Abstract
Despite extensive use of the renovascular/Goldblatt model of hypertension—2K-1C, and the use of renal denervation to treat drug resistant hypertensive patients, autonomic mechanisms that underpin the maintenance of this hypertension are important yet remain unclear. Our aim was to analyse cardiovascular autonomic function by power spectral density analysis of both arterial pressure and pulse interval measured continuously by radio telemetry for 6 weeks after renal artery clipping. Mean arterial pressure increased from 106 ± 5 to 185 ± 2 mm Hg during 5 weeks post clipping when it stabilized. A tachycardia developed during the 4th week, which plateaued between weeks 5 and 6. The gain of the cardiac vagal baroreflex decreased immediately after clipping and continued to do so until the 5th week when it plateaued (from − 2.4 ± 0.09 to − 0.8 ± 0.04 bpm/mm Hg; P < 0.05). A similar time course of changes in the high frequency power spectral density of the pulse interval was observed (decrease from 13.4 ± 0.6 to 8.3 ± 0.01 ms2; P < 0.05). There was an increase in both the very low frequency and low frequency components of systolic blood pressure that occurred 3 and 4 weeks after clipping, respectively. Thus, we show for the first time the temporal profile of autonomic mechanisms underpinning the initiation, development and maintenance of renovascular hypertension including: an immediate depression of cardiac baroreflex gain followed by a delayed cardiac sympathetic predominance; elevated sympathetic vasomotor drive occurring after the initiation of the hypertension but coinciding during its mid-development and maintenance.
Keywords: Autonomic nervous system, Spectral analysis, 2K-1C hypertension
1. Introduction
Arterial hypertension (AH) is found in a significant percentage of the population (Ye et al., 2000). There is evidence that AH is associated with autonomic nervous system dysfunction, elevated sympathetic outflow and alterations in baroreceptor reflex sensitivity. These characteristics are observed in patients with essential hypertension (Mary and Stoker, 2003; Hogarth et al., 2007), secondary hypertension in chronic kidney disease (Remuzzi, 1999) and in a variety of hypertensive animal models such as the renovascular Goldblatt model (Katholi et al., 1982; Head and Burke, 2003; Peotta et al., 2007; Oliveira-Sales et al., 2008; Souza et al., 2008; Zhu et al., 2009), spontaneously hypertensive rats (SHR) (Waki et al., 2003; Li and Pan, 2007; Simms et al., 2007), salt-sensitive hypertensive rats (Fujita et al., 2007), obesity-induced hypertensive rats (Stocker et al., 2007) and Angiotensin II (Ang II) induced hypertension (Malpas et al., 1997; Barrett et al., 2005).
Renovascular hypertension affects 1–4% of the hypertensive population and represents the second-leading cause of secondary hypertension (Hansen et al., 2002). Given the reciprocating communication between the kidney and brain and the introduction of renal denervation as a treatment for drug-resistant hypertension (Krum et al., 2009; Esler et al., 2010), renovascular hypertension and models used to gain insight into the mechanisms of the hypertension, are particular pertinent currently. Since its invention (Goldblatt et al., 1934), there have been many studies using the 2 kidney-1clip (2K-1C) or Goldblatt model of hypertension but information regarding the temporal changes in the balance of cardiovascular sympathetic and parasympathetic activity as well as baroreceptor reflex gain during the development of hypertension is limited to specific and single time points in any given study (McElroy and Zimmerman, 1989; Nakada et al., 1996; Head and Burke, 2003; Wang et al., 2005; Nobre et al., 2006). Thus, there is a paucity of data illustrating the initiation and subsequent temporal profile of autonomic cardiovascular indices post clipping in the 2K-1C model. This would be most informative as it would guide precisely time points for intervention (surgical or pharmacological) as well as indicating the time course of different mechanisms underpinning the development versus maintenance phases of hypertension in this animal model. Thus, the objective of the present study was to analyse cardiovascular autonomic function by power spectral density analysis of arterial pressure and pulse interval measured by radio telemetry as well as spontaneous cardiac baroreceptor reflex gain in conscious freely-moving rats for 6 weeks after renal artery clipping. Herein, we describe the first temporal profile of the underlying cardiovascular autonomic changes that occur in the Goldblatt rat model of hypertension.
2. Materials and methods
Procedures were carried out according to the United Kingdom Home Office Guidelines on Animals (Scientific Procedures) Act of 1986. The animals were housed individually, allowed normal rat chow and drinking water ad libitum, and kept on a 12-hour light/12-hour dark cycle. Animals were divided in the following groups: Control (CT), n = 6 and Hypertensive (2K-1C), n = 6.
2.1. Goldblatt model of hypertension (two kidney one clip, 2K-1C)
Male Wistar rats (150–180 g) were anaesthetized with ketamine (60 mg/kg) and medetomidine (250 μg/kg) intramuscularly. The level of anaesthesia was checked frequently by testing limb withdrawal reflexes to noxious pinching and doses of 0.1 mL were administered (i.m.) as required. The temperature of the rat was maintained at 37 °C using a feedback controlled heating blanket. Via a midline laparotomy, the left renal artery was obstructed partially with a silver clip of 0.2 mm width (Bergamaschi et al., 1995). The CT animals were submitted to the same surgical procedure without partial renal artery occlusion. Anaesthesia was reversed with atipamezole (1 mg/kg).
2.2. Telemetric recording of arterial pressure
2.2.1. Recording system
We used a telemetry system (Data Sciences International) for recording arterial pressure as described previously (Waki et al., 2003). Briefly, the system consists of three basic elements: (1) a transmitter for monitoring arterial pressure (TA11PA-C40); (2) a receiver (RPC-1); and (3) an adapter (R11CPA) with an ambient pressure monitor (APR-1) to output analogue signals of arterial pressure. The system is calibrated relative to atmospheric pressure. A computer-based data acquisition system (Maclab/8s, AD Instruments and PowerBook 3400c, Apple Computer Inc.) was used to acquire, display, store and analyse the telemetered data.
