Abstract
Background
Long-term outcomes of drug-eluting stents (DES) versus bare-metal stents (BMS) in patients with ST-segment elevation myocardial infarction (STEMI) remain uncertain.
Objective
To investigate long-term outcomes of drug-eluting stents (DES) versus bare-metal stents (BMS) in patients with ST-segment elevation myocardial infarction (STEMI).
Methods
We performed search of MEDLINE, EMBASE, the Cochrane library, and ISI Web of Science (until February 2013) for randomized trials comparing more than 12-month efficacy or safety of DES with BMS in patients with STEMI. Pooled estimate was presented with risk ratio (RR) and its 95% confidence interval (CI) using random-effects model.
Results
Ten trials with 7,592 participants with STEMI were included. The overall results showed that there was no significant difference in the incidence of all-cause death and definite/probable stent thrombosis between DES and BMS at long-term follow-up. Patients receiving DES implantation appeared to have a lower 1-year incidence of recurrent myocardial infarction than those receiving BMS (RR = 0.75, 95% CI 0.56 to 1.00, p= 0.05). Moreover, the risk of target vessel revascularization (TVR) after receiving DES was consistently lowered during long-term observation (all p< 0.01). In subgroup analysis, the use of everolimus-eluting stents (EES) was associated with reduced risk of stent thrombosis in STEMI patients (RR = 0.37, p=0.02).
Conclusions
DES did not increase the risk of stent thrombosis in patients with STEMI compared with BMS. Moreover, the use of DES did lower long-term risk of repeat revascularization and might decrease the occurrence of reinfarction.
Keywords: Drug-eluting stents, Bare-metal stents, Acute myocardial infarction, Long-term outcomes, Meta-analysis
Introduction
The use of bare-metal stents (BMS) has showed the benefit in reducing the risk of reocclusion of the ischemia-related artery and the need for repeat revascularization in ST-segment elevation myocardial infarction (STEMI) as compared with balloon angioplasty1. However, more than 20% subjects with STEMI who received BMS implantation during primary percutaneous coronary intervention suffered from in-stent restenosis2. Currently, drug-eluting stents (DES) are increasingly used for treatment of STEMI and remedy the above drawback of BMS3,4.
However, concerns have arisen regarding a potentially higher risk of stent thrombosis with DES related to the reduced endothelialization and healing5, especially in the setting of STEMI patients with the higher possible thrombotic coronary lesions6. The long-term follow-up of several pivotal studies showed an increased risk of stent thrombosis associated with DES implantation in STEMI subjects compared with BMS7,8, but this result has not been confirmed by other studies3,9. Current clinical evidence based on registry studies and randomized controlled trials (RCTs) focusing on this issue delivering conflicting results. These inconsistent findings confused interventional cardiologists' stent selection decisions in these specific subjects. Initial meta-analyses showed the efficacy and safety of DES placement at short-term follow-up in the setting of STEMI10,11, with no safety issues. However, the longer-term treatment effect of stent implantation on these high-risk patients remains uncertain. Therefore, here we performed a meta-analysis on basis of the available data from RCTs to elucidate the long-term clinical outcomes of DES versus BMS in patients with STEMI.
Methods
Eligible criteria
A study was included if 1) patients with STEMI were randomly assigned to DES (everolimus- [EES], zotarolimus-, sirolimu- [SES], or paclitaxe-eluting stent [PES]) versus BMS; 2) the data on efficacy or safety endpoints was available; 3) follow-up duration was no less than 12 months. We restricted our analyses to the DES approved by the US Food and Drug Administration (FDA). Trials would be excluded if the data on patient or procedural characteristics was not available, and post-hoc analyses of RCTs were also excluded.
Study identification
We systematically searched MEDLINE, EMBASE, the Cochrane library, and ISI Web of Science for the eligible trials (until July 2013) using the following terms: everolimus-eluting stent, zotarolimus-eluting stent, drug-eluting stent, sirolimus-eluting stent, paclitaxel-eluting stent, bare-metal stent, uncoated stent, ST-segment elevation myocardial infarction, ST-segment elevation acute coronary syndrome. We checked the reference lists of review articles, meta-analyses, and original studies identified by the electronic searches to find other eligible trials. The search was restricted to English-language literature.
Study enrollment, data collection, and quality assessment
Two investigators independently assessed trial eligibility using predefined eligibility criteria. The data, such as participant characteristics, lesion and procedural characteristics, and follow-up duration, were extracted. The information on clinical outcomes (e.g. all-cause death, recurrent myocardial infarction, target vessel revascularization [TVR], or definite/probable stent thrombosis) was also recorded independently. Any disagreements were resolved through consensus. The quality of the trials was assessed according to concealment of treatment allocation; blinding of patients, investigators, or clinical outcome assessors; and the proportion of patients with complete clinical follow-up12. Additionally, a numerical score between 0 and 5 was assigned as a measure of study design and reporting quality based on Jadad scale13.
Statistical analyses
The pooling analyses were performed using Review Manager 5.1 software (Cochrane Collaboration, Copenhagen, Denmark). We pooled treatment effects and calculated risk ratios (RRs) with 95% confidence intervals (CI) for all end points by using random-effects model. Statistical homogeneity was quantified with the I2 statistic with a scale of 0% to 100% (>75% represented very large between-study inconsistency)14. Subgroup analysis was performed to test the potential influence of clinical factors including the type of DES, time from pain to angioplasty, dual antiplatelet therapy duration, and the percentage of use of glycoprotein IIb/IIIa inhibitors. Overall estimates in subgroup analyses were calculated based on the longest observations when a trial reported follow-up findings at different time points. For verification of the robustness of the results, sensitivity analyses were conducted by omitting each trial at a time from analysis and then calculating overall estimates for the remaining studies. Publication bias among the enrolled studies was qualitatively assessed using funnel plot method. The significance level was set at p<0.05. This work was organized as the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)15.
