Abstract
Background
The effects of modern therapy on functional recovery after acute myocardial infarction (AMI) are unknown.
Objectives
To evaluate the predictors of systolic functional recovery after anterior wall AMI in patients undergoing modern therapy (reperfusion, aggressive platelet antiaggregant therapy, angiotensin-converting enzyme inhibitors and beta-blockers).
Methods
A total of 94 consecutive patients with AMI with ST-segment elevation were enrolled. Echocardiograms were performed during the in-hospital phase and after 6 months. Systolic dysfunction was defined as ejection fraction value < 50%.
Results
In the initial echocardiogram, 64% of patients had systolic dysfunction. Patients with ventricular dysfunction had greater infarct size, assessed by the measurement of total and isoenzyme MB creatine kinase enzymes, than patients without dysfunction. Additionally, 24.5% of patients that initially had systolic dysfunction showed recovery within 6 months after AMI. Patients who recovered ventricular function had smaller infarct sizes, but larger values of ejection fraction and E-wave deceleration time than patients without recovery. At the multivariate analysis, it can be observed that infarct size was the only independent predictor of functional recovery after 6 months of AMI when adjusted for age, gender, ejection fraction and E-wave deceleration time.
Conclusion
In spite of aggressive treatment, systolic ventricular dysfunction remains a frequent event after the anterior wall myocardial infarction. Additionally, 25% of patients show functional recovery. Finally, infarct size was the only significant predictor of functional recovery after six months of acute myocardial infarction.
Keywords: Myocardial infarction, Heart failure, Ventricular dysfunction, Recovery of function
Introduction
Many factors determine the outcome of patients after an Acute Myocardial Infarction (AMI)1,2. Among these prognostic factors, heart failure is highlighted due to left ventricular dysfunction. In fact, cardiac dysfunction after AMI increases the risk of death by three to four-fold3.
Epidemiological studies report that signs and symptoms of heart failure after infarction occur in approximately 25% of patients with AMI. Additionally, large clinical trials report that approximately 40% of AMI cases are accompanied by left ventricular systolic dysfunction, suggesting that functional deterioration is a common event after AMI4.
An important aspect to be considered, however, is that a considerable percentage of patients with systolic dysfunction in the acute phase of myocardial infarction show functional recovery over time. However, the current prevalence and risk factors for prediction of functional recovery are not completely understood.
Thus, this study aimed to evaluate the prevalence and predictors of systolic functional recovery after AMI in patients undergoing modern therapy after anterior myocardial infarction.
Methods
Design
The present was a prospective, observational study carried out in the coronary care unit of our institution. Consecutive patients of both genders that had the first episode of anterior AMI from December 2008 to December 2010 were included in the study.
The diagnosis of anterior AMI was established by a history of chest pain lasting more than 20 minutes and the presence of ST-segment elevation in at least two contiguous precordial leads (V1-V4) or the presence of new complete left bundle branch block at the electrocardiogram. Exclusion criteria were: congenital heart disease, significant primary valve disease, atrial fibrillation, inadequate condition for the completion of first echocardiogram during hospitalization, inadequate echocardiographic window, cancer, autoimmune disease, chronic renal failure (creatinine clearance ≤ 30 mL/min), liver failure and chronic immunosuppressive therapy.
The study protocol was approved by the Ethics Committee of our institution and patients were enrolled after signing the free and informed consent form. During hospitalization, patients were evaluated daily and submitted to the first echocardiogram; the time of follow-up after hospital discharge was 6 months and at 6 months, patients underwent clinical reassessment and a new echocardiogram.
Clinical variables
Data related to the clinical profile of patients were obtained from patient history and physical examination on admission. Blood samples were obtained according to the routine of the Coronary Care Unit. Electrolytes, renal function and blood count were measured on admission and measurement of blood glucose and lipids was performed on samples obtained after a 12-hour fast. The levels of total (CPK) and isoenzyme MB creatine phosphokinase (CK-MB) were assessed at admission and every 6 hours until levels started to decrease. Two measurements of troponin I were performed (on admission and 90 minutes after patient arrival).