2.2.2. Implantation of transmitters
The radio transmitters were implanted on the same day as renal artery clipping. Via a midline laparotomy, the intestines were carefully reflected to expose the abdominal aorta. The blood flow was transiently stopped using a ligature and using a 21 gauge needle a small hole in the aorta just above its iliac bifurcation was made. The radio transmitter catheter was inserted towards heart and fixed in place with a cellulose patch and Vetbond™ glue. The transmitter casing was sutured to the ventral wall of the abdominal cavity and the incision repaired. The anaesthesia was reversed with a subcutaneous injection of atipamezole (1 mg/kg). Rats were returned to their home cage to recover from the surgery. Animals were supplied with standard rat chow and water ad libitum and kept in a climate controlled room on a 12 hour light/dark cycle. At 7 days post surgery baseline recordings of blood pressure, were recorded and from this heart rate and pulse interval computed (Waki et al., 2003).
2.3. Spectral analysis of blood pressure
A computer-based acquisition system — Hey Presto telemetry software (Waki et al., 2006) was used for acquiring, displaying, storing and analysing the data (acquisition at 2 kHz). Using Fast Fourier transform (FFT) spectral variations in arterial pressure and heart rate were computed. The 3 frequency bands were: high frequency (HF), low frequency (LF) and very low frequency (VLF) at 0.75–3.0 Hz, 0.25–0.75 Hz and 0.01–0.25 Hz, respectively. We recorded 5 min of each hour during 24 h of day. So we had an average by day of each animal. And each point is a final average of all animals by day. Changes in VLF of systolic blood pressure (SBP) reflect changes in sympathetic outflow related to thermoregulation, hormonal activity or changes in blood flow to meet local metabolic demands (Akselrod et al., 1985; Cerutti et al., 1991), while LF of SBP is indicative of the level of sympathetic vasoconstrictor activity (deBoer et al., 1987; Madwed et al., 1989). We have acknowledged that this is a caveat of the study and that this is at best an indirect measure of global change in sympathetic activity. HF of heart rate (HR) is thought to be representative of cardiac parasympathetic tone, while the ratio of LF to HF of HR gives an index of cardiac sympathovagal balance (Pagani et al., 1986). In addition, SBP, diastolic blood pressure (DBP) and mean blood pressure (MBP) were computed while HR was derived from the inter-pulse interval. Spontaneous cardiac baroreceptor reflex gain (sBRG) was calculated based on a method by Oosting et al. (1997), which involves a sequence technique allowing alterations in arterial pressure to be divided by reflex changes in heart rate or pulse interval at a delay of 3–5 cardiac cycles.
2.4. Data analysis
Results presented are the mean ± Standard Error (SEM). The data were evaluated using two-way ANOVA followed by Tukey's post-test with statistical software Graphpad Prism 4.0. The level of statistical significance was defined as P < 0.05.
3. Results
Table 1 shows control values of all parameters measured for the CT and 2K-1C rats. For all cardiovascular parameters recorded there was no change in the CT group (see Figs. 1–3). All variables monitored peaked by day 35 and no further significant changes were seen at day 42 post clipping. Thus, the 6th week post-clipping determined the end point of our investigation. The following provides a description of the temporal profile of the changes measured over these six weeks post renal artery clipping.
Table 1.
Control values of all parameters measured for the CT and 2K-1C rats.
| Parameters | CT | 2K-1C |
|---|---|---|
| SBP (mm Hg) | 125 ± 8 | 124 ± 8 |
| DBP (mm Hg) | 89 ± 6 | 97 ± 8 |
| MBP (mm Hg) | 101 ± 6 | 106 ± 8 |
| VLF of SBP (mm Hg2) | 4 ± 0.8 | 4.7 ± 0.8 |
| LF of SBP (mm Hg2) | 2.6 ± 0.8 | 2.8 ± 0.8 |
| HF of SBP (mm Hg2) | 4.5 ± 4.3 | 4.6 ± 0.4 |
| PI (ms) | 156 ± 3 | 150 ± 3 |
| LF of PI (ms2) | 3.9 ± 0.2 | 3.1 ± 0.2 |
| HF of PI (ms2) | 10.3 ± 0.4 | 13.5 ± 0.9 |
| HR (bpm) | 376 ± 9 | 406 ± 9 |
| sBRG of HR (bpm/mm Hg) | − 2.4 ± 0.1 | − 2.4 ± 0.09 |
| sBRG of PI (ms/mm Hg) | 1.0 ± 0.05 | 0.9 ± 0.04 |
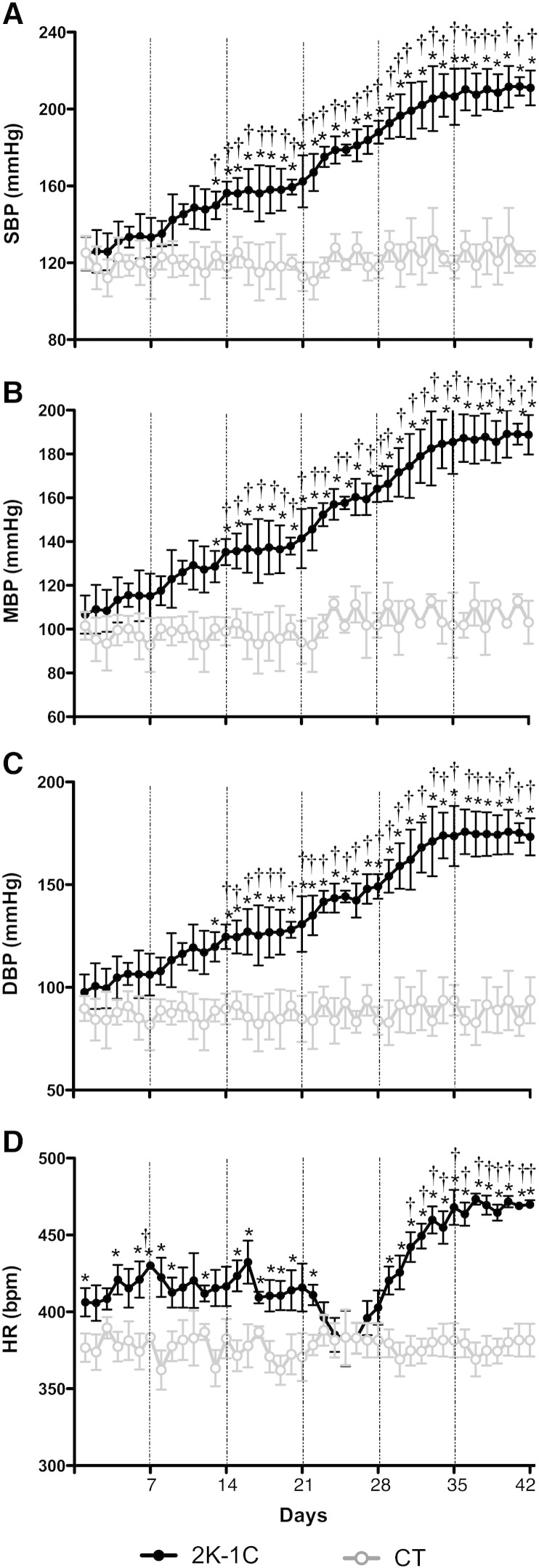
Fig. 1.
