Abstract
Over the last two decades, there have been numerous technical and methodological advances available to clinicians and researchers to better understand attention deficit hyperactivity disorder (ADHD) and its etiology. Despite the growing body of literature investigating the disorder’s pathophysiology, ADHD remains a complex psychiatric disorder to characterize. This chapter will briefly review the literature on ADHD, with a focus on its history, the current genetic insights, neurophysiologic theories, and the use of neuroimaging to further understand the etiology. We address some of the major concerns that remain unclear about ADHD, including subtype instability, heterogeneity, and the underlying neural correlates that define the disorder. We highlight that the field of ADHD is rapidly evolving; the descriptions provided here will hopefully provide a sturdy foundation for which to build and improve our understanding of the disorder.
Keywords: Attention, Attention deficit hyperactivity disorder, Brain development, Executive function, FMRI, Functional connectivity, Heritability, Heterogeneity, Hyperactivity, Impulsivity, Neuropsychiatric disorders, Reward system
1 Introduction
Attention deficit hyperactivity disorder (ADHD) is a prevalent and persistent psychiatric disorder that emerges early in childhood, with a current prevalence rate of 5 % in children 4–17-years old. The disorder is classically characterized by symptoms of inattention, impulsivity, and hyperactivity. ADHD is believed to typically onset in early childhood, although diagnosis is typically determined in the school age years. The disorder is particularly relevant in today’s society as it is one of the most common diagnoses in educational and children’s mental health settings. Furthermore, many children diagnosed with the disorder go on to have problems related to education, social functioning, and/or other mental illness as adolescents and young adults (Klein et al. 2012). Thus the disorder is one of high individual and societal costs to the population (Pelham et al. 2007).
2 History of ADHD
It is interesting to speculate whether ADHD is actually a psychiatric condition of somewhat recent historical onset that may have been very rare in ancient times (like anorexia), or a disorder that seems to have followed our species for several thousands of years (like schizophrenia). Filling this gap in historical knowledge would put useful constraints on theories of what causes ADHD. There is no obvious description of ADHD in the ancient literature, despite the distinct personality types described by Galen, the prominent Roman physician and philosopher. The modern history of ADHD-like medical description traces back over 200 years and is provided in more detail by Taylor (2010), Barkley and Peters (2012), and Nigg and Barkley (In press). Descriptions analogous to either ADHD or ADHD without hyperactivity are found sporadically in the European literature from the late 1700s to early 1900s, and in the United States from the early 1800s onward.
While disputed with regard to its importance to the concept of ADHD, we note that after the great encephalitis epidemics of 1915–1920, it was observed that some children who survived brain infections had many problems with defiance, impulsivity, and over-activity; and while these descriptions do not perfectly match ADHD, they have notable similarities (Ebaugh 1923; Hohman 1922; Stryker 1925). The field eventually adopted the term “brain-injured child syndrome” (Strauss and Lehtinen 1947), often associated with mental retardation, but was also applied to children who today would be labeled with ADHD. The concept of “brain-injured child syndrome” gradually evolved into that of “minimal brain damage” and eventually to “minimal brain dysfunction” (MBD). Children with hyperactive behavior and other attributes of MBD showed remarkable improvement in response to stimulant medication (Benzedrine) (Bradley 1937). Although physicians did not begin regularly prescribing stimulants for MBD until the 1950s and 1960s, this serendipitous discovery probably also affected concepts, and shifted interest away from cognitive and learning problems toward hyperactivity (Taylor 2011).
By the late 1950s, labels such as “hyperkinetic impulse disorder” or “hyperactive child syndrome” were also in use (Burks 1960; Chess 1960). Conceptual theories ranged from theories of cortical overstimulation or cortical under-arousal to psychoanalytic and psychosocial theories. In the Diagnostic and Statistical Manual of Mental Disorders, second edition (DSM-II), childhood disorders were introduced for the first time, and were all described as “reactions” (implying psychological defense or adaptation driving behavior), including “hyperkinetic reaction of childhood” (Association 1968). The fundamental tension between viewing these children as having either a neurobiological or a behavioral/psychosocial problem is apparent in arguments about ADHD to varying extents to the present day, and is partly reflected in distinct diagnostic approaches in Europe and the United States.
By the 1970s, inspired by progress in cognitive and experimental psychology in operationally defining attention, researchers emphasized problems with sustained attention and impulse control in addition to hyperactivity (Douglas 1972). Douglas’s model, which was quite influential and also highlighted an inclination to seek immediate reinforcement, contributed to the renaming of the disorder as attention deficit disorder (ADD) in 1980 in DSM-III (Association 1980). At that time, the syndrome was redefined in narrower terms than what had previously been MBD, and the term MBD was abandoned. In 1987, the disorder was renamed “attention-deficit hyperactivity disorder” in DSM-III, revised edition, (DSM-III- R) (Association AP 1987), and a single list of items incorporating all three symptoms (hyperactivity, inattentiveness, impulsivity) was specified that had better statistical validity than the factors in DSM-III.
The 1980s and 1990s saw extensive research on the information and reward-processing features associated with ADHD. Further psychometric work suggested that the problems with hyperactivity and impulsivity formed a single dimension of behavior for all practical purposes (Achenbach and Edelbrock 1983; Goyette et al. 1978; Lahey et al. 1988). As a result, ADHD was described in DSM-IV as reflecting two distinct yet correlated dimensions or domains of behavior: a set of symptoms for inattention and a set of symptoms for hyperactive–impulsive behavior (Association 1994). Unlike DSM-III-R, DSM-IV once again permitted the diagnosis of a subtype of ADHD that consisted principally of problems with attention, and for the first time provided specific diagnostic criteria for this group—although the conceptual definition was somewhat distinct from that in DSM-III, which had allowed these children to be impulsive, but not hyperactive. DSM-IV also permitted, for the first time, the distinction of a subtype of ADHD that consisted chiefly of hyperactive–impulsive behavior without significant inattention. Children having significant problems from both item lists were described as having ADHD-combined type.
Several developments in the literature were notable in the first decade of the twenty-first century and are likely to influence future characterization of the disorder. Particularly notable was the emphasis on “multiple pathway models” of ADHD, which suggests that both attention-related theories and motivation-related theories contribute to the disorder. These considerations are discussed below in Sect. 4 when future directions in the field are discussed [also see Sonuga-Barke (2005)]. Almost 20 years after DSM-IV, the publication of DSM-V occurred in 2013. During that 20-year period, in addition to developments in theory, technology revolutionized research on child psychopathology. ADHD was, for the first time, associated with a massive and rapidly expanding literature on structural and functional brain imaging as well as molecular genetic studies. This literature provides new and tantalizing evidence of biological correlates of ADHD. Despite numerous potential improvements to the criteria, few substantive changes were made to DSM-V.1 The same two behavioral domains (inattention and hyperactivity– impulsivity) are still in force. Indeed, the literature of the last 20 years provides powerful evidence for the clinical utility and validity of distinguishing these two symptom domains that, despite being highly correlated predict different impairments and likely have different neural correlates (Willcutt et al. 2012; Fair et al. 2012b) (Fig. 1). In particular, symptoms of inattention-disorganization tend to predict academic problems, certain driving difficulties, and peer neglect, for instance. Symptoms of hyperactivity–impulsivity tend to predict aggression, peer rejection, and speeding citations among other difficulties. Nonetheless, issues of subtype instability (Willcutt et al. 2012), and difficulties in identifying the genetic and neurobiological underpinnings of the disorder (Nigg et al. 2005) are likely pointing to a significantly complex characterization of the disorder that will need to be further clarified to improve outcomes.
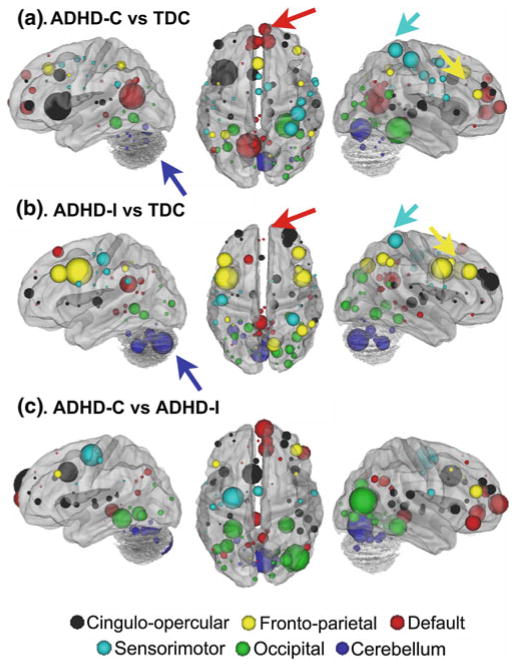
Fig. 1.
Using multivariate pattern analysis to compare the two most common subtypes of ADHD (ADHD-Inattentive and ADHD-Combined), this report showed relatively strong classification for single subjects (Fair et al. 2012b). Up to 77.0 % accuracy was attained for ADHD-C compared to typically developing controls (TDC), and up to 80.8 % accuracy for ADHD-I compared to TDC. Note that the features, or connections, that contributed most strongly to these predictions showed distributed patterns of atypical connectivity relative to TDC, measured by “differential” node strength (nodes with many connections that differentiated groups). Node strength for ADHD-C versus TDC shows strong differentiation in regions (a) somewhat different from those found in ADHD-I versus TDC (b). c Comparisons between the subtypes show similar trends. Node colors: red, default; blue, cerebellum; yellow, fronto-parietal; black, cingulo-opercular; green, occipital; cyan, sensorimotor
3 The Genetics of ADHD
3.1 Heritability
There are now many lines of evidence that highlight the genetic aspect of ADHD. Family and twin studies reveal that ADHD exhibits high heritability (Faraone et al. 2005). Twin studies estimate the heritability of ADHD to be 70–80 % (Burt 2009), while family studies show there is a 2–8 fold increase in the risk for ADHD in parents and siblings of children with ADHD (Biederman et al. 1990b, 1992; Faraone et al. 1991). Other studies have shown that 30–35 % of the full siblings of children with ADHD also had ADHD, compared to the base rate in the population, which was considerably less (Biederman et al. 1990a; Faraone et al. 2000a). There is also an increased incidence among first- and second-degree relatives of individuals with ADHD (Faraone et al. 1994, 2000b). A study of twin boys showed that genetic factors were important in the expression of different dimensions of ADHD, with heritability of inattention at 0.39 and heritability of impulsivity–hyperactivity at 0.69 (Sherman et al. 1997).
Adoption studies further support a genetic etiology in ADHD. A study by Sprich et al. (2000) showed that adoptive relatives of children with ADHD had similar rates of ADHD to relatives of typically developing children, while having lower rates of ADHD than biological relatives of non-adopted children with ADHD. Alberts-Corush et al. (1986) show that adoptive relatives of children with ADHD perform better in measures of attention than biological relatives of children with ADHD.
Although the putative subtypes in DSM-IV (like presentations in DSM-V) tend to be transmitted in families at above chance rates, the effect size is sufficiently small that both subtypes often occur in the same families (Stawicki, von Eye, & Nigg 2005), further supporting both the need to consider heterogeneity and to revise the current definitions. This also would suggest that risk genes may predispose individuals to ADHD, while there may be other factors that influence the ADHD phenotype.
