Abstract
Integrins are a large family of transmembrane receptors that, in addition to mediating cell adhesion, modulate cell proliferation. The β1C integrin is an alternatively spliced variant of the β1 subfamily that contains a unique 48–amino acid sequence in its cytoplasmic domain. We have shown previously that in vitro β1C inhibits cell proliferation and that in vivo β1C is expressed in nonproliferative, differentiated epithelium and is selectively downregulated in prostatic adenocarcinoma. Here we show, by immunohistochemistry and immunoblotting analysis, that β1C is coexpressed in human prostate epithelial cells with the cell-cycle inhibitor p27kip1, the loss of which correlates with poor prognosis in prostate cancer. In the 37 specimens analyzed, β1C and p27kip1 are concurrently expressed in 93% of benign and 84%–91% of tumor prostate cells. Forced expression of β1C in vitro is accompanied by an increase in p27kip1 levels, by inhibition of cyclin A–dependent kinase activity, and by increased association of p27kip1 with cyclin A. β1C inhibitory effect on cell proliferation is completely prevented by p27kip1 antisense, but not mismatch oligonucleotides. β1C expression does not affect either cyclin A or E levels, or cyclin E–associated kinase activity, nor the mitogen-activated protein (MAP) kinase pathway. These findings show a unique mechanism of cell growth inhibition by integrins and point to β1C as an upstream regulator of p27kip1 expression and, therefore, a potential target for tumor suppression in prostate cancer.
Introduction
Integrins are a large family of transmembrane receptors composed of an α and a β subunit that, in addition to mediating cell adhesion to the extracellular matrix (ECM), have been shown to regulate cell growth, survival, and differentiation (1, 2). Considerable effort has been devoted to elucidate the intracellular signaling events modulated by integrins, in particular the activation of intracellular protein kinases, including members of the mitogen-activated protein (MAP) kinase family (3–5). The MAP kinase family is composed of serine/threonine kinases that, in addition to integrin engagement, are activated by mitogens and modulate gene expression (4) and, ultimately, cell proliferation.
The β1C integrin is an alternatively spliced variant of the β1 subfamily that contains a unique 48–amino acid sequence in its cytoplasmic domain (6). In vivo, β1C is expressed in nonproliferative and differentiated epithelium (7). In the prostate, β1C is found in benign glandular epithelial cells and is selectively downregulated in adenocarcinoma (8). Previous studies have shown that forced expression of either β1C or its cytoplasmic domain inhibits proliferation of both tumorigenic (specifically PC3 prostate cancer and Chinese hamster ovary [CHO]) and nontumorigenic (specifically 10T1/2) cells (7, 9, 10) without affecting cytoskeletal or focal adhesion organization (10).
It is well established that cell-cycle progression is regulated by cyclin-dependent kinases (CDKs) (11), whose activity is controlled by cyclin binding, phosphorylation/dephosphorylation, and association with a group of CDK-inhibitory proteins, designated CKIs (12). A member of a CKI family, p27kip1, controls cell-cycle progression by specific binding to cyclin D-, E-, and A-CDK complexes. This inhibitor is highly expressed in nonproliferative, quiescent cells and its levels are increased by growth-inhibitory signals (12). Furthermore, its forced overexpression is sufficient to inhibit cell proliferation (12). The pathophysiological relevance of p27kip1 regulated expression is suggested by recent studies showing that in prostate cancer, as well as in breast or colorectal carcinomas, loss of p27kip1 is an adverse prognostic factor that correlates with poor patient survival (13–17).
Several studies have shown that cell adhesion to the ECM is required for cell-cycle progression and proliferation in different cell types (18). Loss of cell anchorage to the ECM recently has been shown to upregulate the expression of p27kip1 and p21cip1/waf1, while at the same time decreasing the levels of cyclin A (19–21). Some studies have also indicated that the expression of cyclin D1 and E is adhesion dependent (19, 21, 22). Anchorage is also required for cyclin E-CDK2 and cyclin D-CDK4/6 activities (20, 21, 23). Changes of p27kip1, p21cip1/waf1, and cyclin A expression levels, as well as cyclin E-CDK2 activity, were also observed in response to structural alterations of collagen matrices and consequent intracellular modifications of cytoskeleton and focal adhesion sites (24). Overall, these studies show that control of cell-cycle molecule expressions and activities is mediated by adhesion- and spreading-dependent events. At this time, however, modulation by integrins of either p27kip1 expression or CDK activities in the absence of changes in cell adhesion or spreading has never been shown.
In this study, we show that in vivo β1C and p27kip1 expressions are concurrently downregulated in neoplastic prostate epithelial cells, thus describing for the first time an in vivo correlation of expression of integrins and a cell-cycle inhibitor. We hypothesized that β1C may function as an upstream regulator of specific CKIs and would increase p27kip1 levels to inhibit cell proliferation. We show that in vitro forced expression of β1C is accompanied by an increase in p27kip1 levels and in its association with cyclin A, and by selective inhibition of cyclin A–dependent, but not cyclin E–dependent, kinase activity. Moreover, p27kip1 antisense, but not mismatch, oligonucleotides prevented inhibition of cell proliferation observed in β1C transfected cells. The study also shows that neither cyclin A nor E expressions, nor the Ras/MAP kinase pathway are affected. These data describe a unique mechanism of cell growth inhibition by integrins and point to β1C as an upstream regulator of p27kip1 expression and, therefore, a target molecule for tumor suppression in prostate cancer.
Methods
Reagents.
Rabbit affinity-purified antibodies specific for the β1C subunit cytoplasmic domain were generated and affinity-purified as described previously (8). The following antibodies were used: mouse monoclonal antibodies (MABs) to p27kip1 and to p130Cas (Transduction Laboratories, Lexington, Kentucky, USA); to β-tubulin (Sigma Chemical Co., St. Louis, Missouri, USA); Ha2/5 to rat β1 integrin (PharMingen, San Diego, California, USA); TS2/16 to human β1 integrin extracellular domain purchased from American Type Culture Collection (Rockville, Maryland, USA) and a kind gift of M.E. Hemler (Dana-Farber Cancer Institute, Boston, Massachusetts, USA); and 12CA5 to hemagglutinin (American Type Culture Collection). Also used were: rabbit affinity-purified antibodies to cyclin E, to cyclin A, and to extracellular signal-regulated kinase-1 and -2 (ERK-1 and -2; Santa Cruz Biotechnology Inc., Santa Cruz, California, USA); rabbit antisera to cyclin A and to CDK2, kindly provided by H. Zhang (Yale University, New Haven, Connecticut, USA); and nonimmune rabbit IgG (Sigma Chemical Co.). Human plasma fibronectin was purified by affinity chromatography on gelatin-Sepharose (25). Human vitronectin was purchased from Life Technologies Inc. (Gaithersburg, Maryland, USA).
Tissue specimens and immunohistochemistry.
