Abstract
Diagnosis, prognosis and evaluation of death risk in Chagas cardiomyopathy still constitute a challenge due to the diversity of manifestations, which determine the importance of using echocardiography, tissue Doppler and biomarkers. To evaluate, within a systematic review, clinical and echocardiographic profiles of patients with chronic chagasic cardiomyopathy, which may be related to worse prognosis and major mortality risk. To perform the systematic review, we used Medline (via PubMed), LILACS and SciELO databases to identify 82 articles published from 1991 to 2012, with the following descriptors: echocardiography, mortality and Chagas disease. We selected 31 original articles, involving diagnostic and prognostic methods. The importance of Chagas disease has increased due to its emergence in Europe and United States, but most evidence came from Brazil. Among the predictors of worse prognosis and higher mortality risk are morphological and functional alterations in the left and right ventricles, evaluated by conventional echocardiography and tissue Doppler, as well as the increase in brain natriuretic peptide and troponin I concentrations. Recently, the evaluations of dyssynchrony, dysautonomia, as well as strain, strain rate and myocardial twisting were added to the diagnostic arsenal for the early differentiation of Chagas cardiomyopathy. Developments in imaging and biochemical diagnostic procedures have enabled more detailed cardiac evaluations, which demonstrate the early involvement of both ventricles, allowing a more accurate assessment of the mortality risk in Chagas disease.
Keywords: Chagas Cardiomyopathy / mortality, Prognosis; Chagas' Disease, Echocardiography
Introduction
Chagas disease remains a serious worldwide, economic and public health problem, endemic in South America and emergent in Europe and the United States1. The World Health Organization (WHO), in 2013, estimated between 7 and 8 million people chronically infected with Trypanosoma cruzi in the world, most of them in 21 Latin American countries, among which more than 30% develop cardiac abnormalities and more than 10% digestive and neurological problems2. Additionally, the WHO estimated there are 25 million people at risk of contracting the disease3.
In Brazil, it is estimated that there are somewhere between 2 to 3 million infected individuals4, which makes it the third main cause of parasitic diseases after malaria and schistosomiasis, and the fourth cause of great damage among communicable diseases in America3.
In the acute phase of Chagas disease, cardiac involvement can occur in up to 90% of cases5. After six to eight weeks, most patients show recovery of the clinical picture6.
In the indeterminate phase, the infestation is only diagnosed by serological or parasitological tests, as there is no evidence of organ damage. Subclinical cardiac involvement may be demonstrated by imaging studies in some patients7. Symptoms will develop in the chronic phase, a long time after the first infestation, with cardiac involvement being mainly responsible for the reduction in life expectancy7.
Several physiopathological hypotheses have been implicated in myocardial damage, ranging from direct aggression by the parasite1,8, sympathetic and parasympathetic dysautonomia9 and coronary microvascular abnormalities9 to autoimmune processes10. The lack of definition of the physiopathology complicates the diagnosis and hence, the early implementation of treatment in patients with Chagas cardiomyopathy11. For these reasons, and because the mortality from Chagas cardiomyopathy is closely related to significant organ involvement, it becomes important to identify prognostic markers related to worse outcomes12.
This article aims to systematically review the clinical and echocardiographic profiles of patients with chronic Chagas' disease, which may be related to worse prognosis and mortality.
Method
A systematic literature review was performed in the following databases: Medical Literature Analysis and Retrieval System Online (MEDLINE) via PubMed, Latin American and Caribbean Literature on Health Sciences (LILACS) and Scientific Electronic Library Online (SciELO). Using the descriptors: echocardiography, Chagas disease and mortality, the following were employed as inclusion criteria for the studies: being original; having as research subjects individuals diagnosed with Chagas disease; addressing diagnostic or prognostic methods of Chagas cardiomyopathy: detailing clear inclusion, exclusion and discontinuation (when relevant) criteria, and objectives for the research subjects, regardless of gender.
The search was not limited by period of time and language. Duplicate articles, those assessing interventions, those performed in patients aged zero to 10 years and in animals, in vitro experiments, case reports and editorials were excluded.
Data Analysis
Article selection was performed in three steps. Firstly, the titles were read and the ones that did not fit any of the study inclusion criteria were excluded. Secondly, summaries of the selected studies in the first step were read and, likewise, the ones that did not fit at least one of the inclusion criteria were excluded. In the third step, all studies that were not excluded in previous studies were read in full to select the ones that would be included in this review.
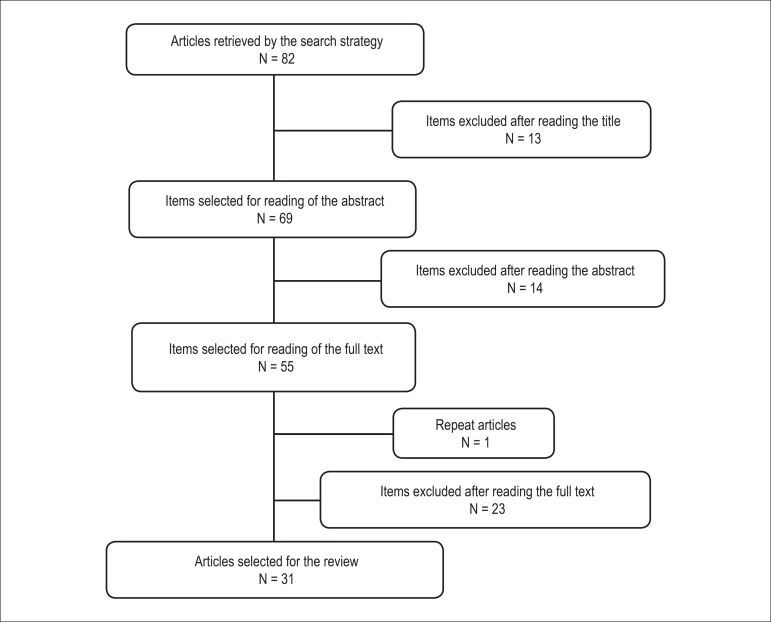
A total of 52 articles were found in the MedLine database via PubMed using the descriptors, of which 13 were excluded after reading the title and eight after reading the abstracts. The remaining 31 articles were read in full and 12 were excluded and 19 included in the review. A total of 30 articles were located in the SciELO and LILACS databases, six of which were excluded after reading the abstracts and 11, after reading the full article; one of these was a duplicate, resulting in the selection of 12 articles. Thus, 31 articles were included in this review, published between 1991 and 2012 (Figure 1).