Clinical variables were obtained: age, gender, ethnicity, symptoms at admission, duration of chest pain (between the onset of pain and the first assessment in the emergency room), prior coronary revascularization, heart rate, systemic blood pressure and signs of pulmonary and systemic congestion5-8.
The following cardiovascular risk factors were investigated: systemic arterial hypertension (SAH), Diabetes Mellitus (DM), dyslipidemia, smoking, obesity and family history of coronary artery disease5-8.
Patients that reported a previous diagnosis of SAH and that had at least one measurement of systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg, or routinely used antihypertensive drugs were considered hypertensive. Patients that reported a previous diagnosis of DM and were receiving regular treatment, or patients that had two fasting glucose levels ≥ 126 mg/dL or a casual plasma glucose ≥ 200 mg/dL associated with classic symptoms of DM were considered as diabetics. Dyslipidemia was considered in patients on regular use of lipid-lowering medication or the presence of serum Low Density Lipoprotein levels (LDL-C) ≥ 160 mg/dL and/or serum levels of high density lipoprotein (HDL-c) ≤ 40 mg/dL (men) or ≤ 50 mg/dL (women) and/or serum triglycerides ≥ 150 mg/dL.
Patients that smoked daily, regardless of the number of cigarettes, were considered smokers. Patients with first-degree relatives with premature coronary artery disease (men < 55 years and women < 65 years) were considered as having a positive family history for CAD. Finally, obesity was diagnosed by the presence of Body Mass Index (BMI) ≥ 30 kg/m2 and central obesity by the presence of waist circumference > 102 cm for men and > 88 cm for women. Weight and height were measured on a Welmy Brazil scale, with a maximum capacity of 200 kg and fixed stadiometer. The waist circumference was measured with an inelastic tape in the mid-distance between the iliac crest and the last rib.
Weight, height and waist circumference were measured in the morning, after fasting, 48 hours after admission with patients wearing the standard hospital gown5-8.
Regarding the treatment of AMI, reperfusion strategy and the following drugs prescribed during the in-hospital phase were evaluated: acetylsalicylic acid (ASA), Clopidogrel, heparin (unfractionated or low-molecular weight), Glycoprotein IIb/IIIa receptor inhibitor (IGP IIb/IIIa) inhibitors, angiotensin-converting enzyme inhibitors (ACEI), beta-blockers, calcium-channel blockers, nitrates, intravenous positive inotropic agents, diuretics, digitalis, statins, spironolactone and warfarin5-8.
Echocardiographic assessment
The post-AMI clinical complications that occurred in the in-hospital phase were defined as follows: post-AMI ischemia as the occurrence of chest pain during appropriate drug therapy and/or acute ischemic alterations in the electrocardiogram (ECG) after the second day of evolution after AMI; arrhythmia with occurrence of sustained ventricular tachycardia or ventricular fibrillation; heart failure as clinical or radiological pulmonary congestion that required intravenous diuretic treatment, presence of hypotension as SBP < 90 mmHg for a period > 30 minutes in euvolemic patients with no signs of tissue hypoperfusion; cardiogenic shock, as SBP < 90 mmHg for a period > 30 min and signs of peripheral hypoperfusion (cold extremities, oliguria, sweating, pallor, restlessness or drowsiness) associated with pulmonary congestion and pericarditis, as occurrences of characteristic chest discomfort and associated with pericardial friction or the presence of diffuse ST-segment elevation on the electrocardiogram5-8.
Morphological and functional evaluation of the left ventricle (LV) was performed by echocardiography. The examinations were performed by three echocardiographists blinded to patients' clinical characteristics and treatment. Echocardiograms were performed during the in-hospital phase and 6 months after the AMI by the same examiner. In our service, the interobserver variability is < 5% for one-dimensional measurements and < 10% for two-dimensional measurements and Doppler-derived time variables; intraobserver variability is <5% for all variables.
Examinations were performed in a Philips HDI-5000 equipment according to standard technique9. Systolic dysfunction was defined as ejection fraction < 50% assessed by Simpson's method10. Recovery of left ventricular systolic function was defined as an increase in LV ejection fraction > 50% in those patients with dysfunction detected between the first and second echocardiograms.