Results
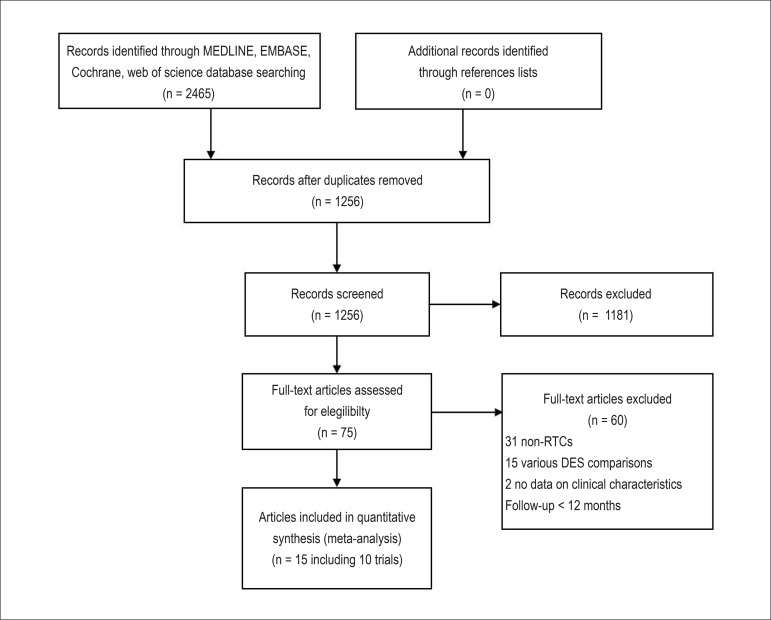
Our initial search yielded 2,465 potential literature citations (Figure 1). Among them, 1,209 were excluded by removing duplicate literatures and through review of title. Abstracts from 1,256 articles were reviewed and an additional 1,181 articles were excluded, leaving 75 for full publication review. Thereafter 60 were excluded (31 non-RCTs; 15 for comparing clinical outcomes of various DES; 2 having no data on clinical characteristics; 12 for follow-up period less than 12 months). Finally, we identified 15 articles reporting 10 studies for analysis3,4,16-28.
Figure 1.
Flowchart of selection of studies for inclusion in meta-analysis. DES: drug-eluting stents; RCTs: randomized controlled trials
A total of 7,592 participants with STEMI in the 10 long-term trials were included (Table 1). Of them, 4,601 were randomly allocated to DES group and 2,991 to BMS group. Among the 10 trials, 9 were two arm trials (six for SES vs. BMS16,18-21,23-26,28; two for PES vs. BMS17,22,27; one for EES vs. BMS3) and the rest one was three arm trial (SES vs. PES vs. BMS)4. Seven trials reported 1-year follow-up clinical outcomes3,4,19,20,22,24,26; 3 reported 2-year data4,17,24; 4 reported 3-year date16,18,25,28; and 3 reported more than 4-year data21,23,27. The majority of participants were male and the mean age ranged from 59 years to 64 years. Total stent length per patient ranged from 19mm to 29mm. Dual antiplatelet therapy duration ranged from 3 months to 12 months, and the percentage of use of glycoprotein IIb/IIIa inhibitors was from 51.75% to 100% in the included studies. Additionally, the level of quality for each article was graded with a score of 3 to 4 according to the Jadad scale (Table 1).
Table 1.
Baseline patient characteristics of randomized controlled trials included in the meta-analysis
| First author, year | Study name | Comparisons | No. enrolled | Mean age | Male, % | Time from symptom to PCI, min | Target vessel, LAD/LCX/ RCA, % | Stent length, mm | DAPT duration, m | Use of GP IIb/ IIIa inhibitors, % | Follow-up, yrs | Jaded score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| van der Hoeven BL 2008, Atary JZ 2010 | MISSION | SES vs. BMS | 158/152 | 59 | 77.7 | 189 | 54.8/15.7/29.5 | 26.4 | 12 | 99.6 | 1, 3 | 4 |
| Dirksen MT 2008, Vink MA 2011 | PASSION | PES vs. BMS | 310/309 | 61 | 76 | 179 | 50/8.1/39.9 | 19 | 6 | 73.8 | 2, 5 | 4 |
| Leibundgut G 2009 | BASKET-AMI | SES vs. BMS | 75/74 | 62.1 | 80 | ND | - | 39 | 6 | 67 | 3 | 3 |
| Lorenzo ED 2009 | PASEO | PES vs. SES vs. BMS | 90/90/90 | 62.5 | 70 | 318 | 50.5/24.5/25 | 21 | 6 | 100 | 1, 2 | 4 |
| Sabate M 2012 | EXAMINATION | EES vs. BMS | 751/747 | 61.1 | 83 | within 48h | 40.5/14.5/43.5 | 23 | 12 | 52.5 | 1 | 4 |
| Spaulding C 2006, 2011 | TYPHOON | SES vs. BMS | 251/250 | 59.2 | 78.6 | 180 | 43.7/14.6/41.3 | 21.2 | 6 | 71.5 | 1, 4 | 4 |
| Stone GW 2009 | HORIZONS-AMI | PES vs. BMS | 2257/749 | 59.6 | 76.5 | 223 | 41/15.5/43.5 | 29 | 6-12 | 51.75 | 1 | 4 |
| Valgimigli M 2007, Tebaldi M 2009 | STRATEGY | SES vs. BMS | 87/88 | 63 | 73 | 180 | 45/19/36 | 23 | 3 | 100 | 1, 2, 5 | 4 |
| Valgimigli M 2011 | MULTISTRATEGY | SES vs. BMS | 372/372 | 64 | 75.9 | 195 | 43.5/15.5/39.3 | 21 | 3 | 100 | 3 | 4 |
| Menichelli M 2007, Violini R 2010 | SESAMI | SES vs. BMS | 160/160 | 62 | 80 | 240 | 49.7/12.8/37.5 | 18 | 12 | 74.9 | 1, 3 | 3 |
DAPT: dual antiplatelet therapy: EES: everolimus-eluting stents; LAD: left anterior descending artery; LCX: left circumflex artery; NA: not available; PES: paclitaxel-eluting stents; RCA: right coronary artery; STEMI: ST-segment elevation myocardial infarction; TIMI: Thrombolysis In Myocardial Infarction; SES: sirolimus-eluting stents.