Specimens from 37 radical prostatectomies, performed for prostatic adenocarcinoma at the Yale-New Haven Hospital (New Haven, Connecticut, USA), were included in this study according to a protocol approved by Yale University School of Medicine Review Board. Hematoxylin and eosin–stained sections were reviewed, and sections showing both neoplastic and benign prostate tissue were selected for evaluation of β1C and p27kip1 immunoreactivity. Serial sections from paraffin-embedded and formalin-fixed tissue specimens stained previously using antibodies to β1C were selected (8). Single-labeling experiments were performed as described previously (7, 8). For double-labeling experiments, tissue sections were first stained using MAB to p27kip1 and then treated sequentially with a biotinylated horse anti–mouse secondary antibody (Vector Laboratories, Burlingame, California, USA) and peroxidase-labeled streptavidin (Boehringer Mannheim, Indianapolis, USA). Development of peroxidase activity was achieved using 3,3′-diaminobenzidine tetrahydrochloride dehydrate (Sigma Chemical Co.) as chromogen. After p27kip1 immunodetection, tissue sections were rinsed in distilled water and stained sequentially with rabbit affinity-purified antibody to β1C, a biotinylated goat anti–rabbit secondary antibody (Vector Laboratories), and the alkaline phosphatase–labeled streptavidin (Kirkegaard & Perry Laboratories Inc., Gaithersburg, Maryland, USA), followed by alkaline phosphatase substrate kit III (Vector Laboratories). The slides were then rinsed in distilled water and mounted using Aqua Mount (Lerner Laboratories, Pittsburgh, Pennsylvania, USA) without dehydration.
Immunostaining evaluation.
Adjacent areas of neoplastic and benign prostate tissue from the same section were evaluated essentially as described (13, 14). The β1C and p27kip1 immunoreactivity, in double-staining experiments, was assessed independently by three investigators (M. Fornaro, G. Tallini, and L.R. Languino) and scoring was performed in a blinded manner. Five high-power fields were randomly chosen and scored for the percentage of cells either showing or lacking β1C and p27kip1 staining; a minimum of 300 cells per specimen were evaluated. β1C and p27kip1 expression in benign or neoplastic cells was scored as positive (+) if more than 30% of the cells were stained and as negative (–) if less than 30% of the cells were stained. The percentage of cells that showed correlation of β1C and p27kip1 expression was calculated as the ratio of number of cells either expressing or lacking both molecules/total cell number counted in five fields (×100). There was 98% concordance among the observers’ scores; in one instance, because of disagreement among the observers, the specimen was discarded. Statistical analysis was performed using Fisher's exact test. In double-staining experiments, hematoxylin counterstain was not used.
Cells and transfections.
Normal nonimmortalized rat prostate epithelial cells, NRP152 (26), were maintained in DMEM-F12 (Life Technologies Inc.) supplemented with 5% FCS (Gemini Bioproducts Inc., Calabasas, California, USA), 2 mM glutamine (Gemini Bioproducts Inc.), 20 ng/ml epidermal growth factor (EGF) (R&D Systems Inc., Minneapolis, Minnesota, USA), 5 μg/ml insulin (Sigma Chemical Co.), 0.1 μM dexamethasone (Sigma Chemical Co.), and 10 ng/ml cholera toxin (Sigma Chemical Co.). CHO cells (American Type Culture Collection) were maintained in DMEM (Life Technologies Inc.) supplemented with 10% FCS, 2 mM glutamine, 100 μg/ml streptomycin, 100 U/ml penicillin (Gemini Bioproducts Inc.), and 0.1 mM nonessential amino acids (Life Technologies Inc.).
The tetracycline-regulated expression system, designed for the inducible expression of exogenous proteins in mammalian cells, consists of two plasmids: the pTet-tTA plasmid contains the neomycin-resistance gene and encodes a transcriptional transactivator (tTA) that drives expression of itself and a target gene inserted into the multiple cloning site of the second plasmid, pTet-Splice (27). To obtain stable transfectants expressing β1C in a tetracycline-regulated system, ClaI-XbaI fragment–encoding full-length human β1C was isolated from Bluescript-β1C (9) and subcloned into ClaI-SpeI sites in the pTet-Splice plasmid, a kind gift of D. Schatz (Yale University), to generate the pTet-β1C construct. NRP152 cells were electroporated using a Genepulser apparatus (Bio-Rad Laboratories Inc., Hercules, California, USA) set at 300 V and 950 μF, using either 20 μg pTet-β1C or pTet-splice, along with 10 μg pTet-tTA. Neomycin-resistant cells were selected using medium containing 0.56 mg/ml G418 (Life Technologies Inc.). G418-resistant clones were isolated in two rounds and screened for cell surface expression of β1C integrin by FACS®, using TS2/16, MAB against human β1 integrin, or 12CA5 MAB, as a negative control, as described (9). Stable transfectants were maintained in growth medium containing 1 μg/ml tetracycline (Boehringer Mannheim) and 0.1 mg/ml G418.
The CHO cells were transfected as described above, either with the pTet-β1C construct or the pTet-splice, along with the pTet-tTA plasmid by electroporation, using a Genepulser apparatus set at 350 V and 950 μF. Neomycin-resistant cells were selected using medium containing 1.4 mg/ml G418. G418-resistant clones were isolated in two rounds and screened for cell surface expression of the human β1C integrin by FACS® analysis, and stable transfectants were maintained as described above.
The CHO cells were also transiently transfected by electroporation using pBJ1-β1C, or pBJ1-β1A, or pBJ1 vector alone, and surface expression of the transfected β1C or β1A integrins was evaluated by FACS® analysis as described (9).
CHO cell adhesion to fibronectin (3 μg/ml), vitronectin (30 μg/ml), BSA (10 mg/ml; Sigma Chemical Co.), and TS2/16 (1:10 dilution of culture supernatant) was performed as described previously (28) using [51Cr]-labeled (Du Pont Nen Research Products, Wilmington, Delaware, USA) cells.
Immunoblotting, immunoprecipitation, and in vitro kinase assay.
NRP152 transfectants were cultured for 72 h in the absence of tetracycline, then cells were detached with 0.05% trypsin/0.53 mM EDTA (Life Technologies Inc.), washed three times, and resuspended in serum-free medium. To engage β1 integrins, NRP152-β1C transfectants were seeded on tissue culture dishes coated with TS2/16, whereas NRP152–mock transfectants were seeded on tissue culture dishes coated with Ha2/5 for 1 h at 37°C, washed three times with serum-free medium, and cultured for 20 h in growth medium. Cells were then lysed, and p27kip1 expression was evaluated by immunoblotting as described below.
