Abstract
Background
Real-time vascular imaging that provides both anatomic and hemodynamic information could greatly facilitate the diagnosis of vascular diseases and provide accurate assessment of therapeutic effects. Here we have developed a novel fluorescence-based all-optical method, named near-infrared II (NIR-II) fluorescence imaging, to image murine hindlimb vasculature and blood flow in an experimental model of peripheral arterial disease, by exploiting fluorescence in the NIR-II region (1000–1400 nm) of photon wavelengths.
Methods and Results
Owing to the reduced photon scattering of NIR-II fluorescence compared to traditional NIR fluorescence imaging and thus much deeper penetration depth into the body, we demonstrated that the mouse hindlimb vasculature could be imaged with higher spatial resolution than in vivo microCT. Furthermore, imaging over 26 days revealed a significant increase in hindlimb microvascular density in response to experimentally induced ischemia within the first 8 days of the surgery (P < 0.005), which was confirmed by histological analysis of microvascular density. Moreover, the tissue perfusion in the ischemic hindlimb could be quantitatively measured by the dynamic NIR-II method, revealing the temporal kinetics of blood flow recovery that resembled microbead-based blood flowmetry and laser Doppler blood spectroscopy.
Conclusions
The penetration depth of millimeters, high spatial resolution and fast acquisition rate of NIR-II imaging makes it a useful imaging tool for murine models of vascular disease.
Keywords: near-infrared II (NIR-II), fluorescent imaging, angiography, hemodynamics, acute limb ischemia, carbon nanotubes, vascular regeneration
Peripheral arterial disease (PAD) is an atherosclerotic arterial occlusive disease of the limbs that affects over 10 million people in the US.1 Novel therapeutic approaches to stimulate vascular regeneration and improve blood perfusion are often tested in the murine model of experimentally induced hindlimb ischemia. The evaluation of new therapies in this model would be aided by non-invasive imaging approaches that provide hemodynamic and anatomical information. We have previously reported the application of near-infrared II (NIR-II) fluorescence-based imaging as a multifunctional approach for quantifying limb blood flow and vessel structural information, achieving higher spatial resolution than conventional microscopic computed tomography (μCT), while the temporal resolution matches that of ultrasonography for tracking blood dynamics in real time.2
The multifunctional imaging capabilities in the NIR-II window originate from the intrinsic fluorescence properties of single-walled carbon nanotubes (SWNTs), which are cylindrical molecules made of rolled-up graphite sheets comprised of carbon.3 Semiconducting SWNTs exhibit strong resonant absorption in the traditional near-infrared region (NIR-I, 750–900 nm) and re-emit fluorescence in the NIR-II region (1000–1400 nm) with a large Stokes shift of ~400 nm, making them novel NIR-II fluorophores for in vivo fluorescence imaging.4, 5 The benefits of using the long wavelength NIR-II fluorescence over traditional NIR-I fluorescence comes from reduced scattering and thus much deeper tissue penetration depth of photons, due to the inverse dependence (~λ−w, w=0.22~1.68) of photon scattering on wavelength in turbid biological tissues.6 Moreover, the large Stokes shift of SWNTs and the absorption and emission of SWNTs in the biologically transparent NIR window also greatly reduce background autofluorescence, which minimizes the interference from indigenous tissue signals.7 When compared with non-optical imaging modalities such as μCT and magnetic resonance imaging (MRI), NIR-II maintains all the strengths of a fluorescence-based optical imaging modality, including easy implementation,2 high diffraction-limited spatial resolution (microns),8 and fast wide-field acquisition rate (tens to hundreds of frames per second).4
Here, we report the use of NIR-II based fluorescence imaging to provide multifunctional information on limb perfusion and collateral vessel formation in an experimental model of PAD, over the course of 26 days. Our results demonstrate the ability of NIR-II imaging to quantify tissue perfusion, with validation by microbead blood flowmetry and laser Doppler blood spectroscopy, as well as reveal the temporal kinetics of collateral vessel formation with a spatial resolution higher than μCT. The high spatial resolution and dynamic recording capability of NIR-II fluorescence imaging allows us to non-invasively image the temporal kinetics of vascular remodeling and formation of collateral vessels, while enabling blood flow quantification, which is not attainable by any single imaging modality as such μCT or ultrasound. Our finding of the transient vascular density increase as a response to acute hindlimb ischemia, followed by the return to normal vascular level, agrees with previous studies and provides a non-invasive and direct way of visualizing collateral formation.
Methods
Unilateral Hindlimb Ischemia
All animal studies were carried out under the approval of the Institutional Animal Care and Use Committees (IACUC) at Stanford University. Female athymic nude mice (Charles River, 13 weeks old) were anesthetized and maintained under 2% isoflurane and oxygen flow rate of 1 L/min. For the 10-day short-term study and the 26-day long-term study, 6 mice each were used for induction of unilateral hindlimb ischemia, which was performed by ligating and excising the femoral artery, according to our previous publications.9, 10 Please note that the time intervals in all experiments refer to post-operative time points.
Laser Doppler Tissue Perfusion Measurement
To measure tissue perfusion of mouse hindlimb using laser Doppler spectroscopy (PeriSCan PIM3, Permed AB), the animals were anesthetized and maintained under 2% isoflurane and oxygen flow rate of 1 L/min. The animals were pre-warmed to 37.5 °C body temperature and placed in the supine position during laser Doppler blood spectroscopy. Recovery of limb blood flow over time was expressed as the relative ratio of perfusion in the ischemic over the non-ischemic limb.9
Preparation of Biocompatible SWNT-Based NIR-II Contrast Agents
High-pressure carbon monoxide conversion (HiPCO) SWNTs (Unidym, Inc.) were suspended in an aqueous solution of sodium deoxycholate at a concentration of 1 wt% by bath sonication for 1 h. This SWNT suspension was ultracentrifuged at 300,000 g, the supernatant was retained and 1.0 mg·ml−1 of DSPE-mPEG(5 kDa) (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol, 5000)], Laysan Bio) was added. The resulting suspension was then dialyzed against 1× PBS in a 3500 Da membrane (Fisher) with pH controlled at 7.4 to remove sodium deoxycholate, leaving the SWNTs coated with the biocompatible DSPE-mPEG only.