3.2 Molecular Findings
Twin studies have concluded that additive genetic effects explain up to 80 % of the variance in the ADHD phenotype (Albayrak et al. 2008; Thapar et al. 1999). Understanding the genetic basis of ADHD may assist in the development of new diagnostic criteria and therapeutic opportunities. A number of genes have been identified that convey risk for ADHD (Faraone et al. 2005; Durston 2008), although the effects of each gene marker are too small to be of clinical utility and explain only a small fraction of the overall genetic influence in ADHD. In a genome-wide scan, where all chromosomes are screened for linkage more often than expected, Fisher et al. (2002) identified four regions (5p12, 10q26, 12q23, and 16p13) with evidence of linkage (log odds ratio scores 1.5) in a study of 126 American affected sibling pairs. Smalley et al. (2002) observed even stronger evidence for the 16p13 region (logs odd ratio score 4) in a sample of 203 families. Other genome studies fromvarious samples identified a region proximal to the dopamine transporter gene (Bakker et al. 2003; Hebebrand et al. 2006; Ogdie et al. 2003). However, a recent meta-analysis found no genome-wide significant associations, suggesting that the effect sizes of common ADHD risk variants are likely to be very small (Neale et al. 2010).
More common than the genome approach in ADHD are candidate gene studies, where genes are chosen on theoretical grounds to associate a particular gene with ADHD. Cook et al. (1995) observed an association between the 480-bp allele (or genotype) in the dopamine transporter (DAT) gene and ADHD. Additional polymorphisms in intron 9 and exon 9 were examined, with a trend for biased transmission of the 480-bp allele of the variable number of tandem repeats (VNTR) polymorphism (Barr et al. 2001). Meta-analysis confirms that genes for the dopamine transporter-1 (DAT1), dopamine D4 and D5 receptors (DRD4, DRD5), serotonin-transporter-linked polymorphic region (5HTTPLR), 5-hydroxytryptamine (serotonin) receptor 1B (HTR1B), and synaptosomal-associated protein 25 (SNAP25) share common markers associated with ADHD (Gizer et al. 2009). Of considerable theoretical interest associated with ADHD is the 7-repeat allele of DRD4 due to the extensive theory and evidence that dopamine functioning is involved in ADHD (Faraone et al. 2001). Some studies also suggest a role for noradrenergic genes particularly the alpha-2 receptor (Rivero et al. 2013).
It is important to note, however, that in the face of ADHD’s high heritability (noted above), it is striking that all the candidate genes identified to date account for less than 5 % of genetic variation in ADHD (Neale et al. 2010). Nonetheless, further genetic variance remains to be explained, and a handful of possibilities need examination. Gene × environment interactions can inflate heritability estimates, and appear to be operating in ADHD as well as in other psychopathologies (Nijmeijer et al. 2010; Nigg 2013). Second, epistasis or gene × gene interactions have not been deeply explored. A third possibility relates to the rare variant hypothesis, or the idea that common disease variants (what has largely been examined to date) is insufficiently targeting the correct genes. Rather, multiple rare variants may be playing a larger role (Williams et al. 2012). Fourth, and of particular interest in the current chapter, is the possibility that ADHD does not necessarily describe one syndrome, but rather is biologically heterogeneous and thus clustering subjects into the same genetic studies based on the clinical phenotype may be limiting what is able to be identified with genetics. A few of these issues will be discussed in more detail throughout the remaining portions of this chapter.
4 Neurophysiologic Theories of ADHD
Over the past two decades, neurobiological theories of ADHD have centered on two common, but not mutually exclusive, models. One model emphasizes top-down, controlled processing problems, such as those associated with cognitive control or executive functioning (Barkley 1997). Another model emphasizes bottom-up, motivational, and incentive or reward response (Sagvolden et al. 2005) [for a review see (Nigg 2005)]. While consensus is yet to be reached on the core neural pathways that lead to the disorder (potentially a result of heterogeneity in the disease—see Sect. 5), there exist several lines of evidence that support both of these models and suggest both types of psychobiological systems are involved in ADHD. We will briefly discuss this work in the following sections.
4.1 Executive (Top-Down) Theories
Typical executive function development
The significant, measurable cognitive changes that occur throughout childhood reflect, in large part, the development of executive control processes. Executive functions are higher order, top-down cognitive processes that allow for the complex organization of behavior through appropriate process selection and set, or goal, maintenance over time (Lyon and Krasnegor 1996; Pennington 1997). From infancy to young adulthood, the development of cognitive functioning is reflected in behavior that is less reflexive and stimulus-bound but rather more goal-directed, self-organized, and flexible (Stuss 1992). Various lines of evidence of cognitive development suggest that typical maturation of controlled processing functions occurs in a multistage progression in which different components and kinds of control develop at different rates, beginning in infancy and continuing into adolescence (Welsh et al. 1991; Klenberg et al. 2001; Becker et al. 1987; Levin et al. 1991; Luria 1966/1980; Passler et al. 1985; Welsh and Pennington 1988).
The functional neuroanatomy linked to executive control has commonly been associated with selective recruitment of specific prefrontal cortical regions. These regions are hypothesized to interact with posterior cortical regions, along with subcortical regions [e.g., caudate nucleus or dorsal striatum (Aron et al. 2004; Eagle and Robbins 2003)] that flexibly configure processing in accordance with behavioral goals (Posner and Petersen 1990; Dosenbach et al. 2006, 2007; Petersen and Posner 2012). Considerable focus has been given to the dorsolateral prefrontal cortex (PFC) (Miller and Cohen 2001), medial superior frontal cortex, the adjacent dorsal anterior cingulate cortex (ACC) (Rushworth et al. 2005), dorsal frontal cortex, and regions in the parietal lobe such as the inferior parietal lobe and intraparietal sulcus (Corbetta and Shulman 2002). More recently, the anterior PFC (lateral Brodmann Area 10) (Braver et al. 2003; Sakai and Passingham 2003, 2006; Gilbert et al. 2006) and regions at the boundary of the anterior insula and the frontal operculum (Crone et al. 2006; Bunge et al. 2002; Dosenbach et al. 2006), sometimes referred to as ventral or ventrolateral PFC [e.g., Bunge et al. (2002)], have also been implicated in control processing. Not surprisingly, the development of these regions in terms of the activation profiles of the individual regions, as well as the interactions between the regions (i.e., functional connectivity), is thought to be implicated in the normal development of control processing (Dosenbach et al. 2006; Sakai et al. 2002; Wager and Smith 2003; Bunge and Wright 2007). These several regions indeed cohere into a small number of distributed neural networks. Along the same lines, the atypical development of these executive systems in terms of brain activity or connectivity has been implicated in ADHD.
Executive functioning in ADHD
Executive or top-down control in ADHD has been discussed as a core dysfunction of the disorder for over a century [see Nigg and Barkley (In press)]. In one of the most well-developed versions of this argument, Barkley (1997) presented what he termed a unifying theory of ADHD, suggesting that symptoms of the disorder, such as atypical behavioral inhibition, were caused by deficits in response inhibition that in turn disrupted four specific executive functions. As the cognitive neurosciences rapidly advanced in the first part of the twenty-first century, others updated this type of model and integrated in it other forms of control processing and their relation to neural systems [e.g., Nigg and Casey (2005) and Castellanos and Tannock (2002)]. Indeed, it has now been repeatedly shown that children with ADHD often show impairments in performance on tasks that measure some form of executive processes.
Also consistent with that idea of Barkley (1997), ADHD appears to be related to impairment in response inhibition (Doyle 2006; Nigg 1999; Nigg 2001; Seidman et al. 1997). Response inhibition is the ability to inhibit or suppress an inappropriate, prepotent response in a certain context in favor of a more appropriate alternative and is believed to involve fronto-striatal and fronto-subthalamic circuits (Aron 2011). It is regarded as a prerequisite for self-control (Muraven and Baumeister 2000), emotional regulation (Eisenberg and Morris 2002), and cognitive flexibility (Arbuthnott and Frank 2000). Response inhibition, along with response selection, is a key factor in facilitating goal-directed behavior, and thus its relationship to ADHD is likely to have key importance. However, response inhibition is not the only executive process atypical in children with ADHD. Other domains, including working memory, set shifting, set maintenance, and planning have also been repeatedly identified as being atypical in the disorder (Barnett et al. 2001; Clark et al. 2000; Karatekin and Asarnow 1998; McLean et al. 2004; Nigg et al. 1998; Pennington and Ozonoff 1996). Importantly, as noted above, it is becoming clear that many of the brain regions and circuits related to the typical development of executive functioning are atypical in children with ADHD (see Sect. 6 below) (Bush et al. 1999; Rubia et al. 1999; Fair et al. 2012b; Mennes et al. 2011).
4.2 Sensory/Reward (Bottom-Up) Theories
While work in ADHD has mostly focused on top-down theories of the disorder, bottom-up, motivation, reward, and emotional regulation theories, which gained emphasis in the 1970s, have recently seen a surge in the literature (Barkley 2009; Luman et al. 2005; Nigg and Casey 2005; Sonuga-Barke 2005). Motivational or reward responding, either related to temporal discounting or to poor regulation of affect, are typically viewed in terms of “approach” behavior, which can also be construed as positive affectivity, or “avoid” behavior, construed as negative affectivity. Although not part of the diagnostic criteria, ADHD is often associated with problems with emotions, such as difficulty with anger, mood, and affect regulation.
Integrated models of ADHD propose conceptual consideration of both top-down cognitive control and bottom-up emotion regulation in understanding ADHD’s complex clinical picture, which is helpful when considering the neural bases of self-regulation (Barkley 2009; Nigg and Casey 2005). While reward anticipation heavily relies on ascending dopamine circuits, the overall neural systems involved in processing emotion and motivation include the limbic system, formed by the amygdala, PFC, ACC, ventral striatum or nucleus accumbens (NAc), and insula. The amygdala is linked to avoidance behavior, while the NAc is often viewed in terms of approach behavior (Nigg and Casey 2005). The PFC projects to both of these subcortical regions, probably through ACC, providing top-down regulation of emotional processes (Barkley 2009; Nigg and Casey 2005). Emotional regulation thus reflects the ability to change or delay the initial emotional response to create a behavior more supportive to the goal. Emotional self-regulation is closely connected to working memory systems and its dysfunction might be more related to inattentive symptoms (Barkley 2009). Emotional inhibition is associated with a skill of suppressing the prepotent emotional reaction to express a more socially accepted emotion or behavior; a deficit in emotional inhibition might be related to hyperactive and impulsive symptoms (Barkley 2009). Emotional problems involving self-regulation and inhibition are important characteristics of ADHD and both interact with each other (Barkley 2009; Nigg and Casey 2005).
Emotional processes are linked to motivation, which is involved with approach-avoidance behavior when experiencing an event or situation (Barkley 2009; Nigg and Casey 2005). Positive emotional valence (or expectation of reward) is related to approach, whereas negative valence (or expectation of non-reward) is associated with avoidance and withdrawal (Davidson et al. 1990; O’Doherty et al. 2003). Individuals with ADHD tend to give disproportionate weight to immediate rewards and are hypersensitive to delayed reward, thus they exhibit a behavior characterized by excessive approach (Nigg and Casey 2005). Besides the unregulated approach, individuals with ADHD also present a failure in avoidance, continuing to show certain behaviors even when it is resulting in negative consequences (Nigg and Casey 2005). Furthermore, studies assessing reward processes found that children with ADHD are more likely to choose small but immediate rewards than larger delayed rewards, when controls tend to choose larger delayed rewards (Luman et al. 2005). As a result of a negative emotion experienced when in a delay-rich environment, ADHD children manifest impulsive and overactive behavior (Sonuga-Barke 2005). Rewards for children with ADHD become atypically low in reinforcing power as the delay to reward becomes longer, as evidenced by over-responsiveness to immediate rewards but under-responsiveness to more distant reward contingencies (Tripp and Alsop 1999).
