Abstract
Background
The applicability of international risk scores in heart surgery (HS) is not well defined in centers outside of North America and Europe.
Objective
To evaluate the capacity of the Parsonnet Bernstein 2000 (BP) and EuroSCORE (ES) in predicting in-hospital mortality (IHM) in patients undergoing HS at a reference hospital in Brazil and to identify risk predictors (RP).
Methods
Retrospective cohort study of 1,065 patients, with 60.3% patients underwent coronary artery bypass grafting (CABG), 32.7%, valve surgery and 7.0%, CABG combined with valve surgery. Additive and logistic scores models, the area under the ROC (Receiver Operating Characteristic) curve (AUC) and the standardized mortality ratio (SMR) were calculated. Multivariate logistic regression was performed to identify the RP.
Results
Overall mortality was 7.8%. The baseline characteristics of the patients were significantly different in relation to BP and ES. AUCs of the logistic and additive BP were 0.72 (95% CI, from 0.66 to 0.78 p = 0.74), and of ES they were 0.73 (95% CI; 0.67 to 0.79 p = 0.80). The calculation of the SMR in BP was 1.59 (95% CI; 1.27 to 1.99) and in ES, 1.43 (95% CI; 1.14 to 1.79). Seven RP of IHM were identified: age, serum creatinine > 2.26 mg/dL, active endocarditis, systolic pulmonary arterial pressure > 60 mmHg, one or more previous HS, CABG combined with valve surgery and diabetes mellitus.
Conclusion
Local scores, based on the real situation of local populations, must be developed for better assessment of risk in cardiac surgery.
Keywords: Thoracic surgery / mortality, Risk factors, Information services, Brazil
Introduction
Currently, there are more than 20 models of risk scores in cardiac surgery1-5. Among the best known are the Parsonnet score, STS risk score, Higgins score, Northern New England score (NNE score), Ambler score, Bernstein-Parsonnet 2000 score and EuroSCORE. The Bernstein Parsonnet 2000 (BP) and EuroSCORE (ES) are differentiated, as they can be used for bypass surgery, valve surgery, or both, plus the capacity to be applied at the bedside. The Parsonnet score was developed in 1989 and is notable for having been a pioneer in systematic risk stratification in cardiac surgery and applicable to different populations1. The Bernstein Parsonnet 2000 corresponds to the revised and simplified Parsonnet score, based on a database of 10,703 patients in New Jersey, in the United States, between 1994 and 19952.
The EuroSCORE (European System for Cardiac Operative Risk Evaluation) is currently one of the most commonly used scores. It was developed from data on 19,030 patients in Europe, in 19956-10. In 2011, the EuroSCORE was revised based on data from approximately 32,000 patients and called EuroSCORE II. Although it was already possible to use it through its official website (www.euroscore.org), the article containing its validation was not available at the time of our article's review.
Both scores are shown in the additive and logistic models. The additive model is more easily applicable. However, the logistic model is more discriminating in high-risk patients5,11,12. One should consider, when evaluating a risk score, its capacity to accurately estimate the in-hospital mortality, in addition to the discriminatory power4,13,14.
In Brazil, data show that postoperative mortality in cardiac surgery is still high6,7. This can be explained in part by the socioeconomic inequalities of our population8 and because the few tertiary centers performing large volumes of cardiac surgeries per year are located in the richest regions of the country6,9.
Given the different characteristics of the population and the scarcity of national scores, we aimed to test the applicability of BP and ES in patients undergoing cardiac surgery in southern Brazil and to identify risk factors.
Methods
A retrospective cohort of patients older than 18 years of age, treated at the Instituto de Cardiologia do Rio Grande do Sul (IC/FUC) from January 2007 to July 2008, submitted to coronary artery bypass graft (CABG) surgery, valve surgery or CABG combined with valve surgery was assessed. Patients with pathologies of the aorta exclusively, congenital heart disease and heart transplants were excluded. The sample consisted of 1,065 patients.
IC/FUC is a reference tertiary care center in cardiology in southern Brazil, where approximately 2,000 heart surgeries are performed each year, including congenital heart surgery, transplants, pacemakers and cardioverter-defibrillator implants, as well as aortic surgery, among others.
Medical records were reviewed manually by the authors. Almost all of the variables in the assessed risk scores were in the charts, which included the standardized preoperative evaluation form, always filled out by the same medical team. Data from anesthetic records and information on postoperative intensive care unit admission were also extracted.
However, information on the specific degree of severity of chronic obstructive pulmonary disease (COPD) and the degree of sequelae after a neurological event were rarely available. The definition of COPD used in the BP score was the same used in the ES, as there is no available definition of this variable in the BP. Patients were considered as having severe COPD only when FEV1 < 50% of the predicted at the spirometry10. Neurological dysfunction was considered as any prior neurological event (cerebrovascular accident [CVA] or transient ischemic attack [TIA]). Congestive heart failure was considered present in patients with functional class III or IV according to the New York Heart Association (NYHA).
Data were recorded on a specific form and typed in by the authors in Microsoft Office Excel database.