Near-Infrared II Fluorescence-Based Blood Perfusion Measurement
Near-infrared II fluorescence-based small animal in vivo imaging was done on a customized imaging setup with optimized parameter settings according to our established protocol.2, 4–6, 11 For dynamic tissue perfusion imaging using NIR-II fluorescence, one mouse was mounted on the imaging stage at a time in its supine position prior to injection. The excitation was provided by an 808-nm laser diode (RMPC lasers) connected to a collimator with a focal length of 4.5 mm (Thorlabs). The excitation light was filtered through an 850-nm short-pass filter (Thorlabs) and a 1000-nm short-pass filter (Thorlabs).2, 4 The average power density of the excitation laser on the imaging stage (140 mW·cm−2) was significantly lower than the safe exposure limit of 329 mW·cm−2 at 808 nm for animals.12 The emitted NIR-II fluorescence was filtered through a 900-nm long-pass filter and an 1100-nm long-pass filter (Thorlabs) and focused onto a indium-gallium-arsenide (InGaAs) 2D detector (Princeton Instruments) through lenses.2, 4 The InGaAs camera started recording images immediately after a 200 μL bolus of NIR-II contrast solution containing 0.10 mg/mL biocompatible single-walled carbon nanotubes (SWNTs) was injected into the mouse tail vein, which was set to be t = 0 s. An exposure time of 100 ms was used for all images in the video. The frame rate was 5.3 frames·s−1 due to an overhead time of 87.5 ms for the acquisition of each frame.
Consecutive video rate images were then loaded into MATLAB software for perfusion analysis. In a typical procedure, same regions of interest (ROI) of both control and ischemic hindlimbs were selected from the video frames in a same way as the laser Doppler method, and the NIR-II fluorescence intensity increase within each ROI was plotted against time from 0 s to 65.625 s (350 frames) post injection (p.i.). The plot featured a linear rising edge followed by a plateau region due to blood saturation of NIR-II contrast agent. The plot was normalized against the saturation level of the control limb, and the linear rising edge (after normalization) was used to fit a line with its slope representing the perfusion level, similar to a previous publication of fluorescence-based perfusion quantification.13 Two slopes were generated for each mouse, one derived from the control limb, and the other derived from the ischemic limb. The slope of the ischemic limb was then normalized against the slope of the control limb to obtain the relative tissue perfusion (RTP) in units of percentage,
where by definition the RTP of the control hindlimb is 100%.
To estimate the absolute blood velocity within the hindlimb, a NIR-II intensity-to-velocity conversion coefficient of 0.0747±0.0019 cm·% −1 for the hindlimb of athymic nude mice was used to translate the percentage NIR-II intensity increase into local blood velocity, as previously described.2 The normalized slope has a unit of %/s, which is multiplied by the conversion coefficient in units of cm/% to give the blood velocity in units of cm/s.
Fluorescent Microbead Perfusion Assay
To validate the NIR-II-based blood perfusion measurements, additional hindlimb ischemia experiments were performed in which the relative fluorescence intensity of systemically-delivered fluorescent microbeads were quantified for up to 10 days after induction of hindlimb ischemia. Prior to euthanasia on days 0, 3, 7, and 10, the anesthesized mice received intracardiac injections of fluorescent microbeads (blue-green FluoSpheres 15μm diameter, 0.5 X 106 beads), followed by intra-cardiac injections of heparin (2000 U) and nitroglycerin (500 mg). Microbead extraction and quantification were performed based on established methods.14 In brief, the ischemic and control hindlimb tissues were excised and digested in KOH (0.5 M) at 52 ºC to extract the microbeads. The microbeads were then dissolved in cellosolve acetate before measuring in a fluorescence plate reader. The data is shown as relative fluorescence intensity between the ischemic and control limb, normalized by tissue weight (n≥4 for each time point).
Vessel Structural Imaging Using Near-Infrared II Fluorescence
To monitor vascular regeneration/remodeling in the ischemic mouse hindlimb, NIR-II imaging was applied at a high magnification with pixel size of ~78 μm. The same excitation source and detector as the dynamic NIR-II tissue perfusion imaging were used except that the distance between the two focusing achromats was adjusted to afford a ~2.5-fold higher magnification. This field of view could only cover one hindlimb at a time due to the magnification.2 All NIR-II images were taken at ~10–15 min p.i.
For vessel width analysis, a line was drawn on each NIR-II image, perpendicular to the femoral vessels. The NIR-II intensity values on this line were extracted using the ImageJ software and plotted against their physical locations on this line. Vessels intersected by the line were represented as peaks in the intensity profile and each peak was fitted using the Origin software into a Gaussian function. The full width half maximum (FWHM) of each peak is related to the Gaussian width w by the following relationship:
Two different methods, line cross-sectional peak counting and fast Fourier transform (FFT), were applied on all NIR-II hindlimb images to calculate microvascular density change over the period of revascularization. For the line cross-sectional peak counting method, four straight lines were drawn on the NIR-II image. In the control hindlimb, two horizontal lines were drawn medially and laterally to the femoral vessels (lines 1 and 2 in Figure S1), and two vertical lines were drawn near the proximal and distal ends of the femoral vessels (lines 3 and 4 in Figure S1). The two horizontal lines were drawn close to the femoral vessels without intersecting them in order to account for as many branch vessels as possible. In the ischemic hindlimb, the femoral artery was excised, but these lines were drawn surrounding the same region.