Similar to the executive top-down models, dopamine plays a key role in modulating the reward circuit, which includes the NAc of the ventral striatum (Wise 1980; Schultz et al. 1993; Robbins and Everitt 1992) and ACC and orbitofrontal cortical regions (Rogers et al. 2004). The circuit also includes the midbrain ventral tegmental area (Schultz 1997) as well as the amygdala, which is considered to provide contextual information to adjust motivational significance of incentives (Baxter and Murray 2002). Unlike with executive functions, the dopamine pathway important for reward processing is the mesolimbic dopamine pathway, which projects from the ventral tegmental area to the NAc. The reward circuit is important for facilitating reward-related incentive learning, appropriate responses to stimuli, and the development of goal-directed behaviors. Dysfunction of the NAc, via selective lesions, induces impulsivity in rats, though lesions to the ACC and medial PFC had no effect on delayed rewards (Cardinal et al. 2001).
5 Heterogeneity in ADHD
Clinical heterogeneity
Clinical presentation of ADHD is heterogeneous, and the phenotypes are extensive. This reality supports the notion that scientific study of ADHD should not simply focus on examining or considering ADHD patients as one homogeneous group, but consider it as a disorder that rides along a continuum. Some key considerations for sources of phenotypic heterogeneity include potential dimensionality of trait domains, comorbid behavioral and psychiatric profile with conduct disorder, anxiety disorder, major depressive disorder, or learning disorder (Jensen et al. 1997), as well as family history of ADHD (Seidman et al. 1995), and gender effects (Hinshaw et al. 2002; Nigg et al. 2002). With respect to comorbid profiles, the debate continues as to whether ADHD with conduct disorder/ aggression represents a different etiological pathway than ADHD without conduct problems (Banaschewski et al. 2003). This debate can also be made with regard to ADHD with and without anxiety (Jensen et al. 2001). Gender-based differences in the heterogeneity of ADHD remain an important issue to address in the field as well. Some studies have indicated that there are significant differences in deficits between girls and boys with ADHD (Gershon 2002; Nigg et al. 2002; Hasson and Fine 2012). Other studies have suggested that girls and boys with ADHD share similar clinical and neuropsychological profiles (Hinshaw et al. 2002).
As mentioned earlier, the DSM-V describes three presentations, identified in accordance with the most prevalent current symptoms (e.g., primarily inattentive, primarily impulsive/hyperactive, and a combination of both). These DSM-V presentations are explicitly not expected to be stable, as children diagnosed with the disorder can change presentation over time from year to year (Lahey et al. 2005). Furthermore, ADHD-inattentive presentation includes children who are sluggish and hypoactive as well as children who are active but shy of reaching criteria for ADHD-combined presentation, and some experts argue that these distinctions may represent etiologically distinct phenomena (Carlson and Mann 2002; Harrington and Waldman 2010; Bauermeister et al. 2012). Yet, ADHD is still heterogeneous. It is well accepted, for example, that the two symptom dimensions that tend to converge in ADHD have distinct neural and clinical correlates (Lahey et al. 2005; Willcutt et al. 2012). ADHD is also associated with cognitive impairments of inhibitory control and executive functions but neuropsychological profiles of subjects with ADHD have shown inter- and intra-individual variability.
Etiological heterogeneity
The clinical heterogeneity of ADHD points to a potential array of causal pathways leading to the development of the disorder. Until recently, causal models of ADHD, as well as other mental disorders, have typically proposed a single core dysfunction (Wakefield 1997). Investigators traditionally compared a group of children with ADHD defined by core symptoms (i.e., the DSM), to a group of control children without the disorder. Statistical group differences based on psychometrics, functional brain imaging, or genetics were then used to inform models of the disorder. This assumption of homogeneity in the case of ADHD has been questioned in numerous theoretical papers (Berger and Posner 2000; Sonuga-Barke 2002, 2005; Sonuga-Barke et al. 2008; Nigg et al. 2004, 2005). These data suggest that while numerous unique neuropsychological measures have been proposed as related to ADHD, each of them applies to only a subset of those with the disorder.
Recently, attention has been given to another form of heterogeneity that may assist in a better characterization of ADHD—heterogeneity in typical populations. While, perhaps, less palpable than heterogeneity in clinical samples, heterogeneity in typical populations may also be interfering with progress in understanding psychiatric illnesses. Investigators have often treated typically developing control populations as a monolithic group. However, there is considerable evidence that individual differences in successful, adaptive psychological styles are essential for human development, functioning, social cohesion, and health outcomes (Buss 1991; Goldman et al. 2005; Chapman and Goldberg 2011; Braver et al. 2010).
A recent report by our group (Fair et al. 2012a) has highlighted this potential. In the report, we applied community detection to a well-characterized data set of 498 children, which included both typically developing control youth (N = 213) and youth who met research criteria for ADHD (N = 285) (Fair et al. 2012a). We found several cognitive profiles that existed in both the ADHD and control populations. Importantly, clarifying what cognitive functions were atypical in any given child depended on the context (i.e., the profile) provided by the control populations (Fig. 2). In other words, a portion of the variation observed across neuropsychological abilities in typically developing populations appeared to be embedded into discrete communities. Perhaps even more importantly, the heterogeneity in individuals with ADHD appears to be “nested” in the normal variation. The authors highlight the importance in identifying mechanisms associated with a mental disorder, such as ADHD, for comparing individuals to well-adjusted persons with the same cognitive style or profile.
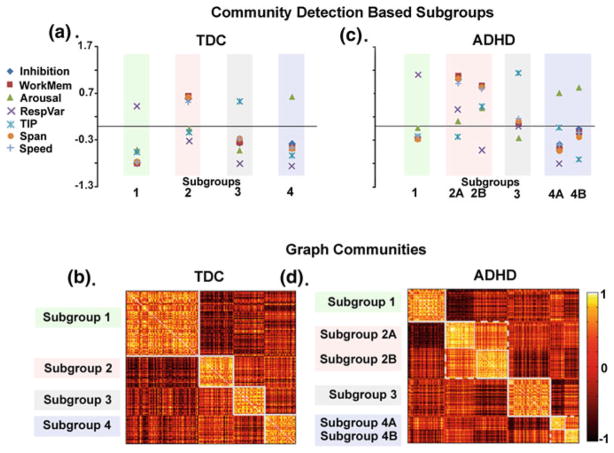
Fig. 2.
In a previous report, community detection was used to identify subgroups in typically developing controls (TDC) and ADHD child samples (Fair et al. 2012a). a Four unique subgroups (i.e., cognitive profiles) were identified in TDC and community structure is depicted by correlation matrices shown in (b). Darker colors on the grid show lower correlations between subjects, while lighter colors reveal positive correlations between subjects. Identified communities are outlined in white. c Applying the community detection algorithm to the ADHD cohort independently shows similar findings as in (a), with correlation matrices presented in (d). The authors highlight that, based on neuropsychological performance, TDC can be classified into distinct subgroups with high precision and the heterogeneity in individuals with ADHD may be “nested” in this normal variation
6 Neuroimaging
Noninvasive neuroimaging techniques have been very important in our understanding of the neural pathways thought to be disrupted in ADHD. While various noninvasive measures are often used to study ADHD (e.g., electroencephalography or EEG), here we focus on the most common, magnetic resonance imaging (MRI) techniques, as they are the most widely used to date. The three most common MRI techniques include structural or morphologic studies, which measures the size and shape of brain structures, diffusion tensor imaging (DTI), which is typically used to provide insight into the integrity of white matter fiber tracts, and functional MRI which can be used to measure task-dependent brain activity or task-independent functional connectivity.
6.1 Morphologic Changes Measured with MRI in Typical Development and ADHD
The earliest and likely most recognized work with regard to morphologic changes in brain development comes from the work of Giedd et al. (1996). These studies measured changes in cortical volume or gray matter thickness throughout development. These descriptions of white and gray matter development with MRI mostly agree with results from earlier histological work (Yakovlev and Lecours 1967; Benes et al. 1994; Paus 2005; Lenroot and Giedd 2006; Toga et al. 2006). The most consistent finding in white matter maturity is generally linear protracted development, which advances into young adulthood (Giedd et al. 1999; Toga et al. 2006; Paus 2005; Casey et al. 2005; Pfefferbaum et al. 1994). In contrast, gray matter development consists of mostly nonlinear changes that vary markedly in rate by brain region. Although studies differ on the details (Paus 2005; Giedd et al. 1999; Sowell et al. 2003; Gogtay et al. 2004; Toga et al. 2006), the general consensus appears to be a differential peak in gray matter volume (or density) between childhood and early adolescence that begins to decline during adolescence. Volume loss occurs earliest in primary sensorimotor areas and latest rostrally in the PFC and caudally/laterally into parietal and temporal cortex (Toga et al. 2006; Paus 2005; Sowell et al. 2001; Gogtay et al. 2004). We note here that this general description of white and gray matter development is only a partial account of a markedly complex process [for review see Toga et al. (2006)].
Developmental changes in brain matter volume are thought to be representative of processes such as synapse formation and myelination early in development (leading to increases in volume), and selective pruning and apoptosis (linked to decreases in volume) later in development. Volume changes throughout the brain are therefore thought to be critical for normal brain development. With that said, one particular challenge with using structural MRI is resolution. Every MRI image is a collection of voxels (usually on the millimeter scale), any one of which consists of a mixture of neurons (axons, dendrites, cell bodies), glia (including myelin), and blood vessels. For gray matter development, this partial volume effect makes it difficult to determine how these properties relate specifically to maturation. For example, increased myelination could potentially be misinterpreted as gray matter loss (Paus 2005; Gogtay et al. 2004). Importantly, the typical structural changes described above have been related to cognitive behaviors. For example, Casey et al. (1997) showed performance improvement in an attention task was correlated with higher ACC volume. Sowell et al. (2004) has shown that cortical thinning of the dorsal frontal and parietal lobes correlates with the verbal portion of the Weschler’s intelligence scale and that left hemispheric thinning was correlated with verbal IQ.
Perhaps not surprisingly, considering the systems involved and the relationships to behavior of morphologic changes over age, atypical trajectories of these changes have been linked to ADHD. A study by Shaw et al. (2006) shows that children with ADHD have cortical thinning throughout the cortex, but particularly in prefrontal regions associated with attention and other executive functions. Other groups have also shown cortical thinning in the frontal regions of individuals with ADHD (Depue et al. 2010; McAlonan et al. 2007; Batty et al. 2010). Depue et al. (2010) also showed that disrupted performance in behavioral tasks in ADHD (such as response inhibition, response variability, and processing speed) are associated with reduced volume in the inferior frontal gyrus. Gray matter reductions have also been reported in ADHD in the parietal, temporal, and occipital cortices (Carmona et al. 2005; Castellanos et al. 2002; Konrad et al. 2010; Shaw et al. 2007; Sowell et al. 2003). Subcortical regions such as the hippocampus and amygdala (Plessen et al. 2006), the basal ganglia, in both size and symmetry (Castellanos et al. 1994, 1996; Filipek et al. 1997; Ellison-Wright et al. 2008), and the cerebellum (Castellanos et al. 2002; Mackie et al. 2007) have been implicated as well.
