Abstract
Natural estrogens such as 17β-estradiol are endogenous vasodilators and have been implicated in the gender differences of hypertension. These hormones activate estrogen receptors ERα and ERβ, which mediate part of estrogen-dependent vasodilation. In addition, a novel G protein-coupled estrogen-binding receptor termed GPER/GPR30 has been identified that is expressed in the cardiovascular system. Using knock-out animals or drugs selectively targeting GPER/GPR30, a significant role for this receptor as a mediator of acute estrogen-dependent vasodilation involving nitric oxide (NO) and blood pressure-lowering activity has been demonstrated. The accumulating evidence that GPER/GPR30 is responsible for control of vascular tone indicates that this receptor may represent a novel drug target for pharmacologic treatment of hypertension in postmenopausal women and possibly also men.
Keywords: Blood Pressure, Endothelium, Hormone Therapy, Hypertension, Menopause, Nitric Oxide, Vasodilation
1. Endogenous Estrogens and Their Receptors in the Cardiovascular System
Globally, more than 25% of women have hypertension, with the prevalence being particularly high in women more than 60 years of age [1]. Whereas blood pressure levels are lower in premenopausal women compared to age-matched men, they markedly increase during the first decade following menopause [2]. In fact, the prevalence of hypertension is higher in women than in men more than 70 years of age [2], which translates into a higher cardiovascular risk [3]. Similarly, whereas the prevalence of coronary artery disease is lower in premenopausal women compared to age-matched men, these gender-based differences narrow after menopause [4]. As a result, cardiovascular disease represents the leading cause of death in men and women alike [5]. These data also suggest that endogenous estrogens confer a protective effect on the development of hypertension and atherosclerotic vascular disease [4, 6].
In line with these epidemiological data, experimental studies have demonstrated a variety of beneficial effects of endogenous estrogens on the cardiovascular system often independent of sex, which include acute and chronic vasodilator activity ultimately lowering blood pressure [4, 6, 7]. However, the underlying mechanisms are still incompletely understood. Estrogens are traditionally referred to as ligands of the classical estrogen receptors α (ERα) and β (ERβ) [4, 7, 8]. These receptors primarily function as ligand-activated nuclear transcription factors modulating expression of hormonally regulated genes. ERα and ERβ also activate rapid intracellular signaling pathways in response to estrogen, which are presumably mediated by plasma membrane-associated subpopulations of the receptors [8, 9]. Via such rapid or “non-genomic” mechanisms, both ERα and ERβ mediate vasodilation that occurs within only a few minutes [10]. In 1997, a seven-transmembrane G protein-coupled receptor (GPR30) was cloned from shear stress-exposed human endothelial cells [11] among other sources, and in 2000 it was demonstrated that this receptor is estrogen-responsive [12]. More recently, binding of estrogen to GPR30 has been shown [13, 14] that results in activation of rapid signaling cascades [12-14]. After establishing GPR30 as a bona fide estrogen-binding receptor, it was renamed GPER by the International Union of Basic and Clinical Pharmacology [15]. Similar to ERα and ERβ [4, 7], GPER/GPR30 is expressed throughout the vascular system in humans and animals of both sexes [11, 16-26]. These findings suggest that in addition to ERα and ERβ, GPER/GPR30 is likely to be involved in the vascular effects of estrogen and to play a physiological role in the control of vascular homeostasis in females and males.
2. Vascular Effects of Non-Selective GPER/GPR30 Agonists
The predominant endogenous human estrogen 17β-estradiol is synthesized primarily in the ovaries, and binds ERα, ERβ, and GPER/GPR30 with high affinity (dissociation constant Kd 0.05-0.09 nM for ERα and ERβ [27], Kd 2.7-6.6 nM for GPER/GPR30 [13, 14]). 17β-Estradiol is a powerful vasodilator of human blood vessels from males and females [16, 28, 29]. In addition, the estrogen-based steroids estrone and estriol have vasodilator properties in certain vascular beds [30, 31], although their binding affinity for GPER/GPR30 is low at physiological concentrations [14]. In addition to gonadal steroid synthesis, estrogens are produced locally at many sites throughout the body including the vascular wall, where the androgens testosterone and androstenedione are converted into 17β-estradiol and estrone, respectively, by the enzyme aromatase [32]. Interestingly, healthy young men taking the aromatase inhibitor anastrozole display impaired flow-mediated vasodilation and reduced plasma 17β-estradiol levels [33]. In line with these findings, short-term exposure to 17β-estradiol improves endothelium-dependent vasodilation in male patients [34], and male mice lacking ERβ develop hypertension with aging [35]. These data indicate a physiologically relevant role of endogenous estrogens as vasodilators even at low concentrations as seen in men and postmenopausal women. Importantly, short-term treatment with 17β-estradiol improves endothelium-dependent vasomotion in early postmenopausal women, whereas in aged menopausal women, hormone therapy abrogates vasodilation yielding vasoconstriction instead [36, 37].