Then the NIR-II intensity on each line was extracted using the ImageJ software and plotted against the physical location on the line, where the peaks in the cross-sectional profile of each line were counted as existing vessels. Duplications in vessels counted by this approach were subtracted from total number of vessels of all four cross-sectional profiles. The total vessel count from the ischemic hindlimb was normalized against the control hindlimb to give relative vessel number (ischemic/control). An automated approach to quantify vessel density using FFT was also used to confirm the results (see SI).
Vessel Structural Imaging Using μCT
Vessel structural imaging using a μCT scanner (MicroCAT II, Siemens Preclinical Solutions) was performed according to our previous publication.2 After induction of hindlimb ischemia, the mice were injected with iodine-based Fenestra VC (Advanced Research Technologies) contrast agent (0.3 ml per 20 g body weight) by tail vein injection. Fenestra was injected slowly into the tail vein of the mice over 30–60 s to prevent embolization. The μCT scans were acquired 30–45 minutes p.i. The animals were placed in prone position in the scanner, anesthetized with 2% isoflurane with a 1 L/min O2 flow rate. Each scan time lasted 40 minutes (80 kVp, 500 μAmps, 576 views, 2 sec/view) per animal. After acquisition of CT images, three-dimensional reconstruction to a 40 μm isotopic voxel size was performed with Cobra (EXXIM Computing) and the data was analyzed using Amira 5.4 (Visualization Sciences Group) for visualization of the vessels.
For vessel width analysis, a line was drawn on each μCT image, perpendicular to the vessels of interest, while avoiding the bone features. Then the contrast profile was analyzed in a manner similar to NIR-II images to obtain vessel widths.
Immunofluorescence Quantification of Microvascular Density
To validate the non-invasive measurements of vessels numbers using NIR-II, additional animal studies were performed in which the microvascular density in the ischemic hindlimbs were quantified by immunofluorescence analysis. At specified time points, the ischemic gastrocnemius tissues were excised for frozen sectioning and immunofluorescence staining for endothelial marker, CD31 (BD Transduction), according to our previous studies (n>4 for each time point).9 At least three sections per animal were quantified. As a basis for comparison, gastrocnemius tissues from normal contralateral limbs were also immunofluorescently stained for CD31 (n=5). High-powered images were photographed for each section, and relative microvascular density was expressed as the average number of CD31-expressing vessels in the ischemic tissue, normalized by the average number of CD31-expressing vessels in normal limbs.
Statistical Analysis
Data shown are mean ± standard deviation. Statistical analysis was performed using Graphpad Prism software 5.0 (2007). For comparison of tissue perfusion and vascular density in the same animals over time, Repeated Measures Analysis of Variance (ANOVA) with a Tukey’s adjustment was used. P-values less than 0.05 were considered statistically significant.
Results
Recovery of Tissue Perfusion in the Ischemic Hindlimb
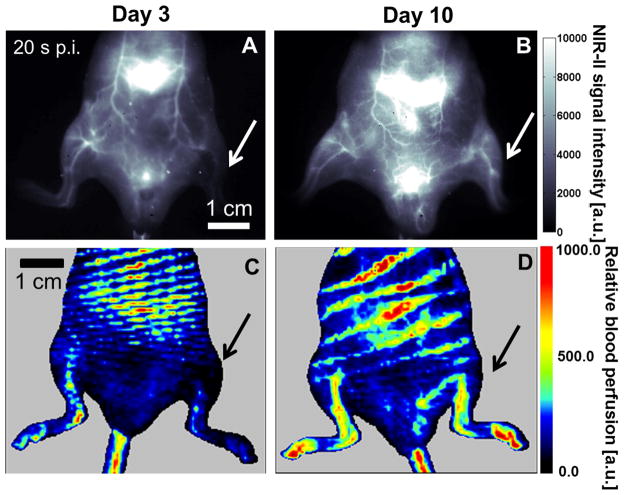
Tissue perfusion was monitored on a group of athymic nude mice (n=6) up to 10 days after induction of hindlimb ischemia. NIR-II fluorescence-based dynamic perfusion imaging resembled laser Doppler-based perfusion measurement in revealing the steady recovery of perfusion in the ischemic hindlimb from day 3 to day 10 post-surgery (Figure 1), as indicated by the increase in intensity units in the ischemic hindlimb on day 10 (Figure 1B&D), compared to day 3 (Figure 1A&C). In comparing the images derived from NIR-II fluorescence microscopy and laser Doppler spectroscopy, NIR-II images provided much higher spatial resolution and discrimination of vascular structures that bear resemblance to angiograms.
Figure 1.
Corresponding NIR-II (A&B) and laser Doppler perfusion images (C&D) on day 3 (A&C) and day 10 (B&D) after surgery-induced hindlimb ischemia, where the arrow denotes the ischemic hindlimb. Note that the NIR-II images in A&B are extracted from Videos 1&2 that dynamically record the tissue perfusion labeled by NIR-II contrast, and that these two images both correspond to the time point of 20 s p.i.