Temporal profile of the increase in blood pressure and heart rate in the Goldblatt model (2K-1C, n = 6/CT, n = 6). [A] Summary of systolic blood pressure (SBP), [B] mean blood pressure (MBP) and [C] diastolic blood pressure (DBP) recorded by radiotelemetry for 42 days. [D] Temporal profile of changes over 42 days in heart rate (HR). Day averages ± SEM are plotted (each point is the mean of 24 recordings, one for each hour). These results showed that the blood pressure increased gradually from 13 days and rose significantly for day 35 reaching a plateau at day 42 in hypertensive animals. *Significantly different (P < 0.05) from CT. †(P < 0.05) within-group difference relative to control levels.
Fig. 2.
[A] Spectral parameters of very low frequency power of systolic blood pressure (VLF of SBP), [B] low frequency power of systolic blood pressure (LF of SBP), [C] high frequency power of systolic blood pressure (HF of SBP), [D] pulse interval (PI), [E] low frequency power of PI (LF of PI) and [F] high frequency power of PI (HF of PI), calculated by Hey-Presto software (39) in the Goldblatt model (2K-1C, n = 6/CT, n = 6). Day averages ± SEM are plotted. Spectral analysis suggests that 2K-1C hypertension coincides with an increase in VLF of SBP and subsequent increased vasomotor sympathetic tone by the 28 days after clipping; both the latter appear important for the maintenance of the hypertension. *Significantly different (P < 0.05) from CT. †(P < 0.05) within-group difference relative to control levels.
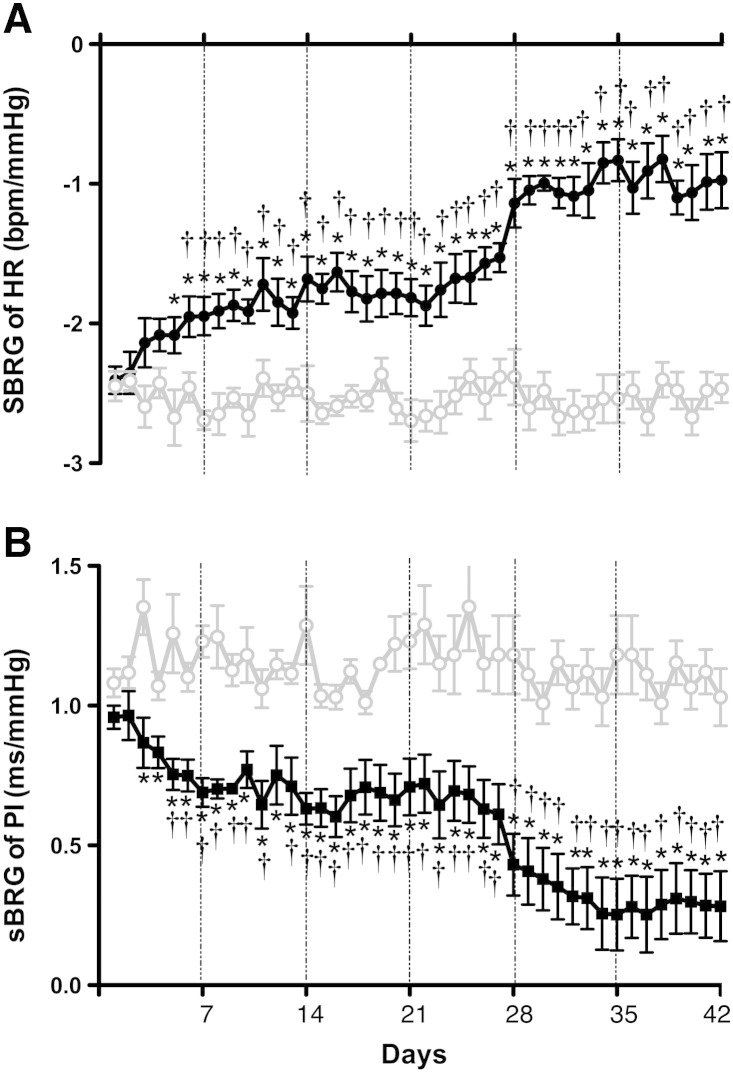
Fig. 3.
