Abstract
Objective
To describe the incidence of low-volume, ultrastage-detected metastases in sentinel lymph nodes (SLNs) identified at surgical staging for endometrial carcinoma and to correlate it with depth of myoinvasion (DMI) and tumor grade.
Methods
We reviewed all patients who underwent primary surgery for endometrial carcinoma with successful mapping of at least one SLN at our institution from 9/2005-12/2011. All patients underwent a cervical injection for mapping. The SLN ultrastaging protocol involved cutting an additional two adjacent 5-μm sections at each of two levels, 50-μm apart, from each paraffin block lacking metastatic carcinoma on routine H&E. At each level, one slide was stained with H&E and with immunohistochemistry (IHC) using anti-cytokeratin AE1:AE3.
Micrometastases (tumor deposits <0.2mm and ≤2mm) and isolated tumor cells (≤0.2mm) were classified as low-volume, ultrastage-detected metastases if pathologic ultrastaging was the only method allowing detection of such nodal disease.
Results
Of 508 patients with successful mapping, 413(81.3%) had endometrioid carcinoma. Sixty-four(12.6%) of 508 patients had positive nodes: routine H&E detected 35 patients(6.9%), ultrastaging detected an additional 23 patients(4.5%) who would have otherwise been missed (4 micrometastases, 19 isolated tumor cells), and 6 patients(1.2%) had metastatic disease in their non-SLNs. The incidence of low-volume, ultrastagedetected nodal metastases in grade 1, 2, and 3 patients was 3.8%, 3.4%, and 6.9%, respectively. The frequency of low-volume, ultrastage-detected metastases in patients with a DMI of 0, <50%, and ≥50% was 0.8%, 8.0%, and 7.4%, respectively. Lymphovascular invasion was present in 20(87%) of the cases containing low-volume, ultrastage-detected metastases in the lymph nodes.
Conclusions
SLN mapping with pathologic ultrastaging in endometrial carcinoma detects additional low-volume metastases(4.5%) that would otherwise go undetected with routine evaluations. Our data support the incorporation of pathologic ultrastaging of SLNs in endometrial carcinoma with any degree of myoinvasion. The oncologic significance of lowvolume nodal metastases requires long-term follow-up.
Keywords: sentinel lymph node, endometrial carcinoma, ultrastaging, micrometastasis, low-volume metastasis
Introduction
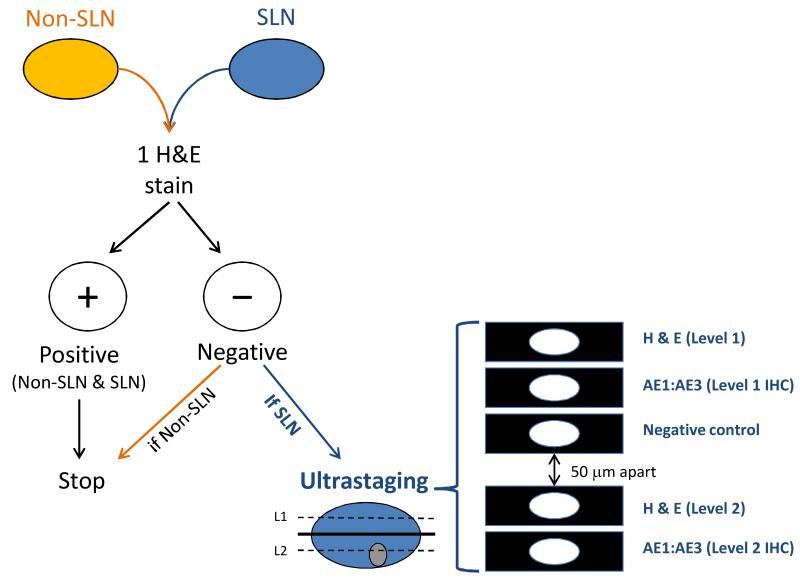
Standard lymph node assessment during the surgical staging of endometrial cancer involves sectioning the node once along the longitudinal axis and staining it with hematoxylin and eosin (H&E) to determine if it contains metastatic tumor cells. For sentinel lymph nodes (SLNs), enhanced pathologic assessment is performed if the initial H&E stain is negative. Ultrastaging involves additional sectioning and staining of the SLN with H&E and immunohistochemistry (IHC) in order to examine the SLN for low-volume metastatic disease. Figure 1 illustrates our institution’s pathologic ultrastaging algorithm for SLNs.