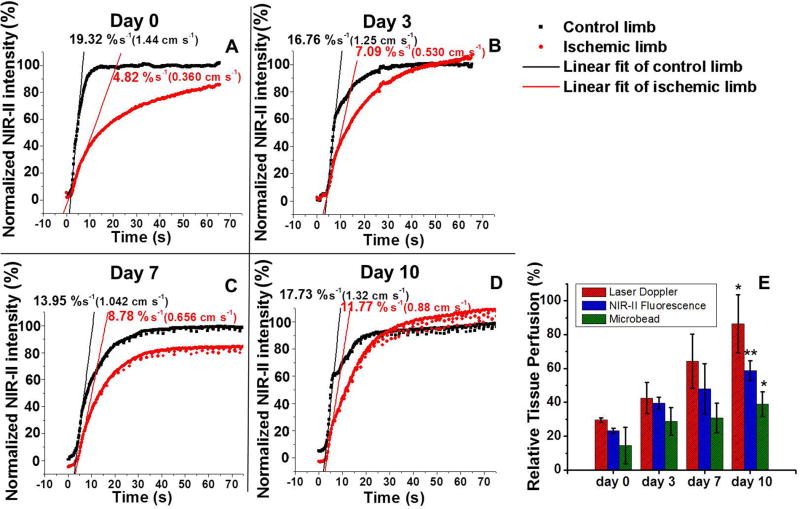
To develop a quantitative method for tissue perfusion measurement based on the fluorescence in the NIR-II, we analyzed the average NIR-II intensity in the control and ischemic hindlimbs as a function of time immediately after injection (Figure 2A–D), taking advantage of the fast dynamic recording capability of NIR-II fluorescence imaging (Videos 1&2). The rising edge of each plot was fitted into a linear function whose slope was related to blood velocity proportionally by an intensity-to-velocity conversion constant, as derived from our previous publication.2 Therefore, for internal comparison, the ratio between the slope derived from the ischemic hindlimb and the control hindlimb could be used as a measure of relative tissue perfusion, similar in concept to the mean perfusion ratio from laser Doppler-based and microbead-based perfusion measurements. The quantitative tissue perfusion values in Figure 2E exhibit a statistically significant increase in tissue perfusion in the ischemic hindlimb postoperatively from day 0 to day 10, as revealed by the NIR-II method (P<0.005). These results were further validated by laser Doppler blood spectroscopy (P<0.05) and microbead perfusion assay (P<0.05), in which a similar increase in blood perfusion could be quantitatively assessed over time.
Figure 2.
Comparison of quantitative tissue perfusion after induction of hindlimb ischemia. (A–D) Normalized NIR-II fluorescence intensity in control and ischemic hindlimbs as a function of time p.i. on post-operative days 0, 3, 7 and 10. (E) A bar chart showing the relative tissue perfusion values (ischemic/control) on day 0, 3, 7 and 10 derived from laser Doppler technique (red bars), NIR-II fluorescence method (blue bars) and microbead measurements (green bars). Statistically significant differences of perfusion are found between days 0 and 10 for all methods (*P < 0.05 and **P < 0.005).
Whereas restoration of blood flow over time could be documented by all three methods, we observed some differences in the magnitude in relative blood perfusion. The comparatively lower perfusion levels by NIR-II than by laser Doppler could be attributed to the difference of photon wavelength utilized for probing blood flow by these two techniques. Since laser Doppler applies red laser light with center wavelength of 650–690 nm, whereas NIR-II exploits photons in the range of 1100–1400 nm, NIR-II probes tissue perfusion within a much deeper region from the skin than laser Doppler6, 15, 16 and thus provides more volumetric perfusion assessment in thicker tissue than laser Doppler. In contrast, the microbead method is capable of measuring tissue perfusion at any depth underneath the skin by sacrificing the animal and resecting the tissue sample. Therefore, NIR-II is a surface-weighted technique in that the deeper tissues generally contribute a lower level of signal in the NIR-II, and that it does not adequately probe perfusion at depths > 3 mm due to severe scattering of photons. This agrees with the fact that the NIR-II tissue perfusion measurement lies between laser Doppler and the microbead method (Figure 2E). Our results show that NIR-II is a good compromise between laser Doppler and microbeads in that it can quantify blood flow at greater penetration depth than laser Doppler, while allowing serial imaging, which cannot be accomplished by microbeads.
Structural Imaging of Angiogenesis with NIR-II and μCT
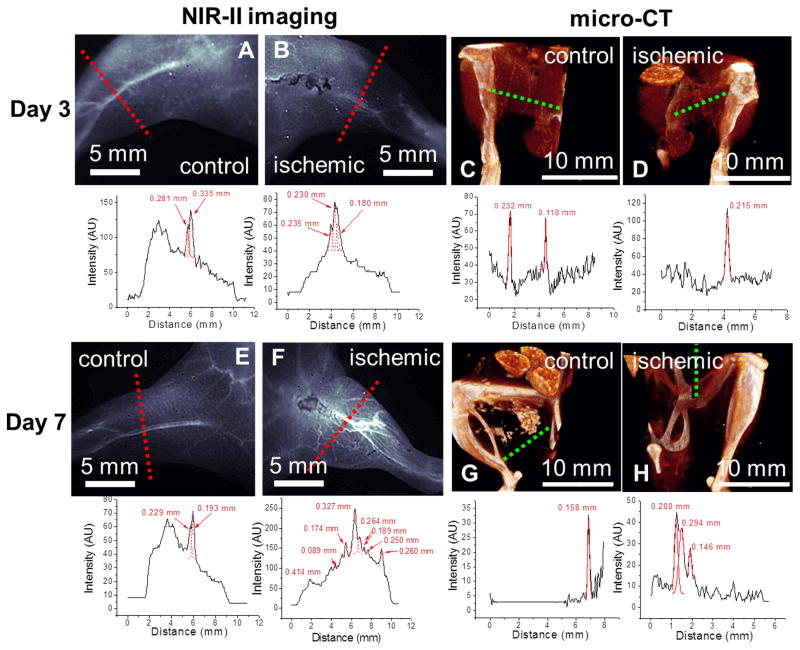
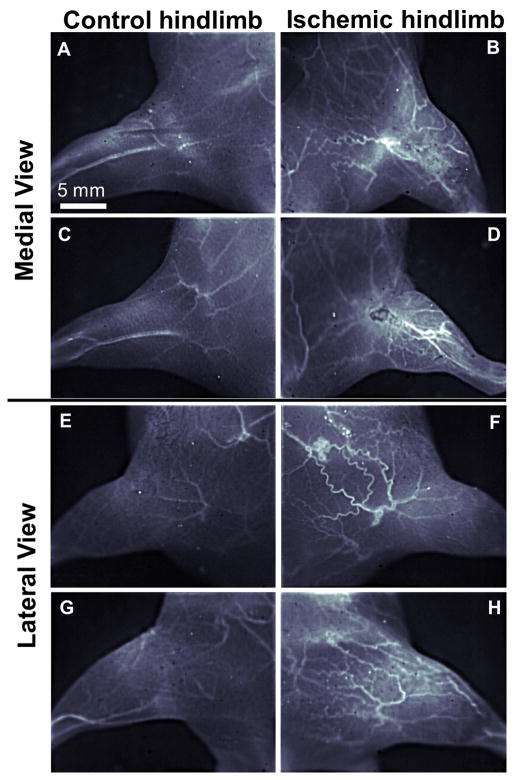