[A] Spontaneous cardiac baroreceptor reflex gain (sBRG of HR) and [B] sBRG of PI in the Goldblatt model (2K-1C, n = 6/CT, n = 6). The results showed that the gain of the parasympathetic component of the cardiac baroreflex decreased from day 5 reaching a plateau at day 42. *Significantly different (P < 0.05) from CT. †(P < 0.05) within-group difference relative to control levels.
3.1. Evolution of arterial blood pressure and heart rate in the Goldblatt rat model
For clarity, SBP data are reported here only; DBP and MAP data are presented in Fig. 1B and C and showed an identical temporal profile in terms of the time point of initiation, development and plateau phases of the hypertension. As shown in Fig. 1A, the earliest time point indicating a significant increase in SBP was day 13 post clipping (from 123.6 ± 7.1 to 149.9 ± 7.1 mm Hg; P < 0.05). SBP continued increasing until day 35 where upon it plateaued and stabilized at 206.4 ± 1.9 mm Hg as recorded at day 42. The levels of blood pressure between days 35 and 42 were not different. In contrast, the baseline levels of SBP, DBP and MBP in the CT group were similar to those of the 2K-1C group prior to clipping and remained unchanged throughout the 6 week observation period (Fig. 1A, B and C). As illustrated in Fig. 1D, the 2K-1C group presented a tachycardia that developed from the first day post-clipping that plateaued by day 35 (i.e. from 406 ± 9 bpm to 468 ± 11 bpm; P < 0.05); this value was not different by day 42.
3.2. Spectral analysis of systolic blood pressure and heart rate
A significant increase in the VLF (SBP) first occurred at 28 days post clipping (from 4.0 ± 0.8 to 6.9 ± 0.2 mm Hg2; P < 0.05) relative to basal levels in the 2K-1C group, which was 15 days after the initial rise in arterial pressure. As with arterial pressure, VLF (SBP) plateaued at day 35 (e.g. 6.8 ± 0.3 mm Hg2) and was not different at day 42. Comparing VLF of SBP between the 2K-1C and CT groups indicated a significant increase after 23 days post-clipping (2K-1C: 5.6 ± 0.6 vs CT: 3.1 ± 0.6 mm Hg2; P < 0.005; Fig. 2A). At the start of 35 days post clipping, we found a significant increase in the LF (SBP), an index of vasomotor sympathetic activity, in the 2K-1C group (from 2.6 ± 0.2 to 5.2 ± 0.3 mm Hg2; P < 0.05), which plateaued between days 35 and 42. Comparing LF (SBP) between the 2K-1C and CT groups also indicated a significant increase at 28 days post-clipping (2K-1C: 5.2 ± 0.3 and CT: 2.6 ± 0.2 mm Hg2; P < 0.004; Fig. 2B). No changes were seen in the HF (SBP) in both the 2K-1C (day 1: 4.6 ± 0.4, day 42: 6.1 ± 0.7 mm Hg2) and CT groups (day 1: 4.5 ± 0.3, day 42: 5.3 ± 0.2 mm Hg2; Fig. 2C). Fig. 2D shows PI changes in the 2K-1C and CT groups. In the 2K-1C group the PI decreased gradually from day 26 to day 42 (from 161.5 ± 0.6 to 128.8 ± 0.7 ms; P < 0.05). Comparing PI between the 2K-1C and CT groups indicated a significant decrease after 32 days post-clipping (2K-1C: 137.7 ± 7.8 and CT: 161.2 ± 2.9 mm Hg2; P < 0.05). No changes were seen in the LF (PI) in both the 2K-1C (day 1: 3.1 ± 0.08, day 42: 2.9 ± 0.6 ms2) and CT groups (day 1: 3.9 ± 0.2, day 42: 3.3 ± 0.1 ms2; Fig. 2E). In contrast, the HF (PI), an index of cardiac parasympathetic activity, decreased from 24 days after clipping becoming significantly lower by day 42 (14.4 ± 1.1 to 8.3 ± 0.01 ms2; P < 0.05) in the 2K-1C animals (Fig. 2F). No changes were observed in PI, LF (PI) and HF (PI) in the CT group over the 6 weeks.
3.3. Changes in spontaneous gain of the cardiac component of the baroreflex
The sBRG (HR) decreased after day 5 post renal artery clipping (P < 0.05) and continued to fall gradually until day 35 (from − 2.0 ± 0.12 to − 0.8 ± 0.1 bpm/mm Hg; P < 0.05) when it plateaued (Fig. 3A). For comparative reasons, we also measured sBRG of PI which showed a similar time profile in response to the sBRG of HR becoming significantly lower than baseline by week 1 (from 0.6 ± 0.05 to 0.2 ± 0.03 ms/mm Hg; P < 0.05) in the 2K-1C animals (Fig. 3B). No changes were observed in sBRG (HR) and sBRG (PI) in the CT group during 6 weeks after clipping.
4. Discussion
The present study shows for the first time that in conscious 2K-1C rats hypertension is associated with: (i) alteration in central autonomic control characterized by decreased cardiac baroreflex sensitivity as early as week 1; (ii) a significant increase in VLF of SBP by 23 days post clipping that coincided with the initiation of the hypertension; (iii) a significant rise in LF of SBP by day 28 post clipping supporting involvement of vasomotor sympathetic activation during the mid-developmental and maintenance phases of the hypertension.