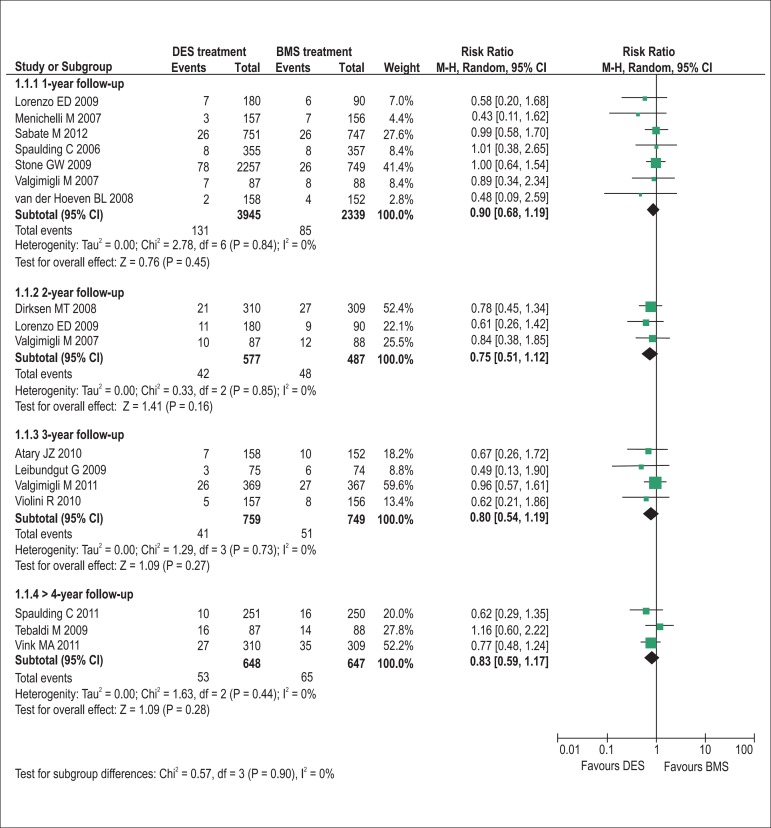
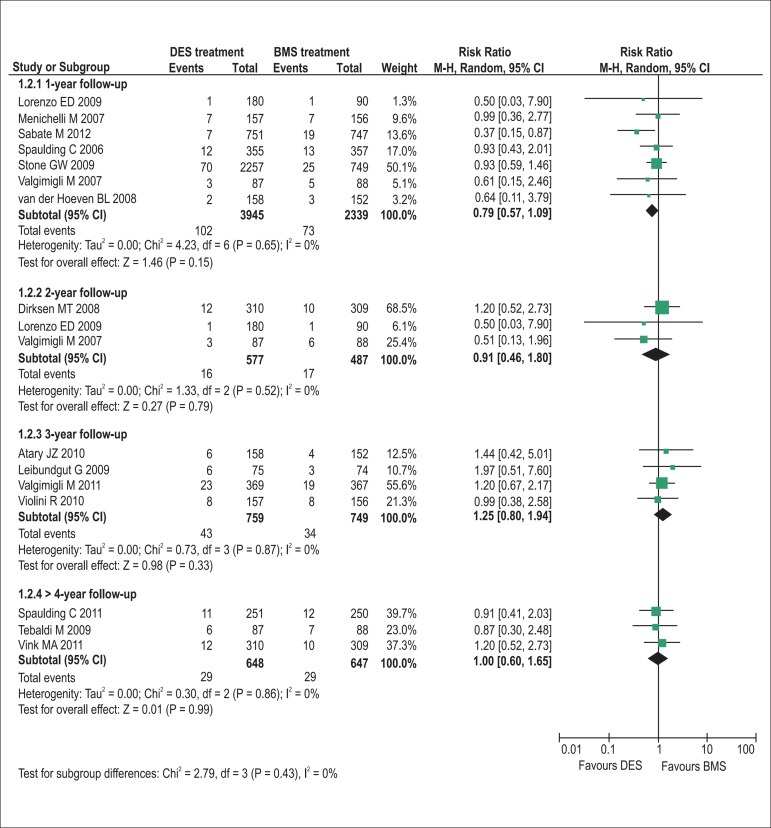
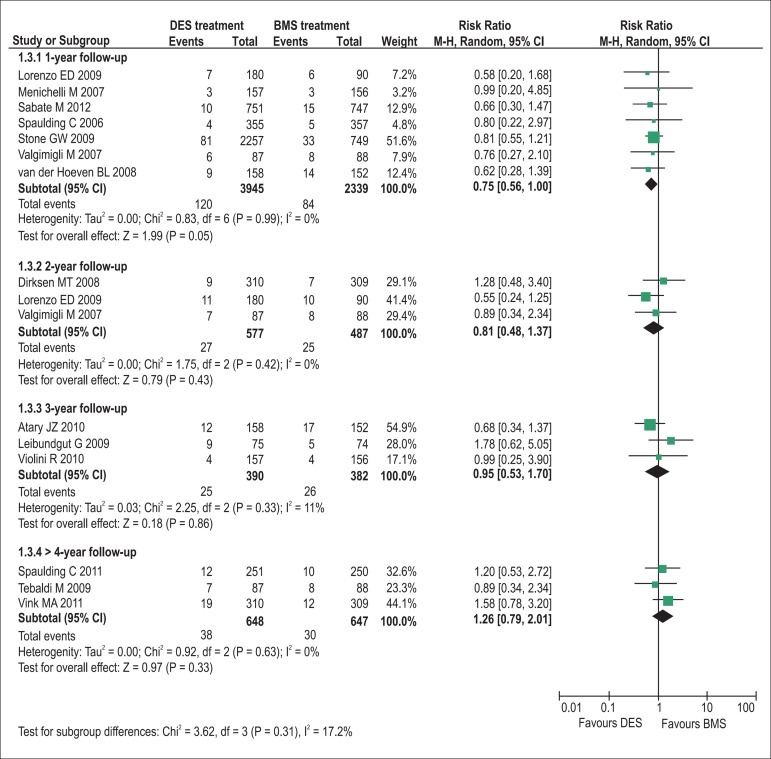
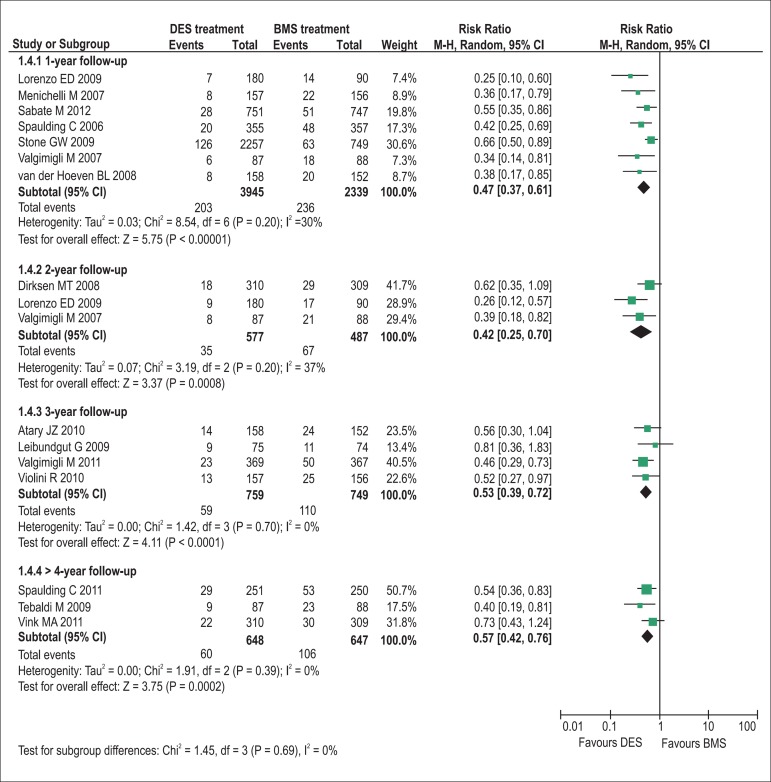
The pooling analyses showed that there were no significant differences in the incidence of all-cause death (1-year follow-up: RR = 0.90, p= 0.45, I2= 0%; 2-year: RR = 0.75, p= 0.16, I2= 0%; 3-year: RR = 0.80, p= 0.27, I2= 0%; >4-year: RR = 0.83, p= 0.28, I2= 0%; Figure 2) and definite/probable stent thrombosis (1-year: RR = 0.79, p= 0.15, I2= 0%; 2-year: RR = 0.91, p= 0.79, I2= 0%; 3-year: RR = 1.25, p= 0.33, I2= 0%; >4-year: RR = 1.00, p= 0.99, I2= 0%; Figure 3) between DES and BMS regardless of follow-up duration in patients with STEMI. Moreover, DES seemed likely to reduce the 1-year occurrence of recurrent myocardial infarction (RR = 0.75, 95% CI 0.56 to 1.00, p= 0.05, I2= 0%; Figure 4). However, the superiority of DES became nonsignificant with the prolongation of observation period (all p > 0.10; Figure 4). Notably, the risk of TVR in STEMI patients receiving DES placement was dramatically lowered compared with that receiving BMS during 1-year to 3-year follow-up (1-year follow-up: RR = 0.47, 95% CI 0.37 to 0.61, p< 0.001, I2= 30%; 2-year: RR = 0.42, 95% CI 0.25 to 0.70, p< 0.001, I2= 37%; 3-year: RR = 0.53, 95% CI 0.39 to 0.72, p< 0.001, I2= 0%), and the favorable effect of DES remained almost constant up to the maximum observed follow-up of more than 4 years (RR = 0.57, 95% CI 0.42 to 0.76, p< 0.001, I2= 0%; Figure 5).
Figure 2.