Statistical analysis
Continuous variables are shown as mean and standard deviation or median and 25% and 75% percentiles in case of non-normal distribution. Proportional variables were analyzed by chi-square test or the Fisher exact test for comparison between groups. Continuous variables were tested for normality; continuous variables with normal distribution were compared by Student's t test, while non-normal continuous variables were compared using the Mann-Whitney test.
Existing associations between the variables and functional recovery after AMI were analyzed by multivariate logistic regression. The occurrence of functional recovery was included as the dependent variable. Variables that showed statistically significant differences in the univariate analysis, plus age and gender, were included as independent variables. The ROC (Receiver Operating Characteristic) curve was used to determine the best infarct size cutoff. The SigmaStat statistical package for Windows 3.5 (Systat Software Inc. - San Jose, CA - USA) was used or statistical analysis. The level of significance was set at 5% for all tests.
Results
During the observation period, 94 patients with anterior AMI were evaluated. However, eight patients died before the second echocardiogram and three patients were lost to follow-up. Thus, our final sample consisted of 83 patients. Of these, 73% were males, mean age of 58 ± 12 years.
In the initial echocardiogram, 64% of the patients had systolic dysfunction. As expected, patients with ventricular dysfunction had larger infarct sizes, assessed by CPK and CPK-MB enzymes, than patients without dysfunction (Table 1). No differences were found for the other analyzed variables.
Table 1.
Clinical, demographic and treatment data of 83 patients with anterior-wall acute myocardial Infarction
| Variables | Ventricular dysfunction | p value | |
|---|---|---|---|
| Yes (n = 53) | No (n = 30) | ||
| Age (years) | 57.9 ± 11.7 | 59.2 ± 13.1 | 0.632 |
| Male, % (n) | 75.5 (40) | 70.0 (21) | 0.777 |
| SAH, % (n) | 50.9 (27) | 73.3 (22) | 0.078 |
| DM, % (n) | 24.5 (13) | 33.3 (10) | 0.545 |
| Dyslipidemia, % (n) | 75.5 (40) | 80.0 (24) | 0.842 |
| BMI (kg/m2) | 26.9 (23.6-28.9) | 28.9 (24.8-32.0) | 0.116 |
| WC (cm) | 94.1 ± 9.8 | 98.1 ± 12.6 | 0.113 |
| CPK (U/L) | 4421 (1.453-7.658) | 1.491 (683-4.116) | 0.018 |
| CPK-MB (U/L) | 445.0 (180.8-734.5) | 178.5 (111.0-324.0) | 0.002 |
| Primary angio, % (n) | 69.8 (37) | 66.7 (20) | 0.960 |
| Reperfusion, % (n) | 84.9 (45) | 86.7 (26) | 1.000 |
| ASA, % (n) | 100 (53) | 100 (30) | - |
| Clopidogrel, % (n) | 100 (53) | 100 (30) | - |
| ARB, % (n) | 1.9 (1) | 6.7 (2) | 0.295 |
| ACEI, % (n) | 92.5 (49) | 93.3 (28) | 1.000 |
| Beta-blocker, % (n) | 94.3 (50) | 100 (30) | 0.550 |
| Spironolactone, % (n) | 22.6 (12) | 13.3 (4) | 0.386 |
SAH: systemic arterial hypertension; DM: diabetes mellitus; BMI: body mass index; WC: waist circumference; CPK: creatine phosphokinase; CPK-MB: creatine phosphokinase-MB fraction; ASA: acetylsalicylic acid; ARB: angiotensin-II receptor blocker; ACEI: angiotensin-converting enzyme inhibitor. Data expressed as mean ± SD or median (including 25th and 75th percentiles).
Regarding baseline echocardiographic data, patients with ventricular dysfunction had higher LV diameters, associated with lower ejection fractions. There were no other differences between patients with or without dysfunction (Table 2).
Table 2.