Figure 1.
Flow chart of the number of articles found and selected after applying the inclusion and exclusion criteria
For a better presentation of the results, we chose to classify methodologically articles selected by the variables: author, year, country, sample size, mean age of the subjects, presence of criteria for subject selection (inclusion and exclusion), of comparison group and results (Chart 1).
Chart 1.
Methodological classification of selected articles
| Author | Year | Country | Type of study | N. of patients | Mean age (years) (range) | Selection criteria | Group of comparison | Results |
|---|---|---|---|---|---|---|---|---|
| Guerrero et al.13 | 1991 | Venezuela | Cohort | 269 | > 18 | Yes | Yes | The higher prevalence of complex ventricular arrhythmias in chronic Chagasic patients, compared to those with primary dilated cardiomyopathy, exposes Chagasic patients to a worse prognosis. |
| Marin-Neto et al.14 | 1998 | Brazil | Prospective | 45 | Yes | Yes | Dysautonomia is evident in chagasic patients with the digestive form, but not with the indeterminate form. There is no causal association with early myocardial damage, apparent only in patients with impaired right ventricular function, which appears to be the mechanism for the predominance of systemic over pulmonary congestion when heart failure occurs. | |
| Without abnormalities = 42.4 ± 11.9 | ||||||||
| Barros et al.15 | 2001 | Brazil | Prospective | 40 | Not informed | Yes | Yes | TDI allowed the identification of higher isovolumic contraction of the septal wall in chagasic patients with the indeterminate form. |
| Barros et al.16 | 2002 | Brazil | Prospective | 30 | 39.9 ± 9.9 | Yes | Yes | TDI allows early identification of the RV dysfunction (increased isovolumic contraction of the septal and lateral walls). |
| Barros et al.17 | 2003 | Brazil | Prospective cross-sectional | 77 | Yes | Yes | Tissue Doppler imaging (TDI) identifies early contractility abnormalities. | |
| Chagasic with abnormal ECG: 43.9 ± 11.1 | ||||||||
| Arias et al.18 | 2003 | Brazil | Prospective cross-sectional | 60 | > 18 | Yes | Yes | Troponin levels are elevated in different clinical presentations of Chagas' disease and detect early inflammation. |
| Nunes et al.19 | 2004 | Brazil | Prospective cross-sectional | 74 | 47.5 ± 12.9 (22-73) | Yes | No | RV dysfunction was significantly associated with LV involvement and pulmonary hypertension, which was more correlated with RV overload than to contractility. |
| Viotti et al.20 | 2004 | Argentina | Cohort | 849 | > 18 | Yes | No | Echocardiography determines the prognosis of chronic Chagasic patients without congestive heart failure (CHF). |
| Talvani et al.21 | 2004 | Brazil | Prospective cross-sectional | 81 | 43.5 ± 11.1 | Yes | Yes | High levels of brain natriuretic peptide (BNP) in Chagasic patients detect heart failure (HF) or severe HF manifestations, generating arrhythmias. |
| Rocha et al.22 | 2004 | Brazil | Cohort | 60 | 47 ± 13 | Yes | No | Left atrial volume (LAV) was an important predictor of death in Chagas disease. |
| Freitas et al.23 | 2005 | Brazil | Cohort | 1220 | 13 ± 72 (45) | Yes | No | Chagasic etiology in HF patients was the main prognostic factor for mortality. |
| Melo et al.24 | 2005 | Brazil | Transversal | 25 | 62.7 ± 7.7 chronic 42.2 ± 11.7 asymptomatic | Yes | Yes (Chagas asymptomatic x symptomatic) | BNP increases with worsening functional class and cardiac area. |
| Pazin-Filho et al.25 | 2006 | Brazil | Prospective cross-sectional | 59 | 37-76 > 55 | Yes | No | In time, slight alterations in the left ventricular ejection fraction (LVEF) lead to the worsening of global LV systolic function. |
| Rassi Jr et al.26 | 2006 | Brazil | Cohort | 424 | 47 ± 11 | Yes | Yes | Development of death risk assessment in chagasic cardiomyopathy based on six factors |
| Chaves et al.27 | 2006 | Colombia | Prospective cross-sectional | 430 | Md = 40.5 (IQR = 36-45) | Yes | Yes | Comparing controls, stage I and stage II using tissue Doppler, it is possible to identify, in stage II, the greater relaxation time, lower velocity of A wave, higher E/A ratio, lower pulmonary A velocity, greater pulmonary/mitral A velocity ratio, greater annulus A wave velocity, and the RV shows increased tricuspid A wave velocity and isovolumic contraction time. |
| Benchimol Barbosa28 | 2007 | Brazil | Cohort | 50 | 34-74 | Yes | No | In the male gender, pathological Q wave, ventricular extrasystoles, ventricular tachycardia and abnormal echocardiogram (diastolic and systolic dysfunction and apical aneurysm) are markers of poorer prognosis. |
| Barbosa et al.29 | 2007 | Brazil | Prospective cross-sectional | 59 | 48 ± 11 | Yes | No | The pro-BNP levels remained a strong correlation with LVEF and duration indices of diastolic dysfunction of left atrial volume, as well as with the more severe forms of the disease, allowing their differentiation from the moderate forms. |