Pooled risk ratios of DES versus BMS for all-cause mortality. BMS: bare-metal stents; CI: confidence intervals; DES: drug-eluting stents; M–H: Mantel-Haenszel.
Figure 3.
Pooled risk ratios of DES versus BMS for definite or probable stent thrombosis. BMS: bare-metal stents; CI: confidence intervals; DES: drug-eluting stents; M–H: Mantel-Haenszel.
Figure 4.
Pooled risk ratios of DES versus BMS for recurrent myocardial infarction. BMS: bare-metal stents; CI: confidence intervals; DES: drug-eluting stents; M–H: Mantel-Haenszel.
Figure 5.
Pooled risk ratios of DES versus BMS for target vessel revascularization. BMS: bare-metal stents; CI: confidence intervals; DES: drug-eluting stents; M–H: Mantel-Haenszel.
In subgroup analyses, the second-generation DES, EES, might provide a benefit in lowering the risk of stent thrombosis in STEMI patients (RR = 0.37, 95% CI 0.15 to 0.87, p=0.02, Table 2), whereas both the first-generation SES and PES did not show the benefit compared with BMS (both p > 0.1, Table 2). Except for this, there were no significant influences of several important clinical factors, such as the type of DES, time from symptom to angioplasty, dual antiplatelet therapy duration, and the percentage of use of glycoprotein IIb/IIIa inhibitors, on the beneficial effect of DES in TVR, with statistically significant differences (all p <0.01, Table 2). Additionally, in sensitivity analysis omission of each trial one at a time from the analysis did not have any relevant influence on other overall results in the meta-analysis. Funnel plots were performed for all outcomes, and essential symmetry regarding overall TVR and stent thrombosis was found, which suggested that there was no publication bias in the meta-analysis.
Table 2.
Subgroup analyses on target vessel revascularization and in-stent thrombosis
| Subgroups | No. of patients | TVR | Stent thrombosis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RR (95% CI) | p value | RR (95% CI) | P value | ||||||
| SES | 2364 | 0.50 [0.40, 0.63] | < 0.001 | 1.12 [0.78, 1.60] | 0.54 | ||||
| PES | 3805 | 0.62 [0.44, 0.88] | 0.007 | 0.98 [0.67, 1.46] | 0.94 | ||||
| EES | 1498 | 0.55 [0.35, 0.86] | 0.008 | 0.37 [0.15, 0.87] | 0.02 | ||||
| Pain to angioplasty ≤ 3h | 1295 | 0.57 [0.42, 0.76] | 0.0002 | 1.00 [0.60, 1.65] | 0.99 | ||||
| Pain to angioplasty > 3h | 6133 | 0.54 [0.44, 0.67] | < 0.001 | 0.90 [0.63, 1.28] | 0.55 | ||||
| DAPT duration ≤ 6 m | 2450 | 0.51 [0.39, 0.67] | < 0.001 | 1.11 [0.77, 1.60] | 0.56 | ||||
| DAPT duration > 6 m | 5127 | 0.60 [0.49, 0.75] | < 0.001 | 0.81 [0.49, 1.33] | 0.40 | ||||
| Use of GP Ilb/IIIa inhibitors ≥ 90% | 1491 | 0.43 [0.32, 0.58] | < 0.001 | 1.13 [0.71, 1.80] | 0.61 | ||||
| Use of GP IIb/IIIa inhibitors < 90% | 6086 | 0.62 [0.52, 0.74] | < 0.001 | 0.89 [0.63, 1.27] | 0.52 | ||||
CI: confidence interval; DAPT: dual antiplatelet therapy; EES: everolimus-eluting stents; GP: glycoprotein; PES: paclitaxel-eluting stents; RR: risk ratios; SES: sirolimus-eluting stents; TVR: target vessel revascularization.
Discussions
The present study revealed that no significant differences in the incidence of all-cause death and definite/probable stent thrombosis were shown between DES and BMS in patients with STEMI during long-term follow-up. Notably, the use of the second-generation DES, EES, offered a favorable effect on reducing the risk of stent thrombosis, whereas both SES and PES did not show the clinical benefit. Moreover, compared with BMS implantation, DES implantation seemed to be associated with reduced 1-year incidence of recurrent myocardial infarction, but the benefit did not maintain more than 2 years. Furthermore, DES showed a consistent benefit in lowering the risk of TVR during long-term observation.
Development of DES was primarily conceived to further improve clinical utility of coronary stent targeting on the potential drawback of BMS, mainly referring to the increased occurrence of in-stent restenosis. As a class of immunosuppressant and antiproliferative agent, sirolimus, paclitaxe, zotarolimus, or everolimus usually used to elute coronary stents exert potent inhibition of growth factor-induced proliferation of vascular intima and vessel smooth muscle cells29. The combination of anti-hyperplasia effect with potent mechanical support for lesion vessel wall in DES yielded a benefit in decreasing the need for repeat revascularization compared with BMS or balloon angioplasty alone during short-term follow-up30. Recently, concerns have been raised regarding the "late catch-up" phenomenon in DES, especially with "limus"-eluting stents, in unselected coronary artery diseases31. A study by Awata et al. using angioscopy showed that SES delayed reendothelialization with immature plaques and accelerated neointimal coverage at 2-year follow-up32. As a consequence, a significant increase in late luminal loss from 2- to 4-year follow-up was indicated33. A randomized trial demonstrated a lower luminal loss at 6 months with EES compared with PES. However, this initial advantage disappeared at 3-year follow-up34, suggesting that delayed luminal loss might occur in DES. However, the profile of the unfavorable phenomenon in higher risk coronary artery diseases (e.g. STEMI) post DES implantation is now not well established. In the current study, a maintained clinical benefit of DES for more than 4 years in terms of reintervention in the previously instrumented artery with no excess of probable or definite stent thrombosis was identified in the current study. Conservatively, the beneficial result did not verify the presence of late catch-up with clinical significance in the setting of STEMI patients receiving DES treatment.