Echocardiographic data of 83 patients with anterior-wall acute myocardial Infarction
| Variables | Ventricular dysfunction | p value | |
|---|---|---|---|
| Yes (n = 53) | No (n = 30) | ||
| LA (mm) | 41.2 ± 4.6 | 40.9 ± 4.8 | 0.776 |
| LVEDD (mm) | 51.2 ± 5.6 | 48.7 ± 4.2 | 0.037 |
| LVESD (mm) | 35.7 ± 5.4 | 31.0 ± 4.0 | < 0.001 |
| PW (mm) | 10.5 ± 1.4 | 10.9 ± 1.8 | 0.309 |
| E/A | 0.76 (0.64-0.92) | 0.79 (0.71-0.88) | 0.624 |
| IVRT (ms) | 116 (100-124) | 114 (104-128) | 0.943 |
| EDT (ms) | 215.3 ± 64.5 | 228.8 ± 60.1 | 0.360 |
| HR (bpm) | 76.4 ± 13.5 | 74.9 ± 13.4 | 0.621 |
| EF | 0.41 ± 0.05 | 0.58 ± 0.05 | < 0.001 |
LA: left atrium; LVEDD: left ventricular-end diastolic diameter; LVESD: left ventricular-end systolic diameter; PW: left ventricular posterior wall thickness; IVRT: isovolumetric relaxation time; EDT: E-wave deceleration time; HR: heart rate; EF: ejection fraction. Data expressed as mean ± SD or median (including 25th and 75th percentiles).
Regarding functional recovery, 24.5% of patients with initial systolic dysfunction showed recovery within 6 months after AMI. Patients that recovered ventricular function had smaller infarct sizes than patients without recovery. There were no differences in relation to other clinical variables (Table 3).
Table 3.
Clinical, demographic and treatment data of patients with ventricular dysfunction
| Variables | Functional recovery | p value | |
|---|---|---|---|
| Yes (n = 13) | No (n = 40) | ||
| Age (years) | 63.4 ± 12.3 | 56.2 ± 11.1 | 0.061 |
| Male, % (n) | 69.2 (9) | 77.5 (31) | 0.712 |
| SAH, % (n) | 38.5 (5) | 55.0 (22) | 0.473 |
| DM, % (n) | 15.4 (2) | 27.5 (11) | 0.480 |
| Dyslipidemia, % (n) | 84.6 (11) | 72.5 (29) | 0.480 |
| BMI (kg/m2) | 25.7 ± 3.5 | 26.8 ± 3.7 | 0.356 |
| WC (cm) | 92.4 ± 9.9 | 94.6 ± 9.8 | 0.483 |
| CPK (U/L) | 1.351 (841-4.167) | 5.587 (2.125-8.269) | 0.002 |
| CPK-MB (U/L) | 168 (80-273) | 579 (254-807) | < 0.001 |
| Primary angio, % (n) | 69.2 (9) | 70.0 (28) | 1.000 |
| Reperfusion, % (n) | 76.9 (10) | 87.5 (35) | 0.389 |
| ASA, % (n) | 100 (13) | 100 (40) | - |
| Clopidogrel, % (n) | 100 (13) | 100 (40) | - |
| ARB, % (n) | 7.7 (1) | 0 (0) | 0.245 |
| ACEI, % (n) | 84.6 (11) | 95.0 (38) | 0.249 |
| Beta-blocker, % (n) | 92.3 (12) | 95 (38) | 1.000 |
| Spironolactone, % (n) | 7.7 (1) | 27.5 (11) | 0.147 |
SAH: systemic arterial hypertension; DM: diabetes mellitus; BMI: body mass index; WC: waist circumference; CPK: creatine phosphokinase; CPK-MB: creatine phosphokinase-MB fraction; ASA: acetylsalicylic acid; ARB: angiotensin-II receptor blocker; ACEI: angiotensin-converting enzyme inhibitor Data expressed as mean ± SD or median (including 25th and 75th percentiles).
Regarding the echocardiographic data, patients that had functional recovery showed higher EDT and ejection fraction than those without recovery. No differences were found for other variables (Table 4).
Table 4.
