometry and characterized as increased bone resorption, low bone formation, and a mineralization defect as previously shown by our group (3–6) and others (7,8). In a previous study, we found that the receptor activator of NF-κB ligand (RANKL) mediated bone resorption in IH patients (6).
Fibroblast growth factor 23 (FGF-23) is produced in bone and acts on kidney as a systemic phosphaturic factor. Although increased serum levels of FGF-23 were found in hypophosphatemic nephrolithiasis patients (9), FGF-23 overexpression in cultured calvarias may suppress bone formation and matrix mineralization irrespective of the phosphate homeostasis (10).
Serum 1,25-dihydroxyvitamin D [1,25(OH)2D3] levels in IH patients are either normal or high (11–14), and vitamin D receptor (VDR) polymorphism has been associated with hypercalciuria (15). In hypercalciuric patients, VDR concentration was normal in fibroblasts (13) but 2-fold higher in PBMCs (14), despite normal serum 1,25(OH)2D3, suggesting an altered tissue vitamin D response. Genetic hypercalciuric stone-forming (GHS) rats exhibit increased VDR coupled with normal levels of 1,25(OH)2D3, resulting in increased functional VDR–1,25(OH)2D3 complexes (16). Finally, increased bone resorption has been observed after calcitriol exposure in GHS cultured calvarias (17).
Another important regulator of bone metabolism produced in osteocytes is sclerostin (Scl), which is codified by the SOST gene and functions as a negative regulator of bone mass and osteoblast differentiation (18,19), but it also promotes osteoclast formation and activity (19).
In the present study, we aimed to evaluate bone expression of FGF-23, VDR, and Scl in IH stone formers and examine their relationship with bone remodeling alterations.
Materials and Methods
In this retrospective study, undecalcified bone fragments obtained from transiliac bone biopsies of 30 Caucasian stone-forming IH patients with normal renal function (previously performed from 1992 to 2002) were included. Only those patients with evidence of uni- or bilateral radio-opaque stones consistent with calcium composition were selected. Exclusion criteria for bone biopsy were hyperparathyroidism, renal tubular acidosis, sarcoidosis, diabetes mellitus, and use of corticosteroids, bisphosphonates, citrate, or diuretics. IH was defined by normocalcemia and 24-hour urinary calcium>250 mg/d (for women) and 300 mg/d (for men) or >4 mg/kg per day in two nonconsecutive samples under unrestricted diet. Histomorphometric parameters, serum levels of calcium, phosphate, creatinine, 1,25(OH)2D3, and intact parathyroid hormone (PTH), obtained near the date of the bone biopsy were retrieved from their medical records; 1,25(OH)2D3 had been determined by radioimmunoassay (Diasorin; Stillwater, MN/Nichols Institute Diagnostics, CA) and PTH by different immunoradiometric assays according to the availability of methods between 1992 and 2002 (3–5). The Ethical Committees of both institutions approved this study, and all patients signed written consent by the time that the biopsies were performed.
Bone Histomorphometry
Histomorphometric parameters had been obtained by transiliac bone biopsies as previously described (20). The control group for static parameters encompassed 33 healthy individuals (18 men and 15 women; 25 Caucasian and 8 non-Caucasian; 32.9±5.4 years) selected from our bone histomorphometry database (20) consisting of bone biopsies obtained immediately after early death from victims of gunshot or knife wounds, trauma, or traffic accidents who were not known to have any disease or be users of anticonvulsant drugs, corticosteroids, or any medication that interfered with bone metabolism based on their medical records. Dynamic parameters were compared with the controls from the work by Melsen and Mosekilde (21), which were obtained from 41 volunteers with similar age: 12 men (mean age=32 years) and 29 women (mean age=29 years).
Immunohistochemistry
Immunostaining for FGF-23, VDR, and Scl was performed in IH patients and controls according to a technique previously described (22). The primary antibodies used were monoclonal anti-human FGF-23 (provided by Susan C. Schiavi, Genzyme, Framingham, MA), rat monoclonal anti-human VDR (ABR-Affinity BioReagents, Inc. Golden, CO), and human monoclonal antibody anti-Scl (R&D Systems, Minneapolis, MN). Counting was performed on 30 microscopic fields at a magnification of ×200 for each bone sample. The area of immunopositivity was determined by the number of positive points in the tissue compared with the total number of points.
Statistical Analyses
Normality of distribution was assessed by the Kolmogorov–Smirnov test. Accordingly, data are expressed as mean±SD or median (25th, 75th percentiles). Differences between groups were analyzed using Mann–Whitney or unpaired t tests depending on the distribution of variables. Correlations between immunohistochemistry and biochemical or histomorphometric parameters were assessed by Pearson or Spearman correlation testing as appropriate, and confidence intervals are presented. The level of significance was set up as P<0.05. Prism software version 4.0 (GraphPad Software Inc., San Diego, CA) was used for statistical analyses.