Figure 1.
Memorial Sloan-Kettering Cancer Center’s Pathologic Ultrastaging Algorithm for Sentinel Lymph Nodes
Low-volume metastatic disease, as defined in the breast cancer literature, includes micrometastases (MMs) and isolated tumor cells (ITCs).1 MMs are defined as a focus of metastatic tumor cells measuring >0.2 mm and ≤2 mm, whereas ITCs are defined as microscopic clusters and single cells measuring ≤0.2 mm. Additionally, with this enhanced pathologic ultrastaging, some SLNs are found to have rare, isolated cytokeratin-positive cells (CKPCs), the appearance of which is not diagnostic of carcinoma. Such cells have been reported to be present on occasion in lymph node sinuses and may be benign mesothelial cells. The clinical significance of finding CKPCs is not yet fully known, and in practice, these nodes are considered benign.
The primary objective of this study was to report the incidence of occult low-volume, ultrastage-detected metastases identified during ultrastaging of SLNs submitted at volume, ultrastage-detected positive SLNs with final depth of myometrial invasion (DMI) and tumor grade.
Methods
Patients and therapeutic interventions
Using an existing Institutional Review Board (IRB)-approved database, we reviewed the results of all patients who underwent primary surgery with SLN mapping for endometrial cancer from September 2005 to December 2011 at Memorial Sloan-Kettering Cancer Center (MSKCC). Surgical staging involved total hysterectomy, bilateral salpingo-oophorectomy, and SLN mapping, with additional full pelvic and/or paraaortic lymph node dissection based on attending discretion. All modalities of surgical assessment were included.
Lymphatic mapping was performed in all cases by injecting 1mL of blue dye into the cervical stroma at superficial and deep levels at the 3 and 9 o’clock positions for a total of 4 mL. Blue nodes were dissected and sent as SLNs for pathologic review, as previously described.2 Any suspicious or grossly enlarged nodes, per surgeon’s assessment, were removed and sent separately as non-SLNs.
Clinical patient characteristics, pathologic results, and operative reports were evaluated using the electronic medical record. Adjuvant chemotherapy and/or radiation was recommended and given as per physician discretion.
Lymph node processing and definition of low-volume, ultrastage-detected disease
All lymph nodes are routinely sectioned and stained with H&E. Our institution’s pathology protocol in assessing SLNs is based on work by Yared et al., who determined that re-examining SLNs at two additional levels with H&E and IHC could reliably detect lowvolume micrometastases.3 SLNs are initially examined by routine H&E staining, and subsequent ultrastaging is performed if the initial H&E assessment is negative. SLN ultrastaging is performed by cutting two adjacent 5-μm sections at each of two levels, 50-μm apart, from each paraffin block lacking metastatic carcinoma. At each level, one slide is stained with H&E and with IHC using the anti-cytokeratin AE1:AE3 (Ventana Medical Systems, Inc., Tucson, AZ) for a total of five slides per block (Figure 1).