To detect cyclin E, cyclin A, CDK2 or p27kip1 stable NRP152 or CHO cell transfectants were lysed with Nonidet P-40 (NP-40) lysis buffer: with 0.5% NP-40 (Calbiochem, San Diego, California, USA), 150 mM NaCl, 50 mM HEPES (pH 7.5), 10% glycerol, 0.1 mM sodium vanadate (Sigma Chemical Co.), 1 mM sodium fluoride (Sigma Chemical Co.), 1 mM PMSF (Life Technologies Inc.), 10 μg/ml aprotinin (Sigma Chemical Co.), 10 μg/ml leupeptin (Calbiochem) for 30 min at 4°C (29). Transiently transfected CHO cells were lysed in 0.1% Tween-20 (American Bioanalytical, Natick, Massachusetts, USA), 150 mM NaCl, 50 mM HEPES (pH 7.5), 10% glycerol, 1 mM EDTA, 2.5 mM EGTA, 0.1 mM sodium vanadate, 1 mM sodium fluoride, 1 mM DTT (Bio-Rad Laboratories Inc.), 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and sonicated (30). Similar results were observed using either NP-40 or Tween-20 lysis buffers (29, 30). To analyze β1C and p27kip1 expression in the prostate, either benign or tumor frozen tissue specimens obtained from radical prostatectomies were homogenized in lysis buffer containing 100 mM Tris, pH 7.5, 150 mM NaCl, 0.1% Triton X-100 (Sigma Chemical Co.), 5% SDS, 1 mM PMSF, 10 μg/ml leupeptin, 1 mM benzamidine (Sigma Chemical Co.), 1 μM D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK) (Boehringer Mannheim), 10 μg/ml soybean trypsin inhibitor (Life Technologies Inc.), using an OMNI 2000 homogenizer (OMNI International Inc., Gainesville, Virginia). The protein content in each lysate was quantitated using the bicinchoninic acid (BCA) protein assay reagent (Pierce Chemical Co., Rockford, Illinois, USA). Immunoblotting of cyclin E, cyclin A, CDK2, p27kip1, and tubulin was performed as described (31); immunoblotting of β1C integrin was carried out as described (8).
Cyclin A or cyclin E were immunoprecipitated (31), and the associated kinase activities were assayed as described (30), using 10 μCi of [γ-32P]ATP (3000 Ci/mmol; Amersham Life Sciences Inc., Arlington Heights, Illinois, USA) and 33 μg/ml histone H1 (Life Technologies Inc.) as a substrate. Phosphorylated histone H1 was observed using autoradiography.
The association of cyclin A with p27kip1 was detected by immunoprecipitation with rabbit antiserum to cyclin A, followed by immunoblotting with MAB to p27kip1, as described (31). In each immunoprecipitation, the applied amounts of cyclin A from each lysate were comparable, as evaluated by densitometric analysis.
In all instances, quantification of immunoreactive bands was performed by densitometric analysis; the values were normalized for protein loading and reported as mean ± SEM. Group differences were compared using Student's t test .
Oligonucleotide treatment and proliferation assay.
CHO-β1C cell transfectants (3.5 × 103) were plated on tissue culture plates in growth medium containing 1 μg/ml tetracycline. After approximately 24 h, tetracycline was removed to induce β1C expression. A mixture of 30 nM oligonucleotides (Gilead Sciences, Foster City, California, USA) (32) and 5 μg/ml GS3815 cytofectin (Gilead Sciences) in OPTI-MEM I (Life Technologies Inc.) was incubated for 15 min at room temperature and added to CHO cell transfectants for 24 h. Cells were rinsed three times with PBS and cultured for 48 h at 37°C, either in the absence or in the presence of 10% FCS, and pulsed with 1 μCi [3H]thymidine per well (5.0 Ci/mmol; Amersham Life Sciences Inc.) during the last 3 h of the 48-h culture. [3H]thymidine incorporation was evaluated as described (9). In each experiment, duplicate or triplicate observations were performed, and the values are reported as mean ± SEM. Group differences were compared using one-way analysis of variance.
By using FITC-labeled oligonucleotides as described (32), we determined that 90%–95% of the cells were positive for oligonucleotide uptake. The sequences of the antisense and mismatch p27kip1 C-5-propyne-modified phosphorothioates used were 5′-UGGCUCUCCUGCGCC-3′ and 5′-UCCCUUUGGCGCGCC-3′, respectively (32).
MAP kinase mobility shift and immunocomplex kinase assay.
Serum-starved CHO cells, transiently transfected either with β1C or β1A integrins, were detached with 0.05% trypsin/0.53 mM EDTA. After trypsin neutralization by 1 mg/ml soybean trypsin inhibitor, cells were washed twice and resuspended in serum-free medium containing 2% BSA. Cells were incubated for 30 min at 37°C, then kept in suspension or plated on tissue culture plates coated with TS2/16, antibody to β1 integrin, either in the absence or in the presence of 1% FCS, or with 10 μg/ml fibronectin in the absence of FCS for 10 min at 37°C. Cell lysis, immunoblotting, and kinase assay were carried out as described (33).
Results
Concurrent expression of β1C and p27kip1 in prostatic adenocarcinoma.
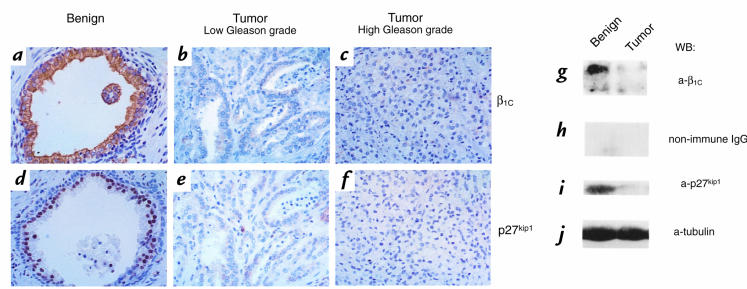
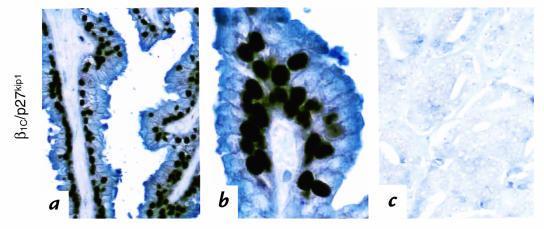
Expression of β1C and p27kip1 was examined by immunohistochemistry and immunoblotting in 37 specimens resected for prostatic adenocarcinoma (Figs. 1 and 2; Table 1). Marked expression of both β1C and, in the nuclei, of p27kip1 was consistently observed in benign glandular epithelial cells (Fig. 1, a and d); whereas downregulation of both molecules was observed in neoplastic tissue (Fig. 1, b, c, e, and f). Double-staining experiments showed a very high correlation of β1C and p27kip1 expression in 93% of benign cells (Fig. 2, a and b; Table 1) and in 84%–91% of neoplastic cells of the 37 specimens analyzed (Fig. 2c and Table 1). Both β1C and p27kip1 were downregulated in tumor areas compared with benign counterparts in 24 cases (75% of the specimens analyzed). They were coexpressed in benign and tumor areas in 7 cases (22% of the specimens analyzed). In only one instance did their expression not correlate (3% of the specimens analyzed; Table 1). Among the 24 specimens showing downregulation of both β1C and p27kip1, two were selected for immunoblotting analysis. The results confirmed downregulation of both molecules in the lysates from both neoplastic tissues compared with their benign counterparts (Fig. 1, g and i; data not shown).
Figure 1.