Results
IH patients with calcium stones (18 men and 12 premenopausal women; 36.3±9.3 years) presented mean urinary calcium of 340.8±70.6 mg/24 h, eGFR of 81.1±12.2 ml/min per 1.73 m2, and serum creatinine, calcium, and phosphorus of 0.92±0.12, 9.6±0.5, and 3.2±0.5 mg/dl, respectively. Two patients were hypophosphatemic, and two patients presented 1,25(OH)2D3 values slightly above normal ranges. All had serum PTH within normal limits: median values for intact PTH were 33.5 (21.5, 49.5) and 20.3 (12.5, 37.5) pg/ml and median values for 1,25(OH)2D3 were 40.7±17.1 and 50.1±16.1 pg/ml according to the method used.
Bone Histomorphometry
As shown in Table 1, IH patients presented significantly lower mean bone volume (BV/TV) and trabecular number (Tb.N) and significantly higher trabecular separation (Tb.Sp). Mineralization lag time (MLT) was significantly higher, and mineralizing surface (MS/BS) was significantly lower. Both eroded surfaces (ES/BS) and osteoclast surfaces (Oc.S/BS) were significantly higher. ES/BS from IH patients did significantly correlate with serum 1,25(OH)2D3 (r=0.55; P<0.01).
Table 1.
Histomorphometric parameters
| Variables | IH Patients (n=30) | Controls (n=33) | P Value |
|---|---|---|---|
| Structure | |||
| BV/TV (%) | 18.78±7.54 | 25.42±6.52 | <0.001 |
| Tb.N (mm) | 1.65 (1.25, 1.84) | 1.90 (1.75, 2.30) | 0.002 |
| Tb.Sp (μm) | 481 (394, 676) | 359 (319, 439) | <0.001 |
| Tb.Th (μm) | 125.13±30.75 | 126.58±24.41 | 0.97 |
| Formation | |||
| OV/BV (%) | 1.62 (0.88, 2.82) | 1.60 (0.98, 2.75) | 0.95 |
| OS/BS (%) | 10.20 (5.60, 16.98) | 9.3 (6.65, 15.60) | 0.93 |
| Ob.S/BS (%) | 1.13 (0.28, 2.92) | 0.70 (0.30, 1.56) | 0.49 |
| MLT (d) | 48.40±32.20 | 23.00±2.40 | <0.001 |
| O.Th (mm) | 12.32±5.08 | 10.75±2.81 | 0.42 |
| MAR (µm/d) | 0.69±0.23 | 0.65±0.12 | 0.40 |
| MS/BS (%) | 6.48±5.13 | 13.75±7.25 | <0.001 |
| BFR/BS (μm3 μm2/d) | 0.07±0.16 | 0.09±0.05 | 0.52 |
| Resorption | |||
| ES/BS (%) | 7.29 (3.17, 14.00) | 2.05 (1.55, 2.90) | <0.001 |
| Oc.S/BS (%) | 0.30 (0, 0.46) | 0 (0, 0.02) | <0.001 |
Mean±SD or median (25th, 75th percentiles). IH, idiopathic hypercalciuria; BV/TV, bone volume; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness; OV/BV, osteoid volume; OS/BS, osteoid surface; Ob.S/BS, osteoblastic surface; MLT, mineralization lag time; O.Th, osteoid thickness; MAR, mineral apposition rate; MS/BS, mineralizing surface; BFR/BS, bone formation rate; ES/BS, eroded surface; Oc.S/BS, osteoclast surface.
Immunohistochemistry
Compared with controls, IH patients did not present statistical differences for bone immunostaining of FGF-23 in osteocytes (6.8±5.9% versus 6.0±5.0%; P=0.49), VDR immunostaining in osteoblasts (21.4±17.8% versus 28.7±20.1%; P=0.14), VDR in osteocytes (13.8±9.6% versus 12.0±8.5%; P=0.52), VDR in the total of both cells (16.4±11.5% versus 18.9±11.1%; P=0.35); VDR immunostaining in osteoclasts was negligible, and Scl bone immunostaining in osteocytes (9.1±11.1% versus 6.1±5.5%; P=0.22; data not shown in tables).
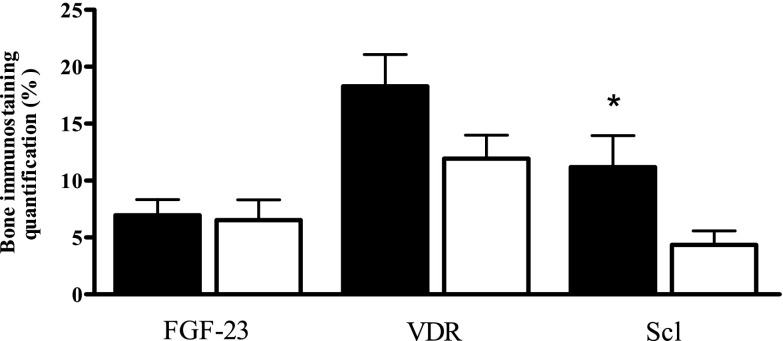
As seen in Figure 1, mean bone FGF-23 immunostaining in IH patients classified as having high bone resorption (ES/BS=1 SD above mean, n=21) did not differ from normal bone resorption (ES/BS<1 SD, n=9). Mean VDR bone immunostaining in the sum of all stained cells was numerically but not statistically higher in patients with high versus normal ES/BS. Mean Scl immunostaining in osteocytes was significantly stronger in high versus normal bone resorption IH patients. The median (25th, 75th percentiles) estimated duration of disease for patients with high versus normal ES/BS (7.0 [5, 17] versus 2.0 [1.5, 10] years) presented a marginal trend to significance (P=0.05; data not shown).