Estrogenic compounds are also synthesized by soy and other plants (phytoestrogens), and mediate numerous vascular effects similar to 17β-estradiol, including vasodilation [38]. One of the most widely studied phytoestrogens, the isoflavone genistein, activates ERs including GPER/GPR30 [39]. Other man-made estrogens (xenoestrogens) and GPER/GPR30 agonists comprise chemical detergents and pesticides such as nonylphenol and DDT [39], and limited experimental data also suggests a role for these compounds in regulation of vascular function [40, 41]. Despite the widespread use of certain xenoestrogens and the subsequent chronic low-level exposure to humans [42], the potential impact of these agents on vascular homeostasis has not been investigated.
Based on their clinical use, the role of selective estrogen receptor modulators (SERMs) for regulation of vascular tone has been evaluated in a variety of studies. These drugs generally act as ER agonists in the cardiovascular system, bone, and liver, and as ER antagonists in breast tissue [43]. Moreover, SERMs such as tamoxifen and raloxifene are also agonists of GPER/GPR30 [44, 45]. These compounds evoke acute endothelium-dependent as well as endothelium-independent vasodilation in porcine coronary arteries and other vascular beds [46-51]. Raloxifene also activates endothelial nitric oxide synthase (eNOS) via ERα-dependent activation of the PI3K/Akt-pathway [52] as has previously been shown for 17β-estradiol [53].
In addition to SERMs, selective estrogen receptor downregulators (SERDs) such as ICI 182,780, which abolish ERα/ERβ signaling regardless of the type of tissue, have been used experimentally and therapeutically [54]. Importantly, ICI 182,780 displays significant binding affinity to GPER/GPR30 [14] and acts as a GPER/GPR30 agonist in breast cancer cells and several other cell lines and tissues [12, 13, 44]. These findings suggest GPER/GPR30 can mediate estrogenic effects even when ERα and ERβ are concomitantly blocked.
In summary, several natural and synthetically generated estrogens that have been implicated in the regulation of vascular function not only activate ERα and ERβ, but also the recently discovered GPER/GPR30. Whereas these substances (including the major human estrogen 17β-estradiol) are nonspecific activators of ERα, ERβ, and GPER/GPR30, selective agonists (G-1 [55]) and antagonists (G15 [56]) of GPER/GPR30 as well as genetically modified animals have been introduced, which aid in the delineation of GPER/GPR30’s specific vascular effects.
3. GPER/GPR30-Dependent Vasodilation
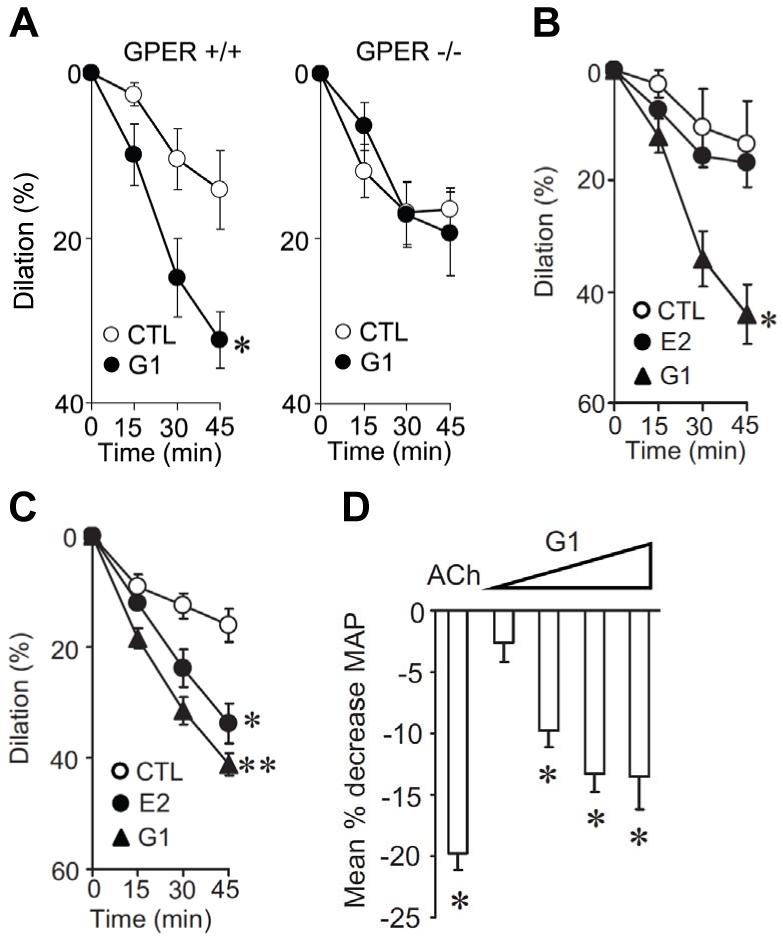
Human endothelial cells exposed to fluid shear stress were used as one of the first experimental approaches to identify and clone a cDNA encoding GPER/GPR30 [11]. Although these experiments suggested a potential role for GPER/GPR30 in vascular regulation, this possibility has been strengthened by recent research using the selective GPER/GPR30 agonist G-1 [55] in several vascular beds. In fact, G-1 acutely dilates human internal mammary and porcine coronary arteries, as well as rodent aorta, carotid, and mesenteric arteries independent of sex, a response that is less potent in the conduit arteries (Figure 1A-1C) [17, 19, 23, 26, 57]. G-1 also indirectly inhibits endothelin- [57], angiotensin II- [19], serotonin- [17], and thromboxane A2 receptor-dependent [23] contractions in certain vascular beds. In GPER/GPR30 knock-out animals as well as after pretreatment with the GPER/GPR30 antagonist G15, the vasodilator effect of G-1 is lacking [17, 26], which further underscores a role for GPER/GPR30 in the control of vasomotor tone (Figure 1A). Interestingly, G-1-dependent relaxation in human internal mammary and murine carotid arteries is even more pronounced than that of the non-selective ER agonist 17β-estradiol (Figure 1B and 1C) [17]. This points to a potential crosstalk between GPER/GPR30, ERα and ERβ, which are all involved in regulation of estrogen-dependent vasodilation.