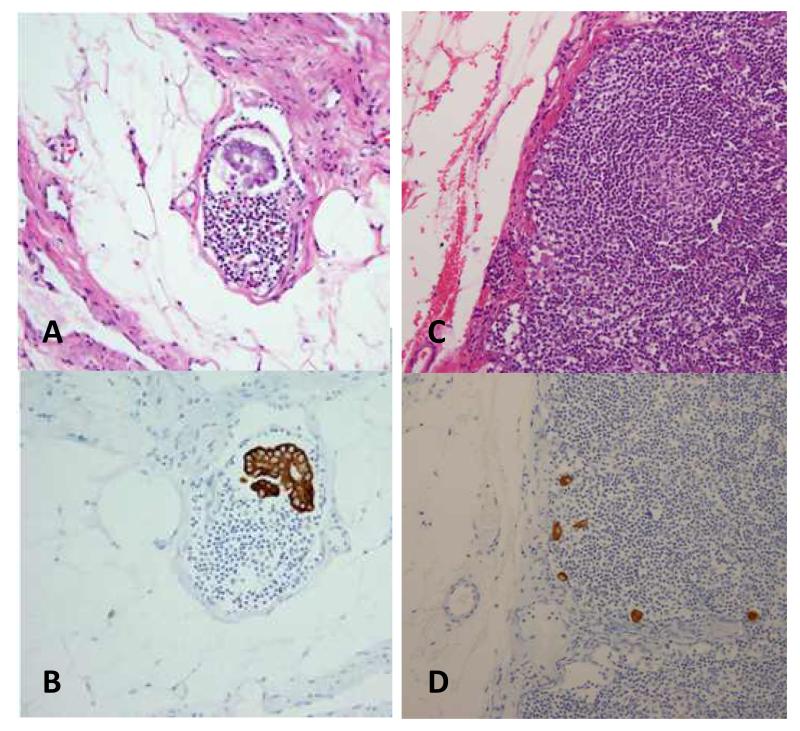
SLNs carrying low-volume, ultrastage-detected disease included both MMs and ITCs, as defined in the breast cancer literature by the American Joint Committee on Cancer (AJCC).1 Macrometastases in lymph nodes were defined as tumor cells >2.0 mm. MMs were reported by our gynecologic pathologists as “metastatic carcinoma in the form of microscopic clusters and single cells, measuring > 0.2 mm to ≤2 mm, identified in ___ of ___ lymph node(s) on additional H E-stained sections and cytokeratin immunohistochemical stains (AE1:AE3)/on additional H&E-stained sections only / on cytokeratin immunohistochemical stains (AE1:AE3) only.” ITCs were reported as “metastatic carcinoma in the form of microscopic clusters and single cells, measuring identified in __ of __ lymph node(s) on additional H&E-stained sections and cy, ≤0.2 mm tokeratin immunohistochemical stains (AE1:AE3) / on additional H&E-stained sections only / on cytokeratin immunohistochemical stains (AE1:AE3) only. While each cluster of carcinoma cells measures less than 0.2 mm, there are several clusters seen dispersed throughout the lymph node(s), spanning an area measuring ____ mm and consisting of about ____ cells.” In contrast, SLNs containing only CKPCs were considered negative for metastatic disease and were not included in the low-volume, ultrastage-detected metastasis category. CKPCs were reported as “rare/isolated/other _______ cytokeratin-positive cells (about ____ cells) are present in ____ of ____ lymph node(s). These cells are / are not identified on the corresponding H&E-stained sections. The clinical significance of this finding is not certain.” Figure 2 shows examples of low-volume, ultrastage-detected disease found in SLNs using IHC compared to standard H&E.
Figure 2.
Low-Volume Metastatic Tumor Cells Seen during Ultrastaging with Immunohistochemistry Micrometastases (MMs) on standard H&E stain (A) compared to MMs as seen on IHC (B). Isolated tumor cells (ITCs) on standard H&E stain (C) compared to ITCs as seen on IHC (D).
Non-SLN status was determined by routine pathologic processing using H&E staining of one section, while SLN status reflected results after ultrastaging of all submitted SLNs. Final lymph node status combined results from both SLNs and non-SLNs together such that lymph nodes were characterized as: (a) negative if no tumor cells were found in all non-SLNs and SLNs, (b) positive if macroscopic tumor cells were found in any non-SLN or on routine H&E staining of SLNs, and (c) low-volume, ultrastage-detected metastases if MMs or ITCs were found by histologic ultrastaging in SLNs and all other non-SLNs did not contain any disease. In four of the cases found to have ITCs on final histology, the pathologists likely identified the ITCs on the original H&E, but the SLN ultrastaging workup was implemented in order to better assess the nodal tumor volume and classify the type of low-volume tumor cells seen as MMs or ITCs.
All demographic, clinicopathologic, SLN mapping details, and pathology results were obtained from the patients’ medical records. As the details on the location and extent of lymphadenectomy are not relevant to this study’s objectives, we will not include this examination in our results. All tumor grades and DMI were based on final pathologic examination; serous endometrial, clear cell, and carcinosarcomas were considered grade 3.