Figure 1.
Bone immunostaining quantification (%) of fibroblast growth factor 23 (FGF-23), vitamin D receptor (VDR), and sclerostin (Scl). Idiopathic hypercalciuria patients with high (black bars; n=21) or normal (white bars; n=9) bone resorption defined by eroded surface (*P=0.03 versus white bars). The quantification of immunostaining was determined by the ratio of positive to total number of stained cells in bone tissue.
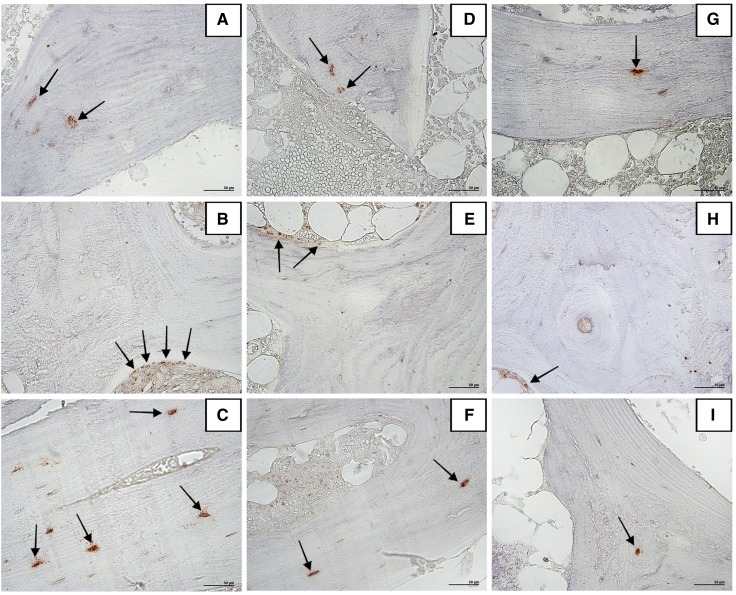
Figure 2 illustrates immunoreactivity localization of FGF-23 in osteocytes, VDR in osteoblasts, and Scl in osteocytes in bone tissue.
Figure 2.
Immunohistochemistry for FGF-23, VDR, and Scl in bone tissue (positive immunostaining is shown in red). No differences between the patterns of FGF-23 immunostaining in osteocytes (arrows) in (A) a patient with high bone resorption, (D) a patient with normal bone resorption, and (G) a control patient are observed. Examples of higher expression of VDR in osteoblasts (arrows) and Scl immunostaining in osteocytes (arrows) from an idiopathic hypercalciuria patient with high bone resorption are given in B and C, respectively, contrasting with the fewer stained cells in an idiopathic hypercalciuria patient with normal bone resorption (E and F for VDR and Scl, respectively) and a control individual (H and I, respectively). Original magnification, ×200.
Table 2 shows significant correlations between serum 1,25(OH)2D3 and bone expression of VDR in osteoblasts and Scl in osteocytes.
Table 2.
Correlation between bone immunostaining of fibroblast growth factor 23, vitamin D receptor, and sclerostin with serum parameters in IH patients
| Parameters | FGF-23 (OC) | VDR | Scl (OC) | |
|---|---|---|---|---|
| OB | OC | |||
| sCa | 0.14 (−0.49 to 0.26) | −0.03 (−0.42 to 0.36) | −0.02 (−0.41 to 0.36) | −0.28 (−0.60 to 0.14) |
| sP | 0.28 (−0.12 to 0.60) | 0.12 (−0.30 to 0.50) | 0.12 (−0.30 to 0.50) | 0.12 (−0.32 to 0.50) |
| PTH | 0.19 (−0.55 to 0.24) | −0.18 (−0.54 to 0.23) | 0.15 (−0.26 to 0.51) | −0.11 (−0.51 to 0.29) |
| 1,25(OH)2D3 | −0.33 (−0.67 to 0.09) | 0.52 (0.13 to 0.77)a | −0.02 (−0.44 to 0.39) | 0.53 (0.16 to 0.79)b |
Data are presented as r (95% confidence interval). FGF-23, fibroblast growth factor 23; OC, osteocyte; VDR, vitamin D receptor; Scl, sclerostin; OB, osteoblast; sCa, serum calcium; sP, serum phosphate; PTH, intact parathyroid hormone; 1,25(OH)2D3, 1,25 dihidroxyvitamin D3.
P=0.01.
P=0.02.
FGF-23 immunostaining did not correlate with any histomorphometric parameter, which is seen in Table 3, nor in the control group (data not shown). ES/BS significantly correlated with VDR bone immunostaining in osteoblasts and the sum of all stained cells from IH but not in the controls (data not shown). Tb.N was positively correlated with VDR bone immunostaining in osteoblasts from IH patients but negatively correlated with VDR in osteoblasts and the sum of all cells from controls (r=−0.46; P=0.008 and r=−0.49; P=0.004, respectively; data not shown). Scl immunostaining in osteocytes was significantly correlated with ES/BS in IH patients but not the control group. In the control group, Scl immunostaining correlated with BV/TV (r=0.47; P<0.01), Tb.N (r=0.48; P<0.01), and Tb.Sp (r=−0.56; P<0.001; data not shown).