(a–j) Downregulation of β1C and p27kip1 expression in prostatic adenocarcinoma. The expression of β1C and p27kip1 in a representative case of benign (a and d) or neoplastic (b, c, e, and f) prostate tissue was evaluated by immunohistochemistry (a–f) using 1.8 μg/ml affinity-purified antibody to β1C (a–c) or 0.6 μg/ml monoclonal antibody to p27kip1 (d–f), and by immunoblotting (g–j). Tumor or benign prostate tissue detergent extracts were electrophoresed on 10% SDS-PAGE under reducing conditions, transferred to nitrocellulose membranes, and immunostained using 2 μg/ml affinity-purified antibody to β1C (g), 2 μg/ml nonimmune rabbit IgG as negative control for β1C (h), or 0.8 μg/ml monoclonal antibody to p27kip1 (i). Monoclonal antibody to tubulin was used to control for protein loading (j). Proteins were viewed by ECL. ECL, enhanced chemiluminescence. The results show that β1C and p27kip1 expressions correlate in benign and neoplastic prostate.
Figure 2.
(a–c) Coexpression of β1C and p27kip1 in benign and neoplastic prostate tissue. Double staining for β1C and p27kip1 is shown in a representative case of benign (a and b) or intermediate Gleason's score neoplastic (c) prostate tissue (blue and dark brown staining for β1C and p27kip1, respectively). Immunohistochemical analysis was performed as in Fig. 1. Hematoxylin counterstain was not used. The results show that β1C and p27kip1 expressions correlate in benign and neoplastic prostate.
Table 1.
Correlation of β1C and p27kip1 expression in benign and neoplastic prostate
Forced β1C expression is accompanied by increased levels of p27kip1.
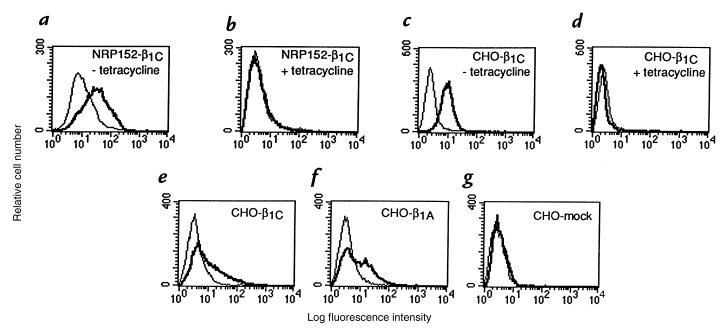
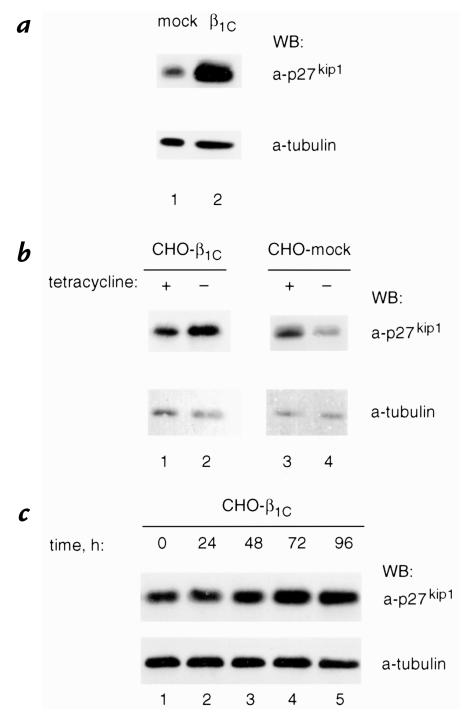
On the basis of these observations, we hypothesized that β1C might have a causal role in regulating p27kip1 levels in vitro. To test this hypothesis, we generated NRP152 or CHO transfectants expressing human β1C under the control of a tetracycline-regulated promoter. β1C expression in NRP152 (Fig. 3, a and b) or CHO (Fig. 3, c and d) cells was analyzed by fluorescence-activated cell sorter (FACS®) using either TS2/16, MAB to human β1 integrin (which does not cross-react with either rat or hamster β1 integrin), or 12CA5 as a negative control; in both cell types, maximal β1C expression was obtained 72 hours after tetracycline removal (not shown). NRP152-β1C stable transfectants were detached and seeded on tissue culture plates coated with TS2/16, whereas mock-transfected cells were plated on Ha2/5, MAB to rat β1 integrin. Immunoblotting analysis showed an increase of p27kip1 expression in NRP152-β1C stable transfectants compared with mock-transfected cells (10.9 ± 2.6-fold increase in two experiments; Fig. 4 a, top). Similar results were confirmed using CHO-β1C stable transfectants. In these experiments, cells were not detached and were allowed to reattach to monoclonal antibodies to β1 integrins, but they were lysed in the tissue culture plate. In these cells, we observed a 4.65 ± 0.65-fold increase in three experiments 72 hours after removal of tetracycline (Fig. 4 b, compare lanes 1 and 2; top) whereas mock-transfected cells showed no increase in the absence of tetracycline (1.28 ± 0.41-fold increase in three experiments; Fig. 4 b, compare lanes 3 and 4; top). The increase of p27kip1 levels in response to β1C expression was time dependent and was observed as early as 48 hours after withdrawal of tetracycline (Fig. 4 c, lane 3), with a maximum at 72 hours (Fig. 4 c, lane 4). The results show that β1C expression is accompanied by increased levels of p27kip1 in both nontumorigenic as well as tumorigenic cells.
Figure 3.
(a–g) Exogenous expression of β1C in NRP152 and CHO cell transfectants. Stable cell transfectants expressing β1C were generated using a tetracycline-regulated expression system. NRP152-β1C or CHO-β1C stable cell transfectants were cultured for 72 h, either in the absence (a and c) or in the presence (b and d) of 1 μg/ml tetracycline and analyzed by FACS® using TS2/16, MAB to human β1 integrin, or 12CA5 as a negative control, followed by FITC-goat anti–mouse IgG. Fluorescence intensity is expressed in arbitrary units. FACS® analysis of a representative β1C clone is shown. CHO cells were transiently transfected using pBJ1-β1C (e), or pBJ1-β1A (f), or pBJ-1 vector (g), and after 44 h, cells were stained and analyzed as described above. Thick line, TS2/16; thin line, 12CA5. MAB, monoclonal antibody.
Figure 4.
β1C expression is accompanied by increased p27kip1 protein levels in NRP152 and CHO cells. (a) NRP152-β1C (lane 2) or NRP152-β1C-mock (lane 1) stable cell transfectants were cultured for 72 h in the absence of tetracycline, detached, and NRP152-β1C was seeded on tissue culture dishes coated with TS2/16, whereas NRP152-mock transfectants were seeded on tissue culture dishes coated with Ha2/5 for 1 h at 37°C, washed three times with serum-free medium, and cultured for 20 h in growth medium. Cells were then lysed and p27kip1 expression levels were evaluated by immunoblotting using 0.8 μg/ml MAB to p27kip1 (top). (b and c) CHO-β1C (b, lanes 1 and 2; c) or CHO-β1C-mock (b, lanes 3 and 4) stable cell transfectants were cultured for 72 h in b and for the indicated times in c, either in the absence (b, lanes 2 and 4; c) or in the presence (b, lanes 1 and 3) of 1 μg/ml tetracycline. In these experiments cells were not detached and allowed to reattach to MAB to β1 integrins, but were lysed in the tissue culture plate, and p27kip1 expression levels were evaluated as described in a. The experiments were repeated three times using two different β1C clones with similar results. Group differences were compared using Student's t test. The differences in p27kip1 expression levels in CHO-β1C, but not in CHO-mock transfectants in the presence or in the absence of tetracycline, are statistically significant (P = 0.03). Control for protein loading was provided by MAB to tubulin (a–c, bottom). Proteins were viewed by ECL. Time refers to the length of time in absence of tetracycline.