The propensity of stent thrombosis after DES implantation has raised safety concern in unselected coronary artery diseases35. However, to date we do not know that whether the thrombosis risk of DES is higher in STEMI patients with the higher possible thrombotic coronary lesions. The implantation of DES in ruptured plaques with a large necrotic core (the lesion substrate responsible for most cases of STEMI) might impair vascular healing responses, and potentially result in increased rates of stent thrombosis36. However, a previous meta-analysis comparing DES versus BMS for acute coronary syndromes did not show an evidence of significantly increased risk of stent thrombosis associated with DES implantation37. Similarly, the current study did not show the significant increase in fatal thrombotic evens and all-cause death associated with DES placement in STEMI patients. Even in a special class of DES, mainly referring to EES, a favorable effect in reducing the risk of stent thrombosis was shown at long-term follow-up. The finding was consistent with the result from a previous large-scale meta-analysis in unselected coronary artery diseases, indicating that EES treatment had the lower rate of stent thrombosis within 2 years of stent implantation than BMS38. Causally, STEMI was characterized by the more possible thrombotic coronary lesions than non-ST segment elevation acute coronary syndrome or stable coronary artery diseases. As thus, we presumed that, in terms of lowering the risk of stent thrombosis, EES might have the more superiority in patients with higher possible thrombotic lesions. Additionally, a favorable tendency toward reduce the risk of recurrent myocardial infarction was achieved in DES treatment group in the first year follow-up, but the potential benefit did not maintain during the longer-term observation. It was notable that the trend of reinfarction related to DES placement was presented substantially consistent with that of stent thrombosis, the risk of which appeared to be lower at 1-year follow-up. The potential benefit of DES in lowering the rate of reinfarction might partially result from their favorable trend to reducing the risk of stent thrombosis.
Methodologically, the use of random-effect model, no publication bias, and relatively low statistical heterogeneities among the included trials corrected the inherent drawback and provided reassurance for making a robust conclusion. Moreover, sensitivity analyses further confirmed the credibility of the meta-analysis estimates. However, it was worthwhile notable that this meta-analysis investigated the long-term clinical outcomes of DES versus BMS in patients with STEMI, the results of which cannot be automatically extrapolated to non-ST-segment elevation acute coronary syndrome. In addition, the power in subgroup analysis on ≥2-year follow-up data might be restricted by the limited study number, and the conclusions should be made carefully. Therefore, more studies with longer-term observation are required to further verify the findings and conclusions in the subgroup analyses of the current study.
Conclusions
This present study has identified the persistent benefit of DES on reducing the need for repeat revascularization in patients with STEMI at long-term follow-up. Although DES did not offer a benefit in reducing all-cause mortality compared with BMS, the procedure did not increase the risk of stent thrombosis, and even EES might markedly decrease the malignant clinical occurrence in these specific patients with the higher possible thrombotic coronary lesions. In addition, DES seems to be as safe as BMS, without evidence of any increased long-term risk of all-cause death and recurrent myocardial infarction. The current study, a meta-analysis based on the newest available data from RCTs comparing DES versus BMS in patients with STEMI, offers important insights into the relative safety and efficacy of DES.
Footnotes
Author contributions
Conception and design of the research: Wang S; Acquisition of data: Wang L, Wang H; Analysis and interpretation of the data: Wang L, Wang H, Li Z, Wang Y; Obtaining financing: Dong P; Writing of the manuscript: Wang L, Wang H, Wang Y; Critical revision of the manuscript for intellectual content: Dong P, Duan N, Zhao Y, Wang S.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any thesis or dissertation work.
References
- 1.De Luca G, Suryapranata H, Stone GW, Antoniucci D, Biondi-Zoccai G, Kastrati A, et al. Coronary stenting versus balloon angioplasty for acute myocardial infarction: a meta-regression analysis of randomized trials. Int J Cardiol. 2008;126(1):37–44. doi: 10.1016/j.ijcard.2007.03.112. [DOI] [PubMed] [Google Scholar]
- 2.Stone GW, Grines CL, Cox DA, Garcia E, Tcheng JE, Griffin JJ, et al. Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) Investigators. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction. N Engl J Med. 2002;346(13):957–966. doi: 10.1056/NEJMoa013404. [DOI] [PubMed] [Google Scholar]
- 3.Sabate M, Cequier A, Iniguez A, Serra A, Hernandez-Antolin R, Mainar V, et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012;380(9852):1482–1490. doi: 10.1016/S0140-6736(12)61223-9. [DOI] [PubMed] [Google Scholar]