| Nunes et al.30 | 2008 | Brazil | Cohort | 158 | 48 ± 12 | Yes | No | Evaluation of systolic and diastolic function using the Tei index (myocardial performance index) and CF showed to be useful prognostic tool in Chagasic cardiomyopathy. |
| Villas-Boas et al.31 | 2008 | Brazil | Prospective cross-sectional | 38 | 51.3 ± 1.6 for chagasic and 53.3 ± 2.3 for control patients | Yes | Yes | The concentration of BNP is increased and correlates with heart failure (direct), functional class (direct) natremia (reverse), blood pressure (reverse), right atrial pressure, R/L ventricular dysfunction, TNF-alpha (demonstrating inflammatory characteristic of the disease). |
| Nunes et al.32 | 2009 | Brazil | Cohort | 192 | 48 ± 12 | Yes | No | LAV provides powerful information to predict mortality regardless of clinical and echocardiographic data. |
| Del Castillo et al.33 | 2009 | Brazil | Prospective cross-sectional | 40 | 55 ± 10 | Yes | Yes | Based on the principle of speckle tracking and employing the assessment of intramyocardial velocity gradients, they identified the reduction in the percentage of deformation (X-strain) and the rate of deformation (strain rate) of the infero-lateral left ventricular wall in Chagas patients unidentifiable by clinical data or conventional echocardiography. |
| Terzi et al.34 | 2010 | Brazil | Prospective cross-sectional | 62 | 58 | Yes | No | Chagasic patients with normal LVEF and normal electrocardiogram had a significant frequency of contractile alterations related with arrhythmias and reduced LVEF. |
| Pereira Nunes et al.35 | 2010 | Brazil | Cohort | 287 | Yes | Yes | Identification of Chagasic etiology resulted in poor prognosis in patients with HF, regardless of clinical and echocardiography data. | |
| Idiopathic: 49.6 ± 15.9 | ||||||||
| Nunes et al.36 | 2010 | Brazil | Cohort | 65 | 48.6 ± 9.1 | Yes | No | RV dysfunction is an important determinant, regardless of exercise capacity, and the systolic valvular velocity of this ventricle was associated with the peak volume of O2 consumed, regardless of age, gender and echocardiographic parameters. |
| Garcia-Alvarez et al.37 | 2010 | Spain | Cohort | 54 | 20-58 (mean 37) | Yes | Yes (4 groups) | The identification of diastolic dysfunction can be achieved by increasing BNP, which correlates with functional class (NYHA), TNF-alpha, PS, endothelin, ANP and reduction in the percentage of longitudinal and radial deformation to assess contractility. |
| Duarte et al.38 | 2011 | Brazil | Cohort | 56 | 56 ± 10 | Yes | No | Although high prevalence of intraventricular dyssynchrony and moderate interventricular dyssynchrony was demonstrated among Chagasic patients, especially in patients without a pacemaker, dyssynchrony did not show to be a risk factor for poor prognosis. |
| Valerio et al.39 | 2011 | Spain | Descriptive | 100 | 38.2 ± 10.2 | Yes | No | The fact that Spain has become a center of migration of Latin Americans in Europe has aroused the concern of Chagas disease becoming an important cause of cardiomyopathy in health services. |
| Nunes et al.40 | 2012 | Brazil | Prospective endpoint | 232 | 48 ± 12 | Yes | Yes (survivors and deaths) | Using tissue Doppler imaging, they identified risk factors for death: functional class III and IV increased RV Tei, increased left atrial volume index and interaction of LV ejection fraction and E / E' index and protectors: E / E ' ratio and reduction in LV ejection fraction. |
| Vasconcelos and Junqueira41 | 2012 | Brazil | Prospective cross-sectional | 15 controls and 13 chagasic | Yes | Yes | The sympathetic and parasympathetic depressions with preserved balance were associated with heart rate variability and increased left ventricular systolic diameter in Chagasic patients with cardiomyopathy, indicating that these depressions may precede and be independently more severe than ventricular dysfunction, with no causal association between this depression and dysfunction. | |
| chagasic = 35-49 | ||||||||
| Del Castillo et al.42 | 2012 | Brazil | Prospective cross-sectional | 20 | 54.8 ± 13.5 | Yes | Yes | Employing the speckle tracking technique, it was possible to prove reduction of twisting and torsional deformation of myocardial fibers in Chagas cardiomyopathy, thus differentiating it from ventricular hypertrophy |
| Melo et al.43 | 2010 | Brazil | Cross-sectional | 150 | Yes | Yes | It was demonstrated that both groups had increased inflammatory activity, but there were no indications of greater prothrombotic status among chagasic patients. | |
| G2: 54.88 ± 0.8787 |
Legend: ANP: Atrial Natriuretic Peptide, BNP: brain natriuretic peptide; DTI: Doppler tissue imaging (Image by tissue Doppler), CF: cardiac function, LVEF, left ventricular ejection fraction, HF: Heart failure; NYHA: New York Heart Association; O2: oxygen; Tei: myocardial performance index, TNF: Tumor Necrosis Factor; LAV: left atrial volume, RV: right ventricle, LV: left ventricle.