RESULTS
Between 9/2005 and 12/2011, 643 patients with endometrial cancer underwent SLN mapping. All patients received a cervical injection of blue dye. Eight patients were excluded in the final analysis as their postoperative tumor histology involved adenosarcoma or undifferentiated carcinoma. As adenosarcomas are mesenchymal tumors, performing IHC of cytokeratins would not be helpful in these cases. Similarly, undifferentiated carcinomas are largely negative for AE1/AE3; thus, performing IHC in these cases would also not be useful. The patient and tumor characteristics are summarized in Table 2. Among the 508 (80.0%) of 635 eligible patients with at least one SLN detected, the median age at the time of surgery was 61 years (range: 34-88), and the median BMI was 29 kg/m2 (range: 16-55). Final tumor histology included 413 (81.3%) endometrioid, 62 (12.2%) serous, 12 (2.4%) clear cell, and 21 (4.1%) carcinosarcomas. Final grade 1, 2, and 3 tumors were found in 261 (51.4%), 116 (22.8%), and 131 (25.8%) patients, respectively. No myometrial invasion was found in 242 patients (47.2%), while 198 patients (39.0%) had <50% myoinvasion, and 68 (13.4%) had ≥50% myoinvasion. A total of 1613 SLNs and 3970 non-SLNs were submitted from the 508 patients. A median of 3 SLNs (range: 1-15) and 4 non-SLNs (range: 0-55) were biopsied per patient. At least one SLN was identified in 315 (62%) cases bilaterally, while in 193 (38.0%) cases, patients mapped only unilaterally, and thus additional side-specific lymphadenectomy was performed.
Table 2. Incidence of H&E Macrometastases in SLNs by Final Histologic Grade and Depth of Myometrial Invasion.
| DMI | Grade 1 | Grade 2 | Grade 3 | Total |
|---|---|---|---|---|
| No Invasion | H&E 0 n = 165 |
H&E 1 n = 39 |
H&E 1 n = 38 |
2/242 = 0.8% |
| < 50% invasion | H&E 6 n = 80 |
H&E 4 n = 62 |
H&E 6 n = 56 |
16/198 = 8.0% |
| ≥ 50% invasion | H&E 6 n = 16 |
H&E 3 n = 15 |
H&E 8 n = 37 |
17/68 = 25.0% |
| Total | 12/261= 4.6% | 8/116= 6.9% | 15/131 = 11.5% | 35/508 = 6.9% |
H&E: hematoxylin and eosin; DMI: depth of myometrial invasion
The SLN was positive for metastatic disease on initial routine H&E in 35 (6.9%) of the 508 successfully mapped cases, 33 (94.3%) of which were in patients whose tumors had evidence of myoinvasion. Table 2 shows the distribution of the H&E-detected positive SLNs by final tumor grade and final DMI. Ultrastaging detected an additional 23/508 (4.5%) patients with low-volume metastasis in their SLNs who would have been missed otherwise; this included 4 MM and 19 ITC cases. Table 3 highlights the distribution of low-volume, ultrastage-detected metastases by final tumor grade and final DMI. Among the 23 patients with low-volume, ultrastage-detected disease in their SLNs, a median of 3 SLNs (range, 1-9) and a median of 3 non-SLNs (range, 0-35) were examined per patient using standard H&E methods. Of the 198 patients with superficial, <50% myoinvasion, 16 (8.0%) were found to have low-volume disease within their SLNs detected by histologic ultrastaging. This is in contrast to the much lower rate of low-volume, ultrastage-detected nodal disease found in only 2/242 (0.8%) cases with no myoinvasion on final pathology. Ultrastaging also found 10 patients (1.9%) with CKPCs in their respective SLNs; these were considered negative nodes.
Table 3. Incidence of Ultrastage-detected, Low-Volume Metastases in SLNs by Final Histologic Grade and Depth of Myometrial Invasion.
| DMI | Grade 1 | Grade 2 | Grade 3 | Total |
|---|---|---|---|---|
| No Invasion | MM 1 ITC 1 n = 165 |
MM 0 ITC 0 n = 39 |
MM 0 ITC 0 n = 38 |
2/242 = 0.8% |
| < 50% invasion | MM 2 ITC 4 n = 80 |
MM 0 ITC 4 n = 62 |
MM 0 ITC 6 n = 56 |
16/198 = 8.0% |
| ≥ 50% invasion | MM 0 ITC 2 n = 16 |
MM 0 ITC 0 n = 15 |
MM 1 ITC 2 n = 37 |
5/68 = 7.4% |
| Total | 10/261= 3.8% | 4/116= 3.4% | 9/131 = 6.9% |
23/508 =
4.5% |
MM: micrometastasis; ITC: isolated tumor cells; DMI: depth of myometrial invasion
There were an additional 6/508 (1.2%) patients who had positive non-SLNs. Five of these 6 patients mapped unilaterally; thus, in 5 cases, the positive non-SLN was found in the contralateral hemi-pelvis after a side-specific lymphadenectomy was performed based on our surgical algorithm.4 In one patient, a unilateral SLN was negative but the contralateral pelvic non-SLN and paraaortic non-SLNs were positive; this patient had a high-grade tumor. The sixth patient was found to have one positive non-SLN on completion lymphadenectomy performed for a known high-grade tumor after SLNs were mapped bilaterally; in this case, the biopsied SLNs were falsely negative for disease.