Figure 1.
GPER/GPR30-dependent regulation of vascular tone. In carotid arteries of wild-type mice (GPER +/+), the selective GPER/GPR30-agonist G-1 causes time-dependent acute dilation, which is absent in GPER/GPR30-knockout animals (GPER −/−, A). In murine carotid (B) and human internal mammary arteries (C), the dilator effect of G-1 is even stronger than that of 17β-estradiol (E2). Injection of G-1 at increasing doses (4.12 ng/kg, 41.2 ng/kg, 412 ng/kg, and 20.6 μg/kg) acutely reduces mean arterial blood pressure (MAP, calculated as 1/3 Max + 2/3 Min, where Max is the systolic pressure and Min the diastolic pressure) in normotensive male rats. For comparison, the response to achetylcholine (ACh, 30 ng/kg) is shown (D). Reproduced from Haas, E., Bhattacharya, I., Brailoiu, E., Damjanovic, M., Brailoiu, G.C., Gao, X., Mueller-Guerre, L., Marjon, N.A., Gut, A., Minotti, R., Meyer, M.R., Amann, K., Ammann, E., Perez-Dominguez, A., Genoni, M., Clegg, D.J., Dun, N.J., Resta, T.C., Prossnitz, E.R., Barton, M., Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity, Circ Res, 104(3), 288-291, ©2009 by the American Heart Association. Figure reproduced with the permission of the publisher.
On the other hand, the GPER/GPR30-dependent vasodilator response does not necessarily depend on the activity of ERα and ERβ as pointed out by studies using the ERα/ERβ-antagonist but GPER/GPR30-agonist ICI 182,780 [12, 14]. In porcine coronary arteries, ICI 182,780 alone evokes rapid relaxation [57]. In line with these findings, ICI 182,780 causes a rapid, nitric oxide (NO)-dependent dilation of pressurized carotid (but not femoral) arteries from ovariectomized mice [58]. Moreover, ICI 182,780 does not block vasodilation in response to 17β-estradiol in several vascular beds of different species [59-63]. Conversely, NO-dependent vasodilatory effects of ICI 182,780 in these arteries are also abolished in animals lacking ERα or ERβ [58], again suggesting potentially complex crosstalk between GPER/GPR30, ERα and ERβ. Further studies using genetically modified animals or selective agonists/antagonists of specific ERs are needed to clarify their individual role in this context.
While several independent investigators have reported vasodilator effects in response to G-1, the mechanisms involved in GPER/GPR30-dependent regulation of vasomotor tone are still scarcely understood. Interestingly, GPER/GPR30 activation abrogates calcium flux induced by the vasoconstrictor serotonin indicating calcium-antagonistic or desensitizing effects [17]. Moreover, G-1-induced relaxation in rat aorta, common carotid, and mesenteric arteries as well as in porcine coronary arteries depends at least partly on the presence of an intact endothelium and is inhibited by the NOS inhibitor L-NAME [23, 26, 57]. This suggests that GPER/GPR30-dependent vasodilation is elicited via release of endothelium-derived NO in these vascular beds. In line with these findings, vasodilation of murine carotid arteries in response of the ERα/ERβ-antagonist but GPER/GPR30-agonist ICI 182,780 is absent in the presence of L-NAME [58]. This suggests that the potential beneficial vascular effects of GPER/GPR30 activation are at least partly mediated by ameliorating endothelial cell dysfunction, a vascular abnormality common to hypertension and atherosclerosis that is characterized by impaired endothelial NO production [64]. Conversely, chronic G-1 treatment of surgically postmenopausal (ovariectomized) mRen2.Lewis rats, a model of postmenopausal hypertension, has no effect on aortic eNOS gene expression [19]. In addition, endothelium-dependent relaxation was not affected by hypertension in these animals [19], although findings from hypertensive animals have some limitations and thus should not be generalized. However, the potential molecular pathways whereby GPER/GPR30 interacts with the NO pathway remain to be determined. Furthermore, preliminary evidence indicates that GPER/GPR30 activation by G-1 and ICI 182,780 also evokes endothelium- / NO-independent coronary vasodilation via BKCa channel-mediated membrane hyperpolarization.1, 2 In particular, endothelium-independent vasodilator effects of G-1 are likely to be present in resistance arteries [26].