Results
Regarding temporal distribution, there was a marked prevalence of publications in the past decade, with only two publications having been published in the penultimate decade (1990-1999) 13-14. Another important point to be considered is the absolute hegemony of studies carried out in Brazil (77% of those included in this review)4,6-9,11,12,14-19,21-26,28-36,38,40-43. Among the five studies performed in other countries, three were carried out in Latin America13,20,27 and two in Europe37,39.
Regarding the use of comparison groups, it was observed that in 18 articles (58%) the authors used the design of comparisons between groups8,13-18,21,24,26,27,31,35,37,33,40-41,43. It is also interesting to note the predominance of adult patients in the selected studies, with only one study including adolescent chagasic patients23.
As for the addressed topics, 10 articles considered prognostic factors of mortality and risk of disease progression using echocardiography13,19,22,23,26,28,30,32,35,36, five studies assessed left ventricular ejection fraction (LVEF)20,25,29,34,39, alone or together with prognostic factors, seven articles assessed systolic and diastolic ventricular function with tissue Doppler15-17,27,33,36,40, five assessed concentrations of brain natriuretic peptide (BNP)21,24,29,31,37, one study assessed the prognostic value of troponin concentrations18, one study evaluated ventricular dyssynchrony38, two studies assessed cardiac dysautonomia14,41, two studies used two-dimensional echocardiography to assess ventricular deformation (strain, strain rate and twisting)33,42 and one study addressed the proinflammatory and prothrombotic factors in Chagas disease 43.
Discussion
The predominance of publications over the last ten years may be related to the fact that in past decades, the prognosis for Chagas disease relied almost exclusively on clinical predictors of mortality, which could be evaluated solely during patient follow-up. This means that the preventive process was restricted to the tertiary level, when treatment aimed only to improve quality of life, as the entire spectrum of cardiomyopathy was already established 7. With the technological development of ultrasound devices, which allow performing more detailed tests capable of providing valuable information on the physiopathology and prognosis of Chagas cardiomyopathy and establish hemodynamic correlation, studies have become more comprehensive.
As for the near hegemony of studies performed in South America, particularly in Brazil, one possible explanation is that our country constitutes a true source of specialized centers and researchers who, for decades, have been devoted to research on Chagas disease, mainly concerning the factors that alone or combined, may be involved with worse prognosis and increased mortality. Among these are Rassi Jr et al.26, Nunes et al. 19,30,32,35,36,40, Marin-Neto et al. 14 Barbosa 28, Barbosa et al.29.
Another possible explanation for the increase in publications in the last decade was the dissemination of T. cruzi beyond the limits of Central and South America. The immigration of Latin Americans resulted in the diagnosis of patients with the indeterminate form, who were blood donors, especially in Europe39, arousing concerns given the frequency at which Chagas cardiomyopathy became an important diagnostic issue in health services, which were not used to managing these patients39.
This concern is also found in other countries, especially with the objective of providing primary and secondary disease prevention26.
Studies with group comparison13-18,21,24,26,27,31,33,35,37,40,41 aimed to evaluate the clinical, hemodynamic and evolution aspects of Chagas cardiomyopathy, while those aimed to identifying morphological and functional characteristics related to prognosis and mortality did not employ this methodology19,20,22,23,25,28-30,32,34,36,38,39. The identification of the predominance of publications aimed to investigate adult patients (between the third and fourth decades of life), when compared to those involving adolescents, seems justified by the physiopathology of the disease. In the chronic phase, symptoms of organ involvement most often manifests after 10 to 30 years since the first infestation. That is why Chagas cardiomyopathy in adolescents is a rarer event, for which the acute phase is the most frequent in epidemiological studies23. It is appropriate to stress that, in cases of vertical transmission of the disease, children and adolescents may exhibit signs of early cardiac impairment, but these cases have been the subject of underreporting, reinforcing the scarcity of cases also in adolescence23 .
In addition to the involvement of young adults, chronic Chagas cardiomyopathy constitutes a serious public health problem, as it is a major cause of heart failure with a high mortality rate23. This fact, which persists a century after the first description of the disease, has motivated many studies on prognostic factors of mortality and risk of disease progression, among which 10 were included in this review10,17,19,20,23,25,27,29,31,37. All these studies recognize the importance of identifying the chagasic etiology of heart failure, given its destructive and overwhelming role due to incessant myocarditis, caused by extensive myocardial fibrosis, as well as being responsible for complex ventricular arrhythmias, which may explain poorer prognosis of chagasic patients when compared to patients with primary dilated cardiomyopathy13,34.
Emphasizing the importance of the etiological diagnosis of heart failure in the context of Chagas disease, another study, which compared 224 patients with chagasic cardiomyopathy and 63 with idiopathic dilated cardiomyopathy, showed that only the identification of Chagas disease conferred poor prognosis for patients with heart failure, regardless of clinical and echocardiographic data35.
In addition to heart failure, other studies have shown that right ventricular involvement may be useful for risk stratification of Chagas cardiomyopathy. The myocardial performance index (Tei index), assessed by echocardiography, adds significant prognostic information and allows analysis of systolic and diastolic function of the right ventricle (RV), very useful and effective in patients with Chagas heart disease30. Another index used to evaluate RV systolic function is the tricuspid annular plane systolic excursion (TAPSE), capable of providing more reliable information33.
Thus, the RV morphological and functional characteristics were also the subject of a study including 74 patients19, demonstrating that such changes are associated with significant left ventricular involvement and pulmonary hypertension, contributing to poor prognosis of Chagas disease and also acting as an independent determinant of reduced exercise capacity36. Among the left ventricular alterations, the presence of apical aneurysm, impaired left ventricular function and ventricular extrasystoles also acted as predictors of poor prognosis28, associated with increased left atrial volume, regardless of the clinical data and conventional echocardiographic parameters20,22,32. Left ventricular wall motion abnormalities in patients with normal systolic function were shown to be a predictor of ventricular function deterioration in chagasic patients with normal ECG and no symptoms or physical signs25 .