Table 3.
Correlations between bone immunostaining of FGF-23, VDR, and Scl with histomorphometric parameters in 30 IH patients
| Parameters | FGF-23 (OC) | VDR | Scl (OC) | ||
|---|---|---|---|---|---|
| OB | OC | Total | |||
| BV/TV (%) | −0.01 (−0.51 to 0.24) | −0.03 (−0.40 to 0.34) | 0.04 (−0.33 to 0.40) | −0.29 (−0.61 to 0.09) | 0.09 (−0.31 to 0.43) |
| Tb.N (mm) | −0.01 (−0.83 to 0.26) | 0.44 (0.03 to 0.73)a | −0.15 (−0.53 to 0.29) | 0.10 (−0.22 to 0.61) | 0.15 (−0.29 to 0.54) |
| Tb.Sp (μm) | 0.11 (−0.26 to 0.58) | −0.42 (−0.72 to −0.00) | 0.05 (−0.38 to 0.46) | −0.25 (−0.65 to 0.12) | −0.18 (−0.56 to 0.26) |
| Tb.Th (μm) | −0.20 (−0.46 to 0.45) | 0.22 (−0.23 to 0.58) | 0.14 (−0.30 to 0.53) | 0.27 (−0.16 to 0.63) | −0.01 (−0.43 to 0.42) |
| OV/BV (%) | −0.22 (−0.60 to 0.11) | 0.32 (−0.05 to 0.61) | −0.09 (−0.44 to 0.28) | 0.17 (−0.23 to 0.50) | −0.08 (−0.43 to 0.29) |
| OS/BS (%) | −0.14 (−0.57 to 0.15) | 0.19 (−0.19 to 0.51) | −0.20 (−0.52 to 0.18) | 0.19 (−0.26 to 0.47) | −0.18 (−0.50 to 0.20) |
| Ob.S/BS (%) | −0.20 (−0.53 to 0.19) | 0.18 (−0.19 to 0.51) | −0.05 (−0.41 to 0.31) | −0.05 (−0.24 to 0.49) | −0.15 (−0.48 to 0.23) |
| MLT (d) | −0.30 (−0.56 to 0.21) | 0.36 (−0.02 to 0.65) | −0.16 (−0.51 to 0.24) | 0.37 (−0.15 to 0.57) | 0.26 (−0.37 to 0.39) |
| MS/BS (%) | −0.35 (−0.67 to 0.14) | 0.13 (−0.32 to 0.53) | −0.21 (−0.59 to 0.25) | −0.28 (−0.66 to 0.14) | −0.39 (−0.50 to 0.24) |
| BFR/BS (μm3 μm2/d) | −0.08 (−0.45 to 0.29) | 0.23 (−0.14 to 0.55) | 0.04 (−0.34 to 0.40) | 0.11 (−0.29 to 0.46) | −0.04 (−0.38 to 0.36) |
| ES/BS (%) | −0.07 (−0.47 to 0.28) | 0.51 (0.19 to 0.74)b | 0.15 (−0.22 to 0.48) | 0.47 (0.12 to 0.71)c | 0.41 (0.07 to 0.68)d |
| Oc.S/BS (%) | −0.08 (−0.52 to 0.22) | −0.09 (−0.44 to 0.28) | −0.05 (−0.40 to 0.32) | −0.22 (−0.50 to 0.20) | −0.12 (−0.47 to 0.24) |
Data are presented as r (95% confidence interval). OC, osteocyte; OB, osteoblast; Total, sum of all stained cells; BV/TV, bone volume; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness; OV/BV, osteoid volume; OS/BS, osteoid surface; Ob.S/BS, osteoblastic surface; MLT, mineralization lag time; MS/BS, mineralizing surface; BFR/BS, bone formation rate; ES/BS, eroded surface; Oc.S/BS, osteoclast surface.
P=0.04.
P=0.004.
P=0.01.
P=0.02.
Discussion
Decreased bone mineral density (BMD) is commonly encountered in IH patients (23), and an increased prevalence of bone fractures has been reported for nephrolithiasis patients (24,25). GHS rats also exhibited reduced BMD and more brittle and fracture-prone bones (26) because of lower trabecular volume and mineralized volume and thickness. Human histomorphometric studies have shown either normal or low bone formation but a unanimous bone mineralization defect (3,5,7,8,27,28).
The exact pathophysiological underlying mechanisms for altered bone remodeling in stone formers remain unknown, but increased production of cytokines by peripheral monocytes of hypercalciuric patients has been reported (29,30). At a bone level, we have previously shown a lower expression of TGF-β in bone immunostains from hypercalciuric stone formers (6), which might have contributed to the lower bone formation and delayed bone mineralization (31).