Initial echocardiographic data of patients with ventricular dysfunction
| Variables | Functional recovery | p value | |
|---|---|---|---|
| Yes (n = 13) | No (n = 40) | ||
| AE (mm) | 39.9 ± 3.0 | 41.6 ± 5.0 | 0.239 |
| LVEDD (mm) | 50.8 ± 3.5 | 51.4 ± 6.2 | 0.737 |
| LVESD (mm) | 34.7 ± 3.9 | 36.0 ± 5.8 | 0.467 |
| PW (mm) | 10.0 (10.0-11.0) | 10.7 (9.4-11.4) | 0.827 |
| E/A | 0.75 (0.64-0.86) | 0.77 (0.62-0.93) | 0.844 |
| IVRT (ms) | 120 (109-126) | 116 (98-125) | 0.443 |
| EDT (ms) | 250.9 ± 77.6 | 203.5 ± 55.7 | 0.020 |
| HR (bpm) | 70.8 ± 12.0 | 78.2 ± 13.6 | 0.097 |
| EF | 0.44 ± 0.06 | 0.40 ± 0.05 | 0.006 |
LA: left atrium; LVEDD: left ventricular-end diastolic diameter; LVESD: left ventricular-end systolic diameter; PW: left ventricular posterior wall thickness; IVRT: isovolumetric relaxation time; EDT E-wave deceleration time; HR: heart rate; EF: ejection fraction. Data expressed as mean ± SD or median (including 25th and 75th percentiles).
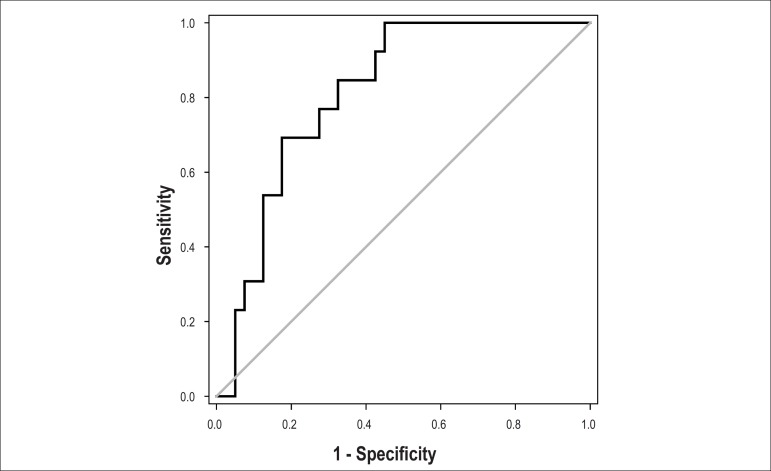
Multivariate analysis showed that infarct size was the only independent predictor of functional recovery 6 months after AMI, when adjusted for age, gender, ejection fraction, and EDT (Table 5). The ROC curve was used to determine the best cutoff for infarct size as assessed by the MB isoform, which determines LV function recovery, with the following result for the area under the curve: 0.814; 95% confidence interval (95% CI): 0.698 to 0.929, p <0.001, with a cutoff of 521U/L (Figure 1).
Table 5.
Logistic regression for predicting left ventricular function recovery 6 months after AMI
| Patient characteristics | Odds Ratio | 95%CI | p value |
|---|---|---|---|
| Gender | 1.719 | 0.236-12.509 | 0.593 |
| Age (years) | 1.062 | 0.986-1.144 | 0.114 |
| EF (%) | 1.137 | 0.895-1.445 | 0.294 |
| EDT (ms) | 1.002 | 0.982-1.002 | 0.845 |
| CPK-MB (U/L) | 0.995 | 0.990-0.999 | 0.015 |
95%CI: 95% confidence interval; EF: ejection fraction; EDT: E-wave deceleration time; CPK-MB: creatine phosphokinase-MB fraction.
Figure 1.
ROC curve of the infarct size measured by peak CK-MB isoform, which determines left ventricular function recovery. Area under the curve: 0.814, 95% confidence interval: 0.698 to 0.929, p value < 0.001, and cutoff of 521U / L.
Discussion
This study aimed to evaluate the prevalence and predictors of systolic functional recovery after AMI in patients undergoing modern therapy after anterior myocardial infarction. Our data suggest that systolic ventricular dysfunction remains a frequent event after AMI, with approximately 25% of patients showing functional recovery. Additionally, infarct size was the only significant predictor of functional recovery 6 months after the acute coronary event.
The first information from our study to be considered is that systolic dysfunction was a common event. In fact, approximately 65% of patients had ventricular dysfunction. Therefore, some factors are worth mentioning.