The evaluation of several mutually related indices demonstrates their potential in determining prognostic factors of mortality and risk of disease progression27, as emphasized in the paper of Rassi Jr. et al.26, wich described six risk factors and attributed a score to constitute a simple and validated score. It should be noted that this score was gradually increased by the adding of other factors identified by echocardiography and tissue Doppler imaging.
Mitral annular tissue Doppler, an established modality of echocardiographic assessment of ventricular diastolic and systolic function, disclosed early abnormalities of myocardial contractility15-17, thus enabling a previous cardiac planning, regarding the institution of early measures to preserve left ventricular function. It also showed to be effective in predicting RV systolic function, correlated with peak oxygen uptake, regardless of the influence of gender and age or other echocardiographic parameters36. Additionally, the E / E' ratio showed high prognostic value for mortality in patients with mild or moderate left ventricular dysfunction40. Tissue Doppler imaging constitutes an easy and reproducible way of systolic and diastolic assessment that must be increasingly used, as it is superior to conventional echocardiography17, identifying overall contractile abnormalities, especially in patients at the indeterminate phase of Chagas disease and with normal ECG15.
Other studies have sought to determine the prognostic markers of cardiac function in Chagas disease. Among them, BNP, a neurohormone of specific cardiac origin, has been the subject of five studies, with the aim of associating its concentration to cardiac dysfunction21,24,29,31,37, with discordant results.
Talvani et al.21, by correlating BNP levels to ventricular function indices in chagasic patients, showed that because it was a simple, fast, relatively inexpensive method, it should be used for diagnosis of heart failure in these patients, to identify the increased risk of severe arrhythmias, sudden death and reduced survival.
Additionally, they recommended the use of a cutoff lower than 60 pg/mL to characterize patients with low risk of severe chagasic cardiomyopathy, for which echocardiography was not necessary. A study carried out in 2005, however, despite confirming the correlation between high concentrations of BNP and cardiac involvement in Chagas disease, did not confirm it when considered ejection fraction. This study showed that individuals with the indeterminate form of Chagas disease had serum BNP levels similar to those found in the general population, i.e., it did not contribute to the diagnosis of this clinical form24.
Subsequent studies, however, have supplemented the significance of BNP measurement in patients with Chagas disease and were also compared to those results. They demonstrated that the assessment of systolic and diastolic functions of the left ventricle and right atrial pressure, together with serum BNP levels, helps to stratify the risk of cardiovascular event development, facilitating the diagnosis of Chagas cardiomyopathy21,29,31. Some studies contrasted to the findings of Melo et al.24, by identifying a strong correlation between BNP levels and ejection fraction29, as well as the validity of BNP to identify patients in the indeterminate form of Chagas disease37. The differences between the findings can be attributed to increased sensitivity and specificity of the test used for BNP measurement, verified since 2006, in response to its large-scale use in many countries.
Considering Chagas disease in the context of inflammatory diseases, another biomarker of heart muscle injury has been evaluated in chagasic patients - troponin I. In chagasic individuals, the concentration of troponin I has been identified in the chronic and indeterminate forms, allowing early detection of myocardial inflammation and consequently, secondary prevention of this injury18. More recent studies have sought prognostic markers of myocardial damage degree and, consequently, risk of death, based on physiological processes. In this context, a study has addressed ventricular dyssynchrony38, while another41 sought to update cardiac dysautonomia, of which importance in Chagas disease had already been highlighted in 199814.
As for the dyssynchrony in chagasic cardiomyopathy, a cohort assessed by echocardiography showed high intraventricular and moderate interventricular prevalence and found that, despite the high prevalence, this phenomenon may not affect prognosis as it is independent from the changes in QRS. The importance of this finding is that dyssynchrony, being a segmental alteration secondary to the presence of areas of fibrosis associated with chronic diffuse inflammatory process, does not behave as a risk factor. However, the authors left the significance of dyssynchrony open to discussion and suggested studies with larger samples so that the evaluation of poor prognosis can be supplemented by other factors38.
In 1998, Marin-Neto et al. 14, based on the premise that the digestive form of Chagas disease is characterized by autonomic dysfunction, essential for the development of megaesophagus and megacolon, admitted that this dysfunction could be involved in ventricular alterations of Chagas cardiomyopathy. Cardiac autonomic function was evaluated using angiography with Tc-99m labeled RBCs, demonstrating that patients with the digestive form had pronounced parasympathetic cardiac injury and right ventricular dysfunction in the absence of diagnosed left ventricular dysfunction. However, cardiac autonomic dysfunction was absent in the indeterminate forms, even in the presence of similar right ventricular involvement14. The importance of this pioneering study was to demonstrate the possibility that the presence of severe right ventricular involvement can constitute the only cardiac dysfunction in asymptomatic chronic chagasic patients with no other clinical signs of cardiac involvement14.
Fourteen years later, a period in which echocardiography was improved by the technological development of equipment and programs (software), allowing early evaluation of morphological and functional disorders, cardiac autonomic function in asymptomatic chronic Chagas cardiomyopathy started to be investigated once again in more detail41.
Cardiac autonomic dysfunction, characterized by marked depression of autonomic modulation of heart rate variability with preserved vagosympathetic balance, was verified regardless of the standing position of the patient. The association of autonomic dysfunction with normality of most ventricular echocardiographic variables suggests that dysautonomia must be a primary phenomenon that may precede ventricular mechanical changes. Thus, its assessment may constitute a precursor of ventricular dysfunction41.