To address if a putative excess of FGF-23 could negatively regulate bone mineralization (10), the first purpose of the present study was to evaluate its bone expression. Bone FGF-23 from IH patients was not different from controls and did not correlate with histomorphometric parameters (nor in the control group). These findings differ from the findings of Pereira et al. (32), who observed bone FGF-23 expression to be inversely correlated with skeletal mineralization in CKD patients. However, given that renal function was not compromised in the present series, the comparison may be not suitable. Although a significant inverse association between serum phosphate and FGF-23 was reported in recurrent stone formers (9), the sample was not exclusively composed of hypercalciuric individuals. In our study, there were no significant correlations between FGF-23 bone immunostaining and serum phosphate or between PTH and vitamin D. The small number of hypophosphatemic patients in the current series might have accounted for the lack of such correlation.
Among the presumed mechanisms responsible for impaired bone formation in IH patients, elevated serum 1,25(OH)2D3 levels may play a role (11–14,33,34) through decreasing bone collagen synthesis (31) or increasing bone resorption (35). A greater increase in urinary calcium with 1,25D in GHS rats fed a low calcium diet has suggested that the additional urinary calcium must result from increased bone resorption because of the increased number of VDR in their bone cells (35). With a few exceptions (27,28), most of the previous histomorphometric studies have shown increased bone resorption among hypercalciuric patients (3,5,7,8). Vitamin D-induced osteoclastogenesis seems to be accomplished through a VDR-mediated increase in RANKL expression in osteoblasts (36).
In the present study, we did not observe an increase in bone expression of VDR in the total of stained bone cells from IH patients compared with controls. However, when we compared the patients who exhibited high versus normal bone resorption, VDR immunostaining was numerically higher but not significantly different in the former group (Figure 1). Moreover, a strong and significant correlation between bone expression of VDR and ES/BS was disclosed in the IH group as a whole, which was attributed to the marked VDR staining in osteoblasts, that was not evidenced in control group. Although we recognize that osteoclasts also express VDR (37), the reasons why we did not observe substantial VDR immunostaining in these cells remain unclear. When we tried to integrate present VDR bone expression with that of RANKL, osteoprotegerin, and TGF-β evidenced in our previous study (6), we found no correlation. However, it is noteworthy that, in the current sample, we could not retrieve sufficient amounts of tissue from six bone specimens derived from the material in the work by Gomes et al. (6), probably because of multiple cuts on the microtome and exposure to chemical reagents in prior immunohistochemical analysis. Unfortunately, all these specimens had originated from patients with high ES/BS, and therefore, it might have compromised the disclosure of a possible correlation between these parameters. In the present series, serum 1,25(OH)2D3 correlated with VDR immunostaining in osteoblasts from IH patients. These findings agree well with experimental data showing a 4-fold increase in the level of VDR in GHS calvarias versus controls and a greater sensitivity to 1,25(OH)2D3 compared with bone from control rats (17,35). The direct correlation that we have found between VDR and Tb.N in osteoblasts in IH further reinforces the contribution of VDR to increase bone resorption. Conversely, Tb.N was negatively correlated with VDR expression in controls. It has already been speculated that even small elevations of serum 1,25(OH)2D3 seem to be sufficient to increase bone resorption when dietary calcium intake is low, indicating that 1,25(OH)2D3 may upregulate its own receptor (14,38). A previous evaluation of stone-forming patients in our laboratory (39) revealed a very low mean calcium intake (around 500 mg/d), and most of the bone tissue samples presently analyzed had been retrieved from patients with up to 60% exhibiting low calcium intake (3,5). In the present study, we observed a trend for a direct association between VDR bone expression and mineralization lag time (r=0.36; P=0.06). Therefore, the increase in bone expression of VDR might have helped to further compromise bone mineralization in our IH patients, because the low calcium intake probably contributed to a negative calcium balance. Indeed, an experimental study by Lieben et al. (40) has shown that, when intestinal calcium absorption decreases, calcium mobilization from bone is elicited and bone matrix mineralization is suppressed because of decreased calcium incorporation in bone by osteoblastic VDR signaling that stimulates mineralization inhibitors.
The signaling pathways through which Scl exerts its effects on bone metabolism are still incompletely understood. Although Scl has emerged as a stimulator of osteoblasts apoptosis (18) and a potent inhibitor of Wnt–β-catenin signaling (hence, decreasing bone formation) (41), an experimental model in which the Wnt pathway was specifically inhibited in osteocytes has shown normal bone formation but a significant increase in osteoclastic bone resorption instead (42). The reduction of the antiosteoclastogenic factor osteoprotegerin (OPG) and the concomitant increase in the osteocytic RANKL/OPG ratio might have contributed to the increased number of osteoclasts and resorption in this model. Additional studies showing a severe osteopetrotic phenotype in mice lacking RANKL specifically in osteocytes (43) reinforced that osteocytes are the major source of RANKL in bone remodeling in vivo. The mechanism whereby Scl controls osteoclast formation and activity seems to occur through a direct increase in mRNA expression of RANKL in osteocytes to a greater extent than the decrease in OPG (19). It has been hypothesized that, in response to catabolic stimulus, Scl expressed by mature mineral embedded, or even apoptosing osteocytes, may act in an autocrine manner, increasing local expression of RANKL relative to OPG, as well as a paracrine manner, inducing such expression in immature osteocytes immediately below the osteoblastic lining cells (44).