6.2 Structural Changes Measured with DTI in Typical Development and ADHD
DTI has been especially useful in understanding typical brain development. DTI provides information about the directionality and coherence of water diffusion. Water molecules inside tissues experience random motion due to thermal energy, which is commonly referred to as Brownian motion (Le Bihan and Karni 1995). Diffusion-weighted MRI is sensitive to this Brownian motion because it magnetically labels moving protons. In the early 1990s, scientists observed that this diffusion coefficient was orientation-dependent in white matter (Douek et al. 1991; Basser et al. 1994). These orientation biases reflect underlying axonal orientation and are now used for mapping axonal bundle trajectories (Conturo et al. 1999; Mori and Barker 1999). Importantly, these diffusion characteristics have implications for the structural integrity, maturation, and organization of myelinated axons in white matter at the microstructural level (Johansen-Berg and Rushworth 2009; Nucifora et al. 2007).
There have been several studies examining the typical development of white matter integrity via DTI. While we will not be exhaustive here, there are a few studies that deserve mention. One study by Liston et al. (2006) used DTI to assess the development of fronto-striatal connectivity. The study revealed that diffusion in fronto-striatal white matter tracts becomes more restricted with age, from 7 to 30 years, paralleled by an age-associated increase in efficiency in cognitive task performance. The authors suggested that based on these data, fronto-striatal connectivity may support developmental and individual differences in the efficient recruitment of cognitive control. Another study by Bava et al. (2010) shows that during adolescence, an essential period of brain maturation and rewiring, changes in diffusion properties occur in the bilateral superior longitudinal fasciculi, superior corona radiata, anterior thalamic radiations, and posterior limb of the internal capsule, suggesting an ongoing refinement of projection and association fibers. The authors also found correlations with cognitive performance in adolescents, further suggesting that behavioral improvement corresponds with white matter changes. DTI has provided the basis for much of this work, demonstrating, for instance, that intellectual functioning in youth is associated with the development of white matter circuitry in bilateral frontal, occipito-parietal, and occipito-temporo- parietal regions (Schmithorst et al. 2005). In addition, the reading skills of children and adolescents improve with white matter changes in the internal capsule, corona radiata, and temporo-parietal regions (Beaulieu et al. 2005; Nagy et al. 2004; Niogi and McCandliss 2006; Qiu et al. 2008), and greater left lateralization of the arcuate fasciculus fibers is associated with improved phonological processing and receptive vocabulary (Lebel and Beaulieu 2009). Visuospatial working memory capacity is linked to a fronto-intraparietal network (Olesen et al. 2003), while better visuospatial construction and psychomotor performance is associated with high corpus callosum fractional anisotropy (Fryer et al. 2008). Faster response inhibition in children is associated with higher fractional anisotropy and lower perpendicular diffusivity in the right inferior frontal gyrus and presupplementary motor cortex (Madsen et al. 2010).
DTI has also informed our understanding of brain circuitry in ADHD (Konrad et al. 2010; Nagel et al. 2011; van Ewijk et al. 2012). Results from a meta-analysis by van Ewijk et al. (2012) show that white matter integrity is disrupted in children, adolescents, and adults with ADHD in regions and tracts such as the inferior and superior longitudinal fasciculus, anterior corona radiata, corticospinal tract, cingulum, corpus callosum, internal capsule, caudate nucleus, and cerebellum Integrity of fronto-striatal structural connectivity in both children and adults using DTI show disturbed connectivity in ADHD subjects compared to controls (Ashtari et al. 2005; Casey et al. 2007; Konrad et al. 2010; Qiu et al. 2011). Casey et al. (2007) showed that disruption in fronto-striatal fiber tracts of subjects with ADHD was correlated with lower performance on a go/no go task, suggesting atypical fronto-striatal circuitry affects cognitive control in children with ADHD. Importantly, some of these findings are strengthened by work showing their heritability (Casey et al. 2007; Lawrence et al. 2013). In a sibling study by Lawrence et al. (2013), individuals with ADHD and their unaffected siblings show similar differences in white matter microstructure, compared to controls, in the anterior thalamic radiation, forceps minor, and superior longitudinal fasciculus.
6.3 fMRI in Typical Development and ADHD
Task-based functional MRI is likely the most recognizable technique that measures brain function, due to its noninvasive nature, easy applicability, and widespread availability. Much of the work investigating the nature of typical and atypical functional brain development is drawn from functional MRI studies (Durston and Casey 2006; Luna and Sweeney 2001). Recent evidence suggests that the general rules of structural maturation (whereby sensory and motor area maturation is followed by association areas) do not necessarily hold true for “functional” maturation. Though association areas mature later, they are not sitting dormant or being unresponsive to specific task demands, but rather they are actively involved in information processing despite their structural immaturity. For example, Brown et al. found multiple types of changes over development for regions involved with lexical processing throughout early processing and higher associative regions (Brown et al. 2005). Work by Luna et al. (2001), Casey et al. (1995) and several others (Turkeltaub et al. 2003; Johnson et al. 2005; Mills and Neville 1997) have shown similar types of dynamics, such that frontal regions, despite being structurally immature, can have adult-like activation patterns for specific tasks. With that said, changes in these areas do occur and the strength of higher order activity in children, and across development, appear to be related to control-related processes. For example, studies have shown that a decrease in fronto-striatal regions was associated with impaired cognitive control (Bush et al. 1999; Durston et al. 2003; Konrad et al. 2006). Further evidence suggests that sensorimotor regions uncorrelated with task performance were recruited less in cognitive control tasks, whereas enhanced recruitment was observed in the ventral PFC correlated with task performance (Durston et al. 2006). Another study by Casey et al. (2002) examined the development of cognitive and neural systems involved in overriding a learned action in favor of a new one, which showed greater activation in the hippocampus and striatum in children than adults along with poorer task performance. The authors also found that adults recruited cortical regions more than children, suggesting the pattern of activation becomes more focused throughout development with maturation and learning.
Functional imaging studies in ADHD have been widespread. Lower activation in the dorsolateral PFC has also been associated with working memory deficits in adolescents with ADHD (Sheridan et al. 2007; Hart et al. 2012). Sheridan et al. (2007) suggest that the ability of individuals to perform a memory task more rapidly while recruiting less PFC can be viewed as efficient. A recent meta-analysis by Hart et al. (2012) shows that there are two main domains that are functionally abnormal in ADHD—attention networks (dorsolateral PFC, parietal cortex, and cerebellum) and inhibitory networks (including the inferior frontal cortex and ACC). These data support the top-down theory of atypical development, as we have described previously. In terms of examining reward (bottom-up) circuitry, children with ADHD have been found to exhibit reduced ventral striatal activity in response to reward, whereas healthy controls show the opposite relationship (Scheres et al. 2007; Plichta and Scheres 2013). It has been suggested that neural hyporesponsivity to anticipated reward may lead to increased reward-seeking behavior, correlated with symptoms of hyperactivity/impulsivity, to compensate for lower activation in the ventral striatum (Scheres et al. 2007). Similarly, adults with ADHD have been shown to have reduced ventral striatum activity during an anticipation of reward gain, while exhibiting enhanced orbitofrontal cortical activation in response to reward outcomes, a neural “dissociation” during reward processing (Strohle et al. 2008).
6.4 Functional Connectivity in Typical Development and ADHD
Typically, fMRI studies identify changes in activated brain regions in response to a stimulus or task compared to a baseline or control condition. To date, much of our understanding about brain function is drawn from task- or stimulus-based studies. However, investigations examining spontaneous brain activity via resting state functional connectivity MRI (rs-fcMRI) are beginning to surge (Biswal et al. 1995). These investigations attempt to characterize regional interactions while subjects are at rest, not performing a task. Rs-fcMRI comes from the discovery that spontaneous low-frequency (< ~0.1 Hz) blood oxygen level-dependent (BOLD) signal fluctuations between functionally related brain regions show strong correlations at rest (Biswal et al. 1995). Low-frequency BOLD fluctuations are thought to relate to spontaneous neural activity (Biswal et al. 1995; Nir et al. 2006; Leopold et al. 2003; Lowe et al. 1998) and cross-correlating the time series of two different regions allows the investigator to determine which regions are “functionally connected.” Rs-fcMRI measures are of interest because they are thought to reflect human anatomical connectivity (Koch et al. 2002; Quigley et al. 2003) and are less burdensome in experimental design, subject compliance, and training demands making it attractive for studies of development and clinical groups (Bokde et al. 2006; Greicius et al. 2004; Rombouts and Scheltens 2005; Tian et al. 2006; Whalley et al. 2005). It should be noted, however, that correlated activity of two brain regions may be a result of multi-synaptic pathways rather than a direct anatomical connection.
Rs-fcMRI has been used to examine systems organization of motor (Biswal et al. 1995), memory (Andrews-Hanna et al. 2007; Hampson et al. 2006a), language (Hampson et al. 2006b), attention (Fox et al. 2006), and control systems (Dosenbach et al. 2007; Fair et al. 2007; Seeley et al. 2007). Rs-fcMRI is becoming a frequently used tool for examining changes in network structure across development (Fair et al. 2007; Fransson et al. 2007; Kelly et al. 2009). This technique has been used to study several disease states including schizophrenia (Tononi and Edelman 2000), autism (Just et al. 2007), Alzheimer’s disease (Greicius et al. 2004), Tourette syndrome (Church et al. 2007), and adult ADHD (Castellanos et al. 2008). With regard to development, cortico–cortical interactions measured with rs-fcMRI have been investigated from birth through adulthood (Supekar et al. 2009; Fair et al. 2007, 2008, 2009, 2010a; Kelly et al. 2009; Fransson et al. 2007). It is largely believed that the changes in connectivity throughout development may contribute to the shift from reflexive, stimulus-bound behavior in childhood, to the goal-directed and more flexible functioning that is found in adulthood.2
Several studies suggest that the manner in which regions are anatomically and functionally connected play a significant role in neurodevelopmental disorders such as ADHD and autism (Castellanos et al. 2008; Just et al. 2007). Indeed, atypical correlated neural spontaneous activity has been tightly linked to ADHD (Castellanos et al. 2008; Fair et al. 2010b; Uddin et al. 2008; Mills et al. 2012; Costa Dias et al. 2013). For example, Castellanos et al. (2008) have utilized rs-fcMRI to characterize atypical cortical connections, showing decreased functional connectivity between the ACC and the precuneus in adults with ADHD. Other investigators have shown atypical functional connectivity of the default mode network in both children and adults with ADHD (Uddin et al. 2008; Fair et al. 2010b). Mills et al. (2012) have also previously shown that resting state functional connectivity between the thalamus and basal ganglia is atypical in children with ADHD. Another study by Costa Dias et al. (2013) found that atypical resting state functional connectivity in the NAc of children with ADHD, as well as increased connectivity between the NAc and the PFC in ADHD was associated with greater impulsivity.
7 Conclusions and Recommendations
Over the last two decades, there have been numerous technical and methodological advances available to clinicians and researchers to better understand ADHD and its etiology. Despite the growing body of literature investigating the disorder’s pathophysiology, ADHD remains a complex psychiatric disorder to characterize. Some of the major issues that remain unclear include subtype instability, heterogeneity (in both typical and atypical populations), and the neural correlates underlying the disorder. Further insight into the heritability of ADHD will be key in understanding the disorder’s development. Identifying endophenotypes, or intermediate phenotypes (measurable components that lie in between genes and observed symptoms), for instance, may improve the prospects of genetic studies. Also, understanding the heterogeneity found within ADHD populations will be critical in characterizing the ADHD phenotype and identifying stable, reliable subtypes. Further work in the heterogeneity and neurobiology of typically developing children will aid in these efforts to investigate the multiple pathways and circuits related to ADHD.