Lymphovascular invasion (LVI) was present in 20/23 (87%) of the cases with low-volume, ultrastage-detected metastatic SLNs; similarly, LVI was present in 31/35 (89%) of the cases that contained macrometastasis found on routine H&E (Table 4). Five of the 6 patients (83%) with positive non-SLNs had evidence of LVI. In contrast, LVI was present in only 76 (17%) of the remaining 444 node-negative cases.
Table 4.
Frequency of Lymphovascular Invasion among Patients with Positive and Negative Metastatic Lymph Nodes
| Patients | LVI present |
LVI absent |
|---|---|---|
| Low-volume SLN mets (N = 23) | 20 (87%) | 3 (13%) |
| Routine H&E SLN mets (N = 35) | 31 (89%) | 4 (11%) |
| Routine H&E non-SLN mets (N = 6) | 5 (83%) | 1 (17%) |
| Negative SLNs (N = 444) | 76 (17%) | 368 (83%) |
LVI: lymphovascular invasion; H&E: hematoxylin and eosin; mets: metastases
Two patients with low-volume, ultrastage-detected disease in their SLNs recurred; both had only ITCs identified in their respective SLNs. One patient was 49 years old at the time of diagnosis, with a grade 2 endometrioid tumor, *lt;50% myoinvasion, LVI, and negative washings. She recurred in her paraaortic region approximately 27 months after being treated with pelvic radiation. The second patient was an 88-year-old woman with carcinosarcoma of the uterus, ≥50% myoinvasion, LVI, and negative washings. She was found to have a recurrence both locally at her vaginal cuff and distantly in her lungs approximately 24 months after being treated with adjuvant chemotherapy and vaginal brachytherapy. She died from an unknown cause less than a month after being diagnosed with recurrent disease and an extensive deep vein thrombus in her pelvic veins that involved her lower inferior vena cava.
DISCUSSION
This is the largest single-institution study to date with standardized specialized pathology review reporting the incidence of low-volume, ultrastage-detected metastases in SLNs during the surgical staging of endometrial cancer.5,6 In this study, we identified low-volume, ultrastage-detected metastatic disease in SLNs, defined as MMs or ITCs, in 23 (4.5%) patients with endometrial cancer in whom no metastatic disease would have otherwise been detected by conventional pathologic processing. These cases were in addition to the 35 (6.9%) patients found to have macrometastases during routine H&E evaluation of their SLNs. Interestingly, in comparing Tables 2 and 3, both macrometastases and low-volume, ultra-stage detected metastases were more commonly found in the cases with some evidence of myometrial invasion. In contrast to tumors with no myoinvasion, in which ultrastage-detected nodal metastases were only found in 0.8% of cases, patients with any myoinvaison had approximately 7-8% low-volume, ultrastage-detected nodal metastases in SLNs. These data argue for continued incorporation of ultrastaging with enhanced pathology evaluation of SLNs in endometrial cancers with any myoinvasion and eliminating this technique in cases of no myoinvasion on final pathology.
Similar to what we know from historical non-SLN data, our rate of finding H&E-positive macrometastases in SLNs increased with increasing DMI.7 H&E detected positive SLNs in 1%, 8%, and 25% of tumors with no myoinvasion, DMI*lt;50%, and DMI ≥50%, respectively.
Low-volume, ultrastage-detected metastasis was not just an occurrence in low-grade tumors. There were twice as many low-volume metastases in grade 3 tumors compared to grade 1-2 tumors. The presence of LVI was also seen in similar percentages in patients with macrometastases and those with low-volume disease, further supporting the observation that these are true tumor cells in nodes. However, the oncologic significance of low-volume, ultrastage-detected disease requires long-term follow-up and cannot be determined from this study alone.