Besides providing dynamic tissue perfusion imaging capability, NIR-II fluorescence imaging method could also be applied to quantify changes in vascular anatomy in the hindlimb region in response to ischemia over a 10-day period after surgery. Using image analysis approaches, we quantified the NIR-II intensity values that intersect the line perpendicular to the femoral vessels (Figure 3). The plots showing NIR-II intensity versus physical location were used to identify collateral vessels, represented by individual peaks. Based on the number of distinctive peaks, the NIR-II images of the mouse hindlimb vasculatures on post-operative days 3 and 7 (Figure 3B&F) revealed a large number of newly recruited collateral vessels in the ischemic hindlimb in response to the surgical excision of the femoral artery, which is further confirmed by internal comparison between the ischemic and control hindlimbs of multiple mice imaged at a higher magnification (Figure 4). In contrast, the vascular anatomy of the healthy control hindlimb remained unchanged over time, based on the similar numbers of individual peaks (Figure 3A&E). These NIR-II fluorescence images were compared with a standard vascular imaging method, μCT. Due to spatial resolution limitations (low contrast-to-noise ratio, high background signal from bone), μCT images (Figure 3C, D, G&H) showed fewer vessel-like structures in all images, and the trend of vessel regeneration in the ischemic hindlimb between day 3 and day 7 was not as clearly revealed as in the NIR-II method.
Figure 3.
Comparison of NIR-II fluorescence (A, B, E, F) and μCT images (C, D, G, H) of mouse hindlimb vasculature (top) and cross-sectional vessel width measurements (bottom) on day 3 (A–D) and day 7 (E–H) after surgery-induced hindlimb ischemia.
Figure 4.
High magnification NIR-II fluorescence images of mouse hindlimb vasculature of two different mice (one mouse is shown in A,B,E,F and the other in C,D,G,H) at day 7 after surgery-induced hindlimb ischemia. Both medial (A–D) and lateral (E–H) aspects of control (A,C,E,G) and ischemic (B,D,F,H) hindlimbs are shown.
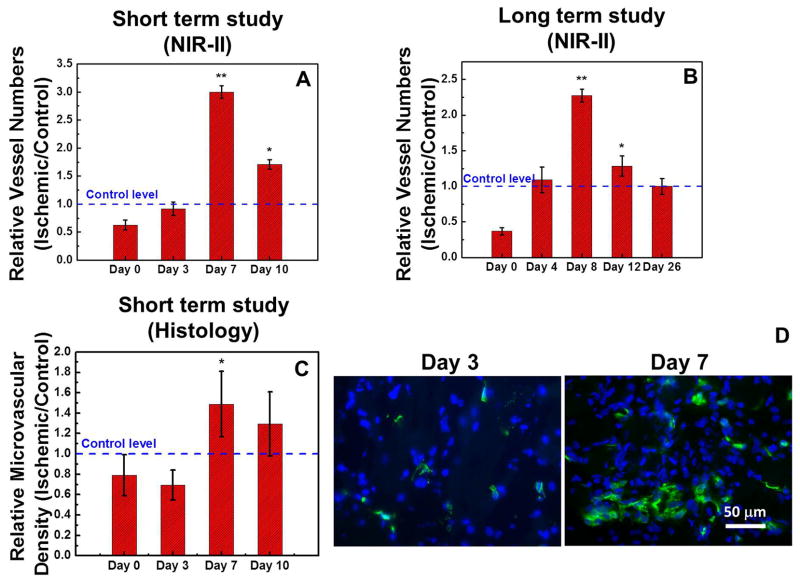
Quantitative Microvascular Density Analysis
In addition to qualitatively demonstrating the increase of collateral vessel formation from day 3 to day 7 in the ischemic hindlimb (Figure 3&4), we further quantified microvascular density in the hindlimb from the NIR-II images by counting the vessels that intersect the surrounding borders of the femoral vessels and normalizing the vessel count in the ischemic hindlimb against the control hindlimb, to reveal the temporal kinetics of microvascular density (Figure S1; see Methods for more detail). The results shown in Figure 5A&B for both the short-term (10-day) and long-term (26-day) studies revealed a statistically significant temporal increase of the relative vessel numbers between days 7 and 12, reaching up to ~6-fold increase compared to immediately after the induction of hindlimb ischemia on day 0 (*P<0.005). This was followed by a return to normal relative vessel numbers by day 26. These results were confirmed by another automated approach to quantify vessel density (Figure S2). The long-term vascular density data is consistent with the histological quantification of microvascular density (Figure 5C&D), and reveals a significant recruitment of collateral vessels in response to ischemia during the first 8 days which exceeds normal values of vascular density. This phase is followed by a gradual return to normal levels of vascularity, possibly due to regression of collateral vessels or resolution of the acute inflammatory response triggered by ischemia.17–19
Figure 5.