Footnotes
The DSM-V changed the age of onset from age 7 to 12, reduced the cut point for diagnosing ADHD in adults from six symptoms to five symptoms, and allowed concurrent diagnosis of ADHD and autism spectrum disorder. These changes are expected to have minimal effects on epidemiology or clinical practice, but make the criteria more congruent with empirical findings.
One of the major hurdles in both fMRI and fcMRI data analysis is head movement. Children often move more than adults and patients more than controls. The typical approaches to movement correction, however, may not be sufficient when processing functional connectivity data. This is particularly relevant for hyperkinetic disorders such as ADHD. Recent reports suggest that traditional motion correction may not be controlling for the changes in signal intensity due to changes in head position (Fair et al. 2012; Power et al. 2012; Satterthwaite et al. 2012; Van Dijk et al. 2012). There have been several new investigations, which are emerging rapidly that propose new methodological approaches toward correcting for movement-related artifacts from the scanner. While it is not clear which measurements are superior per se, it is clear that additional steps need to be taken in fcMRI preprocessing (and fMRI data for that matter) to correct for so called “micro-movements,” and that our current knowledge of atypical brain activity in ADHD needs to be carefully scrutinized.
Contributor Information
Marguerite Matthews, Department of Behavioral Neuroscience, Oregon Health and Science University, 3181 SW Sam Jackson Park Road, L470 Portland, OR 97239, USA.
Joel T. Nigg, Department of Behavioral Neuroscience, Oregon Health and Science University, 3181 SW Sam Jackson Park Road, L470 Portland, OR 97239, USA. Department of Psychiatry, Oregon Health and Science University, Portland, OR, USA. Department of Pediatrics, Oregon Health and Science University, Portland, OR, USA
Damien A. Fair, Email: faird@ohsu.edu, Department of Behavioral Neuroscience, Oregon Health and Science University, 3181 SW Sam Jackson Park Road, L470 Portland, OR 97239, USA. Department of Psychiatry, Oregon Health and Science University, Portland, OR, USA. Advanced Imaging Research Center, Oregon Health and Science University, Portland, OR, USA
References
- Achenbach T, Edelbrock C. Manual for the child behavior checklist and revised behavior profile. University of Vermont Department of Psychiatry; Burlington: 1983. [Google Scholar]
- Albayrak O, Friedel S, Schimmelmann BG, Hinney A, Hebebrand J. Genetic aspects in attention-deficit/hyperactivity disorder. J Neural transm. 2008;115(2):305–315. doi: 10.1007/s00702-007-0839-9. [DOI] [PubMed] [Google Scholar]
- Alberts-Corush J, Firestone P, Goodman JT. Attention and impulsivity characteristics of the biological and adoptive parents of hyperactive and normal control children. Am J orthopsychiatry. 1986;56(3):413–423. doi: 10.1111/j.1939-0025.1986.tb03473.x. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnott K, Frank J. Executive control in set switching: residual switch cost and task-set inhibition. Can J Exp Psychol. 2000;54(1):33–41. doi: 10.1037/h0087328. (Revue canadienne de psychologie experimentale) [DOI] [PubMed] [Google Scholar]
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69(12):e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Kumra S, Bhaskar SL, Clarke T, Thaden E, Cervellione KL, Rhinewine J, Kane JM, Adesman A, Milanaik R, Maytal J, Diamond A, Szeszko P, Ardekani BA. Attentiondeficit/ hyperactivity disorder: a preliminary diffusion tensor imaging study. Biol Psychiatry. 2005;57(5):448–455. doi: 10.1016/j.biopsych.2004.11.047. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and statistical manual of mental disorders. 2. American Psychiatric Association; Washington DC: 1968. [Google Scholar]
- Association AP. Diagnostic and statistical manual of mental disorders. 3. American Psychiatric Association; Washington DC: 1980. [Google Scholar]
- Association AP. Diagnostic and statistical manual of mental disorders. 3. American Psychiatric Association; Washington DC: 1987. Revised. [Google Scholar]
- Association AP. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; Washington DC: 1994. [Google Scholar]
- Bakker SC, van der Meulen EM, Buitelaar JK, Sandkuijl LA, Pauls DL, Monsuur AJ, van ‘t Slot R, Minderaa RB, Gunning WB, Pearson PL, Sinke RJ. A whole-genome scan in 164 Dutch sib pairs with attention-deficit/hyperactivity disorder: suggestive evidence for linkage on chromosomes 7p and 15q. Am J Hum Genet. 2003;72(5):1251–1260. doi: 10.1086/375143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaschewski T, Brandeis D, Heinrich H, Albrecht B, Brunner E, Rothenberger A. Association of ADHD and conduct disorder – brain electrical evidence for the existence of a distinct subtype. J Child Psychol Psychiatry. 2003;44(3):356–376. doi: 10.1111/1469-7610.00127. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Advances in the diagnosis and subtyping of attention deficit hyperactivity disorder: what may lie ahead for DSM-V. Revista de neurologia. 2009;48(Suppl 2):S101–S106. [PubMed] [Google Scholar]
- Barkley RA, Peters H. The earliest reference to ADHD in the medical literature? Melchior Adam Weikard’s description in 1775 of “attention deficit” (Mangel der Aufmerksamkeit, Attentio Volubilis) J Attention Disord. 2012;16(8):623–630. doi: 10.1177/1087054711432309. [DOI] [PubMed] [Google Scholar]
- Barnett R, Maruff P, Vance A, Luk ES, Costin J, Wood C, Pantelis C. Abnormal executive function in attention deficit hyperactivity disorder: the effect of stimulant medication and age on spatial working memory. Psychol Med. 2001;31(6):1107–11015. doi: 10.1017/s0033291701004172. [DOI] [PubMed] [Google Scholar]
- Barr CL, Xu C, Kroft J, Feng Y, Wigg K, Zai G, Tannock R, Schachar R, Malone M, Roberts W, Nothen MM, Grunhage F, Vandenbergh DJ, Uhl G, Sunohara G, King N, Kennedy JL. Haplotype study of three polymorphisms at the dopamine transporter locus confirm linkage to attention-deficit/hyperactivity disorder. Biol Psychiatry. 2001;49(4):333–339. doi: 10.1016/s0006-3223(00)01053-2. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty MJ, Liddle EB, Pitiot A, Toro R, Groom MJ, Scerif G, Liotti M, Liddle PF, Paus T, Hollis C. Cortical gray matter in attention-deficit/hyperactivity disorder: a structural magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49(3):229–238. doi: 10.1016/j.jaac.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauermeister JJ, Barkley RA, Bauermeister JA, Martinez JV, McBurnett K. Validity of the sluggish cognitive tempo, inattention, and hyperactivity symptom dimensions: neuropsychological and psychosocial correlates. J Abnorm Child Psychol. 2012;40(5):683–697. doi: 10.1007/s10802-011-9602-7. [DOI] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3(7):563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Plewes C, Paulson LA, Roy D, Snook L, Concha L, Phillips L. Imaging brain connectivity in children with diverse reading ability. NeuroImage. 2005;25(4):1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Becker MG, Isaac W, Hynd GW. Neuropsychological development of nonverbal behaviors attributed to “frontal lobe” functioning. Dev neuropsychol. 1987;3:275–298. [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51(6):477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Berger A, Posner MI. Pathologies of brain attentional networks. Neurosci Biobehav Rev. 2000;24(1):3–5. doi: 10.1016/s0149-7634(99)00046-9. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Keenan K, Benjamin J, Krifcher B, Moore C, Sprich-Buckminster S, Ugaglia K, Jellinek MS, Steingard R, et al. Further evidence for family-genetic risk factors in attention deficit hyperactivity disorder. Patterns of comorbidity in probands and relatives psychiatrically and pediatrically referred samples. Arch Gen Psychiatry. 1992;49(9):728–738. doi: 10.1001/archpsyc.1992.01820090056010. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Keenan K, Knee D, Tsuang MT. Family-genetic and psychosocial risk factors in DSM-III attention deficit disorder. J Am Acad Child Adolesc Psychiatry. 1990a;29(4):526–533. doi: 10.1097/00004583-199007000-00004. [DOI] [PubMed] [Google Scholar]
- Biederman J, Keenan K, Faraone SV. Parent-based diagnosis of attention deficit disorder predicts a diagnosis based on teacher report. J Am Acad Child Adolesc Psychiatry. 1990b;29(5):698–701. doi: 10.1097/00004583-199009000-00004. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Lopez-Bayo P, Meindl T, Pechler S, Born C, Faltraco F, Teipel SJ, Moller HJ, Hampel H. Functional connectivity of the fusiform gyrus during a face-matching task in subjects with mild cognitive impairment. Brain : a journal of neurology. 2006;129(Pt 5):1113–1124. doi: 10.1093/brain/awl051. [DOI] [PubMed] [Google Scholar]
- Bradley C. The behaviour of children receiving Benzedrine. Am J Psychiatry. 1937;94:577–585. [Google Scholar]
- Braver TS, Cole MW, Yarkoni T. Vive les differences! Individual variation in neural mechanisms of executive control. Curr Opin Neurobiol. 2010;20(2):242–250. doi: 10.1016/j.conb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39(4):713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15:275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33(2):301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Curr Opin Neurobiol. 2007;17(2):243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Burks H. The hyperkinetic child. Exceptional Children. 1960;27:18–28. [Google Scholar]
- Burt SA. Rethinking environmental contributions to child and adolescent psychopathology: a meta-analysis of shared environmental influences. Psychol Bull. 2009;135(4):608–637. doi: 10.1037/a0015702. [DOI] [PubMed] [Google Scholar]
- Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol Psychiatry. 1999;45(12):1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Buss DM. Evolutionary personality psychology. Annu Rev Psychol. 1991;42:459–491. doi: 10.1146/annurev.ps.42.020191.002331. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292(5526):2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Carlson CL, Mann M. Sluggish cognitive tempo predicts a different pattern of impairment in the attention deficit hyperactivity disorder, predominantly inattentive type. J Clin Child Adolesc Psychol (the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53) 2002;31(1):123–129. doi: 10.1207/S15374424JCCP3101_14. [DOI] [PubMed] [Google Scholar]
- Carmona S, Vilarroya O, Bielsa A, Tremols V, Soliva JC, Rovira M, Tomas J, Raheb C, Gispert JD, Batlle S, Bulbena A. Global and regional gray matter reductions in ADHD: a voxel-based morphometric study. Neurosci Lett. 2005;389(2):88–93. doi: 10.1016/j.neulet.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, Giedd J, Kaysen D, Hertz-Pannier L, Rapoport JL. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. NeuroImage. 1995;2(3):221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Tonev ST, Spicer J, Niogi S, Millner AJ, Reiss A, Garrett A, Hinshaw SP, Greenhill LL, Shafritz KM, Vitolo A, Kotler LA, Jarrett MA, Glover G. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. The American journal of psychiatry. 2007;164(11):1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Davidson MC, Kunz K, Franzen PL. Dissociating striatal and hippocampal function developmentally with a stimulus-response compatibility task. J Neurosci (the official journal of the Society for Neuroscience) 2002;22(19):8647–8652. doi: 10.1523/JNEUROSCI.22-19-08647.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. J Cogn Neurosci. 1997;9(6):835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Eckburg P, Marsh WL, Vaituzis AC, Kaysen D, Hamburger SD, Rapoport JL. Quantitative morphology of the caudate nucleus in attention deficit hyperactivity disorder. Am J Psychiatry. 1994;151(12):1791–1796. doi: 10.1176/ajp.151.12.1791. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vauss YC, Snell JW, Lange N, Kaysen D, Krain AL, Ritchie GF, Rajapakse JC, Rapoport JL. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53(7):607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. J Am Med Assoc, JAMA. 2002;288(14):1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly AMC, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJS, Rotrosen J, Adler LA, Milham MP. Cingulate-precuneus interactions: A new locus of dysfunction in adult attention-deficit/ hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Chapman BP, Goldberg LR. Replicability and 40-year predictive power of childhood ARC types. J Pers Soc Psychol. 2011;101(3):593–606. doi: 10.1037/a0024289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess S. Diagnosis and treatment of the hyperactive child. NY State J Med. 1960;60:2379–2385. [PubMed] [Google Scholar]