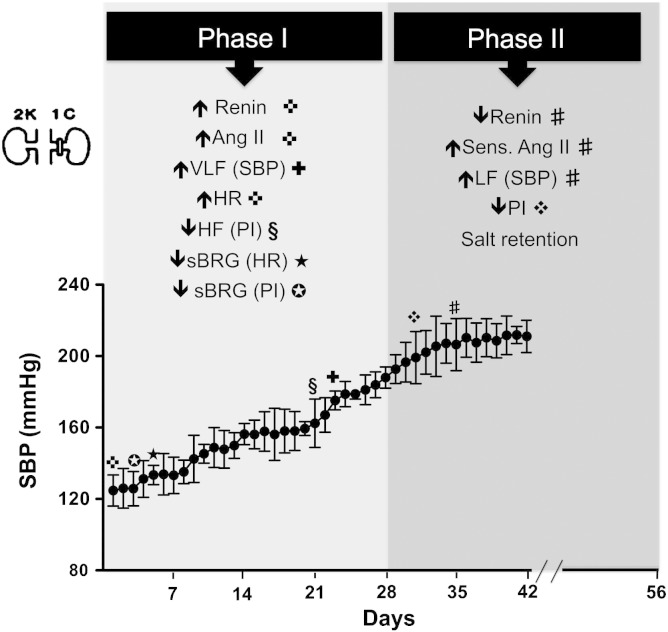
We have described the temporal profile of the development of renovascular hypertension using the 2K-1C procedure in rats. We found that arterial pressure increased gradually and significantly over a 5 week period reaching a plateau level between the 5th and 6th weeks. Our work is consistent with that of Martinez-Maldonado (1991) and provides additional mechanistic insight. Fig. 4 details the phases of hypertension and summarises the mechanisms reported by Martinez-Maldonado (1991) and the new autonomic data described herein.
Fig. 4.
Temporal profile of changes over 6 weeks in the Goldblatt model. ✜Changes of renin, Angiotensin II and heart rate (HR) immediately after clipping — adapted from Martinez-Maldonado (1991). Changes of spontaneous cardiac baroreceptor reflex gain (✪sBRG of PI and ★sBRG of HR). ✚Changes of very low frequency power of systolic blood pressure (VLF of SBP) from day 23. §Changes of high frequency power of PI (HF of PI) from day 21. ❖Changes of pulse interval (PI) from day 31. #Changes of renin, sens. of Angiotensin II and low frequency power of systolic blood pressure (LF of SBP) from day 35 — adapted from Martinez-Maldonado (1991).
Martinez-Maldonado (1991) reported three theoretical phases of experimental (and clinical) Goldblatt renovascular hypertension. Phase I is approximately 4 weeks; phase II, 5–8 weeks; phase III, 9 weeks or more (Fig. 4). Immediately after clipping blood pressure rises and is associated with increases in plasma renin activity (PRA) and increased circulating Ang II concentration; this demarks phase I. This hormonal associated increase in arterial pressure is consistent with our finding of an elevation in VLF SBP. Moreover, the early reduction in cardiac baroreflex gain may be mediated by circulating angiotensin II, as proposed previously (Wong et al., 2002; Paton et al., 2007; Tan et al., 2007), and important for the “escape” of arterial pressure to higher levels. Angiotensin I converting enzyme (ACE) inhibition or clip removal within 7–10 days after clipping causes a fast reversal of arterial pressure back to control levels during phase I. During phase II, which is a salt-retention phase, arterial pressure may remain stable or continue to rise despite a fall in PRA. During this phase, there is increased sensitivity to infusions of exogenous Ang II for evoking a rise in arterial pressure indicating upregulation of angiotensin II type 1 receptors. Increased plasma volume and total exchangeable sodium are found in stage II. In this phase, removal of the clip or treatment with ACE inhibitors reduces blood pressure to normal, but a longer time is required to achieve a full recovery of blood pressure to control levels compared to phase I. The time-dependent depression of cardiac baroreflex gain and elevated LF SBP (sympathetic vasomotor drive) will both assist with the elevation of arterial pressure. If sympathetic activity is increased to the proximal tubules this together with any sympathetically mediated haemodynamic alterations within the kidney could favour salt reabsorption. The increase in extracellular blood volume and heart rate would increase cardiac output further raising arterial pressure. In phase III, whether blood pressure has remained as high or higher than in phase I, PRA and plasma Ang II fall but clip removal and administration of doses of ACE inhibition equal to those used in phases I and II do not return arterial pressure to normal levels.
For the first time we present a temporal profile of the underlying cardiovascular autonomic changes that occur in the Goldblatt model of hypertension during 6 weeks in conscious condition. Some studies have suggested a role for changes in renal sympathetic tone in the 2K-1C hypertension. Indeed, renal denervation has been reported to ameliorate hypertension in Goldblatt animals (Katholi et al., 1982). Nakada et al. (1996) reported that suppression of sympathetic activity appears to depress the development of 2K-1C Goldblatt hypertension mainly after 4 weeks post-clipping, which is consistent with our data of elevations in sympathetic activity at this time. Indeed, there is evidence in renovascular hypertension that decreased renal blood flow leads to elevated sympathetic nerve activity in humans (Johansson et al., 1999). This is analogous to human data indicating a role for renal afferents in exciting sympathetic efferent activity in refractory hypertension (Krum et al., 2009) Recently, we reported that 6 weeks after renal artery clipping in rats, the 2K-1C group showed a significant increase in renal sympathetic nerve activity (RSNA) (and arterial pressure) when compared with the control group in urethane-anaesthetized rats (Oliveira-Sales et al., 2008).