Although the prognostic significance of detecting low-volume, ultrastage-detected disease in lymph nodes during endometrial cancer staging surgery is not known, the relevance of these findings may be important in determining adjuvant treatment and follow-up strategy for these patients. Whereas standard H&E evaluation of lymph nodes occurs at one level, ultrastaging increases the ability to detect low-volume tumor cells as it re-evaluates a presumed negative SLN at two additional levels with additional IHC stains. Prior studies have shown that MMs and ITCs are often undetectable by routine H&E8,9,10; however, after performing ultrastaging on thousands of SLNs in endometrial cancer, our dedicated gynecologic pathologists have experienced an improved understanding of the location and pattern of these low-volume metastases in SLNs. Their extensive experience in pathologic ultrastaging has allowed them to recognize MMs and ITCs during routine H&E staining and thus increased their scrutiny of routine H&E-stained sections, particularly in the presence of myometrial invasion and LVI. This observation that experience may improve the recognition of MMs and ITCs using original H&E slides will require further validation from other investigators. In our series, ultrastaging was still performed in an effort to provide a more definitive assessment of the lesion’s size so that distinctions between ITCs, MMs, and macrometastases were more definitive.
The significance of low-volume, ultrastage-detected metastatic disease in SLNs is still controversial in the breast literature. Some studies have noted that CKPCs found in lymph nodes using IHC may represent artificial displacements of benign epithelial cells during manipulation or biopsy,11,12,13 whereas others have demonstrated that these findings are not a result of instrumentation alone.14,15 We have evaluated this concept previously in endometrial cancer and found that the presence of MMs or ITCs in SLNs was not an artifact of preoperative biopsy or intraoperative uterine manipulation.16
Yabushita el al. reported that IHC expression of cytokeratin in lymph nodes with undetected metastases by routine H&E staining is a risk factor for recurrence in early-stage endometrial cancer (P=0.01).6 In our cohort, there were 2 women who were found to have ITCs that recurred. Despite being the largest single-institution study of low-volume metastatic disease in SLNs biopsied during endometrial cancer surgery, our series is limited by the overall small number of patients found to have low-volume, ultrastagedetected disease. Additionally, the retrospective nature of this study prevents us from drawing broad conclusions without long-term survival data. Since the overall incidence of MMs to lymph nodes is relatively low, it will require long-term follow-up to better evaluate the oncologic significance of this low-volume, ultrastage-detected disease.
As we continue to learn and improve our understanding of the role of SLNs in endometrial cancer staging, pathologic ultrastaging remains a valuable component to SLN mapping and biopsy. The increased detection of low-volume nodal metastases appears to be most notable in endometrial cancers with myoinvasion on final pathology. Pathologic ultrastaging of SLNs may be eliminated in endometrial cancer cases with no myoinvasion on final pathology.
Table 1.