One hundred and fifty-three patients had no preoperative assessment of the left ventricular ejection fraction (LVEF) and 22 had no serum creatinine level measurements. The amount of missing data, which accounted for 14% of the data, was statistically included using SPSS version 19.0 by multiple imputation13,14.
The assessed outcome was in-hospital mortality (IHM), defined as death from any cause, both during surgery and in the postoperative period, before discharge.
Statistical Analysis
The difference between proportions of independent samples was calculated to compare prevalence in the sample and in the scores' populations. Pearson's chi-square test was used to compare risk factors in relation to clinical outcomes. Odds ratio (OR) and 95% confidence interval (95%CI) were used to calculate the magnitude of the effect. The level of significance was set at α = 0.05. SPSS software version 19.0 and PEPI 4.0 were used for the statistical analyses.
Multivariate logistic regression models were applied to the sample to identify independent mortality predictors of the scores. The variables assessed in two scores that showed higher prevalence than 2% in the sample were included, as suggested by Parsonnet et al1, such as age, gender, diabetes mellitus, hypertension, previous heart surgery, more than one previous heart surgery, aortic valve replacement, mitral valve replacement, valve surgery combined with CABG, pulmonary hypertension, active endocarditis, serum creatinine, ejection fraction, left main coronary lesion, carotid artery disease, peripheral vascular disease and neurological disease.
The scores were applied to the sample in their additive and logistic forms, according to the algorithms of the original articles. In the BP score, the sample was divided into deciles and mortality was assessed in each decile so it would be possible to compare it with the ES, as the latter is presented as risk groups (low, medium and high risk). In the BP, a score of 0-3 was considered low risk, 3.1 to 17.5, medium risk, and ≥ 17.5, high risk. In the ES, a score of 0-2 was considered low risk, 3-5, medium risk, and ≥ 6, high risk.
The score accuracy was assessed using the Hosmer-Lemeshow test, which compares the observed and expected mortality rates in same-size deciles. The difference between observed and expected mortality rates was also assessed by the standardized mortality ratio (SMR), which is obtained dividing the observed mortality by the expected one and confidence intervals, calculated as suggested by Breslow & Day15. The H-L test is considered the most adequate to evaluate the differences between the observed and expected mortalities; however, we also chose to use the SMR, as it is possible to apply it to risk groups.
The ROC (Receiver Operating Characteristic) curve evaluated the discriminatory power of the scores and the areas under the ROC curve (AUC) - C statistics - of both models; they were compared using STATA 9.2 software in their additive and logistic forms. The software was also used for chart preparation.
Ethical Considerations
The study was submitted to the analysis and approval by the Research Ethics Committee of Instituto de Cardiologia do RS, according to resolution of the National Health Council.
The researchers undertake to comply with the rules of Resolution 196/96 of the National Health Council, referenced in IV.1-g ("the guarantee of confidentiality that guarantees the privacy of subjects regarding confidential data involved in the research").
Results
The mean age was 61.4 years (± 11.8 years), 38% were women, 60.3% of patients underwent CABG, 32.7%, valve surgery, and 7.0%, CABG combined with valve surgery.
The sample overall mortality rate was 7.8%. Patients undergoing CABG had a mortality rate of 5.9%. In valve surgery (aortic and/or mitral and/or tricuspid), mortality was 8.6% and in CABG associated with valve surgery, 20.0%. Mean mortality in the original BP and ES were 5.3% and 4.7%, respectively. The mean BP score in the sample was 14.69 (minimum zero and maximum 84.5) and in the ES, 4.52 (minimum zero and maximum 16).
Baseline characteristics of patients were significantly different from those of the BP and ES populations (Tables 1,2 and 3). In relation to BP, our sample had a predominance of the female gender and higher prevalence of arterial hypertension, pulmonary hypertension, asthma, morbid obesity, left main coronary artery lesion and previous heart surgery. Aortic, mitral and tricuspid valve surgery were also more frequent. The number of patients with preoperative intra-aortic balloon was significantly lower in our population. Regarding the ES, the female gender also predominated in our sample. We observed a higher prevalence of COPD, neurological dysfunction, previous heart surgery, unstable angina and pulmonary hypertension.
Table 1.
Prevalence of risk factors in the sample and in the Bernstein Parsonnet 2000 population
| Risk factor | Prevalence in sample (%) (n = 1,065) | Prevalence in BP (%) (n = 10,703) | p value |
|---|---|---|---|
| Female gender | 38.7 | 31.3 | < 0.001 |
| > 80 | 4.7 | 7.8 | < 0.001 |
| Diabetes mellitus | 23.6 | 29.4 | < 0.001 |
| < 30% | 4.8 | 8.4 | < 0.001 |
| Arterial hypertension | 73.1 | 63.1 | < 0.001 |
| Congestive heart failure | 19.4 | 18.9 | 0.720 |
| Morbid obesity | 19.6 | 8.9 | < 0.001 |
| Severe COPD | 0.1 | 2.5 | < 0.001 |
| Previous heart surgery | 10.0 | 7.0 | 0.001 |
| Two or more previous heart surgeries | 2.1 | 0.4 | < 0.001 |
| Aortic valve replacement | 18.8 | 12.1 | < 0.001 |
| Mitral valve replacement | 13.4 | 7.1 | < 0.001 |
| CABG with valvular surgery | 7.0 | 8.5 | 0.093 |
| LMC lesion | 23.8 | 16.9 | < 0.001 |
| Preoperative IAB | 0.6 | 8.7 | < 0.001 |
LMC: Left main coronary; IAB: Intra-aortic balloon; BP: Bernstein Parsonnet 2000; CABG: Coronary artery bypass surgery; COPD: Chronic obstructive pulmonary disease; LVEF: Left ventricular ejection fraction.