Recently, new echocardiographic tools have been employed to allow earlier diagnosis of ventricular alterations in chagasic patients, as, although echocardiography is considered adequate for analysis of ventricular contractility and regional myocardial function, it does not allow ventricular deformation assessment. Two-dimensional X-strain echocardiography is an easy-to-apply technique that allows differentiating passive from active deformation of the ventricular cavity walls, through the assessment of tangential and longitudinal deformation. Del Castillo et al.33, using X-strain and strain rate determination, showed the presence of significant reduction in the percentage of deformation and the rate of deformation of the infero-lateral left ventricular wall of patients with Chagas disease, especially in the middle and apical portions, regions that show segmental contractility alterations in the cardiac form of Chagas disease.
This study was improved42 with the use of the speckle tracking technique, which allows determination of basal and apical myocardial rotation, as well as the twisting, i.e., the angular difference resulting from the clockwise rotation of the apical region and of the basal in the counterclockwise direction, during ventricular systole. These new echocardiographic parameters were employed42 in the differentiation of chagasic patients and those with left ventricular hypertrophy. By identifying the reduction in global longitudinal strain, radial strain, twisting and myocardial rotation in patients with chagasic cardiomyopathy, as well as the increase in these parameters in ventricular hypertrophy, the authors42 recommend the use of this modality as a differentiation tool.
In addition to the search for new methodologies for cardiac function assessment, the association between inflammatory and prothrombotic activity in Chagasic cardiomyopathy has been investigated in an attempt to better characterize the disease, as to attain better care of these patients43. Such studies are relevant to the elucidation of the damage of chronic Chagas cardiomyopathy, so that it will no longer be included in the group of neglected diseases, considering that after 100 years of its discovery, it is still a serious public health problem. Chronic Chagas disease still leads individuals to heart failure as result of the disease, incapacitating them for work and for life, increasing public expenses, notably with incapacity to lead a productive life, cardio-stimulation (pacemakers, implantable cardioverter-defibrillators and cardiac resynchronizers), drug therapy and heart transplantation.
Conclusion
The evidence shown in this review allows us to state that the possibility of early etiological diagnosis of chronic chagasic cardiomyopathy has increased over time, enabling us to predict the reduction of mortality in these patients. By demonstrating that ventricular impairment, not only left as it has been emphasized, but also right, occurs since the indeterminate phase of the disease, when symptoms are mild and do not prevent patients from performing activities of daily living, opens up new possibilities that can translate in prolonged life with quality.
New methods of cardiac assessment for the detailing of ventricular impairment, associated with the use of prognostic scores for risk of death in the future tend to be incorporated into cardiologic routine, facilitating the differential diagnosis of Chagas cardiomyopathy while still in its early phase.
Footnotes
Author contributions
Conception and design of the research, Acquisition of data, Analysis and interpretation of the data, Statistical analysis, Writing of the manuscript and Critical revision of the manuscript for intellectual content: Pereira Júnior CB, Markman Filho B; Obtaining financing: Markman Filho B.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This article is part of the thesis of Master submitted by Clodoval de Barros Pereira Jr from Universidade Federal de Pernambuco
References
- 1.Carod-Artal FJ, Gascon J. Chagas disease and stroke. Lancet Neurol. 2010;9(5):533–542. doi: 10.1016/S1474-4422(10)70042-9. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. (WHO). [Acesso em 2013 abr 4];Chagas disease (American trypanosomiasis) 2013 Disponível em: http://www.who.int/mediacentre/factsheets/fs340/en.
- 3.Completion of national laboratory inventories for wild poliovirus containment: WHO Region of the Americas, March 2010. Wkly Epidemiol Rec. 2010;85(34):329–333. [PubMed] [Google Scholar]
- 4.Martins-Melo FR, Alencar CH, Ramos AN Jr, Heukelbach J. Epidemiology of mortality related to Chagas' disease in Brazil, 1999-2007. PLoS Negl Trop Dis. 2012;6(2): doi: 10.1371/journal.pntd.0001508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponukollu G, Gowda RM, Khan IA, Navarro VS, Vasavada BC. Clinical aspects of the Chagas' heart disease. Int J Cardiol. 2007;115(3):279–283. doi: 10.1016/j.ijcard.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Schmunis GA. Epidemiology of Chagas disease in non-endemic countries: the role of international migration. Mem Inst Oswaldo Cruz. 2007;102(Suppl 1):75–85. doi: 10.1590/s0074-02762007005000093. Erratum in Mem Inst Oswaldo Cruz. 2007;102(8):2 p following 1009. [DOI] [PubMed] [Google Scholar]