In summary, current evidence suggests that GPER/GPR30 is a mediator of estrogen-induced vasodilation, involving both endothelium-dependent and -independent mechanisms. The GPER/GPR30-dependent responses depend on distinct vascular beds in different species and the time-course of estrogen administration, which may be reflected by functional ER crosstalk between GPER/GPR30, ERα and ERβ.
4. Effects of GPER/GPR30 Activation on Blood Pressure
Estrogens have been implicated in the gender differences of hypertension, since blood pressure is lower in premenopausal women compared to age-matched men [6]. In line with their acute vasodilatory effects, the loss of endogenous estrogens following menopause is associated with a pronounced increase in blood pressure levels [6]. Increased vascular resistance is a key feature of arterial hypertension in women and men alike [6], and is likely to be modulated by GPER/GPR30 activation [26]. Intravenous injection of G-1 into normotensive male rats acutely reduces mean arterial blood pressure (Figure 1D) [17]. Moreover, in hypertensive ovariectomized mRen2.Lewis rats, treatment with G-1 for 2 weeks lowers blood pressure and reduces gene expression of angiotensin II type 1 receptor and angiotensin-converting enzyme, although G-1 has no effect in estrogen-intact female or in male littermates [19, 65]. G-1 also inhibits angiotensin II receptor binding and angiotensin II-induced intracellular calcium increase in mesenteric smooth muscle cells of female mRen2.Lewis rats,3 suggesting that G-1 lowers blood pressure by attenuating vascular angiotensin II signaling. Interestingly, genetic linkage studies have indicated that the locus of the GPER/GPR30 gene is associated with low-renin hypertension. Indeed, in a model of GPER/GPR30-deficient adult female mice, higher mean arterial blood pressure has been reported than in age-matched controls, although absolute values were similar compared to younger GPER/GPR30-knockout and wildtype animals [66]. Moreover, G-1 reduces left-ventricular hypertrophy and myocyte size, and ameliorates diastolic dysfunction in an estrogen-intact animal model of salt-induced hypertensive cardiomyopathy [65]. Thus, GPER/GPR30 is likely involved in the estrogen-mediated beneficial effects on blood pressure as well as subsequent cardiac hypertrophy and remodeling. This is important in view of the high prevalence of hypertension and heart failure with normal ejection fraction (i.e. diastolic heart failure) in postmenopausal women [6, 67].
5. Implications for Research and Possible Therapeutic Application
Recent studies have shown that GPER/GPR30 mediates both acute and chronic vasodilatory effects in males and females with similar efficacy compared to ERα and ERβ. Thus, the beneficial blood pressure-lowering effects that protect premenopausal women from developing hypertension [6] likely result from activation of (at least) three different estrogen-binding receptors. This also implicates that predicting the cellular response to non-selective ER activation becomes increasingly complex due to functional crosstalk between ERα, ERβ, and GPER/GPR30, which ultimately affects multiple rapid signaling pathways as well as gene transcription [8]. With the availability of knockout animals and selective agonists/antagonists of the different ERs, future studies should aim to better characterize the individual role of GPER/GPR30, ERα, and ERβ for the control of vascular tone and blood pressure. Interestingly, estrogen-independent activation of these receptors by antihypertensive drugs such as olmesartan and nebivolol may also play a role in the regulation of vascular homeostasis [68-70].
Contrary to the clear-cut experimental and epidemiological evidence, many clinical trials using conjugated equine estrogens and medroxyprogesterone acetate for postmenopausal hormone therapy failed to prove a therapeutic benefit on cardiovascular outcomes and were associated with significant adverse effects [71, 72]. Although the design of these studies has been widely criticized for issues such as timing and type of treatment [37, 73], the unfavorable outcome may also have resulted from concomitant activation of multiple beneficial and harmful estrogen signaling pathways and the use of the toxic medroxyprogesterone acetate [37, 73]. Indeed, a very recent analysis of the Women’s Health Initiative Estrogen-Alone Trial suggests that in younger hysterectomized postmenopausal women monotherapy with equine estrogens reduces cardiovascular risk compared to placebo (HR 0.59, 95% CI 0.38-0.90) [74]. Moreover, additional recent reports point to a reduction in the risk of cardiovascular events in younger postmenopausal women treated with the SERM and GPER/GPR30 agonist raloxifene, whereas lasofoxifene demonstrated even greater effects [75-78]. How much of this risk reduction is due to GPR30/GPER activation remains unclear at this point, but future therapeutic approaches should include strategies selectively targeting ERs that mediate beneficial vascular activity. The fact that a GPER/GPR30-selective agonist such as G-1 largely recapitulates the beneficial cardiovascular effects of estrogen(s) without the latter’s feminizing effects suggests that activation of this receptor may evolve as new therapeutic strategy in the treatment of vascular disease, possibly in a gender-independent fashion.