In the current study, mean Scl bone immunostaining in the IH group did not differ from control but was significantly higher in patients with high versus normal bone resorption. Moreover, there has been a significantly positive correlation of Scl bone expression with ES/BS in the entire IH group, which was not observed in the controls. Interestingly, a significant negative correlation of Scl immunostaining with the mineralization surface was evidenced in the subgroup with high bone resorption (r=−0.63; P=0.02) but not in the group with normal bone resorption (r=0.50; P=0.18; data not shown), further suggesting that, within the IH group, the higher bone resorption and compromised mineralization exhibited by most of the patients are associated with bone expression of Scl. These findings agree well with a report about a more significant rise in bone resorption (CrossLaps) than formation (b-AP) in patients with immobilization-induced bone loss (45). Additionally, in a very recent randomized, placebo-controlled trial using a neutralizing antibody to Scl (Romosozumab) in postmenopausal women, the levels of serum β-isomer of the C-terminal telopeptide of collagen, a bone resorption marker, presented a significant and sustained reduction after 12 months, whereas the increase in procollagen N-terminal propeptide, a bone formation marker, was only transitory (46). We did not find an association between bone expression of Scl and RANKL from our previous study (6), but of note, the immunostaining had not been performed in osteocytes (6). At present, we did detect a significant positive correlation between Scl immunostaining and serum 1,25(OH)2D3 in IH patients, which is in accordance with literature data showing that 1,25(OH)2D3 may increase Scl expression in human osteoblastic cells (47). These findings led us to hypothesize that the increased bone resorption seen in some IH patients could be mediated by an increase in vitamin D signaling, stimulating Scl synthesis and leading to an imbalance in the OPG/RANKL ratio.
In the control group, Scl immunostaining correlated positively with BV/TV and Tb.N and negatively with Tb.Sp. Once again, given that Scl inhibits osteoblastic activity, a negative correlation would be expected. However, some studies have also found direct correlations between serum Scl and BMD (48,49) or BV/TV in healthy populations (50).
A limitation of the present study is that, given its retrospective design and the lack of available blood samples, serum levels of Scl (and FGF-23) could not be determined. Nevertheless, Durosier et al. (50) have shown that Scl levels are markedly different according to the immunoassay used, jeopardizing its interpretation. Although the current number of patients is relatively low, this study comprises one of the largest and substantial series in the literature for a bone biopsy study.
In summary, although other investigators had found increased VDR in monocytes (14), no other study in humans has hitherto evaluated VDR expression in bone tissue from IH patients and its relation with bone resorption. It is possible that a longer duration of disease might have contributed to increase bone resorption and influenced the immunohistochemical pattern presently disclosed.
In conclusion, present findings suggest the contribution of VDR and Scl to increase bone resorption in IH but do not implicate FGF-23 in the bone alterations seen in these patients. Additional studies are warranted to determine whether antiresorptive therapy will correct these bone abnormalities. Targeting VDR and Scl may be a new therapeutic approach to prevent bone loss in IH.
Disclosures
None.
Acknowledgments
We thank Susan Schiavi for providing fibroblast growth factor 23–antibody; Rosimeire A.B. Costa, Rita Martin, Luciene M. dos Reis, Fabiana G. Graciolli, and Wagner V. Dominguez for technical assistance; Maria Aparecida da Glória and Maria Teresa de Seixas Alves for invaluable suggestions on immunohistochemistry; and Ana M. Misael and Fellype C. Barreto for histomorphometric data.
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) Grant 08/10515-0.
Portions of this study were presented at the 50th European Dialysis and Transplant Association Meeting, May 18–21, 2013, in Istanbul, Turkey.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Worcester EM, Coe FL: New insights into the pathogenesis of idiopathic hypercalciuria. Semin Nephrol 28: 120–132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakhaee K, Maalouf NM, Kumar R, Pasch A, Moe OW: Nephrolithiasis-associated bone disease: Pathogenesis and treatment options. Kidney Int 79: 393–403, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heilberg IP, Martini LA, Szejnfeld VL, Carvalho AB, Draibe SA, Ajzen H, Ramos OL, Schor N: Bone disease in calcium stone forming patients. Clin Nephrol 42: 175–182, 1994 [PubMed] [Google Scholar]