- 7.Biolo A, Ribeiro AL, Clausell N. Chagas cardiomyopathy - where do we stand after a hundred years? Prog Cardiovasc Dis. 2010;52(4):300–316. doi: 10.1016/j.pcad.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Marin-Neto JA, Simões MA, Sarabanda AV. Cardiopatia chagásica. Arq Bras Cardiol. 1999;72(3):247–263. doi: 10.1590/s0066-782x1999000300001. [DOI] [PubMed] [Google Scholar]
- 9.Marin-Neto JA, Cunha-Neto E, Maciel BC, Simões MV. Pathogenesis of chronic Chagas heart disease. Circulation. 2007;115(9):1109–1123. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 10.Yacoub S, Mocumbi AO, Yacoub MH. Neglected tropical cardiomyopathies: I. Chagas disease: myocardial disease. Heart. 2008;94(2):244–248. doi: 10.1136/hrt.2007.132316. [DOI] [PubMed] [Google Scholar]
- 11.Abuhab A, Trindade E, Aulicino GB, Fujii S, Bocchi EA, Bacal F. Chagas' cardiomyopathy: the economic burden of an expensive and neglected disease. Int J Cardiol. 2013;168(3):2375–2380. doi: 10.1016/j.ijcard.2013.01.262. [DOI] [PubMed] [Google Scholar]
- 12.Nunes MC, Barbosa MM. Valor prognóstico da disfunção diastólica em pacientes com miocardiopatia dilatada chagásica. Rev Bras Ecocardiogr. 2004;17(4):15–22. [Google Scholar]
- 13.Guerrero L, Carrasco H, Parada H, Molina C, Chuecos R. Ventricular mechanics and cardiac arrhythmias in patients with chagasic and primary dilated cardiomyopathy. Echo-electrocardiographic follow-up. Arq Bras Cardiol. 1991;56(6):465–469. [PubMed] [Google Scholar]
- 14.Marin-Neto JA, Bromberg-Marin G, Pazin-Filho A, Simões MV, Maciel BC. Cardiac autonomic impairment and early myocardial damage involving the right ventricle are independent phenomena in Chagas' disease. Int J Cardiol. 1998;65(3):261–269. doi: 10.1016/s0167-5273(98)00132-6. [DOI] [PubMed] [Google Scholar]
- 15.Barros MV, Rocha MO, Ribeiro AL, Machado FS. Doppler tissue imaging to evaluate early myocardium damage in patients with undetermined form of Chagas' disease and normal echocardiogram. Echocardiography. 2001;18(2):131–136. doi: 10.1046/j.1540-8175.2001.00131.x. [DOI] [PubMed] [Google Scholar]
- 16.Barros MV, Machado FS, Ribeiro AL, Da Costa Rocha MO. Detection of early right ventricular dysfunction in Chagas' disease using Doppler tissue imaging. Pt 2J Am Soc Echocardiogr. 2002;15(10):1197–1201. doi: 10.1067/mje.2002.122966. [DOI] [PubMed] [Google Scholar]
- 17.Barros MV, Ribeiro AL, Machado FS, Rocha MO. Doppler tissue imaging to assess systolic function in Chagas' disease. Arq Bras Cardiol. 2003;80(1):36–40. doi: 10.1590/s0066-782x2003000100004. [DOI] [PubMed] [Google Scholar]
- 18.Arias R, Bastos C, Mota G, Sodré F, Moreira A, Tavares A, et al. Troponin in Chagas disease. Braz J Infect Dis. 2003;7(6):358–359. doi: 10.1590/s1413-86702003000600001. [DOI] [PubMed] [Google Scholar]
- 19.Nunes Mdo C, Barbosa Mde M, Brum VA, Rocha MO. Morphofunctional characteristics of the right ventricle in Chagas' dilated cardiomyopathy. Int J Cardiol. 2004;94(1):79–85. doi: 10.1016/j.ijcard.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Viotti RJ, Vigliano C, Laucella S, Lococo B, Petti M, Bertocchi G, et al. Value of echocardiography for diagnosis and prognosis of chronic Chagas disease cardiomyopathy without heart failure. Heart. 2004;90(6):655–660. doi: 10.1136/hrt.2003.018960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talvani A, Rocha MO, Cogan J, Maewal P, de Lemos J, Ribeiro AL, et al. Brain natriuretic peptide and left ventricular dysfunction in chagasic cardiomyopathy. Mem Inst Oswaldo Cruz. 2004;99(6):645–649. doi: 10.1590/s0074-02762004000600020. [DOI] [PubMed] [Google Scholar]
- 22.Rocha ES, Nunes MC, Talvani A, Rocha MO, Cogan J, Maewal P, de Lemos J, Ribeiro AL, Barbosa MM. Volume de átrio esquerdo como preditor de morte em miocardiopatia chagásica dilatada. Rev Bras Ecocardiogr. 2004;17(4):29–36. [Google Scholar]
- 23.Freitas HF, Chizzola PR, Paes AT, Lima AC, Mansur AJ. Risk stratification in a Brazilian hospital-based cohort of 1220 outpatients with heart failure: role of Chagas' heart disease. Int J Cardiol. 2005;102(2):239–247. doi: 10.1016/j.ijcard.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Melo RB, Parente GB, Victor EG. Determinação do peptídio natriurético cerebral humano em portadores de doença de Chagas. Arq Bras Cardiol. 2005;84(2):137–140. doi: 10.1590/s0066-782x2005000200008. [DOI] [PubMed] [Google Scholar]
- 25.Pazin-Filho A, Romano MM, Almeida-Filho OC, Furuta MS, Viviani LF, Schmidt A, et al. Minor segmental wall motion abnormalities detected in patients with Chagas' disease have adverse prognostic implications. Braz J Med Biol Res. 2006;39(4):483–487. doi: 10.1590/s0100-879x2006000400008. [DOI] [PubMed] [Google Scholar]