Quantification of collateral vessel formation in the ischemic limb during revascularization. NIR-II fluorescence based results of short-term (A) and long-term (B) revascularization studies are expressed relative to the vessel numbers in the control limb, and histology based results of a short-term study (C) are expressed in microvascular density. (D) Fluorescence microscopy images of ischemic hindlimb tissues resected on day 3 (left) and day 7 (right) after the induction of acute hindlimb ischemia with CD31 staining (green) and nucleus staining (blue). Statistically significant difference in comparison to day 0 is shown as *P < 0.05 and **P < 0.005.
Discussion
The salient finding of this study is that NIR-II fluorescence imaging enables dynamic tissue perfusion measurement and high-resolution structural vascular imaging in an experimental model of PAD. This multifunctional approach combines traditional imaging modalities of laser Doppler, magnetic resonance angiography, and μCT in a single modality. Using this method, we are able to non-invasively track the recovery of limb perfusion after the induction of hindlimb ischemia by quantifying tissue perfusion and microvascular density simultaneously. This approach is an attractive alternative to performing invasive histological quantification of microvascular density and performing invasive microbead blood flowmetry for tissue perfusion measurement. NIR-II based-fluorescence imaging also revealed vascular branching and structural information that was superior to laser Doppler method, owing to a much higher spatial resolution (Figure 1) and μCT imaging owing to a much higher contrast-to-noise ratio (Figure 3&4).
NIR-II imaging revealed the temporal kinetics of collateral vessel growth. We noted that after the initial decline in vascular density induced by femoral artery excision, there was a substantial increase in microvascular density up to 8 days after the surgery. This increase in vascular density was followed by the return to normal vascular density values by day 26. This pattern in vascular response is consistent with our own histological analysis of microvascular density as well as Hershey et al. who demonstrated enhanced vascular density in rabbit hindlimb at 5 days after induction of hindlimb ischemia, followed by the return to pre-operative levels by day 20.20 Hoefer et al. used post-mortem angiography to quantify collateral arteries in the ischemic hindlimb and reported a transient increase after 1 week, followed by the return to pre-operative levels by 3 weeks.18 It is likely that the transient enhancement in microvascular density may be associated with acute remodeling in response to ischemia, leading to the initial recruitment of collateral vessels, followed by a gradual decline due to their regression.
Dynamic tissue perfusion by MRI has been recently reported.21 Although MRI can assess tissue perfusion with deeper penetration depth into tissue, its temporal resolution is limited to ~2 s/frame, which is significantly lower than dynamic NIR-II imaging method (~0.18 s/frame). Magnetic resonance angiography has also been used in the setting of rabbit hindlimb ischemia, but its resolution was limited to the voxel size of ~0.5 mm, approximately 6 times larger than our NIR-II imaging method (78 μm).22 Based on the results from a direct comparison to μCT, as well as the reported resolution of MRI, NIR-II imaging provides superior spatial and temporal resolution.
NIR-II fluorescence compares favorably to traditional NIR-based fluorescence imaging with respect to the depth of penetration and degree of light scatter. As photons travel through a turbid medium such as the tissue and skin, the overall number of photons can be substantially attenuated due to scattering, while the scattering coefficient for different types of animal tissues scales with ~ λ−w (w=0.22–1.68) that decreases dramatically for emission with longer-wavelength λ.23, 24 Therefore, the longer wavelength of fluorescence is used for imaging, the deeper penetration depth can be achieved with less loss of image fidelity. On the other hand, animal tissues generally have less autofluorescence at longer wavelengths than shorter ones. Traditional NIR-I fluorescence in the 750–900 nm region has been extensively used for vascular imaging. However, due to the immense scattering and lingering autofluorescence, the imaging depth of in vivo NIR-I fluorescence imaging is usually ~0.5 mm.25, 26 In order to use short-wavelength NIR-I fluorescence to image features deep inside the body, usually the skin and surrounding tissue above the vessels of interest are removed to reduce scattering and improve the resolution of the vascular images.27–29 However, these invasive interventions during image acquisition increase the complexity of imaging and the morbidity of subjects. Therefore NIR-II imaging provides a solution to non-invasive through-tissue imaging of vasculatures at a depth of 1~3 mm (Figure S3) without the need of removing the surrounding tissues, while maintaining the high image fidelity. Owing to the deep penetration depth of NIR-II photons, we envisage the use of NIR-II fluorescence in a CT scanner for fluorescence-mediated tomography, which might help resolve overlapping vessels in the z-stack that were not resolvable by a single projection in our current epifluorescence imaging setup.
NIR-II fluorophores may be more powerful than NIR-I fluorophores for quantification of tissue perfusion and vascularization, owing to higher spatial resolution even at deeper depths. Currently, indocyanine green (ICG) is one of the few NIR-based fluorescence dyes that is approved by the FDA for clinical applications in blood flow measurement.30 Choi et al. reported the use of the ICG for calculation of perfusion rate in terms of %/min for ischemic and control hindlimbs based on the dynamics of NIR-I fluorescence intensity.31 However, due to the high degree of scattering of photons in the NIR-I region, the reported perfusion analysis has limited spatial resolution and therefore does not provide crisp angiographic images at the same time, whereas NIR-II-based contrast dyes can provide perfusion analysis and high resolution angiography simultaneously (Figures 1&2).