Selective inhibition of cyclin A–dependent kinase activity and increased p27kip1 association with cyclin A in β1C transfectants.
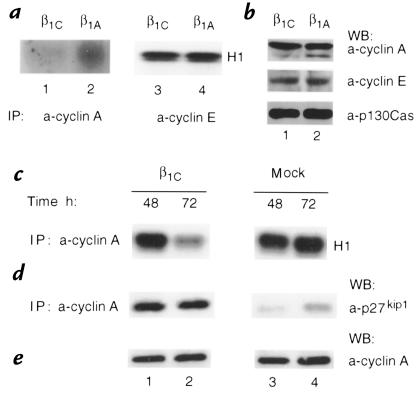
Because p27kip1 binds to and inhibits the activity of cyclin-CDK complexes (12), we investigated whether specific cyclin-associated kinase activities were inhibited in β1C transfectants. Either transient transfectants or two independent stable clones expressing β1C were used as controls for potential clonal variability. Cyclin A or cyclin E were immunoprecipitated from CHO cell lysates transiently expressing β1C or β1A integrins (Fig. 3, e and f), and the complexes were tested for their ability to phosphorylate histone H1. Cyclin A–associated kinase activity was reduced (53 ± 3% decrease in five experiments) in β1C transfectants as compared with β1A transfectants (Fig. 5a, compare lanes 1 and 2), whereas cyclin E–associated kinase activity was unaffected (1.5 ± 0.98% increase in two experiments; Fig. 5a, lanes 3 and 4). A 70.5 ± 1.5% decrease in two experiments in cyclin A–associated kinase activity was also observed in stable transfectants upon maximal induction of β1C expression at 72 hours (Fig. 5c, lane 2), whereas no effect was seen in mock-transfected cells (Fig. 5c, lane 4). A strong inhibition of cyclin A–associated kinase activity was also observed in NRP152-β1C transfectants (data not shown). Immunoblotting analysis of cyclin E, cyclin A, and CDK2 showed that β1C expression had no effect on the levels of these proteins (Fig. 5b, top and middle, e; data not shown).
Figure 5.
Cyclin A–associated kinase activity is inhibited in CHO-β1C cell transfectants. (a) CHO cells were transiently transfected using pBJ1-β1C (lanes 1 and 3) or pBJ1-β1A (lanes 2 and 4). Total cell lysates, obtained as described in Methods, were immunoprecipitated using 1 μg/ml rabbit affinity-purified antibodies to cyclin A (lanes 1 and 2) or to cyclin E (lanes 3 and 4), and the associated kinase activity was assayed in vitro using histone H1 as a substrate. Phosphorylated histone H1 was observed by autoradiography. The experiments were repeated two to five times with consistent results. (b) β1C expression does not affect cyclin E or cyclin A protein levels. Total cell lysates, obtained as described in a, were immunoblotted with 1 μg/ml rabbit affinity-purified antibodies to cyclin A (top) or to cyclin E (middle). Control for protein loading was provided by MAB to p130Cas (bottom). The experiments were repeated at least twice with consistent results. (c–e) CHO-β1C (lanes 1 and 2) or CHO-mock (lanes 3 and 4) stable cell transfectants were cultured in the absence of tetracycline for the indicated times. In c, total cell lysates were immunoprecipitated using rabbit antiserum to cyclin A, and the associated kinase activity was assayed as described above. The experiments were repeated twice using two different β1C clones with similar results. (d) Expression of β1C is accompanied by increased p27kip1 association with cyclin A. Total cell lysates were immunoprecipitated using rabbit antiserum to cyclin A, and the associated p27kip1 was detected by immunoblotting. (e) Total cell lysates were immunoblotted with rabbit affinity-purified antibody to cyclin A, as described above. In d and e, the experiments were repeated three times using two different β1C clones with similar results. In b, d, and e, proteins were viewed by ECL. Group differences were compared using Student's t test. In a and c, the differences between cyclin A-CDK activity in CHO-β1C versus CHO-β1A transfectants are statistically significant (in a, P = 0.0001; in c, P = 0.0069).
Because cyclin A–associated kinase activity can be inhibited by increased association of p27kip1 to cyclin A-CDK complexes, we analyzed the amount of p27kip1 associated with these complexes in β1C transfectants. Comparable amounts of cyclin A were immunoprecipitated from total cell lysates of either β1C or mock-stable transfectants cultured in the absence of tetracycline for 48 or 72 hours, and p27kip1 association was analyzed by immunoblotting. A substantial increase in the amount of p27kip1 associated with cyclin A was found in β1C transfectants (Fig. 5d, lanes 1 and 2) versus mock-transfected cells (Fig. 5 d, lanes 3 and 4) at both 48 and 72 hours (8-fold and 3.6-fold increase, respectively, in the shown experiment; in two additional experiments, which are not shown, the increase at 72 hours was higher than 10-fold). These results suggest that the inhibition of cyclin A–associated kinase activity observed in β1C transfectants is not a consequence of a decrease in cyclin A expression, but is likely a reflection of its increased association with p27kip1. Expression of β1C did not affect cell adhesion to ECM proteins, specifically β1 integrin ligands such as fibronectin and vitronectin, or to integrin-binding antibodies such as TS2/16 (data not shown). These data suggest that β1C could exert its growth-inhibitory effect via an increase of p27kip1 and reduction of cyclin A–associated kinase activity, without affecting cell adhesion.
p27kip1 antisense oligonucleotides prevent β1C effect on cell proliferation.
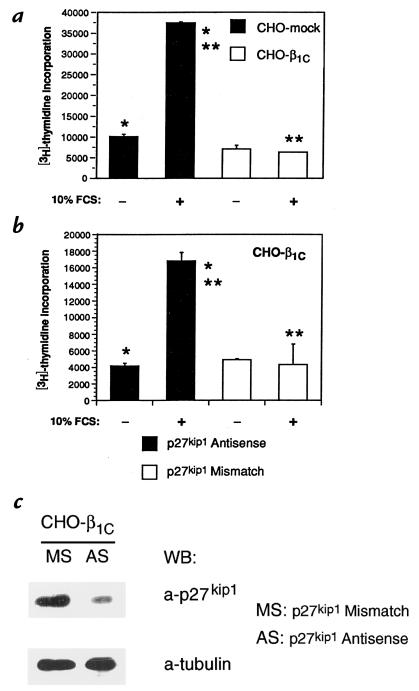
To evaluate the role of p27kip1 in mediating β1C inhibitory effect on cell growth we used p27kip1 antisense oligonucleotides to downregulate its expression. Treatment of CHO-β1C transfectants with p27kip1 antisense (AS) oligonucleotides resulted in a strong reduction of p27kip1 protein expression levels compared with mismatch (MS) oligonucleotide-treated cells (Fig. 6c, top). In agreement with our previous published data (9), induction of β1C expression in CHO stable transfectants resulted in a strong inhibition of [3H]thymidine incorporation in response to serum, whereas [3H]thymidine incorporation was stimulated 3.7-fold in mock-transfected cells (Fig. 6a). p27kip1 antisense oligonucleotides, but not mismatch oligonucleotides, significantly restored DNA synthesis (4.2-fold increase in [3H]thymidine incorporation) in CHO-β1C stable transfectants in response to serum (Fig. 6b). These results show that p27kip1 mediates β1C-dependent growth inhibition.