Table 2.
Prevalence of risk factors in the sample and in the Bernstein Parsonnet 2000 population
| Exceptional situations | Prevalence in sample (%) (n = 1,065) | Prevalence in BP (%) (n = 10,703) | p value |
|---|---|---|---|
| Cardiogenic shock | 0.8 | 1.8 | 0.017 |
| Active Endocarditis | 1.0 | 0.4 | 0.005 |
| Treated Endocarditis | 0.8 | 0.8 | 0.915 |
| LV aneurysm surgery | 0.8 | 0.5 | 0.194 |
| Tricuspid valve surgery | 1.6 | 0.4 | < 0.001 |
| Pace-maker dependence | 1.3 | 1.5 | 0.604 |
| VT/VF | 0.5 | 3.3 | 0.03 |
| Asthma | 7.9 | 2.5 | < 0.001 |
| Preoperative OTI | 0.5 | 1.3 | 0.017 |
| Pulmonary hypertension | 16.6 | 10.7 | <0.001 |
| Cirrhosis | 0 | 0.2 | <0.001 |
| Dialysis | 0.5 | 0.9 | 0.151 |
| Acute or chronic renal failure | 5.1 | 4.6 | 0.457 |
| Asymptomatic abdominal aortic aneurysm | 0.8 | 1.3 | 0.128 |
| Carotid disease (bilateral or unilateral occlusion) | 3.0 | 2.6 | 0.612 |
| Peripheral vascular disease | 6.9 | 9.1 | 0.013 |
| Previous transfusion reaction | 0 | 0.2 | < 0.001 |
| Severe neurological disease (paraplegia, hemiparesis, muscular dystrophy) | 7.6 | 8.4 | 0.364 |
| Unsuccessful PTCA | 1.2 | 2.3 | 0.014 |
| Drug abuse | 0 | 2.1 | < 0.001 |
| AMI < 48 h | 0.9 | 5.4 | < 0.001 |
| Acute VSD | 0.1 | -- | -- |
| Idiopathic thrombocytopenic purpura | 0 | 0.3 | < 0.001 |
PTCA: Percutaneous transluminal coronary angioplasty; BP: Bernstein Parsonnet 2000; VSD: Ventricular septal defect (no cases in the sample); AMI: Acute myocardial infarction; Preoperative OTI: Preoperative orotracheal intubation; VT/ VF: Ventricular tachycardia / ventricular fibrillation; LV: Left ventricle.
Table 3.
Prevalence of risk factors in the sample and in the EuroSCORE population
| Risk factors | Prevalence in sample (%) (n =1.065) | Prevalence in EuroSCORE (%) (n = 19.030) | p value |
|---|---|---|---|
| > 75 | 13.3 | 9.6 | < 0.001 |
| Female gender | 38.7 | 27.8 | < 0.001 |
| COPD | 7.9 | 3.9 | < 0.001 |
| Extra-cardiac arteriopathy | 9.0 | 11.3 | 0.019 |
| Neurological dysfunction | 5.7 | 1.4 | < 0.001 |
| Previous heart surgery | 11.9 | 7.3 | < 0.001 |
| Serum creatinine >2.26 mg/dL | 1.4 | 1.8 | 0.344 |
| Active endocarditis | 1.0 | 1.1 | 0.034 |
| Critical preoperative status | 2.7 | 4.1 | 0.022 |
| Unstable Angina | 17.1 | 8.0 | < 0.001 |
| LVEF= 30-50% | 15.8 | 25.6 | < 0.001 |
| LVEF < 30% | 4.8 | 5.8 | 0.168 |
| AMI < 90 days | 1.2 | 9.7 | < 0.001 |
| Pulmonary hypertension (sPAP > 60 mmHg) | 5.2 | 2.0 | < 0.001 |