- Church JA, Fair DA, Dosenbach NU, Petersen SE, Schlaggar BL. Using functional connectivity MRI to study cognitive development: the abnormal structure of distinct task control networks in Tourette syndrome. Society for Neuroscience; San Diego: 2007. [Google Scholar]
- Clark C, Prior M, Kinsella GJ. Do executive function deficits differentiate between adolescents with ADHD and oppositional defiant/conduct disorder? A neuropsychological study using the six elements test and Hayling sentence completion test. J Abnorm Child Psychol. 2000;28(5):403–414. doi: 10.1023/a:1005176320912. [DOI] [PubMed] [Google Scholar]
- Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder A, Shimony JS, McKinstry RC, Burton H, Raichle ME. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci USA. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook EH, Jr, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, Leventhal BL. Association of attention-deficit disorder and the dopamine transporter gene. Am J Hum Genet. 1995;56(4):993–998. [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Costa Dias TG, Wilson VB, Bathula DR, Iyer SP, Mills KL, Thurlow BL, Stevens CA, Musser ED, Carpenter SD, Grayson DS, Mitchell SH, Nigg JT, Fair DA. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur neuropsychopharmacol (the journal of the European College of Neuropsychopharmacology) 2013;23(1):33–45. doi: 10.1016/j.euroneuro.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2006;16(4):475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Ekman P, Saron CD, Senulis JA, Friesen WV. Approach-withdrawal and cerebral asymmetry: emotional expression and brain physiology. I. J Pers Soc Psychol. 1990;58(2):330–341. [PubMed] [Google Scholar]
- Depue BE, Burgess GC, Bidwell LC, Willcutt EG, Banich MT. Behavioral performance predicts grey matter reductions in the right inferior frontal gyrus in young adults with combined type ADHD. Psychiatry Res. 2010;182(3):231–237. doi: 10.1016/j.pscychresns.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douek P, Turner R, Pekar J, Patronas N, Le Bihan D. MR color mapping of myelin fiber orientation. J Comput Assist Tomogr. 1991;15(6):923–929. doi: 10.1097/00004728-199111000-00003. [DOI] [PubMed] [Google Scholar]
- Douglas VI. Stop, look, and listen: the problem of sustained attention and impulse control in hyperactive and normal children. Can J Behav Sci. 1972;4:259–282. [Google Scholar]
- Doyle AE. Executive functions in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(8):21–26. [PubMed] [Google Scholar]
- Durston S. Converging methods in studying attention-deficit/hyperactivity disorder: what can we learn from neuroimaging and genetics? Dev Psychopathol. 2008;20(4):1133–1143. doi: 10.1017/S0954579408000539. S0954579408000539 [pii] [DOI] [PubMed] [Google Scholar]
- Durston S, Casey BJ. What have we learned about cognitive development from neuroimaging? Neuropsychologia. 2006;44(11):2149–2157. doi: 10.1016/j.neuropsychologia.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Durston S, Mulder M, Casey BJ, Ziermans T, van Engeland H. Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60(10):1062–1070. doi: 10.1016/j.biopsych.2005.12.020. S0006-3223(06)00389-1 [pii] [DOI] [PubMed] [Google Scholar]
- Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, Ulug AM, Casey BJ. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry. 2003;53(10):871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. Lesions of the medial prefrontal cortex or nucleus accumbens core do not impair inhibitory control in rats performing a stop-signal reaction time task. Behav Brain Res. 2003;146(1–2):131–144. doi: 10.1016/j.bbr.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Ebaugh FG. Neuropsychiatric sequelae of acute epidemic encephalitis in children. Am J Dis Child. 1923;25:89–97. doi: 10.1177/1087054707305340. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Morris AS. Children’s emotion-related regulation. Adv Child Dev Behav. 2002;30:189–229. [PubMed] [Google Scholar]
- Ellison-Wright I, Ellison-Wright Z, Bullmore E. Structural brain change in attention deficit hyperactivity disorder identified by meta-analysis. BMC psychiatry. 2008;8:51. doi: 10.1186/1471-244x-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Mills KL, Dias TG, Blythe MS, Zhang D, Snyder AZ, Raichle ME, Stevens AA, Nigg JT, Nagel BJ. Maturing thalamocortical functional connectivity across development. Front Syst Neurosci. 2010a;4:10. doi: 10.3389/fnsys.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc Natl Acad Sci USA. 2012a;109(17):6769–6774. doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain’s default network. PNAS. 2008;105(10):4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA. 2007;104(33):13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NU, Schlaggar BL, Mennes M, Gutman D, Bangaru S, Buitelaar JK, Dickstein DP, Di Martino A, Kennedy DN, Kelly C, Luna B, Schweitzer JB, Velanova K, Wang YF, Mostofsky S, Castellanos FX, Milham MP. Distinct neural signatures detected for ADHD subtypes after controlling for micromovements in resting state functional connectivity MRI data. Front Syst Neurosci. 2012b;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Posner J, Nagel BJ, Bathula D, Dias TG, Mills KL, Blythe MS, Giwa A, Schmitt CF, Nigg JT. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010b;68(12):1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Keenan K, Tsuang MT. Separation of DSM-III attention deficit disorder and conduct disorder: evidence from a family-genetic study of American child psychiatric patients. Psychol Med. 1991;21(1):109–121. doi: 10.1017/s0033291700014707. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Mick E, Williamson S, Wilens T, Spencer T, Weber W, Jetton J, Kraus I, Pert J, Zallen B. Family study of girls with attention deficit hyperactivity disorder. Am J Psychiatry. 2000a;157(7):1077–1083. doi: 10.1176/appi.ajp.157.7.1077. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Milberger S. An exploratory study of ADHD among second-degree relatives of ADHD children. Biol Psychiatry. 1994;35(6):398–402. doi: 10.1016/0006-3223(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Monuteaux MC. Toward guidelines for pedigree selection in genetic studies of attention deficit hyperactivity disorder. Genet Epidemiol. 2000b;18(1):1–16. doi: 10.1002/(SICI)1098-2272(200001)18:1<1::AID-GEPI1>3.0.CO;2-X. [pii] [DOI] [PubMed] [Google Scholar]
- Faraone SV, Doyle AE, Mick E, Biederman J. Meta-analysis of the association between the 7-repeat allele of the dopamine D(4) receptor gene and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158(7):1052–1057. doi: 10.1176/appi.ajp.158.7.1052. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. S0006-3223(04)01226-0 [pii] [DOI] [PubMed] [Google Scholar]
- Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48(3):589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- Fisher SE, Francks C, McCracken JT, McGough JJ, Marlow AJ, MacPhie IL, Newbury DF, Crawford LR, Palmer CG, Woodward JA, Del’Homme M, Cantwell DP, Nelson SF, Monaco AP, Smalley SL. A genomewide scan for loci involved in attention-deficit/ hyperactivity disorder. Am J Hum Genet. 2002;70(5):1183–1196. doi: 10.1086/340112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, Lagercrantz H, Aden U. Resting-state networks in the infant brain. Proc Natl Acad Sci USA. 2007;104(39):15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Frank LR, Spadoni AD, Theilmann RJ, Nagel BJ, Schweinsburg AD, Tapert SF. Microstructural integrity of the corpus callosum linked with neuropsychological performance in adolescents. Brain Cogn. 2008;67(2):225–233. doi: 10.1016/j.bandc.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon J. A meta-analytic review of gender differences in ADHD. J Attention Disord. 2002;5(3):143–154. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6(4):551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18(6):932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet. 2009;126(1):51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6(7):521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Goyette CH, Conners CK, Ulrich RF. Normative data on revised conners parent and teacher rating scales. J Abnorm Child Psychol. 1978;6(2):221–236. doi: 10.1007/BF00919127. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci (the official journal of the Society for Neuroscience) 2006a;26(51):13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Sun Z, Schafer RJ, Skudlarski P, Gore JC, Constable RT. Connectivity-behavior analysis reveals that functional connectivity between left BA39 and Broca’s area varies with reading ability. NeuroImage. 2006b;31(2):513–519. doi: 10.1016/j.neuroimage.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Harrington KM, Waldman ID. Evaluating the utility of sluggish cognitive tempo in discriminating among DSM-IV ADHD subtypes. J Abnorm Child Psychol. 2010;38(2):173–184. doi: 10.1007/s10802-009-9355-8. [DOI] [PubMed] [Google Scholar]
- Hart H, Radua J, Mataix-Cols D, Rubia K. Meta-analysis of fMRI studies of timing in attention-deficit hyperactivity disorder (ADHD) Neurosci Biobehav Rev. 2012;36(10):2248–2256. doi: 10.1016/j.neubiorev.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Hasson R, Fine JG. Gender differences among children with ADHD on continuous performance tests: a meta-analytic review. J Attention Disord. 2012;16(3):190–198. doi: 10.1177/1087054711427398. [DOI] [PubMed] [Google Scholar]
- Hebebrand J, Dempfle A, Saar K, Thiele H, Herpertz-Dahlmann B, Linder M, Kiefl H, Remschmidt H, Hemminger U, Warnke A, Knolker U, Heiser P, Friedel S, Hinney A, Schafer H, Nurnberg P, Konrad K. A genome-wide scan for attention-deficit/hyperactivity disorder in 155 German sib-pairs. Mol Psychiatry. 2006;11(2):196–205. doi: 10.1038/sj.mp.4001761. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, Carte ET, Sami N, Treuting JJ, Zupan BA. Preadolescent girls with attention-deficit/ hyperactivity disorder: II. Neuropsychological performance in relation to subtypes and individual classification. J Consult Clin Psychol. 2002;70(5):1099–1111. doi: 10.1037//0022-006x.70.5.1099. [DOI] [PubMed] [Google Scholar]
- Hohman LB. Post-encephalitic behavior disorders in children. Johns Hopkins Hosp Bull. 1922;33:372–375. [Google Scholar]
- Jensen PS, Hinshaw SP, Kraemer HC, Lenora N, Newcorn JH, Abikoff HB, March JS, Arnold LE, Cantwell DP, Conners CK, Elliott GR, Greenhill LL, Hechtman L, Hoza B, Pelham WE, Severe JB, Swanson JM, Wells KC, Wigal T, Vitiello B. ADHD comorbidity findings from the MTA study: comparing comorbid subgroups. J Am Acad Child Adolesc Psychiatry. 2001;40(2):147–158. doi: 10.1097/00004583-200102000-00009. [DOI] [PubMed] [Google Scholar]