- 4.Heilberg IP, Martini LA, Teixeira SH, Szejnfeld VL, Carvalho AB, Lobão R, Draibe SA: Effect of etidronate treatment on bone mass of male nephrolithiasis patients with idiopathic hypercalciuria and osteopenia. Nephron 79: 430–437, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Misael da Silva AM, dos Reis LM, Pereira RC, Futata E, Branco-Martins CT, Noronha IL, Wajchemberg BL, Jorgetti V: Bone involvement in idiopathic hypercalciuria. Clin Nephrol 57: 183–191, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Gomes SA, dos Reis LM, Noronha IL, Jorgetti V, Heilberg IP: RANKL is a mediator of bone resorption in idiopathic hypercalciuria. Clin J Am Soc Nephrol 3: 1446–1452, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steiniche T, Mosekilde L, Christensen MS, Melsen F: A histomorphometric determination of iliac bone remodeling in patients with recurrent renal stone formation and idiopathic hypercalciuria. APMIS 97: 309–316, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Heller HJ, Zerwekh JE, Gottschalk FA, Pak CY: Reduced bone formation and relatively increased bone resorption in absorptive hypercalciuria. Kidney Int 71: 808–815, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Rendina D, Mossetti G, De Filippo G, Cioffi M, Strazzullo P: Fibroblast growth factor 23 is increased in calcium nephrolithiasis with hypophosphatemia and renal phosphate leak. J Clin Endocrinol Metab 91: 959–963, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Yoshiko Y, Yamamoto R, Minamizaki T, Kozai K, Tanne K, Aubin JE, Maeda N: Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J Bone Miner Res 23: 939–948, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Insogna KL, Broadus AE, Dreyer BE, Ellison AF, Gertner JM: Elevated production rate of 1,25-dihydroxyvitamin D in patients with absorptive hypercalciuria. J Clin Endocrinol Metab 61: 490–495, 1985 [DOI] [PubMed] [Google Scholar]
- 12.Breslau NA, Preminger GM, Adams BV, Otey J, Pak CY: Use of ketoconazole to probe the pathogenetic importance of 1,25-dihydroxyvitamin D in absorptive hypercalciuria. J Clin Endocrinol Metab 75: 1446–1452, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Zerwekh JE, Reed BY, Heller HJ, González GB, Haussler MR, Pak CY: Normal vitamin D receptor concentration and responsiveness to 1, 25-dihydroxyvitamin D3 in skin fibroblasts from patients with absorptive hypercalciuria. Miner Electrolyte Metab 24: 307–313, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Favus MJ, Karnauskas AJ, Parks JH, Coe FL: Peripheral blood monocyte vitamin D receptor levels are elevated in patients with idiopathic hypercalciuria. J Clin Endocrinol Metab 89: 4937–4943, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Ferreira LG, Pereira AC, Heilberg IP: Vitamin D receptor and calcium-sensing receptor gene polymorphisms in hypercalciuric stone-forming patients. Nephron Clin Pract 114: c135–c144, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Li XQ, Tembe V, Horwitz GM, Bushinsky DA, Favus MJ: Increased intestinal vitamin D receptor in genetic hypercalciuric rats. A cause of intestinal calcium hyperabsorption. J Clin Invest 91: 661–667, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krieger NS, Stathopoulos VM, Bushinsky DA: Increased sensitivity to 1,25(OH)2D3 in bone from genetic hypercalciuric rats. Am J Physiol 271: C130–C135, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Moester MJC, Papapoulos SE, Löwik CWGM, van Bezooijen RL: Sclerostin: Current knowledge and future perspectives. Calcif Tissue Int 87: 99–107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ: Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS ONE 6: e25900, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dos Reis LM, Batalha JR, Muñoz DR, Borelli A, Correa PH, Carvalho AB, Jorgetti V: Brazilian normal static bone histomorphometry: Effects of age, sex, and race. J Bone Miner Metab 25: 400–406, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Melsen F, Mosekilde L: Tetracycline double-labeling of iliac trabecular bone in 41 normal adults. Calcif Tissue Res 26: 99–102, 1978 [DOI] [PubMed] [Google Scholar]
- 22.Gomes SA, dos Reis LM, de Oliveira IB, Noronha IL, Jorgetti V, Heilberg IP: Usefulness of a quick decalcification of bone sections embedded in methyl methacrylate [corrected]: An improved method for immunohistochemistry. J Bone Miner Metab 26: 110–113, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Heilberg IP, Weisinger JR: Bone disease in idiopathic hypercalciuria. Curr Opin Nephrol Hypertens 15: 394–402, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Melton LJ, 3rd, Crowson CS, Khosla S, Wilson DM, O’Fallon WM: Fracture risk among patients with urolithiasis: A population-based cohort study. Kidney Int 53: 459–464, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Lauderdale DS, Thisted RA, Wen M, Favus MJ: Bone mineral density and fracture among prevalent kidney stone cases in the Third National Health and Nutrition Examination Survey. J Bone Miner Res 16: 1893–1898, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Grynpas M, Waldman S, Holmyard D, Bushinsky DA: Genetic hypercalciuric stone-forming rats have a primary decrease in BMD and strength. J Bone Miner Res 24: 1420–1426, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Vernejoul MC, Hioco D, Villiaumey J, Chanzy MO, Voisin MC, Canalia AM: Bone histomorphometric study in idiopathic hypercalciuria. Rev Rhum Mal Osteoartic 48: 389–395, 1981 [PubMed] [Google Scholar]
- 28.Malluche HH, Tschoepe W, Ritz E, Meyer-Sabellek W, Massry SG: Abnormal bone histology in idiopathic hypercalciuria. J Clin Endocrinol Metab 50: 654–658, 1980 [DOI] [PubMed] [Google Scholar]