- 26.Rassi A Jr, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG, et al. Development and validation of a risk score for predicting death in Chagas' heart disease. N Engl J Med. 2006;355(8):799–808. doi: 10.1056/NEJMoa053241. [DOI] [PubMed] [Google Scholar]
- 27.Chaves AM, Villar JC, Luengas CA, Villamizar MC, Hernández L, Celis A, et al. Función diastólica en sujetos con serología positiva para enfermedad de Chagas procedentes del estudio CHICAMOCHA. Rev Colomb Cardiol. 2006;13(2):79–84. [Google Scholar]
- 28.Benchimol Barbosa PR. Noninvasive prognostic markers for cardiac death and ventricular arrhythmia in long-term follow-up of subjects with chronic Chagas' disease. Braz J Med Biol Res. 2007;40(2):167–178. [PubMed] [Google Scholar]
- 29.Barbosa MM, Nunes Mdo C, Ribeiro AL, Barral MM, Rocha MO. N-terminal proBNP levels in patients with Chagas disease: a marker of systolic and diastolic dysfunction of the left ventricle. Eur J Echocardiogr. 2007;8(3):204–212. doi: 10.1016/j.euje.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Nunes Mdo C, Rocha MO, Ribeiro AL, Colosimo EA, Rezende RA, Carmo GA, et al. Right ventricular dysfunction is an independent predictor of survival in patients with dilated chronic Chagas' cardiomyopathy. Int J Cardiol. 2008;127(3):372–379. doi: 10.1016/j.ijcard.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Vilas-Boas F, Feitosa GS, Soares MB, Pinho-Filho JA, Nascimento T, Barojas MM, et al. Invasive and noninvasive correlations of B-type natriuretic peptide in patients with heart failure due to Chagas cardiomyopathy. Congest Heart Fail. 2008;14(3):121–126. doi: 10.1111/j.1751-7133.2008.08166.x. [DOI] [PubMed] [Google Scholar]
- 32.Nunes MC, Barbosa MM, Ribeiro AL, Colosimo EA, Rocha MO. Left atrial volume provides independent prognostic value in patients with Chagas cardiomyopathy. J Am Soc Echocardiogr. 2009;22(1):82–88. doi: 10.1016/j.echo.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Del Castillo JM, Herszkowicz N, Rego LC, Silva YA, Moro DR, Maia AM, et al. Strain bidimensional do ventrículo esquerdo na forma indeterminada da doença de Chagas. Rev Bras Ecocardiogr Imagem Cardiovasc. 2009;22(1):31–35. [Google Scholar]
- 34.Terzi FV, Siqueira Filho AG, Nascimento EM, Pereira Bde B, Pedrosa RC. Regional left ventricular dysfunction and its association with complex ventricular arrhythmia, in chagasic patients with normal or borderline electrocardiogram. Rev Soc Bras Med Trop. 2010;43(5):557–561. doi: 10.1590/s0037-86822010000500017. [DOI] [PubMed] [Google Scholar]
- 35.Pereira Nunes Mdo C, Barbosa MM, Ribeiro AL, Amorim Fenelon LM, Rocha MO. Factores predictivos de la mortalidad en pacientes con miocardiopatía dilatada: importancia de la enfermedad de Chagas como etiología. Rev Esp Cardiol. 2010;63(7):788–797. doi: 10.1016/s1885-5857(10)70163-8. [DOI] [PubMed] [Google Scholar]
- 36.Nunes Mdo C, Beloti FR, Lima MM, Barbosa MM, Pinto MM, Filho, de Barros MV, et al. Functional capacity and right ventricular function in patients with Chagas heart disease. Eur J Echocardiogr. 2010;11(7):590–595. doi: 10.1093/ejechocard/jeq022. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Alvarez A, Sitges M, Pinazo MJ, Regueiro-Cueva A, Posada E, Poyatos S, et al. Chagas cardiomyopathy: the potential of diastolic dysfunction and brain natriuretic peptide in the early identification of cardiac damage. PLoS Negl Trop Dis. 2010;4(9): doi: 10.1371/journal.pntd.0000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duarte Jde O, Magalhães LP, Santana OO, Silva LB, Simões M, Azevedo DO, et al. Prevalence and prognostic value of ventricular dyssynchrony in Chagas cardiomyopathy. Arq Bras Cardiol. 2011;96(4):300–306. doi: 10.1590/s0066-782x2011005000037. [DOI] [PubMed] [Google Scholar]
- 39.Valerio L, Roure S, Sabria M, Balanzo X, Valles X, Seres L. Clinical, electrocardiographic and echocardiographic abnormalities in Latin American migrants with newly diagnosed Chagas disease 2005-2009, Barcelona, Spain. Euro Surveill. 2011;16(38):19971–19971. doi: 10.2807/ese.16.38.19971-en. [DOI] [PubMed] [Google Scholar]
- 40.Nunes MP, Colosimo EA, Reis RC, Barbosa MM, Silva JL, Barbosa F, et al. Different prognostic impact of the tissue Doppler-derived E/e' ratio on mortality in Chagas cardiomyopathy patients with heart failure. J Heart Lung Transplant. 2012;31(6):634–641. doi: 10.1016/j.healun.2012.01.865. [DOI] [PubMed] [Google Scholar]
- 41.Vasconcelos DF, Junqueira LF Jr. Funções autonômica cardíaca e mecânica ventricular na cardiopatia chagásica crônica assintomática. Arq Bras Cardiol. 2012;98(2):111–119. doi: 10.1590/s0066-782x2012005000002. [DOI] [PubMed] [Google Scholar]
- 42.Del Castillo JM, Silveira CA, Albuquerque ES. Rotação, twisting e torção miocárdica avaliados pela ecocardiografia bidimensional (speckle tracking) Rev Bras Ecocardiogr Imagem Cardiovasc. 2012;25(3):206–213. [Google Scholar]
- 43.Melo LM, Souza GE, Valim LR, Moreira FP, Damico EA, Rocha TR, et al. Estudo de fatores pró-trombóticos e pró-inflamatórios na cardiomiopatia chagásica. Arq Bras Cardiol. 2010;95(5):655–662. doi: 10.1590/s0066-782x2010005000146. [DOI] [PubMed] [Google Scholar]