- Jensen PS, Martin D, Cantwell DP. Comorbidity in ADHD: implications for research, practice, and DSM-V. J Am Acad Child Adolesc Psychiatry. 1997;36(8):1065–1079. doi: 10.1097/00004583-199708000-00014. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF. Using diffusion imaging to study human connectional anatomy. Annu Rev Neurosci. 2009;32:75–94. doi: 10.1146/annurev.neuro.051508.135735. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Griffin R, Csibra G, Halit H, Farroni T, de Haan M, Tucker LA, Baron-Cohen S, Richards J. The emergence of the social brain network: evidence from typical and atypical development. Dev Psychopathol. 2005;17(3):599–619. doi: 10.1017/S0954579405050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17(4):951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatekin C, Asarnow RF. Working memory in childhood-onset schizophrenia and attention-deficit/hyperactivity disorder. Psychiatry Res. 1998;80(2):165–176. doi: 10.1016/s0165-1781(98)00061-4. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19(3):640–657. doi: 10.1093/cercor/bhn117. bhn117 [pii] [DOI] [PubMed] [Google Scholar]
- Klein RG, Mannuzza S, Olazagasti MA, Roizen E, Hutchison JA, Lashua EC, Castellanos FX. Clinical and functional outcome of childhood attention-deficit/hyperactivity disorder 33 years later. Arch Gen Psychiatry. 2012;69(12):1295–1303. doi: 10.1001/archgenpsychiatry.2012.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenberg L, Korkman M, Lahti-Nuuttila P. Differential development of attention and executive functions in 3- to 12-year-old Finnish children. Dev Neuropsychol. 2001;20(1):407–428. doi: 10.1207/S15326942DN2001_6. [DOI] [PubMed] [Google Scholar]
- Koch MA, Norris DG, Hund-Georgiadis M. An investigation of functional and anatomical connectivity using magnetic resonance imaging. NeuroImage. 2002;16(1):241–250. doi: 10.1006/nimg.2001.1052. [DOI] [PubMed] [Google Scholar]
- Konrad A, Dielentheis TF, El Masri D, Bayerl M, Fehr C, Gesierich T, Vucurevic G, Stoeter P, Winterer G. Disturbed structural connectivity is related to inattention and impulsivity in adult attention deficit hyperactivity disorder. Eur J Neurosci. 2010;31(5):912–919. doi: 10.1111/j.1460-9568.2010.07110.x. [DOI] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Hanisch C, Fink GR, Herpertz-Dahlmann B. Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: evidence from an event-related functional magnetic resonance imaging study. Biol Psychiatry. 2006;59(7):643–651. doi: 10.1016/j.biopsych.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Pelham WE, Loney J, Lee SS, Willcut E. Instability of the DSM-IV subtypes of ADHD from preschool through elementary school. Arch Gen Psychiatry. 2005;62(8):896–902. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Pelham WE, Schaughency EA, Atkins MS, Murphy HA, Hynd G, Russo M, Hartdagen S, Lorys-Vernon A. Dimensions and types of attention deficit disorder. J Am Acad Child Adolesc Psychiatry. 1988;27(3):330–335. doi: 10.1097/00004583-198805000-00011. [DOI] [PubMed] [Google Scholar]
- Lawrence KE, Levitt JG, Loo SK, Ly R, Yee V, O’Neill J, Alger J, Narr KL. White matter microstructure in subjects with attention-deficit/hyperactivity disorder and their siblings. J Am Acad Child Adolesc Psychiatry. 2013;52(4):431–440. e434. doi: 10.1016/j.jaac.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D, Karni A. Applications of magnetic resonance imaging to the study of human brain function. Curr Opin Neurobiol. 1995;5(2):231–237. doi: 10.1016/0959-4388(95)80031-x. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Hum Brain Mapp. 2009;30(11):3563–3573. doi: 10.1002/hbm.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Murayama Y, Logothetis NK. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb Cortex. 2003;13(4):422–433. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- Levin HS, Culhane KA, Hartmann J, Evankovich K, Mattson AJ, Harward H, Ringholz G, Ewing-Cobbs L, Fletcher JM. Developmental changes in performance on tests of purported frontal lobe functioning. Dev Neuropsychol. 1991;7:377–395. [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex. 2006;16(4):553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage. 1998;7(2):119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin Psychol Rev. 2005;25(2):183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. Studies of brain and cognitive maturation through childhood and adolescence: a strategy for testing neurodevelopmental hypotheses. Schizophr Bull. 2001;27(3):443–455. doi: 10.1093/oxfordjournals.schbul.a006886. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. NeuroImage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Luria AR. Higher cortical functions in man (trans: Haigh B) 2. Basic Books; New York: 1966/1980. [Google Scholar]
- Lyon G, Krasnegor N. Attention, memory, and executive function. Brookes Publishing; Baltimore: 1996. [Google Scholar]
- Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF, 3rd, Sharp WS, Giedd JN, Rapoport JL. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry. 2007;164(4):647–655. doi: 10.1176/appi.ajp.164.4.647. [DOI] [PubMed] [Google Scholar]
- Madsen KS, Baare WF, Vestergaard M, Skimminge A, Ejersbo LR, Ramsoy TZ, Gerlach C, Akeson P, Paulson OB, Jernigan TL. Response inhibition is associated with white matter microstructure in children. Neuropsychologia. 2010;48(4):854–862. doi: 10.1016/j.neuropsychologia.2009.11.001. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Cheung V, Cheung C, Chua SE, Murphy DG, Suckling J, Tai KS, Yip LK, Leung P, Ho TP. Mapping brain structure in attention deficit-hyperactivity disorder: A voxel-based MRI study of regional grey and white matter volume. Psychiatry Res. 2007;154(2):171–180. doi: 10.1016/j.pscychresns.2006.09.006. [DOI] [PubMed] [Google Scholar]
- McLean A, Dowson J, Toone B, Young S, Bazanis E, Robbins TW, Sahakian BJ. Characteristic neurocognitive profile associated with adult attention-deficit/hyperactivity disorder. Psychol Med. 2004;34(4):681–692. doi: 10.1017/S0033291703001296. [DOI] [PubMed] [Google Scholar]
- Mennes M, Vega Potler N, Kelly C, Di Martino A, Castellanos FX, Milham MP. Resting state functional connectivity correlates of inhibitory control in children with attention-deficit/ hyperactivity disorder. Front Psychiatry. 2011;2:83. doi: 10.3389/fpsyt.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mills DL, Neville HJ. Electrophysiological studies of language and language impairment. Semin Pediatr Neurol. 1997;4(2):125–134. doi: 10.1016/s1071-9091(97)80029-0. [DOI] [PubMed] [Google Scholar]
- Mills KL, Bathula D, Dias TG, Iyer SP, Fenesy MC, Musser ED, Stevens CA, Thurlow BL, Carpenter SD, Nagel BJ, Nigg JT, Fair DA. Altered cortico-striatal-thalamic connectivity in relation to spatial working memory capacity in children with ADHD. Front Psychiatry. 2012;3:2. doi: 10.3389/fpsyt.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Barker PB. Diffusion magnetic resonance imaging: its principle and applications. Anat Rec. 1999;257(3):102–109. doi: 10.1002/(SICI)1097-0185(19990615)257:3<102::AID-AR7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: does self-control resemble a muscle? Psychol Bull. 2000;126(2):247–259. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Bathula D, Herting M, Schmitt C, Kroenke CD, Fair D, Nigg JT. Altered white matter microstructure in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(3):283–292. doi: 10.1016/j.jaac.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Neale BM, Medland SE, Ripke S, Asherson P, Franke B, Lesch KP, Faraone SV, Nguyen TT, Schafer H, Holmans P, Daly M, Steinhausen HC, Freitag C, Reif A, Renner TJ, Romanos M, Romanos J, Walitza S, Warnke A, Meyer J, Palmason H, Buitelaar J, Vasquez AA, Lambregts-Rommelse N, Gill M, Anney RJ, Langely K, O’Donovan M, Williams N, Owen M, Thapar A, Kent L, Sergeant J, Roeyers H, Mick E, Biederman J, Doyle A, Smalley S, Loo S, Hakonarson H, Elia J, Todorov A, Miranda A, Mulas F, Ebstein RP, Rothenberger A, Banaschewski T, Oades RD, Sonuga-Barke E, McGough J, Nisenbaum L, Middleton F, Hu X, Nelson S, Psychiatric GCAS. Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(9):884–897. doi: 10.1016/j.jaac.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. The ADHD response-inhibition deficit as measured by the Stop Task: replication with DSM-IV combined type, extension, and qualification. J Abnorm Child Psychol. 1999;27(5):393–402. doi: 10.1023/a:1021980002473. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Is ADHD a disinhibitory disorder? Psychol Bull. 2001;127(5):571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biol Psychiatry. 2005;57(11):1424–1435. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Attention-deficit/hyperactivity disorder and adverse health outcomes. Clin Psychol Rev. 2013;33(2):215–228. doi: 10.1016/j.cpr.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Barkley RA. Attention deficit hyperactivity disorder. In: Mash E, Barkley RA, editors. Developmental psychopathology. Guilford Press; New York: In press. [Google Scholar]
- Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD. Neuropsychological executive functions and DSM-IV ADHD subtypes. J Am Acad Child Adolesc Psychiatry. 2002;41(1):59–66. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 2005;17(3):785–806. doi: 10.1017/S0954579405050376. S0954579405050376 [pii] [DOI] [PubMed] [Google Scholar]
- Nigg JT, Goldsmith HH, Sachek J. Temperament and attention deficit hyperactivity disorder: the development of a multiple pathway model. J Clin Child Adolesc Psychol (the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53) 2004;33(1):42–53. doi: 10.1207/S15374424JCCP3301_5. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Hinshaw SP, Carte ET, Treuting JJ. Neuropsychological correlates of childhood attention-deficit/hyperactivity disorder: explainable by comorbid disruptive behavior or reading problems? J Abnorm Psychol. 1998;107(3):468–480. doi: 10.1037/0021-843X.107.3.468. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/ hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol Psychiatry. 2005;57(11):1224–1230. doi: 10.1016/j.biopsych.2004.08.025. S0006-3223(04)00939-4 [pii] [DOI] [PubMed] [Google Scholar]
- Nijmeijer JS, Hartman CA, Rommelse NN, Altink ME, Buschgens CJ, Fliers EA, Franke B, Minderaa RB, Ormel J, Sergeant JA, Verhulst FC, Buitelaar JK, Hoekstra PJ. Perinatal risk factors interacting with catechol O-methyltransferase and the serotonin transporter gene predict ASD symptoms in children with ADHD. J Child Psychol Psychiatry. 2010;51(11):1242–1250. doi: 10.1111/j.1469-7610.2010.02277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi SN, McCandliss BD. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia. 2006;44(11):2178–2188. doi: 10.1016/j.neuropsychologia.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Nir Y, Hasson U, Levy I, Yeshurun Y, Malach R. Widespread functional connectivity and fMRI fluctuations in human visual cortex in the absence of visual stimulation. NeuroImage. 2006;30(4):1313–1324. doi: 10.1016/j.neuroimage.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Nucifora PG, Verma R, Lee SK, Melhem ER. Diffusion-tensor MR imaging and tractography: exploring brain microstructure and connectivity. Radiology. 2007;245(2):367–384. doi: 10.1148/radiol.2452060445. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38(2):329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Ogdie MN, Macphie IL, Minassian SL, Yang M, Fisher SE, Francks C, Cantor RM, McCracken JT, McGough JJ, Nelson SF, Monaco AP, Smalley SL. A genomewide scan for attention-deficit/hyperactivity disorder in an extended sample: suggestive linkage on 17p11. Am J Hum Genet. 2003;72(5):1268–1279. doi: 10.1086/375139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain Res Cogn Brain Res. 2003;18(1):48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Passler MA, Isaac W, Hynd GW. Neuropsychological development of behavior attributed to frontal lobe functioning in children. Dev Neuropsychol. 1985;1:349–370. [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9(2):60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Foster EM, Robb JA. The economic impact of attention-deficit/hyperactivity disorder in children and adolescents. J Pediatr Psychol. 2007;32(6):711–727. doi: 10.1093/jpepsy/jsm022. [DOI] [PubMed] [Google Scholar]