| Emergency heart surgery | 1.2 | 4.9 | < 0.001 |
| Another heart surgery associated or not to CABG CABG | 39.7 | 36.4 | 0.029 |
| Surgery involving thoracic aorta | 2.3 | 2.4 | 0.834 |
| VSD post-AMI | 0.1 | 0.2 | 0.467 |
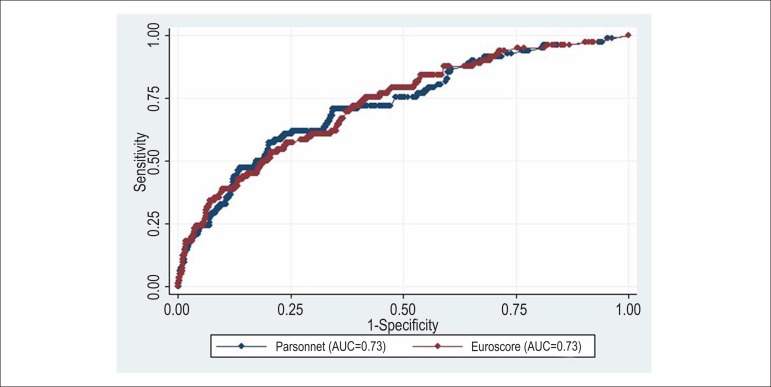
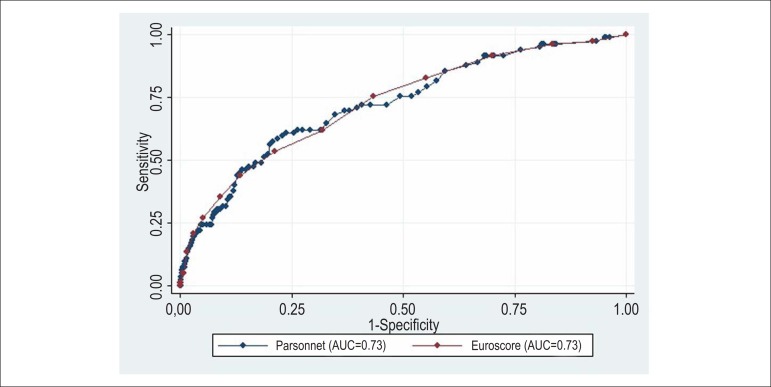
AUCs of the additive and logistic BP were 0.72 (95% CI: 0.66-0.78), and AUCs of additive and logistic ES were 0.73 (95%CI: 0.67-0.78). There was no statistically significant difference when comparing the AUCs of additive (p = 0.74) and logistic (p = 0.80) models of the two risk scores (Figures 1 and 2).
Figure 1.
Comparison of areas under the ROC curve of logistic Bernstein Parsonnet 2000 and EuroSCORE - STATA 9.2 software. AUC of the logistic Bernstein Parsonnet 2000, 0.73 (95%CI: 0.66 to 0.78) and logistic EuroSCORE, 0.73 (95%CI: 0.67-0.78). Chi-square = 0.06, p (x2) = 0.79.
Figure 2.
Comparison of areas under the ROC curve of additive Bernstein Parsonnet 2000 and EuroSCORE - STATA 9.2 software. AUC of additive Bernstein Parsonnet 2000, 0.73 (95% CI: 0.66 to 0.78) and additive EuroSCORE, 0.73 (95% CI: 0.67-0.78). Chi-square = 0.10, p (x2) = 0.74.
The Hosmer-Lemeshow (HL) test showed poor score calibration: x2 = 87.61 (8 gl), p < 0.001 in BP and x2 = 24.20 (8 gl), p = 0.01 in the ES. The calculation of the overall SMR showed that the scores tend to underestimate mortality in the sample, whereas in the additive and logistic BP SMR was 1.59 (95% CI: 1.27 to 1.99). In the additive and logistic ES, the SMR was 1.43 (95% CI: 1.14 to 1.79) -Tables 4 and 5.
Table 4.
Observed and expected mortality in the additive and logistic Bernstein Parsonnet 2000 in risk groups
| Expected mortality | Observed mortality | SMR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BP | N | N | % | N | % | Estimate | LL | UL | ||
| Additive | ||||||||||
| 0 a 3 | 157 | 1 | 0.7 | 3 | 1.9 | 2.7 | 0.5 | 8.1 | ||
| 3.1 a 17.5 | 596 | 10 | 1.7 | 28 | 4.7 | 2.7 | 1.8 | 4.0 | ||
| > 17.5 | 311 | 41 | 13.21 | 52 | 16.7 | 1.3 | 0.9 | 1.7 | ||
| Total | 1.064 | 52 | 4. | 83 | 7.8 | 1.6 | 1.3 | 1.9 | ||
| Logistic BP | ||||||||||
| ≤ 0.0137 | 399 | 4 | 0.9 | 12 | 3.0 | 3.2 | 1.7 | 5.6 | ||
| 0.0138 a 0.0304 | 335 | 7 | 2.0 | 19 | 5.7 | 2.8 | 1.7 | 4.3 | ||
| > 0.0304 | 338 | 42 | 12.6 | 52 | 15.8 | 1.2 | 0.9 | 1.6 | ||
| Total | 1.064 | 52 | 4.9 | 83 | 7.8 | 1.6 | 1.3 | 1.9 | ||
LL: Lower limit; UL: Upper limit; SMR: Standardized Mortality Rate.
Table 5.