Figure 6.
p27kip1 antisense oligonucleotides prevent β1C inhibitory effect on CHO cell proliferation. The experiments were repeated twice using two different β1C clones with similar results. (a) CHO-mock or -β1C stable cell transfectants were cultured for 24 h in tetracycline-free medium, washed three times with PBS, and then incubated for 48 h either in the absence or in the presence of 10% FCS. 1 μCi/well [3H]thymidine was added during the last 3 h of the 48-h culture. (b) CHO-β1C stable cell transfectants were transfected using 30 nM of either p27kip1 antisense or mismatch oligonucleotides. After 24 h, the cells were cultured for additional 48 h either in the absence or in the presence of 10% FCS. 1 μCi/well [3H]thymidine was added during the last 3 h of the 48-h culture. Results are mean ± SEM values of duplicate determinations. Group differences were compared using one-way analysis of variance. In a, the differences in proliferation between CHO-mock and CHO-β1C transfectants in the presence of FCS (**) or between CHO-mock cells in the presence of FCS and CHO-mock cells in the absence of FCS (*) are statistically significant (P < 0.05). In b, the differences in proliferation between antisense- and mismatch-treated transfectants in the presence of FCS (**) or between antisense-treated transfectants in the presence and in the absence of FCS (*) are statistically significant (P < 0.05). Consistent results were obtained in a separate experiment where triplicate observations were performed. (c) CHO-β1C cells were transfected using 30 nM of either p27kip1 antisense (AS) or mismatch (MS) oligonucleotides in the absence of tetracycline. After 24 h, the cells were lysed and p27kip1 expression levels were evaluated by immunoblotting using 0.8 μg/ml MAB to p27kip1 (top). Control for protein loading was provided by MAB to tubulin (bottom). Proteins were viewed by ECL.
Forced β1C expression does not affect MAP kinase activation.
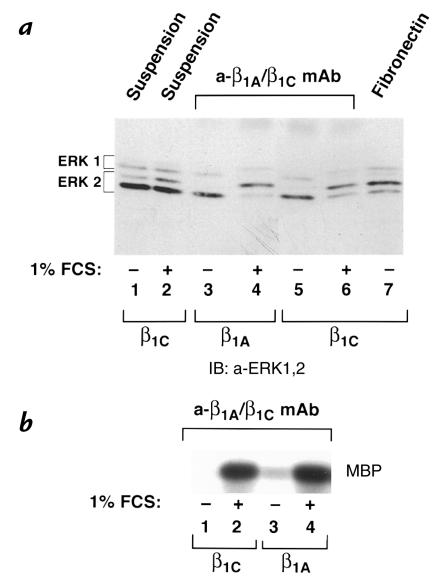
Synergistic activity of integrins and mitogenic stimuli leads to activation of some members of the MAP kinase family, specifically of ERK-1 and ERK-2 (5, 34). To investigate the ability of β1C to modulate ERK-1 and/or ERK-2 activation, transient CHO cell transfectants expressing β1C or β1A integrins were used. The cells were kept in suspension or seeded on tissue culture dishes coated with TS2/16 or fibronectin, and MAP kinase activation was analyzed either in the absence or in the presence of 1% fetal calf serum (FCS). The phosphorylation state of ERK-1 and ERK-2 was analyzed by mobility shift (Fig. 7a) and by in vitro kinase assays (Fig. 7b). A synergistic activation of ERK-1 and ERK-2 by β1C or β1A engagement and serum was observed when β1C or β1A transfectants were plated on TS2/16 in the presence of 1% FCS (Fig. 7 a, lanes 4 and 6; b, lanes 2 and 4). Activation of ERK-1 and ERK-2 was not observed when β1C or β1A transfectants were plated on TS2/16 in absence of FCS (Fig 7 a, lanes 3 and 5; b, lanes 1 and 3), whereas β1C transfectants plated on fibronectin were able to activate ERK-1 and ERK-2 (Fig. 7 a, lane 7). Cells held in suspension either in the absence or in the presence (Fig. 7 a, lanes 1 and 2, respectively) of 1% FCS showed very low levels of ERK-1 and ERK-2 activation. These data show that β1C engagement does not prevent ERK-1 and ERK-2 activation in response to mitogenic stimuli or to fibronectin.
Figure 7.
Synergistic activation of ERK-1 and ERK-2 by β1C engagement and serum stimulation. (a and b) Serum-starved CHO cells were transiently transfected using pBJ1-β1C or pBJ1-β1A. The cells were kept in suspension or seeded on tissue culture dishes coated with TS2/16 (a-β1A/β1C MAB) or fibronectin for 10 min at 37°C, and MAP kinase activation was analyzed either in the absence or in the presence of 1% FCS. The phosphorylation state of both ERK-1 and ERK-2 was examined by mobility shift assay (a) and by in vitro kinase assay (b). In a, detergent cell extracts were analyzed by immunoblotting using 0.1 μg/ml affinity-purified antibody to ERK-1 and ERK-2. Proteins were viewed by ECL. The positions of nonphosphorylated and phosphorylated ERK-1 and ERK-2 are indicated by brackets. In b, ERK-1 was immunoprecipitated from total cell lysate using 0.5 μg affinity-purified antibody to ERK-1, and its kinase activity was analyzed using MBP as a substrate. Phosphorylated MBP was seen with autoradiography. The experiments were repeated at least three times with consistent results. In a, lanes 1–2, 5–7, CHO-β1C cells; lanes 3 and 4, CHO-β1A cells. In b, lanes 1 and 2, CHO-β1C cells; lanes 3 and 4, CHO-β1A cells. MAP, mitogen-activated protein; MBP, myelin basic protein; ERK, extracellular signal-regulated kinase.
Discussion
In this report it is shown, first, that in vivo expression of the β1C integrin and of the CKI, p27kip1, correlates in benign and neoplastic prostate epithelial cells; second, that forced expression of β1C in vitro is accompanied by increased levels of p27kip1 and by selective inhibition of cyclin A–dependent, but not of cyclin E–dependent, kinase activity; third, that increased p27kip1 association with cyclin A is observed as a consequence of β1C expression; and fourth, that p27kip1 mediates β1C-dependent growth inhibition. This is the first report showing an in vivo correlation between integrins and cell-cycle inhibitors in benign and neoplastic prostate tissue; thus, it brings new insights into the molecular mechanisms underlying prostate cancer progression. Furthermore, this study shows a unique mechanism of regulation of cell growth by integrins.