Our results indicate that VLF (SBP) gradually increased by day 23 and after that remained stable suggesting an overall increased level of vasomotor tone induced by humoral agents (Cerutti et al., 1991). In addition, the LF (SBP) showed a significant increase by day 28 indicative of raised vasomotor sympathetic tone (deBoer et al., 1987). Findings by Ponchon and Elghozi (1996) show that the LF (SBP) was raised after 6 weeks post-clipping in the rat. Indeed, they showed that the reduction of the slow fluctuations in arterial pressure following the combined blockade of the kallikrein–kinin and the renin–angiotensin systems reflected the contribution of these humoral systems to this LF (SBP). Other studies have shown that increased LF oscillations ranging from 0.2 to 0.75 Hz were related to an increased sympathetic influence after 15 days (Nobre et al., 2006) and 6 weeks (Wang et al., 2005) post clipping. Why there is such variation in the onset of changes in the neuro-humoral system and differences to our results presented here is not clear but rat strain differences could explain it. Certainly there are differences in methodology including the use of catheter based monitoring of arterial pressure in awake rats during a 2 h period, which may give different data compared to non-invasive, remote 24 h monitoring via radio-telemetry. We believe that the latter method as used in the present study is more sensitive to detect changes in cardiovascular variability.
We acknowledge that spectral analysis reflects a summative response of all vascular beds. It is known that sympathetic outflow can be regulated differentially between different vascular beds (Ninomiya et al., 1971; Ninomiya and Fujita, 1976; McAllen et al., 1995). Spectral analysis may lack sensitivity to detect preferential effects to a single vascular bed. Thus, changes in SNA to a particular vascular bed could be occurring earlier than 4 weeks as found in the present study. This makes a comparison of results from studies that use spectral analysis versus direct sympathetic nerve activity (SNA) recordings difficult.
The present study showed that the sBRG, an index of the baroreceptor reflex activity, decreased gradually from week 1 post clipping to the 5th week when it plateaued. These results may explain the increase in heart rate that occurred concurrently. The tachycardia was also associated with a decrease in HF of PI indicative of reduced levels of parasympathetic drive to the heart, perhaps secondary to the reduced sBRG as baroreceptor reflex pathways provide a major excitatory drive to cardiac vagal motoneurones. A reduced baroreceptor reflex gain might also contribute to the increase in sympathetic drive and hypertension (see Lohmeier et al., 2004) but this remains speculative, as we have no data on this limb of the reflex. However, we fully acknowledge the limitations of the sBRG as it does not provide a full baroreceptor reflex function curve but it does allow comment on shifts in the operating/physiological point on the curve.
The mechanisms by which baroreflex sensitivity is reduced in 2K-1C hypertension are not known. In the study by Salgado and Krieger (1973) arterial baroreceptors were reset to operate at higher pressure levels in 2K-1C hypertension. Rapid (acute) resetting occurs within the first few minutes after elevation of arterial pressure, but this is only partial because the increased threshold for baroreceptor activation represents only 25–50% of the arterial pressure increase. Therefore, complete resetting occurs when the increase in pressure threshold equals the increase in arterial pressure; in the rat this was present after 48 h of 2K-1C hypertension. A possible role of increased circulating Ang II level in baroreceptor re-setting cannot be ruled out. In fact, previous studies showed that there is a correlation between increased Ang II level and baroreceptor dysfunction (Michelini and Bonagamba, 1988; Paton et al., 2006). However, further studies are necessary for a fuller understanding of the role of baroreceptors in the chronic control of cardiovascular system and their causal role in Goldblatt hypertension.
Our findings indicate that the 2K-1C Goldblatt model is associated with the loss of baroreflex sensitivity accompanied with an apparent withdrawal of vagal tone (HF of PI), an increase in VLF of SBP that accompanies the initiation of the hypertension followed by a large increase in the LF of SBP suggesting that sympathetic vasomotor drive is elevated during both the mid-developmental and maintenance phases of the hypertension.
Funding
This work was supported by the Coordenação de Aperfeiçoamento de pessoal de Nivel Superior CAPES (Brazil), the British Heart Foundation and the NIH. JFRP was in receipt of a Royal Society Wolfson Research Merit Award.
Competing interests
None.
References
- Akselrod S., Gordon D., Madwed J.B., Snidman N.C., Shannon D.C., Cohen R.J. Hemodynamic regulation: investigation by spectral analysis. Am. J. Physiol. 1985;249:H867–H887. doi: 10.1152/ajpheart.1985.249.4.H867. [DOI] [PubMed] [Google Scholar]
- Barrett C.J., Guild S.J., Ramchandra R., Malpas S.C. Baroreceptor denervation prevents sympathoinhibition during angiotensin II-induced hypertension. Hypertension. 2005;46:168–172. doi: 10.1161/01.HYP.0000168047.09637.d4. [DOI] [PubMed] [Google Scholar]