- 29.Weisinger JR, Alonzo E, Bellorín-Font E, Blasini AM, Rodriguez MA, Paz-Martínez V, Martinis R: Possible role of cytokines on the bone mineral loss in idiopathic hypercalciuria. Kidney Int 49: 244–250, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Pacifici R, Rothstein M, Rifas L, Lau KH, Baylink DJ, Avioli LV, Hruska K: Increased monocyte interleukin-1 activity and decreased vertebral bone density in patients with fasting idiopathic hypercalciuria. J Clin Endocrinol Metab 71: 138–145, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Raisz LG, Kream BE, Smith MD, Simmons HA: Comparison of the effects of vitamin D metabolites on collagen synthesis and resportion of fetal rat bone in organ culture. Calcif Tissue Int 32: 135–138, 1980 [DOI] [PubMed] [Google Scholar]
- 32.Pereira RC, Jüppner H, Azucena-Serrano CE, Yadin O, Salusky IB, Wesseling-Perry K: Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone 45: 1161–1168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broadus AE, Insogna KL, Lang R, Ellison AF, Dreyer BE: Evidence for disordered control of 1,25-dihydroxyvitamin D production in absorptive hypercalciuria. N Engl J Med 311: 73–80, 1984 [DOI] [PubMed] [Google Scholar]
- 34.Coe FL, Favus MJ, Crockett T, Strauss AL, Parks JH, Porat A, Gantt CL, Sherwood LM: Effects of low-calcium diet on urine calcium excretion, parathyroid function and serum 1,25(OH)2D3 levels in patients with idiopathic hypercalciuria and in normal subjects. Am J Med 72: 25–32, 1982 [DOI] [PubMed] [Google Scholar]
- 35.Frick KK, Asplin JR, Krieger NS, Culbertson CD, Asplin DM, Bushinsky DA: 1,25(OH)2D3 - Enhanced hypercalciuria in genetic hypercalciuric stone-forming rats fed a low calcium diet. Am J Physiol Renal Physiol 305: F1132–F1138, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto Y, Yoshizawa T, Fukuda T, Shirode-Fukuda Y, Yu T, Sekine K, Sato T, Kawano H, Aihara K, Nakamichi Y, Watanabe T, Shindo M, Inoue K, Inoue E, Tsuji N, Hoshino M, Karsenty G, Metzger D, Chambon P, Kato S, Imai Y: Vitamin D receptor in osteoblasts is a negative regulator of bone mass control. Endocrinology 154: 1008–1020, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Ryan JW, Reinke D, Kogawa M, Turner AG, Atkins GJ, Anderson PH, Morris HA: Novel targets of vitamin D activity in bone: Action of the vitamin D receptor in osteoblasts, osteocytes and osteoclasts. Curr Drug Targets 14: 1683–1688, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Maierhofer WJ, Gray RW, Cheung HS, Lemann J, Jr.: Bone resorption stimulated by elevated serum 1,25-(OH)2-vitamin D concentrations in healthy men. Kidney Int 24: 555–560, 1983 [DOI] [PubMed] [Google Scholar]
- 39.Martini LA, Cuppari L, Colugnati FA, Sigulem DM, Szejnfeld VL, Schor N, Heilberg IP: High sodium chloride intake is associated with low bone density in calcium stone-forming patients. Clin Nephrol 54: 85–93, 2000 [PubMed] [Google Scholar]
- 40.Lieben L, Masuyama R, Torrekens S, Van Looveren R, Schrooten J, Baatsen P, Lafage-Proust MH, Dresselaers T, Feng JQ, Bonewald LF, Meyer MB, Pike JW, Bouillon R, Carmeliet G: Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J Clin Invest 122: 1803–1815, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaffler MB, Kennedy OD: Osteocyte signaling in bone. Curr Osteoporos Rep 10: 118–125, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB, Feng JQ, Bonewald LF, Kneissel M: Osteocyte Wnt/β-catenin signaling is required for normal bone homeostasis. Mol Cell Biol 30: 3071–3085, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H: Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17: 1231–1234, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Atkins GJ, Findlay DM: Osteocyte regulation of bone mineral: A little give and take. Osteoporos Int 23: 2067–2079, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Gaudio A, Pennisi P, Bratengeier C, Torrisi V, Lindner B, Mangiafico RA, Pulvirenti I, Hawa G, Tringali G, Fiore CE: Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab 95: 2248–2253, 2010 [DOI] [PubMed] [Google Scholar]
- 46.McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG: Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 370: 412–420, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Sutherland MK, Geoghegan JC, Yu C, Winkler DG, Latham JA: Unique regulation of SOST, the sclerosteosis gene, by BMPs and steroid hormones in human osteoblasts. Bone 35: 448–454, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Amrein K, Amrein S, Drexler C, Dimai HP, Dobnig H, Pfeifer K, Tomaschitz A, Pieber TR, Fahrleitner-Pammer A: Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults. J Clin Endocrinol Metab 97: 148–154, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Polyzos SA, Anastasilakis AD, Bratengeier C, Woloszczuk W, Papatheodorou A, Terpos E: Serum sclerostin levels positively correlate with lumbar spinal bone mineral density in postmenopausal women—the six-month effect of risedronate and teriparatide. Osteoporos Int 23: 1171–1176, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Durosier C, van Lierop A, Ferrari S, Chevalley T, Papapoulos S, Rizzoli R: Association of circulating sclerostin with bone mineral mass, microstructure, and turnover biochemical markers in healthy elderly men and women. J Clin Endocrinol Metab 98: 3873–3883, 2013 [DOI] [PubMed] [Google Scholar]