Observed and expected mortality in the additive and logistic EuroSCORE in risk groups
| Expected Mortality | Observed Mortality | SMR | ||||||
|---|---|---|---|---|---|---|---|---|
| EuroSCORE | N | N | % | N | % | Estimate | LL | UL |
| Additive | ||||||||
| 0 a 2 | 303 | 4 | 1.3 | 7 | 2.3 | 1.8 | 0.7 | 3.7 |
| 3 a 5 | 395 | 11 | 2.8 | 24 | 6.1 | 2.1 | 1.3 | 3.1 |
| ≥ 6 | 365 | 42 | 11.5 | 51 | 14.0 | 1.2 | 0.9 | 1.6 |
| Total | 1.063 | 57 | 5.4 | 82 | 7.7 | 1.4 | 1.1 | 1.8 |
| Logistic | ||||||||
| ≤ 0,02 | 354 | 5 | 1.3 | 10 | 2.8 | 2.1 | 1.0 | 3.8 |
| 0,021 a 0,05 | 358 | 11 | 3.5 | 22 | 6.1 | 2.0 | 1.2 | 3.0 |
| ≥ 0,06 | 351 | 41 | 11.8 | 50 | 14.2 | 1.2 | 0.9 | 1.6 |
| Total | 1063 | 57 | 5.4 | 82 | 7.7 | 1.4 | 1.1 | 1.8 |
LL: Lower limit; UL: Upper limit; SMR: Standardized Mortality Rate.
In the multivariate logistic regression (Table 6), the following showed to be independent risk predictors for in-hospital mortality: diabetes mellitus (OR = 2.28; 95%CI: 1.34 to 3.88), CABG combined with valve surgery (OR = 2.38; 95% CI: 1.18 to 4.80), age between 76 and 79 years (OR = 2.49; 95%CI: 1.12 to 5.54), previous heart surgery (OR = 2.68; 95%CI: 1.40 to 5.12), two or more previous heart surgeries (OR = 3.34; 95% CI: 1.16 to 9.63), systolic pulmonary artery pressure (sPAP)> 60 mmHg (OR = 3.44; 95%CI: 1.60 to 7.39), age > 80 years (OR = 3.61; 95%CI: 1.54 to 8.45), active endocarditis (OR = 4.388; 95%CI: 1.00 to 19.17) and serum creatinine > 2.26 mg/dL (OR = 4.71; 95%CI: 1.28 to 17.34).
Table 6.
Independent predictors for in-hospital mortality in the sample according to logistic regression
| Risk factors | Odds ratio (95%CI) |
|---|---|
| Diabetes mellitus | 2.28 (1.34-3.88) |
| CABG combined with valvular surgery | 2.38 (1.18-4.80) |
| Age 76-79 years | 2.49 (1.12-5.54) |
| Previous cardiac surgery | 2.68 (1.40-5.12) |
| Two or more previous cardiac surgeries | 3.34 (1.16-9.63) |
| Systolic pulmonary artery pressure > 60 mmHg | 3.44 (1.60-7.39) |
| Age > 80 years | 3.61 (1.54-8.45) |
| Active endocarditis | 4.38 (1.00-19.17) |
| Serum creatinine > 2.26 mg/dL | 4.71 (1.28-17.34) |
CABG: Coronary artery bypass grafting
Discussion
In our sample, a North-American score (Bernstein Parsonnet 2000) and a European one (EuroSCORE) showed good discriminatory power, but underestimated the in-hospital mortality. This result contradicts what has been observed when applying BP and ES in developed countries. In a systematic review of the applicability of the ES in cardiac surgery, six studies from Japan, Belgium, France, Turkey and the UK were included, involving 16,000 patients. The additive ES was applied and it was observed that in patients with ES < 6, mortality was overestimated, while in patients with ES > 10, it was underestimated16. When validating the ES in Australia17, in 8,331 patients undergoing cardiac surgery, the expected overall mortality rate for this population was 5.31% for the additive model and 8.76% for the logistic model, while the observed mortality was 3, 20%. In Germany18, in 26,501 patients undergoing isolated CABG, the expected mortality at the logistic ES was 5.2%, while the observed one was 2.6%.
The BP score has been previously compared with ES. In 2006, a prospective study carried out in Tel Aviv by Berman et al19 compared the additive forms of the two scores in a sample of 1,639 patients undergoing cardiac surgery. The mortality rate in this population was 4.83%. The patients were divided into five risk groups. In the BP: 0 to 10; 10.5 to 20; 20.5 to 30; 30.5 to 40 and > 40. In the ES: 0 to 2; 3 to 5; 6 to 8; 9 to 11 and > 12. There were no significant differences between the observed and expected mortalities in both scores. The AUC was 0.83 in BP and 0.73 in ES. In 2004, the work by Syed et al20 compared the initial BP score with ES using retrospective data from only 194 patients undergoing cardiac surgery at a single center in Saudi Arabia. The AUC was 0.685 in BP and 0.766 in ES.