- Bergamaschi C., Campos R.R., Schor N., Lopes O.U. Role of the rostral ventrolateral medulla in maintenance of blood pressure in rats with Goldblatt hypertension. Hypertension. 1995;26:1117–1120. doi: 10.1161/01.hyp.26.6.1117. [DOI] [PubMed] [Google Scholar]
- Cerutti C., Gustin M.P., Paultre C.Z., Lo M., Julien C., Vincent M., Sassard J. Autonomic nervous system and cardiovascular variability in rats: a spectral analysis approach. Am. J. Physiol. 1991;261:H1292–H1299. doi: 10.1152/ajpheart.1991.261.4.H1292. [DOI] [PubMed] [Google Scholar]
- deBoer R.W., Karemaker J.M., Strackee J. Hemodynamic fluctuations and baroreflex sensitivity in humans: a beat-to-beat model. Am. J. Physiol. 1987;253:H680–H689. doi: 10.1152/ajpheart.1987.253.3.H680. [DOI] [PubMed] [Google Scholar]
- Esler M.D., Krum H., Sobotka P.A., Schlaich M.P., Schmieder R.E., Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Symplicity HTN-2 Investigators. Lancet. 2010;376(9756):1903–1909. doi: 10.1016/S0140-6736(10)62039-9. (4) [DOI] [PubMed] [Google Scholar]
- Fujita M., Ando K., Nagae A., Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in salt-sensitive hypertension. Hypertension. 2007;50:360–367. doi: 10.1161/HYPERTENSIONAHA.107.091009. [DOI] [PubMed] [Google Scholar]
- Goldblatt H., Lynch J., Hanzal R.F., Summerville W.W. Studies on experimental hypertension. 1. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J. Exp. Med. 1934;59:347–378. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K.J., Edwards T.E., Craven T.E., Cherr G.S., Jackson S.A., Appel R.G., Burke G.L., Dean R.H. Prevalence of renovascular disease in the elderly: a population-based study. J. Vasc. Surg. 2002;36:443–451. doi: 10.1067/mva.2002.127351. [DOI] [PubMed] [Google Scholar]
- Head G.A., Burke S.L. Are centrally acting imidazoline agents appropriate therapy for renovascular hypertension? Ann. N. Y. Acad. Sci. 2003;1009:234–243. doi: 10.1196/annals.1304.029. [DOI] [PubMed] [Google Scholar]
- Hogarth A.J., Mackintosh A.F., Mary D.A. The effect of gender on the sympathetic nerve hyperactivity of essential hypertension. J. Hum. Hypertens. 2007;21:239–245. doi: 10.1038/sj.jhh.1002132. [DOI] [PubMed] [Google Scholar]
- Johansson M., Elam M., Rundqvist B., Eisenhofer G., Herlitz H., Lambert G., Friberg P. Increased sympathetic nerve activity in renovascular hypertension. Circulation. 1999;99:2537–2542. doi: 10.1161/01.cir.99.19.2537. [DOI] [PubMed] [Google Scholar]
- Katholi R.E., Winternitz S.R., Oparil S. Decrease in peripheral sympathetic nervous system activity following renal denervation or unclipping in the one-kidney one clip Goldblatt hypertensive rat. Clin. Invest. 1982;69:55–62. doi: 10.1172/JCI110441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krum H., Schlaich M., Whitbourn R., Sobotka P.A., Sadowski J., Bartus K., Kapelak B., Walton A., Sievert H., Thambar S., Abraham W.T., Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- Li D.P., Pan H.L. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension. 2007;49:916–925. doi: 10.1161/01.HYP.0000259666.99449.74. [DOI] [PubMed] [Google Scholar]
- Lohmeier T.E., Irwin E.D., Rossing M.A., Serdar D.J., Kieval R.S. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension. 2004;43:306–311. doi: 10.1161/01.HYP.0000111837.73693.9b. [DOI] [PubMed] [Google Scholar]
- Madwed J.B., Albrecht P., Mark R.G., Cohen R.J. Low frequency oscillations in arterial pressure and heart rate: a simple computer model. Am. J. Physiol. 1989;256(H1573):H1579. doi: 10.1152/ajpheart.1989.256.6.H1573. [DOI] [PubMed] [Google Scholar]
- Malpas S.C., Groom A.S., Head G.A. Baroreflex control of heart rate and cardiac hypertrophy in angiotensin II-induced hypertension in rabbits. Hypertension. 1997;29:1284–1290. doi: 10.1161/01.hyp.29.6.1284. [DOI] [PubMed] [Google Scholar]
- Martinez-Maldonado M. Pathophysiology of renovascular hypertension. Hypertension. 1991;17:707–719. doi: 10.1161/01.hyp.17.5.707. [DOI] [PubMed] [Google Scholar]
- Mary D.A., Stoker J.B. The activity of single vasoconstrictor nerve units in hypertension. Acta Physiol. Scand. 2003;177:367–376. doi: 10.1046/j.1365-201X.2003.01082.x. [DOI] [PubMed] [Google Scholar]
- McAllen R.M., May C.N., Shafton A.D. Functional anatomy of sympathetic premotor cell groups in the medulla. Clin. Exp. Hypertens. 1995;17(1–2):209–221. doi: 10.3109/10641969509087066. [DOI] [PubMed] [Google Scholar]
- McElroy N.D., Zimmerman B.G. Characterization of intrarenal arterial adrenergic receptors in renovascular hypertension. Hypertension. 1989;13:851–858. doi: 10.1161/01.hyp.13.6.851. [DOI] [PubMed] [Google Scholar]
- Michelini L.C., Bonagamba L.G. Baroreceptor reflex modulation by vasopressin microinjected into the nucleus tractus solitarii of conscious rats. Hypertension. 1988;11:175–179. doi: 10.1161/01.hyp.11.2_pt_2.i75. [DOI] [PubMed] [Google Scholar]
- Nakada T., Kubota Y., Suzuki H., Sasagawa I., Watanabe M., Ishigooka M. Suppression of sympathetic nervous system attenuates the development of two-kidney, one-clip Goldblatt hypertension. J. Urol. 1996;156:1480–1484. [PubMed] [Google Scholar]
- Ninomiya I., Fujita S. Reflex effects of thermal stimulation on sympathetic nerve activity to skin and kidney. Am. J. Physiol. 1976;230:271–278. doi: 10.1152/ajplegacy.1976.230.2.271. [DOI] [PubMed] [Google Scholar]
- Ninomiya I., Nisimaru N., Irisawa H. Sympathetic nerve activity to the spleen, kidney, and heart in response to baroceptor input. Am. J. Physiol. 1971;221:1346–1351. doi: 10.1152/ajplegacy.1971.221.5.1346. [DOI] [PubMed] [Google Scholar]
