Abstract
Estrogen is an important vasoprotective molecule that causes the rapid dilation of blood vessels by activating endothelial nitric oxide synthase (eNOS) through an unknown mechanism. In studies of intact ovine endothelial cells, 17β-estradiol (E2) caused acute (five-minute) activation of eNOS that was unaffected by actinomycin D but was fully inhibited by concomitant acute treatment with specific estrogen receptor (ER) antagonists. Overexpression of the known transcription factor ERα led to marked enhancement of the acute response to E2, and this was blocked by ER antagonists, was specific to E2, and required the ERα hormone-binding domain. In addition, the acute response of eNOS to E2 was reconstituted in COS-7 cells cotransfected with wild-type ERα and eNOS, but not by transfection with eNOS alone. Furthermore, the inhibition of tyrosine kinases or mitogen-activated protein (MAP) kinase kinase prevented the activation of eNOS by E2, and E2 caused rapid ER-dependent activation of MAP kinase. These findings demonstrate that the short-term effects of estrogen central to cardiovascular physiology are mediated by ERα functioning in a novel, nongenomic manner to activate eNOS via MAP kinase–dependent mechanisms.
Introduction
The hormone estrogen classically exerts its effects by modifying gene expression (1–3). However, there are also important rapid, presumably nongenomic, effects of estrogen and other related steroid hormones in a variety of tissues including the vasculature, brain, and bone (4–9). Recent evidence suggests that the vascular effects of estrogen play a critical role in the atheroprotective properties of the hormone (10, 11). Premenopausal women have very little coronary artery disease compared to men, the incidence of the disease rises markedly after menopause, and hormone replacement therapy reduces the risks to premenopausal levels (12–15). In addition, acute estrogen administration rapidly restores the endothelium-dependent dilation of atherosclerotic arteries in primate models, and it acutely improves endothelium-dependent responses in healthy postmenopausal women (16–19). The rapid vasodilatory effect of estrogen is at least partially related to its ability to enhance the bioavailability of nitric oxide (NO) (10, 11, 16–19), which is a potent regulator of blood pressure, platelet aggregation, leukocyte adhesion, and vascular smooth muscle mitogenesis (20). Endothelial NO is produced by the endothelial isoform of NO synthase (eNOS) upon the conversion of the substrate L-arginine to L-citrulline (20). To better understand the mechanism(s) by which estrogen acutely increases endothelial NO production, the present experiments were performed in cultured endothelial cells to determine the potential role of estrogen receptor (ER) α in this process. We and others have recently shown that estrogen rapidly stimulates eNOS activity in endothelial cells and that ERα is expressed in endothelium (21–24). ERα and the second, more recently discovered ER isoform, ERβ, are known to function as transcription factors mediating estrogen-induced gene expression in both reproductive and nonreproductive tissues (10, 11, 25). However, ER may also be involved in acute, nongenomic physiologic responses because the rapid effects of estrogen in certain cell types are inhibited by ER antagonists (4, 5, 7, 8).
Methods
Cell culture.
Pulmonary artery endothelial cells (PAEC) were obtained from the intrapulmonary arteries of fetal lambs at 125–135 days gestation (term = 144 days) by collagenase digestion and were propagated as described previously (26). These cells have been used previously to evaluate the acute effects of varying oxygenation on eNOS activation (26). Animal care and euthanasia procedures were approved by the Institutional Review Board for Animal Research. Near-confluent PAEC were studied at passage 4–6. COS-7 cells (American Type Culture Collection, Rockville, Maryland, USA) were grown in DMEM (Life Technologies Inc., Grand Island, New York, USA) supplemented with 10% heat-inactivated FBS plus 200 U/ml penicillin and 200 μg/ml streptomycin.
eNOS activation in whole cells.
eNOS activation was assessed in whole cells by measuring [3H]L-arginine conversion to [3H]L-citrulline during acute incubations, using methods reported previously (21). This procedure provides a direct evaluation of the acute activation of existing eNOS, keeping signal transduction mechanisms intact (27). Adherent cells grown in 24-well plates were placed in L-arginine–deficient, serum-free endothelial-SFM Growth Media (Life Technologies Inc.) for 6 h and then preincubated in PBS (pH 7.4) containing 120 mM NaCl, 4.2 mM KCl, 2.5 mM CaCl2, 1.3 mM MgSO4, 7.5 mM glucose, 10 mM HEPES, 1.2 mM Na2HPO4, and 0.37 mM KH2PO4 for 15 min at 37°C. The incubation for eNOS activity was initiated by replacing the preincubate solution with PBS containing 1.5 μCi/ml [3H]L-arginine. After 5–15 min, the reaction was stopped by adding 1 N TCA, the cells were freeze-fractured in liquid nitrogen and scraped with a rubber spatula, the contents of each well were ether extracted, and the [3H]L-citrulline generated was isolated using Dowex AG50WX-8 columns (Sigma Chemical Co., St. Louis, Missouri, USA) and quantified by liquid scintillation spectroscopy. In individual experiments, four to six wells were used for each treatment group. All findings were confirmed in at least three independent studies. Basal [3H]L-citrulline generation over 15 min ranged from 4 to 7 fmol/100,000 cells on different experimental days. Results are expressed as the percent of basal eNOS activity determined in the same 24-well plate. To study the acute effects of estrogen on eNOS in PAEC, [3H]L-arginine conversion to [3H]L-citrulline was measured in whole cells either under basal conditions or in the presence of 10–8 M estradiol-17β (E2). In previous experiments, the maximal effect of E2 was obtained at 10–8 M, and the threshold concentration was 10–10 M (21). The activation of eNOS was also evaluated in the presence of estradiol-17α (10–12 to 10–6 M) or in the presence of the known eNOS agonist acetylcholine (10–6 M) or the calcium ionophore A23187 (10–5) (21). Acetylcholine has been used previously in this cell culture model to mimic mechanisms in intact arteries (21, 28), and it has also been used by others in studies of nitric oxide (NO)-release processes in a variety of endothelial cell types (29–31). Both basal and stimulated eNOS activity were fully inhibited by 2.0 mM nitro-L-arginine methyl ester. To determine if the effect of E2 on PAEC eNOS is mediated at the level of gene transcription, cells were treated with 25 μg/ml actinomycin D for 120 min, and eNOS activation was then determined in the continued presence of actinomycin D for 15 min. Studies of cyclooxygenase type 1 and malate dehydrogenase gene expression have revealed that such treatment fully inhibits gene transcription in the PAEC (32). The role of ER in the rapid response to E2 was determined during 15-min incubations done in the absence or presence of 10–8 M E2, with or without 10–6 M tamoxifen or 10–5 M ICI 182,780 added simultaneously (33).