In Brazil, there are few records on the profile of patients undergoing cardiac surgery. The available data are recorded by the Hospital Information System of the Unified Health System, which does not include clinical data. In 2006, Ribeiro et al6 performed a review of available data in Datasus related to 115,021 cardiac surgeries performed between 2000 and 2003. Prevalence of isolated CABG was 57.1%, and of valve replacement, 33.7%. Overall mortality was 8.0% in this sample, of which 7.0% in patients submitted to CABG, 8.9% in those undergoing valve replacement and 16.5% in those submitted to complex cardiac surgery.
The multivariate analysis showed higher mortality among older patients, women, those submitted to valve and/or complex surgeries and patients admitted to hospitals with fewer surgeries. In hospitals that performed 1-130 surgeries a year, mortality was 9.7%, while in those that performed more than 341 surgeries a year, mortality was 5.8%. Previous studies had already shown an inverse association between the number of procedures performed a year and mortality21.
Compared to the United States, Brazil performs almost six times fewer surgeries per 100,000 inhabitants/year. Another review of Datasus data7 showed that in the years 2005, 2006 and 2007, 63,529 isolated CABG surgeries were performed in 191 hospitals. The recorded in-hospital mortality was 6.22%. The regions with higher number of procedures, which are also the wealthiest ones and the ones that have the highest number of referral centers, tend to have lower mortality. For instance, whereas in the southern region 15,985 procedures were performed with a mortality of 6.52%, in the northern region, 1,354 procedures were carried out, with a mortality rate of 7.24%.
We believe it is very important to study the variables that involve surgery in Brazil, considering that the postoperative mortality is even higher when compared to Europe and the United States. The overall mortality rate in the sample was 7.8%, close to the observed mortality in SUS6, whereas in the BP population, it was 5.3%, and in the ES, 4.7%.
We could attribute this difference to the higher prevalence of rheumatic fever as the pathogenesis of valve disease, in addition to Chagas disease, which require complex surgical procedures and often more than one surgery.
Grinberg et al22, in 2011, prompted by the Brazilian reality, which is different from that of Europe and the United States, proposed that the approach of the patient with valvular disease follow a strategy called "valvulopathy resolution script" (RESOLVA). In this script, which consists of four stages, the application of an international risk score is only one of the elements that assist in decision-making. The score result should be evaluated together with the conceptual benefit of the procedure given by scientific knowledge, with the autonomy of the team and patient in decision-making and discussion of two ethical principles (negligence and malpractice).
On the other hand, Guaragna et al23, in 2010, created a local risk score for patients undergoing valve surgery, eliminating the use of international scores. Advanced age, surgical priority, female gender, ejection fraction < 45%, concomitant CABG, pulmonary hypertension, NYHA functional class III or IV, creatinine (1.5 to 2.49 and > 2.5 mg/dL or dialysis) were identified as risk predictors.
Similarly, Mejía et al24, in 2013, developed a score based on data from patients undergoing cardiac surgery at a single referral center for cardiac surgery. Patients with indication for valve surgery and CABG were included. The score comprised ten variables: age > 70 years, female gender, valve surgery combined with CABG, acute myocardial infarction < 90 days, reoperation, surgical treatment of the aortic valve, tricuspid valve surgery, creatinine > 2 mg / dL, EF < 30% and events. This local score was compared with the ES and the BP, and its performance was similar to theirs, with good discriminatory power (AUC of 0.79 versus 0.81 and 0.82, respectively).
Although some studies have tested the applicability of the ES in valvular surgery, in a meta-analysis of 12 studies with 26,621 patients, Parolari et al25 observed that this score had low discriminatory power in this type of surgery, as it overestimates mortality. Once again it should be noted that the mortality rate in valvular surgery in these studies was around 4.5%, whereas in our sample it was 8.6% and in the population studied by Guaragna et al23, 11.8%.
Patients with pulmonary hypertension (sPAP > 60 mmHg) - a condition associated with valvular diseases - were more prevalent than in the initial ES population (5.2% versus 2.0%) and had higher risk of in-hospital mortality (OR = 3.44; 95%CI: 1.60 to 7.39). Another limitation of risk scores studied in patients with valvular disease is the fact that specific surgical procedures were not evaluated, e.g., combined aortic and mitral valve surgeries.
Our institution treats patients coming from public health centers, with significant socioeconomic problems, which lead to medication misuse, poor health status and advanced stage of disease at surgery. This may explain in part the higher prevalence of combined CABG and valve surgery procedure with high mortality in our sample (20%), which showed to be an independent risk factor in the multivariate analysis. A study carried out in Spain by Pons et al26 in 1994 showed that postoperative in-hospital mortality in public hospitals was higher than that of private hospitals (11.7% versus 6.7%, respectively).
Another aspect to consider is that the higher difference between the expected and observed mortalities occurred in patients at moderate risk, both in the additive BP and ES. One might consider that score applicability was compromised in our sample by evaluating different populations, both due to the profile of patients, as well as the socioeconomic aspects and the higher prevalence of valvular surgery.
It is important to consider that the small number of patients who used intra-aortic balloon (IAB) preoperatively also indicates the possible under-utilization of this resource in more severe patients, contributing to increased mortality. Another relevant fact is that the logistic regression showed DM as an independent risk factor for mortality in our sample, and that this diagnosis was not scored in ES. However, the new ES model that is being proposed has included this variable27.