Although NIR-II fluorescence imaging method can provide dual functions of both vessel structural imaging and dynamic tissue perfusion imaging, there are limitations of this new vascular imaging technique that need to be considered. First, although NIR-II fluorescence in the long wavelength region of 1100–1400 nm can afford penetration depth of millimeters in a scattering medium,2, 6 much deeper than the ~0.5 mm penetration depth of traditional fluorescence imaging methods,25 the penetration depth is only sufficient in small animal model experiments. The imaging depth for large experiment animal or humans would still be confined near the surface. Like in laser Doppler spectroscopy, hair interferes with NIR-II fluorescence imaging, but we have found in our experiments that simple depilation before imaging generates images with comparable quality to athymic nude mice. Even though NIR-II imaging compares favorably to μCT in terms of higher spatial resolution and faster image acquisition time, the NIR-II fluorescence technique lacks the deep penetration depth for most human tomographic imaging where μCT and MRI are widely used. Second, the NIR-II contrast agent used in this study is SWNTs, nanoscopic materials that have a delayed clearance time, but such SWNTs have little toxicity due to their biocompatible surface coating.32 Efforts to produce a small molecule-based NIR-II contrast agent, comparable to other fluorescent dyes widely used in medicine such as fluorescein isothiocyanate (FITC), are under way. It is anticipated that such small molecule-based NIR-II contrast agents can make NIR-II imaging a clinically applicable imaging modality. Lastly, the use of InGaAs camera for NIR-II detection is limited compared to silicon based cameras that are commonly used to detect traditional short-wavelength photons. However, we believe that the many advantages of NIR-II fluorescence imaging in various biomedical applications could lead to more widespread use of infrared detectors and promote the development of new types of detection techniques.
Conclusion
In conclusion, we used a biocompatible carbon nanotube-based blood pool contrast agent with NIR-II fluorescence for dynamic tissue perfusion measurement and structural vascular imaging in murine model of PAD. NIR-II imaging is a novel technique providing simultaneous anatomic and hemodynamic information not achieved by conventional imaging modalities such as laser Doppler or μCT. The high temporal resolution of NIR-II allows us to dynamically track tissue perfusion. Moreover the high spatial resolution of NIR-II fluorescence imaging enables this platform to reveal vascular remodeling in the mouse hindlimb after acute ischemia.
Supplementary Material
Acknowledgments
Sources of Funding
This study was supported in part by grants from the National Cancer Institute of the US National Institutes of Health to H.D. (5R01CA135109-02), the National Heart, Lung and Blood Institute of the US National Institutes of Health to J.P.C. (U01HL100397, RC2HL103400) and N.F.H. (R00HL098688), the National Science Foundation to N.F.H. (1249008), the Department of Defense to N.F.H. (W81XWH-12-C-0111), the Stanford Cardiovascular Institute to N.F.H, and a Stanford Graduate Fellowship to G.H.
Footnotes
Disclosures
JPC, NFH, HD, GH and JCL are inventors on patents, owned by Stanford University, which are related to the imaging technology described in this manuscript.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y, Stroke AHASC. Heart disease and stroke statistics - 2008 update - a report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2008;117:E25–E146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Hong G, Lee JC, Robinson JT, Raaz U, Xie L, Huang NF, Cooke JP, Dai H. Multifunctional in vivo vascular imaging using near-infrared ii fluorescence. Nat Med. 2012;18:1841–1846. doi: 10.1038/nm.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iijima S, Ichihashi T. Single-shell carbon nanotubes of 1-nm diameter. Nature. 1993;363:603–605. [Google Scholar]
- 4.Welsher K, Liu Z, Sherlock SP, Robinson JT, Chen Z, Daranciang D, Dai HJ. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat Nanotechnol. 2009;4:773–780. doi: 10.1038/nnano.2009.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson JT, Hong GS, Liang YY, Zhang B, Yaghi OK, Dai HJ. In vivo fluorescence imaging in the second near-infrared window with long circulating carbon nanotubes capable of ultrahigh tumor uptake. J Am Chem Soc. 2012;134:10664–10669. doi: 10.1021/ja303737a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welsher K, Sherlock SP, Dai HJ. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. P Natl Acad Sci USA. 2011;108:8943–8948. doi: 10.1073/pnas.1014501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Splinter R, Hooper BA. An introduction to biomedical optics. New York: Taylor & Francis; 2007. [Google Scholar]
- 9.Huang NF, Niiyama H, Peter C, De A, Natkunam Y, Fleissner F, Li ZJ, Rollins MD, Wu JC, Gambhir SS, Cooke JP. Embryonic stem cell-derived endothelial cells engraft into the ischemic hindlimb and restore perfusion. Arterioscl Throm Vas. 2010;30:984–991. doi: 10.1161/ATVBAHA.110.202796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rufaihah AJ, Huang NF, Jame S, Lee JC, Nguyen HN, Byers B, De A, Okogbaa J, Rollins M, Reijo-Pera R, Gambhir SS, Cooke JP. Endothelial cells derived from human ipscs increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscl Throm Vas. 2011;31:E72–U44. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong GS, Robinson JT, Zhang YJ, Diao S, Antaris AL, Wang QB, Dai HJ. In vivo fluorescence imaging with ag2s quantum dots in the second near-infrared region. Angew Chem Int Edit. 2012;51:9818–9821. doi: 10.1002/anie.201206059. [DOI] [PubMed] [Google Scholar]