- Nobre F., Silva C.A., Coelho E.B., Salgado H.C., Fazan R. Antihypertensive agents have different ability to modulate arterial pressure and heart rate variability in 2K1C rats. Am. J. Hypertens. 2006;19:1079–1083. doi: 10.1016/j.amjhyper.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Oliveira-Sales E.B., Dugaich A.P., Abreu N.P., Carillo B.A., Abreu N.P., Boim M.A., Bergamaschi C.T., Campos R.R. Oxidative stress supports blood pressure and sympathetic activity in renovascular hypertension. Am. J. Hypertens. 2008;21:98–104. doi: 10.1038/ajh.2007.12. [DOI] [PubMed] [Google Scholar]
- Oosting J., Struijker-Boudier H.A., Janssen B.J. Validation of a continuous baroreceptor reflex sensitivity index calculated from spontaneous fluctuations of blood pressure and pulse interval in rats. J. Hypertens. 1997;15:391–399. doi: 10.1097/00004872-199715040-00010. [DOI] [PubMed] [Google Scholar]
- Pagani M., Lombardi F., Guzzetti S., Rimoldi O., Furlan R., Pizzinelli P., Sandrone G., Malfatto G., Dell'Orto S., Piccaluga E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Paton J.F., Lonergan T., Deuchars J., James P.E., Kasparov S. Detection of angiotensin II mediated nitric oxide release within the nucleus of the solitary tract using electron-paramagnetic resonance (EPR) spectroscopy. Auton. Neurosci. 2006;30(126–127):193–201. doi: 10.1016/j.autneu.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Paton J.F., Waki H., Abdala A.P., Dickinson J., Kasparov S. Vascular-brain signaling in hypertension: role of angiotensin II and nitric oxide. Curr. Hypertens. Rep. 2007;3:242–247. doi: 10.1007/s11906-007-0043-1. [DOI] [PubMed] [Google Scholar]
- Peotta V.A., Gava A.L., Vasquez E.C., Meyrelles S.S. Evaluation of baroreflex control of heart rate in renovascular hypertensive mice. Can. J. Physiol. Pharmacol. 2007;85:761–766. doi: 10.1139/y07-067. [DOI] [PubMed] [Google Scholar]
- Ponchon P., Elghozi J.L. Contribution of the renin–angiotensin and kallikrein–kinin systems to short-term variability of blood pressure in two-kidney, one-clip hypertensive rats. Eur. J. Pharmacol. 1996;297:61–70. doi: 10.1016/0014-2999(95)00721-0. [DOI] [PubMed] [Google Scholar]
- Remuzzi G. Sympathetic overactivity in hypertensive patients with chronic renal disease. N. Engl. J. Med. 1999;340:1360–1361. doi: 10.1056/NEJM199904293401711. [DOI] [PubMed] [Google Scholar]
- Salgado H.C., Krieger E.M. Reversibility of baroreceptor adaptation in chronic hypertension. Clin. Sci. Mol. Med. 1973;45:123s–126s. doi: 10.1042/cs045123s. [DOI] [PubMed] [Google Scholar]
- Simms A.E., Paton J.F., Pickering A.E. Hierarchical recruitment of the sympathetic and parasympathetic limbs of the baroreflex in normotensive and spontaneously hypertensive rats. J. Physiol. 2007;579:473–486. doi: 10.1113/jphysiol.2006.124396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza H.C., Martins-Pinge M.C., Dias da Silva V.J., Borghi-Silva A., Gastaldi A.C., Blanco J.H., Tezini G.C. Heart rate and arterial pressure variability in the experimental renovascular hypertension model in rats. Auton. Neurosci. 2008;139:38–45. doi: 10.1016/j.autneu.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Stocker S.D., Meador R., Adams J.M. Neurons of the rostral ventrolateral medulla contribute to obesity-induced hypertension in rats. Hypertension. 2007;49:640–646. doi: 10.1161/01.HYP.0000254828.71253.dc. [DOI] [PubMed] [Google Scholar]
- Tan P.S., Killinger S., Horiuchi J., Dampney R.A. Baroreceptor reflex modulation by circulating angiotensin II is mediated by AT1 receptors in the nucleus tractus solitarius. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293(6):R2267–R2278. doi: 10.1152/ajpregu.00267.2007. [DOI] [PubMed] [Google Scholar]
- Waki H., Kasparov S., Wong L.F., Murphy D., Shimizu T., Paton J.F.R. Chronic inhibition of endothelial nitric oxide synthase activity in nucleus tractus solitarii enhances baroreceptor reflex in conscious rats. J. Physiol. 2003;546:233–242. doi: 10.1113/jphysiol.2002.030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki H., Katahira K., Polson J.W., Kasparov S., Murphy D., Paton J.F.R. Automation of analysis of cardiovascular autonomic function from chronic measurements of arterial pressure in conscious rats. Exp. Physiol. 2006;91:201–213. doi: 10.1113/expphysiol.2005.031716. [DOI] [PubMed] [Google Scholar]
- Wang D.S., Xie H.H., Shen F.M., Cai G.J., Su D.F. Blood pressure variability, cardiac baroreflex sensitivity and organ damage in experimentally hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2005;32:545–552. doi: 10.1111/j.1440-1681.2005.04229.x. [DOI] [PubMed] [Google Scholar]
- Wong L.F., Polson J.W., Murphy D., Paton J.F., Kasparov S. Genetic and pharmacological dissection of pathways involved in the angiotensinII-mediated depression of baroreflex function. FASEB J. 2002;16(12):1595–1601. doi: 10.1096/fj.02-0099com. [DOI] [PubMed] [Google Scholar]
- Ye S., Mozayeni P., Gamburd M., Zhong H., Campese V.M. Interleukin-1β and neurogenic control of blood pressure in normal rats and rats with chronic renal failure. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H2786–H2796. doi: 10.1152/ajpheart.2000.279.6.H2786. [DOI] [PubMed] [Google Scholar]
- Zhu G.Q., Xu Y., Zhou L.M., Li Y.H., Fan L.M., Wei W., Gao X.Y., Chen Q. Enhanced cardiac sympathetic afferent reflex involved in sympathetic overactivity in renovascular hypertensive rats. Exp. Physiol. 2009;94:785–794. doi: 10.1113/expphysiol.2008.046565. [DOI] [PubMed] [Google Scholar]