The present study has limitations that should be considered. At the time of the manuscript writing, EuroSCORE I was still used, which has been currently updated and replaced by EuroSCORE II27. Mortality in 30 days or long-term survival was not assessed. Another limitation is that our sample consisted of a very heterogeneous population, which may have allowed biases in the results. Moreover, our study has limitations inherent to retrospective studies, with 14% of missing data. However, some risk models described in the literature were developed from pre-existing databases2,28-30. Some variables evaluated subjectively, such as the degree of COPD, degree of neurological damage and heart failure, had their definitions adapted similarly to what was performed in other studies17,31.
When comparing the Society of Thoracic Surgeons risk score (STS) with the ES in an institution in Sweden, the definition of recent acute myocardial infarction was 21 days and not 90 days, as proposed by ES31. Even with this adjustment, the ES had significantly better discriminatory power than the STS (AUC 0.84 versus 0.71). In the initial Parsonnet study, COPD was considered a risk factor that was difficult to obtain and was removed from the score model, even though it was associated with postoperative mortality1. For the same reason, emergency surgery and chronic heart failure were removed from the ES5.
Conclusions
The use of the Berstein Parsonnet 2000 and EuroSCORE underestimated the intra-hospital mortality, showing them to be inappropriate in the preoperative evaluation of patients undergoing cardiac surgery at our institution. Independent predictors of in-hospital mortality in our sample were: age, serum creatinine > 2.26 mg/dL, active endocarditis, pulmonary artery pressure > 60 mmHg, one or more previous heart surgeries, CABG combined with valve surgery and diabetes mellitus. Our study reinforces the need for the development of local scores, based on the reality of the populations, for better risk assessment in cardiac surgery.
Footnotes
Author contributions
Conception and design of the research, Analysis and interpretation of the data, Statistical analysis and Writing of the manuscript: Garofallo SB, Portal VL; Acquisition of data: Garofallo SB, Machado DP, Rodrigues CG, Bordim Jr. O; Critical revision of the manuscript for intellectual content: Garofallo SB, Kalil RAK, Portal VL.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This article is part of the thesis of master submitted by Silvia Bueno Garofallo, from Instituto de Cardiologia/Fundação Universitária de Cardiologia.
References
- 1.Parsonnet V, Dean D, Bernstein AD. A method of uniform stratification of risk for evaluating the results of surgery in acquired adult heart disease. Pt 2Circulation. 1989;79(6):I3–12. [PubMed] [Google Scholar]
- 2.Bernstein AD, Parsonnet V. Bedside estimation of risk as an aid for decision-making in cardiac surgery. Ann Thorac Surg. 2000;69(3):823–828. doi: 10.1016/s0003-4975(99)01424-1. [DOI] [PubMed] [Google Scholar]
- 3.Michel P, Roques F, Nashef SA, EuroSCORE Project Group. Logistic or additive EuroSCORE for high-risk patients? Eur J Cardiothorac Surg. 2003;23(5):684–687. doi: 10.1016/s1010-7940(03)00074-5. [DOI] [PubMed] [Google Scholar]
- 4.Roques F, Nashef SA, Michel P, EuroSCORE Project Group Regional differences in surgical heart valve disease in Europe: comparison between northern and southern subsets of the EuroSCORE database. J Heart Valve Dis. 2003;12(1):1–6. [PubMed] [Google Scholar]
- 5.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16(1):9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro AL, Gagliardi SP, Nogueira JL, Silveira LM, Colosimo EA, Lopes do Nascimento CA. Mortality related to cardiac surgery in Brazil, 2000-2003. J Thorac Cardiovasc Surg. 2006;131(4):907–909. doi: 10.1016/j.jtcvs.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Piegas LS, Bittar OJ, Haddad N. Myocardial revascularization surgery (MRS): results from national health system (SUS) Arq Bras Cardiol. 2009;93(5):555–560. doi: 10.1590/s0066-782x2009001100018. [DOI] [PubMed] [Google Scholar]
- 8.Shibata MC, Flather MD, de Arenaza DP, Wang D, O'Shea JC. Potential impact of socioeconomic differences on clinical outcomes in international clinical trials. Am Heart J. 2001;141(6):1019–1024. doi: 10.1067/mhj.2001.115529. [DOI] [PubMed] [Google Scholar]
- 9.Peterson ED, Coombs LP, DeLong ER, Haan CK, Ferguson TB. Procedural volume as a marker of quality for CABG surgery. JAMA. 2004;291(2):195–201. doi: 10.1001/jama.291.2.195. [DOI] [PubMed] [Google Scholar]