Firstly, we observed that our patients received the recommended treatment for patients with AMI11. Thus, more than 85% of patients were submitted to reperfusion therapy - most by primary angioplasty - 100% of patients received dual antiplatelet therapy and over 90% received beta-blockers and ACE inhibitors. However, we concluded that in most of our patients, this aggressive treatment was not capable of preventing the occurrence of ventricular dysfunction.
Another fact to be considered refers to the fact that the definition of systolic dysfunction is controversial, with different values of ejection fraction (≤ 35%, <40% or <45%) being considered in clinical trials. Our study determined that only patients with ejection fraction > 50% were considered as having completely normal systolic function, in agreement with a recent guideline10. This might have contributed to the high prevalence of dysfunction in our analysis.
To better understand functional recovery after AMI, one must consider the potential mechanisms of dysfunction after infarction. After the ischemic injury, the main physiopathological mechanisms that explain ventricular dysfunction include: loss of contractile capacity dependent on the affected amount of muscle, hearts affected by other comorbidities, mechanical complications, stunned myocardium and cardiac remodeling process4.
However, of the aforementioned mechanisms, the stunned myocardium is the one that best explains functional recovery over time12,13. Corroborating this hypothesis, we consider that currently, a significant number of patients with AMI are submitted to reperfusion therapy, which increases the likelihood of stunned myocardium and functional recovery14-17. Therefore, our study assumes that the stunned myocardium plays a crucial role in functional recovery after coronary occlusion. However, questions remain about the impact of more contemporary therapy on functional improvement and what the predictors of functional recovery are.
In the most recent study, which assessed patients from the HEART study, more than 50% of patients showed functional improvement after reperfusion and 24% had complete recovery within 2 weeks. It is important to emphasize that in this study, 65% of patients underwent thrombolytic therapy, whereas only 15% were submitted to primary angioplasty18. In our study, more than 65% of patients underwent primary angioplasty. However, our rate of complete functional recovery was similar to that observed in the HEART study. Thus, one can infer that our more aggressive treatment strategy had little additional impact on functional recovery of patients with AMI.
Finally, our study, in agreement with a previous one18, suggests that infarct size is the only predictor of functional recovery, therefore being superior to functional variables. Additionally, our study suggests that CPK-MB levels < 521U/L, a simple method for assessing infarct size, could be incorporated into clinical practice to predict functional recovery in patients after anterior wall AMI with ST elevation.
Conclusion
In conclusion, our study indicates that, despite aggressive treatment, systolic ventricular dysfunction remains a frequent event after AMI with ST-segment elevation. Additionally, 25% of patients show functional recovery. Finally, infarct size is the only significant predictor of functional recovery 6 months after the acute coronary event.
Footnotes
Author contributions
Conception and design of the research: Zornoff LAM; Acquisition of data: Fusco DR, Cogni AL, Azevedo PS, Okoshi K, Zanati SG, Paiva SAR, Zornoff LAM; Analysis and interpretation of the data: Fusco DR, Cogni AL, Azevedo PS, Okoshi K, Zanati SG, Paiva SAR; Obtaining financing: Zornoff LAM; Statistical analysis: Zornoff LAM; Writing of the manuscript: Zornoff LAM; Critical revision of the manuscript for intellectual content: Azevedo PS, Paiva SAR, Zornoff LAM.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any thesis or dissertation work.