- 12.Matthes R, Cain CP, Courant D, Freund DA, Grossman BA, Kennedy PA, Lund DJ, Mainster MA, Manenkov AA, Marshall WJ, McCally R, Rockwell BA, Sliney DH, Smith PA, Stuck BE, Tell SA, Wolbarsht ML, Zheltov GI, Cheney F, McLin L, Ness J, Schulmeister K, Steinman RM, Sutter E, Zwick H Protect ICN-IR. Revision of guidelines on limits of exposure to laser radiation of wavelengths between 400 nm and 1.4 mu m. Health Phys. 2000;79:431–440. [PubMed] [Google Scholar]
- 13.Zimmermann A, Roenneberg C, Reeps C, Wendorff H, Holzbach T, Eckstein HH. The determination of tissue perfusion and collateralization in peripheral arterial disease with indocyanine green fluorescence angiography. Clin Hemorheol Micro. 2012;50:157–166. doi: 10.3233/CH-2011-1408. [DOI] [PubMed] [Google Scholar]
- 14.Serrat MA. Measuring bone blood supply in mice using fluorescent microspheres. Nat Protoc. 2009;4:1749–1758. doi: 10.1038/nprot.2009.190. [DOI] [PubMed] [Google Scholar]
- 15.Lim YT, Kim S, Nakayama A, Stott NE, Bawendi MG, Frangioni JV. Selection of quantum dot wavelengths for biomedical assays and imaging. Mol Imaging. 2003;2:50–64. doi: 10.1162/15353500200302163. [DOI] [PubMed] [Google Scholar]
- 16.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Arroyo AG, Iruela-Arispe ML. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc Res. 2010;86:226–235. doi: 10.1093/cvr/cvq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoefer IE, van Royen N, Buschmann IR, Piek JJ, Schaper W. Time course of arteriogenesis following femoral artery occlusion in the rabbit. Cardiovasc Res. 2001;49:609–617. doi: 10.1016/s0008-6363(00)00243-1. [DOI] [PubMed] [Google Scholar]
- 19.Gounis MJ, Spiga MG, Graham RM, Wilson A, Haliko S, Lieber BB, Wakhloo AK, Webster KA. Angiogenesis is confined to the transient period of vegf expression that follows adenoviral gene delivery to ischemic muscle. Gene Ther. 2005;12:762–771. doi: 10.1038/sj.gt.3302481. [DOI] [PubMed] [Google Scholar]
- 20.Hershey JC, Baskin EP, Glass JD, Hartman HA, Gilberto DB, Rogers IT, Cook JJ. Revascularization in the rabbit hindlimb: Dissociation between capillary sprouting and arteriogenesis. Cardiovasc Res. 2001;49:618–625. doi: 10.1016/s0008-6363(00)00232-7. [DOI] [PubMed] [Google Scholar]
- 21.Luo YP, Mohning KM, Hradil VP, Wessale JL, Segreti JA, Nuss ME, Wegner CD, Burke SE, Cox BF. Evaluation of tissue perfusion in a rat model of hind-limb muscle ischemia using dynamic contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging. 2002;16:277–283. doi: 10.1002/jmri.10169. [DOI] [PubMed] [Google Scholar]
- 22.Jaspers K, Versluis B, Leiner T, Dijkstra P, Oostendorp M, van Golde JM, Post MJ, Backes WH. Mr angiography of collateral arteries in a hind limb ischemia model: Comparison between blood pool agent gadomer and small contrast agent gd-dtpa. Plos One. 2011;6:e16159. doi: 10.1371/journal.pone.0016159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bashkatov AN, Genina EA, Kochubey VI, Tuchin VV. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J Phys D Appl Phys. 2005;38:2543–2555. [Google Scholar]
- 24.Troy TL, Thennadil SN. Optical properties of human skin in the near infrared wavelength range of 1000 to 2200 nm. J Biomed Opt. 2001;6:167–176. doi: 10.1117/1.1344191. [DOI] [PubMed] [Google Scholar]
- 25.Brown EB, Campbell RB, Tsuzuki Y, Xu L, Carmeliet P, Fukumura D, Jain RK. In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy (vol 7, pg 864, 2001) Nat Med. 2001;7:864–868. doi: 10.1038/89997. [DOI] [PubMed] [Google Scholar]
- 26.Vakoc BJ, Lanning RM, Tyrrell JA, Padera TP, Bartlett LA, Stylianopoulos T, Munn LL, Tearney GJ, Fukumura D, Jain RK, Bouma BE. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat Med. 2009;15:1219–U1151. doi: 10.1038/nm.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ku T, Choi C. Noninvasive optical measurement of cerebral blood flow in mice using molecular dynamics analysis of indocyanine green. Plos One. 2012;7:e48383. doi: 10.1371/journal.pone.0048383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamp MA, Slotty P, Turowski B, Etminan N, Steiger HJ, Hanggi D, Stummer W. Microscope-integrated quantitative analysis of intraoperative indocyanine green fluorescence angiography for blood flow assessment: First experience in 30 patients. Neurosurgery. 2012;70:65–73. doi: 10.1227/NEU.0b013e31822f7d7c. discussion 73–64. [DOI] [PubMed] [Google Scholar]
- 29.Detter C, Wipper S, Russ D, Iffland A, Burdorf L, Thein E, Wegscheider K, Reichenspurner H, Reichart B. Fluorescent cardiac imaging: A novel intraoperative method for quantitative assessment of myocardial perfusion during graded coronary artery stenosis. Circulation. 2007;116:1007–1014. doi: 10.1161/CIRCULATIONAHA.106.655936. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto M, Sasaguri S, Sato T. Assessing intraoperative blood flow in cardiovascular surgery. Surg Today. 2011;41:1467–1474. doi: 10.1007/s00595-010-4553-0. [DOI] [PubMed] [Google Scholar]
- 31.Kang Y, Choi M, Lee J, Koh GY, Kwon K, Choi C. Quantitative analysis of peripheral tissue perfusion using spatiotemporal molecular dynamics. Plos One. 2009;4:e4275. doi: 10.1371/journal.pone.0004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Davis C, Cai WB, He L, Chen XY, Dai HJ. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by raman spectroscopy. P Natl Acad Sci USA. 2008;105:1410–1415. doi: 10.1073/pnas.0707654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.