- 10.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 11.Roques F, Michel P, Goldstone AR, Nashef SA. The logistic EuroSCORE. Eur Heart J. 2003;24(9):881–882. doi: 10.1016/s0195-668x(02)00799-6. [DOI] [PubMed] [Google Scholar]
- 12.Nashef SA, Roques F, Michel P, Cortina J, Faichney A, Gams E, et al. Coronary surgery in Europe: comparison of the national subsets of the European system for cardiac operative risk evaluation database. Eur J Cardiothorac Surg. 2000;17(4):396–399. doi: 10.1016/s1010-7940(00)00380-8. [DOI] [PubMed] [Google Scholar]
- 13.Moons KG, Donders RA, Stijnen T, Harrell Jr FE. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. 2006;59(10):1092–1101. doi: 10.1016/j.jclinepi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59(10):1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Breslow NE, Day NE. Statistical methods in cancer research. Lyon: International Agency for Research on Cancer; 1987. [Google Scholar]
- 16.Gogbashian A, Sedrakyan A, Treasure T. EuroSCORE: a systematic review of international performance. Eur J Cardiothorac Surg. 2004;25(5):695–700. doi: 10.1016/j.ejcts.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Yap CH, Reid C, Yii M, Rowland MA, Mohajeri M, Skillington PD, et al. Validation of the EuroSCORE model in Australia. Eur J Cardiothorac Surg. 2006;29(4):441–446. doi: 10.1016/j.ejcts.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 18.Gummert JF, Funkat A, Osswald B, Beckmann A, Schiller W, Krian A, et al. EuroSCORE overestimates the risk of cardiac surgery: results from the national registry of the German Society of Thoracic and Cardiovascular Surgery. Clin Res Cardiol. 2009;98(6):363–369. doi: 10.1007/s00392-009-0010-8. [DOI] [PubMed] [Google Scholar]
- 19.Berman M, Stamler A, Sahar G, Georghiou GP, Sharoni E, Brauner R, et al. Validation of the 2000 Bernstein-Parsonnet score versus the EuroSCORE as a prognostic tool in cardiac surgery. Ann Thorac Surg. 2006;81(2):537–540. doi: 10.1016/j.athoracsur.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Syed AU, Fawzy H, Farag A, Nemlander A. Predictive value of EuroSCORE and Parsonnet scoring in Saudi population. Heart Lung Circ. 2004;13(4):384–388. doi: 10.1016/j.hlc.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 22.Grinberg M, Tarasoutchi F, Sampaio RO. Roteiro para resolução de valvopatia (Resolva) Arq Bras Cardiol. 2011;97(4):e86–e90. [PubMed] [Google Scholar]
- 23.Guaragna JC, Bodanese LC, Bueno FL, Goldani MA. Proposta de escore de risco pré-operatório para pacientes candidatos à cirurgia cardíaca valvar. Arq Bras Cardiol. 2010;94(4):541–548. doi: 10.1590/s0066-782x2010005000026. [DOI] [PubMed] [Google Scholar]
- 24.Mejía AO, Lisboa LB, Puig LB, Moreira LF, Dallan LA, Pomerantzeff PM, et al. InsCor: um método simples e acurado para avaliação do risco em cirurgia cardíaca. Arq Bras Cardiol. 2013;100(3):246–254. doi: 10.5935/abc.20130043. [DOI] [PubMed] [Google Scholar]
- 25.Parolari A, Pesce LL, Trezzi M, Cavallotti L, Kassem S, Loardi C, et al. EuroSCORE performance in valve surgery: a meta-analysis. Ann Thorac Surg. 2010;89(3):787-93, 793.e1-2. doi: 10.1016/j.athoracsur.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 26.Pons J, Moreno V, Borras J, Espinas J, Almazan C, Granados A. Open heart surgery in public and private practice. J Health Serv Res Policy. 1999;4(2):73–78. doi: 10.1177/135581969900400204. [DOI] [PubMed] [Google Scholar]
- 27.Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, et al. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41(4):734–744. doi: 10.1093/ejcts/ezs043. [DOI] [PubMed] [Google Scholar]
- 28.Kuduvalli M, Grayson AD, Au J, Grotte G, Bridgewater B, Fabri BM. A multi-centre additive and logistic risk model for in-hospital mortality following aortic valve replacement. Eur J Cardiothorac Surg. 2007;31(4):607–613. doi: 10.1016/j.ejcts.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 29.Ambler G, Omar RZ, Royston P, Kinsman R, Keogh BE, Taylor KM. Generic, simple risk stratification model for heart valve surgery. Circulation. 2005;112(2):224–231. doi: 10.1161/CIRCULATIONAHA.104.515049. [DOI] [PubMed] [Google Scholar]
- 30.Magovern JA, Sakert T, Magovern GJ, Benckart DH, Burkholder JA, Liebler GA, et al. A model that predicts morbidity and mortality after coronary artery bypass graft surgery. J Am Coll Cardiol. 1996;28(5):1147–1153. doi: 10.1016/S0735-1097(96)00310-5. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson J, Algotsson L, Hoglund P, Luhrs C, Brandt J. Early mortality in coronary bypass surgery: the EuroSCORE versus The Society of Thoracic Surgeons risk algorithm. Ann Thorac Surg. 2004;77(4):1235–1239. doi: 10.1016/j.athoracsur.2003.08.034. [DOI] [PubMed] [Google Scholar]