Patient and Tumor Characteristics of Cases Successfully Mapped for Sentinel Lymph Nodes (n=508)
| Characteristics | Patients n=508(%) |
|---|---|
|
| |
| Age at diagnosis (yr) | |
| Median (range) | 61 (34-88) |
|
| |
| BMI (kg/m2) | |
| Median (range) | 29 (16-55) |
|
| |
| Type of surgery | |
| Laparotomy | 107 (21.1) |
| Laparoscopy | 178 (35.0) |
| Robotic-assisted | 223 (43.9) |
|
| |
| Final Histology | |
| Endometrioid | 413 (81.3) |
| Serous | 62 (12.2) |
| Clear cell | 12 (2.4) |
| Carcinosarcoma | 21 (4.1) |
|
| |
| Final Tumor Grade | |
| 1 | 261 (51.4) |
| 2 | 116 (22.8) |
| 3 | 131 (25.8) |
|
| |
| Depth of Myoinvasion | |
| None | 242 (47.6) |
| < 50% | 198 (39.0) |
| ≥ 50% | 68 (13.4) |
|
| |
| Lymphovascular invasion | |
| No | 376 (74.0) |
| Yes | 132 (26.0) |
|
| |
| Total # SLNs biopsied | 1613 |
| Total # non-SLNs biopsied | 3970 |
|
| |
| SLNs biopsied per pt | |
| Median (range) | 3 (1-15) |
| Non-SLNs biopsied per pt | |
| Median (range) | 4 (0-55) |
SLN: sentinel lymph node
References
- 1.Greene Fl, Page DL, Irvin D, et al., editors. AJCC Cancer Staging Manual. Sixth Edition Springer-Verlag; New York: 2003. pp. 223–240. [Google Scholar]
- 2.Abu-Rustum NR, Khoury-Collado F, Gemignani ML. Techniques of sentinel lymph node identification for early-stage cervical and uterine cancer. Gynecol Oncol. 2008;111:S44–50. doi: 10.1016/j.ygyno.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Yared MA, Middleton LP, Smith TL, et al. Recommendations for sentinel lymph node processing in breast cancer. Am J Surg Path. 2002;26:377–382. doi: 10.1097/00000478-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Barlin JN, Khoury-Collado F, Kim CH, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecol Oncol. 2012;125:531–535. doi: 10.1016/j.ygyno.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Kang S, Yoo HJ, Hwang JH, et al. Sentinel lymph node biopsy in endometrial cancer: Meta-analysis of 26 studies. Gynecol Oncol. 2011;123:522–527. doi: 10.1016/j.ygyno.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Bezu C, Coutant C, Ballester M, et al. Ultrastaging of lymph node in uterine cancers. J Exp Clin Cancer Res. 2010;29:5. doi: 10.1186/1756-9966-29-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer: a gynecologi oncology group study. Cancer. 1987;60:2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Fishman A, Klein A, Zemer R, et al. Detection of micrometastasis by cytokeratin-20 (reverse transcription polymerase chain reaction) in lymph nodes of patients with endometrial cancer. Gynecol Oncol. 2000;77:399–404. doi: 10.1006/gyno.2000.5781. [DOI] [PubMed] [Google Scholar]
- 9.Yabushita H, Shimazu M, Yamada H, et al. Occult lymph node metastases detected by cytokeratin immunohistochemistry predict recurrence in node-negative endometrial cancer. Gynecol Oncol. 2001;80:139–44. doi: 10.1006/gyno.2000.6067. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez Bosquet J, Keeney GL, Mariani A, et al. Cytokeratin staining of resected lymph nodes may improve the sensitivity of surgical staging for endometrial cancer. Gynecol Oncol. 2003;91:518–525. doi: 10.1016/j.ygyno.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Youngson BJ, Liberman L, Rosen PP. Displacement of carcinomatous epithelium in surgical breast specimens following stereotaxic core biopsy. Am J Clin Pathol. 1995;103:598–602. doi: 10.1093/ajcp/103.5.598. [DOI] [PubMed] [Google Scholar]
- 12.Carter BA, Jensen RA, Simpson JF, Page DL. Benign transport of breast epithelium into axillary lymph nodes after biopsy. Am J Clin Pathol. 2000;113:259–265. doi: 10.1309/7EF8-F1W7-YVNT-H8H5. [DOI] [PubMed] [Google Scholar]
- 13.Moore KH, Thaler HT, Tan LK, et al. Immunohistochemically detected tumor cells in the sentinel lymph nodes of patients with breast carcinoma: biologic metastasis or procedural artifact? Cancer. 2004;100:929–934. doi: 10.1002/cncr.20035. [DOI] [PubMed] [Google Scholar]
- 14.King TA, Ganaraj A, Fey JV, et al. Cytokeratin-positive cells in sentinel lymph nodes in breast cancer are not random events: experience in patients undergoing prophylactic mastectomy. Cancer. 2004;101:926–933. doi: 10.1002/cncr.20517. [DOI] [PubMed] [Google Scholar]
- 15.de Boer M, van Deurzen CH, van Dijck JA, et al. Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med. 2009;361:L653–663. doi: 10.1056/NEJMoa0904832. [DOI] [PubMed] [Google Scholar]
- 16.Frimer M, Khoury-Collado F, Murray MP, et al. Micrometastasis of endometrial cancer to sentinel lymph nodes: Is it artifact of uterine manipulation? Gynecol Oncol. 2010;199:496–499. doi: 10.1016/j.ygyno.2010.08.030. [DOI] [PubMed] [Google Scholar]