Acknowledgements
Supported by Swiss National Science Foundation (SNF) grants PBZHP3-135874 (to M.R.M.), 3200-108528/1 and K-33KO-122504/1 (to M.B.), and National Institutes of Health (NIH) grants CA116662, CA118743, and CA12773 (to E.R.P.).
List of Abbreviations
- BKCa channel
Large-conductance Ca2+- and voltage-activated K+ channel
- eNOS
Endothelial nitric oxide synthase
- ER
Estrogen receptor
- GPER
G protein-coupled estrogen receptor
- GPR30
G protein-coupled receptor 30
- NO
Nitric oxide
- PI3K
Phosphatidylinositol 3-kinase
- SERD
Selective estrogen receptor downregulator
- SERM
Selective estrogen receptor modulator
Footnotes
Han, G.; Barman, S.A.; White, R.E. Rapid estrogen signaling via GPR30 in coronary artery smooth muscle. Faseb J, 2009, 23 (Meeting Abstract Supplement), abstract 968.5.
Han, G.; Ma, H.; Barman, S.A.; Sellers, M.; Yu, X.; Stallone, J.N.; White, R.E. Rapid estrogen signaling via GPER in human coronary artery smooth muscle. Faseb J, 2010, 24 (Meeting Abstract Supplement), abstract 957.1.
Lindsey, S.H.; Bhat, M.; Aileru, A.; Chappell, M.C. GPR30 attenuates functional AT1 receptor expression in rat mesenteric smooth muscle cells. Faseb J, 2011, 25 (Meeting Abstract Supplement), abstract 1088.8.
Conflict of Interest
None.
REFERENCES
- [1].Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- [2].Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension. 1995;25(3):305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- [3].Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- [4].Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension. 2006;47(6):1019–1026. doi: 10.1161/01.HYP.0000223064.62762.0b. [DOI] [PubMed] [Google Scholar]
- [5].Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- [6].Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension. 2009;54(1):11–18. doi: 10.1161/HYPERTENSIONAHA.108.120022. [DOI] [PubMed] [Google Scholar]
- [7].Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340(23):1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- [8].Meyer MR, Haas E, Prossnitz ER, Barton M. Non-genomic regulation of vascular cell function and growth by estrogen. Mol Cell Endocrinol. 2009;308(1-2):9–16. doi: 10.1016/j.mce.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Moriarty K, Kim KH, Bender JR. Minireview: estrogen receptor-mediated rapid signaling. Endocrinology. 2006;147(12):5557–5563. doi: 10.1210/en.2006-0729. [DOI] [PubMed] [Google Scholar]
- [10].Traupe T, Stettler CD, Li H, Haas E, Bhattacharya I, Minotti R, Barton M. Distinct roles of estrogen receptors alpha and beta mediating acute vasodilation of epicardial coronary arteries. Hypertension. 2007;49(6):1364–1370. doi: 10.1161/HYPERTENSIONAHA.106.081554. [DOI] [PubMed] [Google Scholar]
- [11].Takada Y, Kato C, Kondo S, Korenaga R, Ando J. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem Biophys Res Commun. 1997;240(3):737–741. doi: 10.1006/bbrc.1997.7734. [DOI] [PubMed] [Google Scholar]
- [12].Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14(10):1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- [13].Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- [14].Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146(2):624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- [15].Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl 2):S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, Heigl A, Lachat M, Genoni M, Barton M. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension. 2007;49(6):1358–1363. doi: 10.1161/HYPERTENSIONAHA.107.089995. [DOI] [PubMed] [Google Scholar]
- [17].Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104(3):288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150(4):1722–1730. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- [19].Lindsey SH, Cohen JA, Brosnihan KB, Gallagher PE, Chappell MC. Chronic treatment with the G protein-coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2.Lewis rats. Endocrinology. 2009;150(8):3753–3758. doi: 10.1210/en.2008-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol. 2009;297(5):H1806–1813. doi: 10.1152/ajpheart.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Filice E, Recchia AG, Pellegrino D, Angelone T, Maggiolini M, Cerra MC. A new membrane G protein-coupled receptor (GPR30) is involved in the cardiac effects of 17beta-estradiol in the male rat. J Physiol Pharmacol. 2009;60(4):3–10. [PubMed] [Google Scholar]