The results highlight the role of β1C as an upstream regulator of p27kip1. Low levels of p27kip1 recently have been shown to predict an increased risk for treatment failure in lymph node–negative prostate cancer patients (13), and the use of p27kip1 to evaluate response to therapy and differential treatment decisions has been recommended. Because in vivo downregulation of β1C is likely to occur at an earlier stage than loss of p27kip1 in the pathogenesis of prostate cancer we expect β1C to be a sensitive prognostic indicator of potentially high clinical value to predict therapy and patient survival. Further studies to investigate this area of research are in progress. The β1A integrin that is identical to β1C, except for a different carboxy-terminal cytodomain, was neither downregulated in prostatic adenocarcinoma (8), nor did it inhibit prostate cell proliferation (7), thus indicating a specificity of effect of the unique β1C sequence. On the basis of these observations, a potential use of specific β1C sequences can be foreseen to prevent tumor growth in vivo.
Incorrect expression of integrins or of their cytodomain in epithelial cells modifies their growth rate in vivo and has been shown to generate pathological phenotypes (35–37). Previous data from our laboratory show that β1C integrins in epithelial cells are found in benign and nonproliferative epithelium and are downregulated in prostatic adenocarcinoma (7, 8). Forced expression of the cytoplasmic domain of β1C has a causal role in inhibiting cell proliferation of tumorigenic and highly metastatic prostate cancer cells (7). Thus, because of the ability of β1C to maintain high cellular levels of p27kip1 it is expected that deregulation of β1C, and consequently of p27kip1 expression in prostate epithelial cells, may be an important step for malignant growth.
The effect of p27kip1 antisense oligonucleotides shown in this study confirms the crucial role of p27kip1 in modulating β1C-dependent growth inhibition. The pathways controlled by β1C specifically affect cyclin A-CDK activities, but not cyclin E-CDK activities, thus indicating that cyclin A-CDKs and p27kip1 are selective downstream targets to this integrin. Although surprising, because of the p27kip1 ability to inhibit both cyclin A- and E-CDKs, the findings are suggestive of a unique mechanism regulated by this integrin. The induction of p27kip1 preceded the observed decrease in cyclin A–associated kinase activity, thus pointing to p27kip1 as the earliest yet identified downstream molecule that links the expression of a specific integrin with cell-cycle regulation. The potential key players and mechanisms necessary to maintain high levels of p27kip1 in response to β1C are being studied at this time. Unlike a previous report, where 10T1/2 fibroblasts expressing β1C were used (10), we did not observe changes in cyclin A expression in CHO cells; this finding may be explained by the different nature of the cell types analyzed in the two studies. In a similar manner, it was shown that cyclin D1 expression levels are regulated in an anchorage-dependent manner in 3T3 cells (19, 21), but not in NRK cells (19).
In a previous study, increased levels of another member of the CKI family, p21cip1/waf1, which leads rectal carcinoma cells into an irreversible apoptotic pathway were observed in response to β4 integrin expression (38). Although we have not tested a potential effect of β1C in stimulating apoptosis, this is unlikely to occur because β1C expression appears to affect cell proliferation in a reversible manner. In fact, addition of tetracycline to the growth medium of β1C transfectants did allow successful reculture of all cells, since cells expressing β1C were 100% viable and were all able to reattach to tissue culture plates (Fornaro, M., and Languino, L.R., unpublished results).
The induction of p27kip1 observed in β1C transfectants was not accompanied by detectable changes in either cell adhesion to integrin ligands or spreading or focal contact organization; neither was dependent on β1C engagement. Other reports have shown that disruption of cell adhesion to the ECM inhibits cell proliferation by altering levels of cell-cycle molecules, including p27kip1 and their activities; in these studies, however, these observations were the result of a complex combination of loss of anchorage, loss of cell spreading, and perturbation of cytoskeletal organization (20–24). Similarly, the decrease in cyclin A–associated kinase activity, shown here, reflects an increased association of p27kip1 with cyclin A rather than a transcriptional cyclin A block in response to loss of cell adhesion, as shown previously by several groups (19, 23, 39). Consistent with these observations, adhesion to integrin ligands or adhesion-dependent events, such as cyclin E expression, cyclin E–associated kinase activity, or MAP kinase activation, were not affected by β1C expression. Specifically, the failure of β1C to prevent MAP kinase activation by fibronectin or by synergistic activities of β1C integrin and serum makes unlikely the possibility that upstream mediators of MAP kinase activation, such as FAK, or Shc, or c-Src (4, 40), are inhibited in β1C transfectants; this, however, remains to be proven.
The integrin cytoplasmic domains are key regulators of integrin and cell functions, as well as of intracellular signaling events (41, 42). Recently, β1D, an additional variant form of the β1 subfamily, has been shown to inhibit cell proliferation (43) and can affect development in vivo (44). Because subtle variations in the integrin cytoplasmic domain affect cell proliferation and development, it is conceivable that the expression of these subunits requires a tight transcriptional and translational regulation. Studies in progress in our laboratory on the mechanisms regulating β1C expression in benign and neoplastic prostate will bring new insight into the understanding of the events that contribute to prostate cancer progression.
Acknowledgments
We thank D. Danielpour, M.E. Hemler, and H. Zhang for providing cells and antibodies, and D. Schatz for plasmids. We thank J.A. Madri and H. Zhang for critical discussion. We also thank A.E. Slear for performing a set of immunostainings, and F. Peracchia, C.A. Steger, and A.S. Woodard for constructive suggestions on the manuscript. We are grateful to Rocco Carbone for support in performing flow cytometric analysis, and Mary Helie for technical advice. This work was supported National Institutes of Health grants CA-71870 and DK-52670, by Army PCRP grant DAMD17-98-1-8506 (to L.R. Languino), by the Donaghue Medical Research Foundation Fellowship Award (to M. Fornaro), and by the American-Italian Cancer Foundation Fellowship Award (to M. Manzotti).