References
- 1.Lee KL, Woodlief LH, Topol EJ, Weaver WD, Betriu A, Col J, et al. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction: results from an international trial of 41.021 patients. GUSTO-I Investigators. Circulation. 1995;91(6):1659–1668. doi: 10.1161/01.cir.91.6.1659. [DOI] [PubMed] [Google Scholar]
- 2.Zornoff LA, Paiva SA, Duarte DR, Spadaro J. Ventricular remodeling after myocardial infarction: concepts and clinical implications. Arq Bras Cardiol. 2009;92(2):157–164. doi: 10.1590/s0066-782x2009000200013. [DOI] [PubMed] [Google Scholar]
- 3.Risk stratification and survival after myocardial infarction. N Engl J Med. 1983;309(6):331–336. doi: 10.1056/NEJM198308113090602. [DOI] [PubMed] [Google Scholar]
- 4.Minicucci MF, Azevedo PS, Polegato BF, Paiva SA, Zornoff LA. Heart failure after myocardial infarction: clinical implications and treatment. Clin Cardiol. 2011;34(7):410–414. doi: 10.1002/clc.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cogni AL, Farah E, Minicucci MF, Azevedo PS, Okoshi K, Matsubara BB, et al. Waist circumference, but not body mass index, is a predictor of ventricular remodeling. Nutrition. 2013;29(1):122–126. doi: 10.1016/j.nut.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Farah E, Cogni AL, Minicucci MF, Azevedo PS, Okoshi K, Matsubara BB, et al. Prevalence and predictors of ventricular remodeling after anterior myocardial infarction in the era of modern medical therapy. Med Sci Monit. 2012;18(5):CR276–CR281. doi: 10.12659/MSM.882732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azevedo PS, Cogni AL, Farah E, Minicucci MF, Okoshi K, Matsubara BB, et al. Predictors of right ventricle dysfunction after anterior myocardial infarction. Can J Cardiol. 2012;28(4):438–442. doi: 10.1016/j.cjca.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Farah E, Fusco DR, Okumoto PR, Minicucci MF, Azevedo PS, Matsubara BB, et al. Impact of ventricular geometric pattern on cardiac remodeling after myocardial infarction. Arq Bras Cardiol. 2013;100(6):518–523. doi: 10.5935/abc.20130104. [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Bierig M, Devereaux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 10.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC Committee for Practice Guidelines ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–1847. doi: 10.1093/eurheartj/ehs104. Erratum in: Eur Heart J. 2013 Jan;34(2):158. [DOI] [PubMed] [Google Scholar]
- 11.O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):529–555. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 12.Christian TF, Behrenbeck T, Pellikka PA, Huber KC, Chesebro JH, Gibbons RJ. Mismatch of left ventricular function and infarct size demonstrated by technetium-99m isonitrile imaging after reperfusion therapy for acute myocardial infarction: identification of myocardial stunning and hyperkinesia. J Am Coll Cardiol. 1990;16(7):1632–1638. doi: 10.1016/0735-1097(90)90313-e. [DOI] [PubMed] [Google Scholar]
- 13.Bolli R. Myocardial 'stunning' in man. Circulation. 1992;86(6):1671–1691. doi: 10.1161/01.cir.86.6.1671. [DOI] [PubMed] [Google Scholar]
- 14.Ito H, Tomooka T, Sakai N, Higashino Y, Fujii K, Katoh O, et al. Time course of functional improvement in stunned myocardium in risk area in patients with reperfused anterior infarction. Circulation. 1993;87(2):355–362. doi: 10.1161/01.cir.87.2.355. [DOI] [PubMed] [Google Scholar]
- 15.Serruys PW, Simoons ML, Suryapranata H, Vermeer F, Wijns W, van den Brand M, et al. Preservation of global and regional left ventricular function after early thrombolysis in acute myocardial infarction. J Am Coll Cardiol. 1986;7(4):729–742. doi: 10.1016/s0735-1097(86)80330-8. [DOI] [PubMed] [Google Scholar]
- 16.Sheehan FH, Doerr R, Schmidt WG, Bolson EL, Uebis R, von Essen R, et al. Early recovery of left ventricular function after thrombolytic therapy for acute myocardial infarction: an important determinant of survival. J Am Coll Cardiol. 1988;12(2):289–300. doi: 10.1016/0735-1097(88)90397-x. [DOI] [PubMed] [Google Scholar]
- 17.Schofer J, Lins M, Mathey DG, Sheehan FH. Time course of left ventricular function and coronary patency after saruplase vs streptokinase in acute myocardial infarction. The PRIMI Trial Study Group. Eur Heart J. 1993;14(7):958–963. doi: 10.1093/eurheartj/14.7.958. [DOI] [PubMed] [Google Scholar]
- 18.Solomon SD, Glynn RJ, Greaves S, Ajani U, Rouleau JL, Menapace F, et al. Recovery of ventricular function after myocardial infarction in the reperfusion era: the healing and early afterload reducing therapy study. Ann Intern Med. 2001;134(6):451–458. doi: 10.7326/0003-4819-134-6-200103200-00009. [DOI] [PubMed] [Google Scholar]