- Pennington BF. Dimensions of executive functions in normal and abnormal development. In: Krasnegor N, Lyon R, Goldman-Rakic P, editors. Development of the prefrontal cortex: evolution, neurobiology, and behavior. Brookes Publishing; Baltimore: 1997. pp. 265–281. [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. J Child Psychol Psychiatry. 1996;37(1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, Martin L, Durkin K, Blair C, Royal J, Hugdahl K, Peterson BS. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63(7):795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Scheres A. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Qiu D, Tan LH, Zhou K, Khong PL. Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. NeuroImage. 2008;41(2):223–232. doi: 10.1016/j.neuroimage.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Qiu MG, Ye Z, Li QY, Liu GJ, Xie B, Wang J. Changes of brain structure and function in ADHD children. Brain Topogr. 2011;24(3–4):243–252. doi: 10.1007/s10548-010-0168-4. [DOI] [PubMed] [Google Scholar]
- Quigley M, Cordes D, Turski P, Moritz C, Haughton V, Seth R, Meyerand ME. Role of the corpus callosum in functional connectivity. AJNR Am J Neuroradiol. 2003;24(2):208–212. [PMC free article] [PubMed] [Google Scholar]
- Rivero O, Sich S, Popp S, Schmitt A, Franke B, Lesch KP. Impact of the ADHD-susceptibility gene CDH13 on development and function of brain networks. Eur Neuropsychopharmacol (the journal of the European College of Neuropsychopharmacology) 2013;23(6):492–507. doi: 10.1016/j.euroneuro.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Functions of dopamine in the dorsal and ventral striatum. Semin Neurosci. 1992;4(2):119–127. [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004;55(6):594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Rombouts S, Scheltens P. Functional connectivity in elderly controls and AD patients using resting state fMRI: a pilot study. Curr Alzheimer Res. 2005;2(2):115–116. doi: 10.2174/1567205053585783. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Bullmore ET. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am J Psychiatry. 1999;156(6):891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Gough PM, Alexander IH, Kyriazis D, McDonald KR, Passingham RE. Attentional selection and action selection in the ventral and orbital prefrontal cortex. J Neurosci (the official journal of the Society for Neuroscience) 2005;25(50):11628–11636. doi: 10.1523/JNEUROSCI.2765-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 2005;28(3):397–419. doi: 10.1017/S0140525X05000075. (discussion 419–368) [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE. Prefrontal interactions reflect future task operations. Nat Neurosci. 2003;6(1):75–81. doi: 10.1038/nn987. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE. Prefrontal set activity predicts rule-specific neural processing during subsequent cognitive performance. J Neurosci (the official journal of the Society for Neuroscience) 2006;26(4):1211–1218. doi: 10.1523/JNEUROSCI.3887-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat Neurosci. 2002;5(5):479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61(5):720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum Brain Mapp. 2005;26(2):139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7(2):191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T, Romo R, Scarnati E. Reward-related activity in the monkey striatum and substantia nigra. Prog Brain Res. 1993;99:227–235. doi: 10.1016/s0079-6123(08)61349-7. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci (the official journal of the Society for Neuroscience) 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Faraone SV, Milberger S, Norman D, Seiverd K, Benedict K, Guite J, Mick E, Kiely K. Effects of family history and comorbidity on the neuropsychological performance of children with ADHD: preliminary findings. J Am Acad Child Adolesc Psychiatry. 1995;34(8):1015–1024. doi: 10.1097/00004583-199508000-00011. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Faraone SV, Weber W, Ouellette C. Toward defining a neuropsychology of attention deficit-hyperactivity disorder: performance of children and adolescents from a large clinically referred sample. J Consult Clin Psychol. 1997;65(1):150–160. doi: 10.1037/0022-006X.65.1.150. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA. 2007;104(49):19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, Giedd J, Castellanos FX, Rapoport J. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63(5):540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Sheridan MA, Hinshaw S, D’Esposito M. Efficiency of the prefrontal cortex during working memory in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(10):1357–1366. doi: 10.1097/chi.0b013e31812eecf7. [DOI] [PubMed] [Google Scholar]
- Sherman DK, Iacono WG, McGue MK. Attention-deficit hyperactivity disorder dimensions: a twin study of inattention and impulsivity-hyperactivity. J Am Acad Child Adolesc Psychiatry. 1997;36(6):745–753. doi: 10.1097/00004583-199706000-00010. [DOI] [PubMed] [Google Scholar]
- Smalley SL, Kustanovich V, Minassian SL, Stone JL, Ogdie MN, McGough JJ, McCracken JT, MacPhie IL, Francks C, Fisher SE, Cantor RM, Monaco AP, Nelson SF. Genetic linkage of attention-deficit/hyperactivity disorder on chromosome 16p13, in a region implicated in autism. Am J Hum Genet. 2002;71(4):959–963. doi: 10.1086/342732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Psychological heterogeneity in AD/HD–a dual pathway model of behaviour and cognition. Behav Brain Res. 2002;130(1–2):29–36. doi: 10.1016/s0166-4328(01)00432-6. S0166432801004326. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57(11):1231–1238. doi: 10.1016/j.biopsych.2004.09.008. S0006-3223(04)00948-5 [pii] [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Sergeant JA, Nigg J, Willcutt E. Executive dysfunction and delay aversion in attention deficit hyperactivity disorder: nosologic and diagnostic implications. Child Adolesc Psychiatric Clin North Am. 2008;17(2):367–384. ix. doi: 10.1016/j.chc.2007.11.008. S1056-4993(07)00122-8 [pii] [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci (the official journal of the Society for Neuroscience) 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci (the official journal of the Society for Neuroscience) 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprich S, Biederman J, Crawford MH, Mundy E, Faraone SV. Adoptive and biological families of children and adolescents with ADHD. J Am Acad Child Adolesc Psychiatry. 2000;39(11):1432–1437. doi: 10.1097/00004583-200011000-00018. [DOI] [PubMed] [Google Scholar]
- Strauss AA, Lehtinen LE. Psychopathology and education of the brain-injured child. Grune and Stratton; New York: 1947. [Google Scholar]
- Strohle A, Stoy M, Wrase J, Schwarzer S, Schlagenhauf F, Huss M, Hein J, Nedderhut A, Neumann B, Gregor A, Juckel G, Knutson B, Lehmkuhl U, Bauer M, Heinz A. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. NeuroImage. 2008;39(3):966–972. doi: 10.1016/j.neuroimage.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Stryker S. Encephalitis lethargica—the behavior residuals. Training Sch Bull. 1925;22:152–157. [Google Scholar]
- Stuss DT. Biological and psychological development of executive functions. Brain Cogn. 1992;20(1):8–23. doi: 10.1016/0278-2626(92)90059-u. [DOI] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7(7):e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor E. Children with ADHD at increased risk of adolescent ADHD, ODD, anxiety or depression and functional impairment. Evid based Ment Health. 2010;13(4):110. doi: 10.1136/ebmh.13.4.110. [DOI] [PubMed] [Google Scholar]
- Taylor E. Antecedents of ADHD: a historical account of diagnostic concepts. Attention Deficit Hyperactivity Disord. 2011;3(2):69–75. doi: 10.1007/s12402-010-0051-x. [DOI] [PubMed] [Google Scholar]
- Thapar A, Holmes J, Poulton K, Harrington R. Genetic basis of attention deficit and hyperactivity. Br J Psychiatry (the journal of mental science) 1999;174:105–111. doi: 10.1192/bjp.174.2.105. [DOI] [PubMed] [Google Scholar]
- Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, Sui M, Cao Q, Hu S, Peng M, Zhuo Y. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett. 2006;400(1–2):39–43. doi: 10.1016/j.neulet.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29(3):148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Edelman GM. Schizophrenia and the mechanisms of conscious integration. Brain Res Brain Res Rev. 2000;31(2–3):391–400. doi: 10.1016/s0165-0173(99)00056-9. [DOI] [PubMed] [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. J Clin Child Psychol. 1999;28(3):366–375. doi: 10.1207/S15374424jccp280309. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler LA, Castellanos FX, Milham MP. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods. 2008;169(1):249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2012;36(4):1093–1106. doi: 10.1016/j.neubiorev.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wakefield JC. When is development disordered? Developmental psychopathology and the harmful dysfunction analysis of mental disorder. Dev Psychopathol. 1997;9(2):269–290. doi: 10.1017/s0954579497002058. [DOI] [PubMed] [Google Scholar]
- Welsh MC, Pennington BF. Assessing frontal lobe functioning in children: views from developmental psychology. Dev Neuropsychol. 1988;4:199–230. [Google Scholar]
- Welsh MC, Pennington BF, Groisser DB. A normative-developmental study of executive function: a window on prefrontal function in children. Dev Neuropsychol. 1991;7:131–149. [Google Scholar]
- Whalley HC, Simonotto E, Marshall I, Owens DG, Goddard NH, Johnstone EC, Lawrie SM. Functional disconnectivity in subjects at high genetic risk of schizophrenia. Brain (a journal of neurology) 2005;128(Pt 9):2097–2108. doi: 10.1093/brain/awh556. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, Loo SK, Carlson CL, McBurnett K, Lahey BB. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J Abnorm Psychol. 2012;121(4):991–1010. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NM, Franke B, Mick E, Anney RJ, Freitag CM, Gill M, Thapar A, O’Donovan MC, Owen MJ, Holmans P, Kent L, Middleton F, Zhang-James Y, Liu L, Meyer J, Nguyen TT, Romanos J, Romanos M, Seitz C, Renner TJ, Walitza S, Warnke A, Palmason H, Buitelaar J, Rommelse N, Vasquez AA, Hawi Z, Langley K, Sergeant J, Steinhausen HC, Roeyers H, Biederman J, Zaharieva I, Hakonarson H, Elia J, Lionel AC, Crosbie J, Marshall CR, Schachar R, Scherer SW, Todorov A, Smalley SL, Loo S, Nelson S, Shtir C, Asherson P, Reif A, Lesch KP, Faraone SV. Genome-wide analysis of copy number variants in attention deficit hyperactivity disorder: the role of rare variants and duplications at 15q13.3. Am J Psychiatry. 2012;169(2):195–204. doi: 10.1176/appi.ajp.2011.11060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Action of drugs of abuse on brain reward systems. Pharmacol Biochem Behav. 1980;13(Suppl 1):213–223. doi: 10.1016/s0091-3057(80)80033-5. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Blackwell Scientific; Oxford: 1967. pp. 3–70. [Google Scholar]