- [22].Ding Q, Gros R, Limbird LE, Chorazyczewski J, Feldman RD. Estradiol-mediated ERK phosphorylation and apoptosis in vascular smooth muscle cells requires GPR 30. Am J Physiol Cell Physiol. 2009;297(5):C1178–1187. doi: 10.1152/ajpcell.00185.2009. [DOI] [PubMed] [Google Scholar]
- [23].Broughton BR, Miller AA, Sobey CG. Endothelium-dependent relaxation by G protein-coupled receptor 30 agonists in rat carotid arteries. Am J Physiol Heart Circ Physiol. 2010;298(3):H1055–1061. doi: 10.1152/ajpheart.00878.2009. [DOI] [PubMed] [Google Scholar]
- [24].Bopassa JC, Eghbali M, Toro L, Stefani E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;298(1):H16–23. doi: 10.1152/ajpheart.00588.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ma Y, Qiao X, Falone AE, Reslan OM, Sheppard SJ, Khalil RA. Gender-specific reduction in contraction is associated with increased estrogen receptor expression in single vascular smooth muscle cells of female rat. Cell Physiol Biochem. 2010;26(3):457–470. doi: 10.1159/000320569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lindsey SH, Carver KA, Prossnitz ER, Chappell MC. Vasodilation in response to the GPR30 agonist G-1 is not different from estradiol in the mRen2.Lewis female rat. J Cardiovasc Pharmacol. 2011;57(5):598–603. doi: 10.1097/FJC.0b013e3182135f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- [28].Silva de Sa MF, Meirelles RS. Vasodilating effect of estrogen on the human umbilical artery. Gynecol Invest. 1977;8(5-6):307–313. doi: 10.1159/000301109. [DOI] [PubMed] [Google Scholar]
- [29].Mügge A, Riedel M, Barton M, Kuhn M, Lichtlen PR. Endothelium independent relaxation of human coronary arteries by 17 beta-oestradiol in vitro. Cardiovasc Res. 1993;27(11):1939–1942. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- [30].Clewell WH, Carson BA, Meschia G. Comparison of uterotrophic and vascular effects of estradiol-17 beta and estriol in the mature organism. Am J Obstet Gynecol. 1977;129(4):384–388. doi: 10.1016/0002-9378(77)90581-6. [DOI] [PubMed] [Google Scholar]
- [31].Rosenfeld CR, Rivera R. Circulatory responses to systemic infusions of estrone and estradiol-17alpha in nonpregnant, oophorectomized ewes. Am J Obstet Gynecol. 1978;132(4):442–448. doi: 10.1016/0002-9378(78)90782-2. [DOI] [PubMed] [Google Scholar]
- [32].Harada N, Sasano H, Murakami H, Ohkuma T, Nagura H, Takagi Y. Localized expression of aromatase in human vascular tissues. Circ Res. 1999;84(11):1285–1291. doi: 10.1161/01.res.84.11.1285. [DOI] [PubMed] [Google Scholar]
- [33].Lew R, Komesaroff P, Williams M, Dawood T, Sudhir K. Endogenous estrogens influence endothelial function in young men. Circ Res. 2003;93(11):1127–1133. doi: 10.1161/01.RES.0000103633.57225.BC. [DOI] [PubMed] [Google Scholar]
- [34].Barton M, Cremer J, Mügge A. 17 Beta-estradiol acutely improves endothelium-dependent relaxation to bradykinin in isolated human coronary arteries. Eur J Pharmacol. 1998;362(1):73–76. doi: 10.1016/s0014-2999(98)00787-0. [DOI] [PubMed] [Google Scholar]
- [35].Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295(5554):505–508. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]
- [36].Sherwood A, Bower JK, McFetridge-Durdle J, Blumenthal JA, Newby LK, Hinderliter AL. Age moderates the short-term effects of transdermal 17beta-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler Thromb Vasc Biol. 2007;27(8):1782–1787. doi: 10.1161/ATVBAHA.107.145383. [DOI] [PubMed] [Google Scholar]
- [37].Barton M, Meyer MR, Haas E. Hormone replacement therapy and atherosclerosis in postmenopausal women: does aging limit therapeutic benefits? Arterioscler Thromb Vasc Biol. 2007;27(8):1669–1672. doi: 10.1161/ATVBAHA.106.130260. [DOI] [PubMed] [Google Scholar]
- [38].Cano A, Garcia-Perez MA, Tarin JJ. Isoflavones and cardiovascular disease. Maturitas. 2010;67(3):219–226. doi: 10.1016/j.maturitas.2010.07.015. [DOI] [PubMed] [Google Scholar]
- [39].Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102(1-5):175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- [40].Hsieh CY, Miaw CL, Hsieh CC, Tseng HC, Yang YH, Yen CH. Effects of chronic 4-n-nonylphenol treatment on aortic vasoconstriction and vasorelaxation in rats. Arch Toxicol. 2009;83(10):941–946. doi: 10.1007/s00204-009-0447-6. [DOI] [PubMed] [Google Scholar]
- [41].Ruehlmann DO, Steinert JR, Valverde MA, Jacob R, Mann GE. Environmental estrogenic pollutants induce acute vascular relaxation by inhibiting L-type Ca2+ channels in smooth muscle cells. FASEB J. 1998;12(7):613–619. doi: 10.1096/fasebj.12.7.613. [DOI] [PubMed] [Google Scholar]