- 4.Di Lorenzo E, De Luca G, Sauro R, Varricchio A, Capasso M, Lanzillo T, et al. The PASEO (PaclitAxel or Sirolimus-Eluting Stent Versus Bare Metal Stent in Primary Angioplasty) Randomized Trial. JACC Cardiovasc Interv. 2009;2(6):515–523. doi: 10.1016/j.jcin.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293(17):2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 6.Daemen J, Tanimoto S, Garcia-Garcia HM, Kukreja N, van de Sande M, Sianos G, et al. Comparison of three-year clinical outcome of sirolimus- and paclitaxel-eluting stents versus bare metal stents in patients with ST-segment elevation myocardial infarction (from the RESEARCH and T-SEARCH Registries) Am J Cardiol. 2007;99(8):1027–1032. doi: 10.1016/j.amjcard.2006.11.070. [DOI] [PubMed] [Google Scholar]
- 7.Kaltoft A, Kelbaek H, Thuesen L, Lassen JF, Clemmensen P, Klovgaard L, et al. Long-term outcome after drug-eluting versus bare-metal stent implantation in patients with ST-segment elevation myocardial infarction: 3-year follow-up of the randomized DEDICATION (Drug Elution and Distal Protection in Acute Myocardial Infarction) Trial. J Am Coll Cardiol. 2010;56(8):641–645. doi: 10.1016/j.jacc.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Piscione F, Piccolo R, Cassese S, Galasso G, De Rosa R, D'Andrea C, et al. Effect of drug-eluting stents in patients with acute ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention: a meta-analysis of randomised trials and an adjusted indirect comparison. EuroIntervention. 2010;5(7):853–860. doi: 10.4244/eijv5i7a143. [DOI] [PubMed] [Google Scholar]
- 9.Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, et al. HORIZONS-AMI Trial Investigators. Heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin monotherapy and paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction (HORIZONS-AMI): final 3-year results from a multicentre, randomised controlled trial. Lancet. 2011;377(9784):2193–2204. doi: 10.1016/S0140-6736(11)60764-2. [DOI] [PubMed] [Google Scholar]
- 10.De Luca G, Stone GW, Suryapranata H, Laarman GJ, Menichelli M, Kaiser C, et al. Efficacy and safety of drug-eluting stents in ST-segment elevation myocardial infarction: a meta-analysis of randomized trials. Int J Cardiol. 2009;133(2):213–222. doi: 10.1016/j.ijcard.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 11.Dibra A, Tiroch K, Schulz S, Kelbaek H, Spaulding C, Laarman GJ, et al. Drug-eluting stents in acute myocardial infarction: updated meta-analysis of randomized trials. Clin Res Cardiol. 2010;99(6):345–357. doi: 10.1007/s00392-010-0133-y. [DOI] [PubMed] [Google Scholar]
- 12.Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282(11):1054–1060. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- 13.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Ioannidis JP, Patsopoulos ND, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335(7626):914–916. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7): [PMC free article] [PubMed] [Google Scholar]
- 16.Atary JZ, van der Hoeven BL, Liem SS, Jukema JW, van der Bom JG, Atsma DE, et al. Three-year outcome of sirolimus-eluting versus bare-metal stents for the treatment of ST-segment elevation myocardial infarction (from the MISSION! Intervention Study) Am J Cardiol. 2010;106(1):4–12. doi: 10.1016/j.amjcard.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Dirksen MT, Vink MA, Suttorp MJ, Tijssen JG, Patterson MS, Slagboom T, et al. Paclitaxel-Eluting Stent versus Conventional Stent; in Myocardial Infarction with ST-Segment Elevation (PASSION) investigators. Two year follow-up after primary PCI with a paclitaxel-eluting stent versus a bare-metal stent for acute ST-elevation myocardial infarction (the PASSION trial): a follow-up study. EuroIntervention. 2008;4(1):64–70. doi: 10.4244/eijv4i1a12. [DOI] [PubMed] [Google Scholar]
- 18.Leibundgut G, Nietlispach F, Pittl U, Brunner-La Rocca H, Kaiser CA, Pfisterer ME. Stent thrombosis up to 3 years after stenting for ST-segment elevation myocardial infarction versus for stable angina--comparison of the effects of drug-eluting versus bare-metal stents. Am Heart J. 2009;158(2):271–276. doi: 10.1016/j.ahj.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Menichelli M, Parma A, Pucci E, Fiorilli R, De Felice F, Nazzaro M, et al. Randomized trial of Sirolimus-Eluting Stent Versus Bare-Metal Stent in Acute Myocardial Infarction (SESAMI). J Am Coll Cardiol. 2007;49(19):1924–1930. doi: 10.1016/j.jacc.2007.01.081. [DOI] [PubMed] [Google Scholar]
- 20.Spaulding C, Henry P, Teiger E, Beatt K, Bramucci E, Carrie D, et al. TYPHOON Investigators. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N Engl J Med. 2006;355(11):1093–1104. doi: 10.1056/NEJMoa062006. [DOI] [PubMed] [Google Scholar]
- 21.Spaulding C, Teiger E, Commeau P, Varenne O, Bramucci E, Slama M, et al. Four-year follow-up of TYPHOON (trial to assess the use of the CYPHer sirolimus-eluting coronary stent in acute myocardial infarction treated with BallOON angioplasty) JACC Cardiovasc Interv. 2011;4(1):14–23. doi: 10.1016/j.jcin.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Stone GW, Lansky AJ, Pocock SJ, Gersh BJ, Dangas G, Wong SC, et al. HORIZONS-AMI Trial Investigators. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009;360(19):1946–1959. doi: 10.1056/NEJMoa0810116. [DOI] [PubMed] [Google Scholar]