References
- 1.Hynes RO. Integrins: versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 2.Ruoslahti E, Reed JC. Anchorage dependence, integrins and apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 4.Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 5.Schlaepfer DD, Hunter T. Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 1998;8:151–157. doi: 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- 6.Languino LR, Ruoslahti E. An alternative form of the integrin β1 subunit with a variant cytoplasmic domain. J Biol Chem. 1992;267:7116–7120. [PubMed] [Google Scholar]
- 7.Fornaro M, et al. β1C integrin in epithelial cells correlates with a nonproliferative phenotype: forced expression of β1C inhibits prostate epithelial cell proliferation. Am J Pathol. 1998;153:1079–1087. doi: 10.1016/s0002-9440(10)65652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fornaro M, Tallini G, Bofetiado CJM, Bosari S, Languino LR. Down-regulation of β1C integrin, an inhibitor of cell proliferation, in prostate carcinoma. Am J Pathol. 1996;149:765–773. [PMC free article] [PubMed] [Google Scholar]
- 9.Fornaro M, Zheng DQ, Languino LR. The novel structural motif Gln795–Gln802 in the integrin β1C cytoplasmic domain regulates cell proliferation. J Biol Chem. 1995;270:24666–24669. doi: 10.1074/jbc.270.42.24666. [DOI] [PubMed] [Google Scholar]
- 10.Meredith J, Jr, Takada Y, Fornaro M, Languino LR, Schwartz MA. Inhibition of cell cycle progression by the alternatively spliced integrin β1C. Science. 1995;269:1570–1572. doi: 10.1126/science.7545312. [DOI] [PubMed] [Google Scholar]
- 11.Hunter T, Pines J. Cyclins and cancer II: cyclin D and CDK inhibitors come of age. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 12.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 13.Tsihlias J, et al. Loss of cyclin-dependent kinase inhibitor p27Kip1 is a novel prognostic factor in localized human prostate adenocarcinoma. Cancer Res. 1998;58:542–548. [PubMed] [Google Scholar]
- 14.Yang RM, et al. Low P27 expression predicts poor disease-free survival in patients with prostate cancer. J Urol. 1998;159:941–945. [PubMed] [Google Scholar]
- 15.Catzavelos C, et al. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: Prognostic implications in primary breast cancer. Nat Med. 1997;3:227–230. doi: 10.1038/nm0297-227. [DOI] [PubMed] [Google Scholar]
- 16.Porter PL, et al. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med. 1997;3:222–226. doi: 10.1038/nm0297-222. [DOI] [PubMed] [Google Scholar]
- 17.Loda M, et al. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 18.Bottazzi ME, Assoian RK. The extracellular matrix and mitogenic growth factors control G1 phase cyclins and cyclin-dependent kinase inhibitors. Trends Cell Biol. 1997;7:348–352. doi: 10.1016/S0962-8924(97)01114-8. [DOI] [PubMed] [Google Scholar]
- 19.Guadagno TM, Ohtsubo M, Roberts JM, Assoian RK. A link between cyclin A expression and adhesion-dependent cell cycle progression. Science. 1993;262:1572–1575. doi: 10.1126/science.8248807. [DOI] [PubMed] [Google Scholar]
- 20.Fang F, Orend G, Watanabe N, Hunter T, Ruoslahti E. Dependence of cyclin E-CDK2 kinase activity on cell anchorage. Science. 1996;271:499–502. doi: 10.1126/science.271.5248.499. [DOI] [PubMed] [Google Scholar]
- 21.Zhu X, Ohtsubo M, Bohmer RM, Roberts JM, Assoian RK. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J Cell Biol. 1996;133:391–403. doi: 10.1083/jcb.133.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Day ML, et al. Cell anchorage regulates apoptosis through the retinoblastoma tumor suppressor/E2F pathway. J Biol Chem. 1997;272:8125–8128. doi: 10.1074/jbc.272.13.8125. [DOI] [PubMed] [Google Scholar]
- 23.Schulze A, et al. Anchorage-dependent transcription of the cyclin A gene. Mol Cell Biol. 1996;16:4632–4638. doi: 10.1128/mcb.16.9.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- 25.Engvall E, Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977;20:1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- 26.Danielpour D, Kadomatsu K, Anzano MA, Smith JM, Sporn MB. Development and characterization of nontumorigenic and tumorigenic epithelial cell lines from rat dorsal-lateral prostate. Cancer Res. 1994;54:3413–3421. [PubMed] [Google Scholar]
- 27.Shockett P, Difilippantonio M, Hellman N, Schatz DG. A modified tetracycline-regulated system provides autoregulatory, inducible gene expression in cultured cells and transgenic mice. Proc Natl Acad Sci USA. 1995;92:6522–6526. doi: 10.1073/pnas.92.14.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Languino LR, et al. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1 dependent pathway. Cell. 1993;73:1423–1434. doi: 10.1016/0092-8674(93)90367-y. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Kobayashi R, Galaktionov K, Beach D. p19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 phase kinase. Cell. 1995;82:915–925. doi: 10.1016/0092-8674(95)90271-6. [DOI] [PubMed] [Google Scholar]
- 30.Matsushime H, et al. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aktas H, Cai H, Cooper GM. Ras links growth factor signaling to the cell cycle machinery via regulation of cyclin D1 and the Cdk inhibitor p27kip1. Mol Cell Biol. 1997;17:3850–3857. doi: 10.1128/mcb.17.7.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coats S, Flanagan WM, Nourse J, Roberts JM. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- 33.Chen Q, Kinch MS, Lin TH, Burridge K, Juliano RL. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- 34.Keely P, Parise L, Juliano R. Integrins and GTPases in tumor cell growth, motility and invasion. Trends Cell Biol. 1998;8:101–106. doi: 10.1016/s0962-8924(97)01219-1. [DOI] [PubMed] [Google Scholar]
- 35.Faraldo MM, Deugnier M-A, Lukashev M, Thiery J-P, Glukhova MA. Perturbation of β1 integrin functions alters the development of murine mammary glands. EMBO J. 1998;17:2139–2147. doi: 10.1093/emboj/17.8.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albelda SM. Biology of disease: role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest. 1993;68:4–17. [PubMed] [Google Scholar]
- 37.Carroll JM, Romero RM, Watt FM. Suprabasal integrin expression in epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell. 1995;83:957–968. doi: 10.1016/0092-8674(95)90211-2. [DOI] [PubMed] [Google Scholar]
- 38.Clarke AS, Lotz MM, Chao C, Mercurio AM. Activation of the p21 pathway of growth arrest and apoptosis by the β4 integrin cytoplasmic domain. J Biol Chem. 1995;270:22673–22676. doi: 10.1074/jbc.270.39.22673. [DOI] [PubMed] [Google Scholar]
- 39.Wu R-C, Schonthal AH. Activation of p53-p21waf1 pathway in response to disruption of cell-matrix interactions. J Biol Chem. 1997;272:29091–29098. doi: 10.1074/jbc.272.46.29091. [DOI] [PubMed] [Google Scholar]
- 40.Schlaepfer DD, Jones KC, Hunter T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase- initiated tyrosine phosphorylation events. Mol Cell Biol. 1998;18:2571–2585. doi: 10.1128/mcb.18.5.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fornaro M, Languino LR. Alternatively spliced variants: a new view of the integrin cytoplasmic domain. Matrix Biol. 1997;16:185–193. doi: 10.1016/s0945-053x(97)90007-x. [DOI] [PubMed] [Google Scholar]
- 42.Wei J, Shaw L, Mercurio AM. Regulation of mitogen-activated protein kinase activation by the cytoplasmic domain of the α6 integrin subunit. J Biol Chem. 1998;273:5903–5907. doi: 10.1074/jbc.273.10.5903. [DOI] [PubMed] [Google Scholar]
- 43.Belkin AM, Retta SF. β1D integrin inhibits cell cycle progression in normal myoblasts and fibroblasts. J Biol Chem. 1998;273:15234–15240. doi: 10.1074/jbc.273.24.15234. [DOI] [PubMed] [Google Scholar]
- 44.Baudoin C, Goumans M-J, Mummery C, Sonnenberg A. Knockout and knockin of the β1 exon D define distinct roles for integrin splice variants in heart function and embryonic development. Genes Dev. 1998;12:1202–1216. doi: 10.1101/gad.12.8.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]