- [42].Jacobs MN, Lewis DF. Steroid hormone receptors and dietary ligands: a selected review. Proc Nutr Soc. 2002;61(1):105–122. doi: 10.1079/pns2001140. [DOI] [PubMed] [Google Scholar]
- [43].Katzenellenbogen BS, Katzenellenbogen JA. Defining the “S” in SERMs. Science. 2002;295(5564):2380–2381. doi: 10.1126/science.1070442. [DOI] [PubMed] [Google Scholar]
- [44].Prossnitz ER, Barton M. Signaling, physiological functions and clinical relevance of the G protein-coupled estrogen receptor GPER. Prostaglandins Other Lipid Mediat. 2009;89(3-4):89–97. doi: 10.1016/j.prostaglandins.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Abdelhamid R, Luo J, VandeVrede L, Kundu I, Michalsen B, Litosh VA, Schiefer IT, Gherezghiher T, Yao P, Qin Z, Thatcher GRJ. Benzothiophene selective estrogen receptor modulators provide neuroprotection by a novel GPR30-dependent mechanism. ACS Chem Neurosci. 2011;2(5):256–268. doi: 10.1021/cn100106a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Figtree GA, Lu Y, Webb CM, Collins P. Raloxifene acutely relaxes rabbit coronary arteries in vitro by an estrogen receptor-dependent and nitric oxide-dependent mechanism. Circulation. 1999;100(10):1095–1101. doi: 10.1161/01.cir.100.10.1095. [DOI] [PubMed] [Google Scholar]
- [47].Hutchison SJ, Chou TM, Chatterjee K, Sudhir K. Tamoxifen is an acute, estrogen-like, coronary vasodilator of porcine coronary arteries in vitro. J Cardiovasc Pharmacol. 2001;38(5):657–665. doi: 10.1097/00005344-200111000-00002. [DOI] [PubMed] [Google Scholar]
- [48].Leung HS, Yung LM, Leung FP, Yao X, Chen ZY, Ko WH, Laher I, Huang Y. Tamoxifen dilates porcine coronary arteries: roles for nitric oxide and ouabain-sensitive mechanisms. Br J Pharmacol. 2006;149(6):703–711. doi: 10.1038/sj.bjp.0706921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Leung FP, Yung LM, Leung HS, Au CL, Yao X, Vanhoutte PM, Laher I, Huang Y. Therapeutic concentrations of raloxifene augment nitric oxide-dependent coronary artery dilatation in vitro. Br J Pharmacol. 2007;152(2):223–229. doi: 10.1038/sj.bjp.0707387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Leung HS, Seto SW, Kwan YW, Leung FP, Au AL, Yung LM, Yao X, Huang Y. Endothelium-independent relaxation to raloxifene in porcine coronary artery. Eur J Pharmacol. 2007;555(2-3):178–184. doi: 10.1016/j.ejphar.2006.10.035. [DOI] [PubMed] [Google Scholar]
- [51].Chan YC, Leung FP, Wong WT, Tian XY, Yung LM, Lau CW, Tsang SY, Yao X, Chen ZY, Huang Y. Therapeutically relevant concentrations of raloxifene dilate pressurized rat resistance arteries via calcium-dependent endothelial nitric oxide synthase activation. Arterioscler Thromb Vasc Biol. 2010;30(5):992–999. doi: 10.1161/ATVBAHA.110.203935. [DOI] [PubMed] [Google Scholar]
- [52].Simoncini T, Genazzani AR, Liao JK. Nongenomic mechanisms of endothelial nitric oxide synthase activation by the selective estrogen receptor modulator raloxifene. Circulation. 2002;105(11):1368–1373. doi: 10.1161/hc1102.105267. [DOI] [PubMed] [Google Scholar]
- [53].Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RG, Shaul PW. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87(11):E44–52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- [54].Shanle EK, Xu W. Selectively targeting estrogen receptors for cancer treatment. Adv Drug Deliv Rev. 2010;62(13):1265–1276. doi: 10.1016/j.addr.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2(4):207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- [56].Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5(6):421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Meyer MR, Baretella O, Prossnitz ER, Barton M. Dilation of epicardial coronary arteries by the G protein-coupled estrogen receptor agonists G-1 and ICI 182,780. Pharmacology. 2010;86(1):58–64. doi: 10.1159/000315497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Guo X, Razandi M, Pedram A, Kassab G, Levin ER. Estrogen induces vascular wall dilation: mediation through kinase signaling to nitric oxide and estrogen receptors alpha and beta. J Biol Chem. 2005;280(20):19704–19710. doi: 10.1074/jbc.M501244200. [DOI] [PubMed] [Google Scholar]
- [59].Sudhir K, Chou TM, Mullen WL, Hausmann D, Collins P, Yock PG, Chatterjee K. Mechanisms of estrogen-induced vasodilation: in vivo studies in canine coronary conductance and resistance arteries. J Am Coll Cardiol. 1995;26(3):807–814. doi: 10.1016/0735-1097(95)00248-3. [DOI] [PubMed] [Google Scholar]
- [60].Teoh H, Leung SW, Man RY. Short-term exposure to physiological levels of 17 beta-estradiol enhances endothelium-independent relaxation in porcine coronary artery. Cardiovasc Res. 1999;42(1):224–231. doi: 10.1016/s0008-6363(98)00265-x. [DOI] [PubMed] [Google Scholar]