- 23.Tebaldi M, Arcozzi C, Campo G, Percoco G, Ferrari R, Valgimigli M, STRATEGY Investigators The 5-year clinical outcomes after a randomized comparison of sirolimus-eluting versus bare-metal stent implantation in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2009;54(20):1900–1901. doi: 10.1016/j.jacc.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Valgimigli M, Campo G, Arcozzi C, Malagutti P, Carletti R, Ferrari F, et al. Two-year clinical follow-up after sirolimus-eluting versus bare-metal stent implantation assisted by systematic glycoprotein IIb/IIIa Inhibitor Infusion in patients with myocardial infarction: results from the STRATEGY study. J Am Coll Cardiol. 2007;50(2):138–145. doi: 10.1016/j.jacc.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 25.Valgimigli M, Campo G, Gambetti S, Bolognese L, Ribichini F, Colangelo S, et al. MULTIcentre evaluation of Single high-dose bolus TiRofiban versus Abciximab with sirolimus eluting sTEnt or Bare Metal Stent in Acute Myocardial Infarction studY Investigators. Three-year follow-up of the MULTIcentre evaluation of Single high-dose Bolus TiRofiban versus Abciximab with Sirolimus-eluting STEnt or Bare-Metal Stent in Acute Myocardial Infarction StudY (MULTISTRATEGY) Int J Cardiol. 2013;165(1):134–141. doi: 10.1016/j.ijcard.2011.07.106. [DOI] [PubMed] [Google Scholar]
- 26.van der Hoeven BL, Liem SS, Jukema JW, Suraphakdee N, Putter H, Dijkstra J, et al. Sirolimus-eluting stents versus bare-metal stents in patients with ST-segment elevation myocardial infarction: 9-month angiographic and intravascular ultrasound results and 12-month clinical outcome results from the MISSION Intervention Study. J Am Coll Cardiol. 2008;51(6):618–626. doi: 10.1016/j.jacc.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 27.Vink MA, Dirksen MT, Suttorp MJ, Tijssen JG, van Etten J, Patterson MS, et al. 5-year follow-up after primary percutaneous coronary intervention with a paclitaxel-eluting stent versus a bare-metal stent in acute ST-segment elevation myocardial infarction: a follow-up study of the PASSION (Paclitaxel-Eluting Versus Conventional Stent in Myocardial Infarction with ST-Segment Elevation) trial. JACC Cardiovasc Interv. 2011;4(1):24–29. doi: 10.1016/j.jcin.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Violini R, Musto C, De Felice F, Nazzaro MS, Cifarelli A, Petitti T, et al. Maintenance of long-term clinical benefit with sirolimus-eluting stents in patients with ST-segment elevation myocardial infarction 3-year results of the SESAMI (sirolimus-eluting stent versus bare-metal stent in acute myocardial infarction) trial. J Am Coll Cardiol. 2010;55(8):810–814. doi: 10.1016/j.jacc.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 29.Schomig A, Kastrati A, Wessely R. Prevention of restenosis by systemic drug therapy: back to the future? Circulation. 2005;112(18):2759–2761. doi: 10.1161/CIRCULATIONAHA.105.583484. [DOI] [PubMed] [Google Scholar]
- 30.Stefanini GG, Holmes DR., Jr Drug-eluting coronary-artery stents. N Engl J Med. 2013;368(3):254–265. doi: 10.1056/NEJMra1210816. [DOI] [PubMed] [Google Scholar]
- 31.Sheiban I, Chiribiri A, Galli S, Biondi-Zoccai G, Montorsi P, Beninati S, et al. Sirolimus-eluting stent implantation for bare-metal in-stent restenosis: is there any evidence for a late catch-up phenomenon? J Cardiovasc Med (Hagerstown) 2008;9(8):783–788. doi: 10.2459/JCM.0b013e3282fb7882. [DOI] [PubMed] [Google Scholar]
- 32.Awata M, Kotani J, Uematsu M, Morozumi T, Watanabe T, Onishi T, et al. Serial angioscopic evidence of incomplete neointimal coverage after sirolimus-eluting stent implantation: comparison with bare-metal stents. Circulation. 2007;116(8):910–916. doi: 10.1161/CIRCULATIONAHA.105.609057. [DOI] [PubMed] [Google Scholar]
- 33.Sousa JE, Costa MA, Abizaid A, Feres F, Seixas AC, Tanajura LF, et al. Four-year angiographic and intravascular ultrasound follow-up of patients treated with sirolimus-eluting stents. Circulation. 2005;111(18):2326–2329. doi: 10.1161/01.CIR.0000164271.01172.1A. [DOI] [PubMed] [Google Scholar]
- 34.Garg S, Serruys P, Onuma Y, Dorange C, Veldhof S, Miquel-Hebert K, et al. SPIRIT II Investigators 3-year clinical follow-up of the XIENCE V everolimus-eluting coronary stent system in the treatment of patients with de novo coronary artery lesions: the SPIRIT II trial (Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions) JACC Cardiovasc Interv. 2009;2(12):1190–1198. doi: 10.1016/j.jcin.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356(10):998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 36.de la Torre-Hernandez JM, Alfonso F, Hernandez F, Elizaga J, Sanmartin M, Pinar E, et al. ESTROFA Study Group Drug-eluting stent thrombosis: results from the multicenter Spanish registry ESTROFA (Estudio ESpanol sobre TROmbosis de stents FArmacoactivos) J Am Coll Cardiol. 2008;51(10):986–990. doi: 10.1016/j.jacc.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 37.Greenhalgh J, Hockenhull J, Rao N, Dundar Y, Dickson RC, Bagust A. Drug-eluting stents versus bare metal stents for angina or acute coronary syndromes. Cochrane Database Syst Rev. 2010 May 12;(5): doi: 10.1002/14651858.CD004587.pub2. [DOI] [PubMed] [Google Scholar]
- 38.Palmerini T, Biondi-Zoccai G, Della Riva D, Stettler C, Sangiorgi D, D'Ascenzo F, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379(9824):1393–1402. doi: 10.1016/S0140-6736(12)60324-9. [DOI] [PubMed] [Google Scholar]