- [61].Shaw L, Taggart MJ, Austin C. Mechanisms of 17 beta-oestradiol induced vasodilatation in isolated pressurized rat small arteries. Br J Pharmacol. 2000;129(3):555–565. doi: 10.1038/sj.bjp.0703084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bracamonte MP, Jayachandran M, Rud KS, Miller VM. Acute effects of 17beta -estradiol on femoral veins from adult gonadally intact and ovariectomized female pigs. Am J Physiol Heart Circ Physiol. 2002;283(6):H2389–2396. doi: 10.1152/ajpheart.00184.2002. [DOI] [PubMed] [Google Scholar]
- [63].Scott PA, Tremblay A, Brochu M, St-Louis J. Vasorelaxant action of 17 -estradiol in rat uterine arteries: role of nitric oxide synthases and estrogen receptors. Am J Physiol Heart Circ Physiol. 2007;293(6):H3713–3719. doi: 10.1152/ajpheart.00736.2007. [DOI] [PubMed] [Google Scholar]
- [64].Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 2009;196(2):193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [PubMed: 19220204] [DOI] [PubMed] [Google Scholar]
- [65].Jessup JA, Lindsey SH, Wang H, Chappell MC, Groban L. Attenuation of salt-induced cardiac remodeling and diastolic dysfunction by the GPER agonist G-1 in female mRen2.Lewis rats. PLoS One. 2010;5(11):e15433. doi: 10.1371/journal.pone.0015433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150(2):687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- [67].Masoudi FA, Havranek EP, Smith G, Fish RH, Steiner JF, Ordin DL, Krumholz HM. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41(2):217–223. doi: 10.1016/s0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- [68].Garban HJ, Buga GM, Ignarro LJ. Estrogen receptor-mediated vascular responsiveness to nebivolol: a novel endothelium-related mechanism of therapeutic vasorelaxation. J Cardiovasc Pharmacol. 2004;43(5):638–644. doi: 10.1097/00005344-200405000-00005. [DOI] [PubMed] [Google Scholar]
- [69].Shimada K, Kitazato KT, Kinouchi T, Yagi K, Tada Y, Satomi J, Kageji T, Nagahiro S. Activation of estrogen receptor-{alpha} and of angiotensin-converting enzyme 2 suppresses ischemic brain damage in oophorectomized rats. Hypertension. 2011;57(6):1161–66. doi: 10.1161/HYPERTENSIONAHA.110.167650. [DOI] [PubMed] [Google Scholar]
- [70].Barton M, Meyer MR, Prossnitz ER. Estrogen-independent activation of estrogen receptors. Hypertension. 2011;57(6):1056–57. doi: 10.1161/HYPERTENSIONAHA.111.173427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E, Heart and Estrogen/progestin Replacement Study (HERS) Research Group Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280(7):605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- [72].Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- [73].Dubey RK, Imthurn B, Barton M, Jackson EK. Vascular consequences of menopause and hormone therapy: importance of timing of treatment and type of estrogen. Cardiovasc Res. 2005;66(2):295–306. doi: 10.1016/j.cardiores.2004.12.012. [DOI] [PubMed] [Google Scholar]
- [74].LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, Margolis KL, Stefanick ML, Brzyski R, Curb JD, Howard BV, Lewis CE, Wactawski-Wende J. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355(2):125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- [76].Collins P, Mosca L, Geiger MJ, Grady D, Kornitzer M, Amewou-Atisso MG, Effron MB, Dowsett SA, Barrett-Connor E, Wenger NK. Effects of the selective estrogen receptor modulator raloxifene on coronary outcomes in the Raloxifene Use for The Heart trial: results of subgroup analyses by age and other factors. Circulation. 2009;119(7):922–930. doi: 10.1161/CIRCULATIONAHA.108.817577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Cummings SR, Ensrud K, Delmas PD, LaCroix AZ, Vukicevic S, Reid DM, Goldstein S, Sriram U, Lee A, Thompson J, Armstrong RA, Thompson DD, Powles T, Zanchetta J, Kendler D, Neven P, Eastell R. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med. 2010;362(8):686–696. doi: 10.1056/NEJMoa0808692. [DOI] [PubMed] [Google Scholar]
- [78].Ensrud K, LaCroix A, Thompson JR, Thompson DD, Eastell R, Reid DM, Vukicevic S, Cauley J, Barrett-Connor E, Armstrong R, Welty F, Cummings S. Lasofoxifene and cardiovascular events in postmenopausal women with osteoporosis: Five-year results from the Postmenopausal Evaluation and Risk Reduction with Lasofoxifene (PEARL) trial. Circulation. 2010;122(17):1716–1724. doi: 10.1161/CIRCULATIONAHA.109.924571. [DOI] [PubMed] [Google Scholar]