Cell transfection studies.
To determine the specific role of ERα in the acute endothelial cell response to E2, the abundance of functional ERα was augmented by transiently transfecting the expression plasmid pCMV3-ERα, or pCMV3 alone, into PAEC using Lipofectamine (Life Technologies Inc.) (34). pCMV3-ERα was constructed by cloning full-length human ERα cDNA (35) as an EcoRI fragment into the CMV-driven plasmid pCDNA 3.1 (Invitrogen Corp., San Diego, California, USA). The construct was confirmed by sequencing in both directions. Cotransfection with a plasmid containing SV40-driven β-galactosidase was performed to normalize for transfection efficiency (34). Transfected cells were placed in phenol red–free, estrogen-free media. Seventy-two hours after transfection, acute E2-stimulated eNOS activation was examined. Enhanced ER-mRNA expression was documented in reverse transcription-PCR assays (21), and greater ERα protein abundance was confirmed by both immunocytochemistry and immunoblot analysis with the mouse monoclonal antibody AER 320 directed against amino acids 495–595 of human ERα (Neomarkers Inc., Fremont, California, USA), using methods described previously (34). Immunocytochemistry for β-galactosidase revealed successful expression of the protein in 15%–20% of cells, and immunostaining for ERα confirmed enhanced expression in 20%–40% of cells transfected with ERα cDNA. To determine the effects of ERα overexpression on estrogen-induced transcriptional transactivation, cotransfection was performed with a luciferase reporter plasmid that contains three copies of the Xenopus vitellogenin estrogen-response element (ERE), ERE-Luc,7 or the control plasmid TK-Luc (34). After transfection, cells were placed in either phenol red–free, estrogen-free media, or phenol red–free media containing 10–8 M E2 for 48 h, and reporter activity was measured (34).
Additional experiments were performed to delineate the role of the hormone-binding domain of ERα. Acute eNOS activation was assessed in PAEC transfected with pCMV3, pCMV3-ERα, or pCMV3-ERα-271-NTF. The latter expression plasmid was constructed by first cloning full-length human ERα cDNA into the pCDNA3.1 plasmid, followed by insertion of the coding sequence for the 8–amino acid “Flag” epitope (NTF), recognized by M2 monoclonal antibody (Kodak, Rochester, New York, USA), immediately after the initiation codon at the 5′ end of the ERα coding sequence to yield pCMV3-ERα-NTF. Then pCMV3-ERα-NTF was digested with Xcm1 and Xho1 to remove all coding sequence distal to amino acid 271, which excludes the hormone-binding domain (HBD), followed by blunting of the construct ends and religation to yield pCMV3-ERα-271-NTF. Expression from these plasmids of both full-length and truncated epitope-tagged proteins of the predicted size was demonstrated by transient transfection of COS-1 cells followed by immunoprecipitation and immunoblot analysis.
To generalize the findings in the native endothelial cells, further studies were done to reconstitute the ERα-mediated activation of eNOS in a cell type that does not constitutively express either ER or eNOS and that is not estrogen responsive. COS-7 cells were cotransfected with human eNOS cDNA (36) and either ERα cDNA or sham plasmid, and the acute effects of E2 were assessed.
Tyrosine kinase–MAP kinase inhibition and measurement of MAP kinase activity.
Additional studies were performed to begin to elucidate the signal transduction mechanisms involved in acute E2 stimulation of eNOS. In nonendothelial cell types, E2 can cause the rapid activation of several signaling pathways, including those involving c-src–related tyrosine kinases and mitogen-activated protein (MAP) kinases (7, 8). Therefore, the effects of specific inhibitors of these signaling pathways on eNOS activation were assessed in intact PAEC. Cells were treated with either the tyrosine kinase inhibitors genistein (50 μM) or herbimycin A (10 μM) for 20 h, or with the MAP kinase kinase (MEK) inhibitor PD98059 (50 μM) for 45 min (37, 38). Acute E2-induced eNOS activation was then evaluated in the continued presence of genistein, herbimycin A, or PD98059 over a 15-min period.
The effect of E2 on MAP kinase activity was also assessed (8). PAEC were treated for 5 min with 10–8 M E2 in the absence or presence of 10–5 M tamoxifen or 10–5 M ICI 182,780, or with serum to serve as a positive control. The cells were solubilized with 20 mM Tris buffer (pH 7.5) containing 10% glycerol, 1% Triton X-100, 137 mM NaCl, 25 mM β-glycerophosphate, 2 mM EDTA, 0.5 mM dithiothreitol, 1 mM sodium orthovanadate, 2 mM sodium pyrophosphate, 10 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride. The extracts were centrifuged at 15,000 g for 20 min at 4°C, and the endogenous kinase was immunoprecipitated from the supernatant by incubation for 2 h at 4°C with polyclonal anti-Erk2 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, California, USA) bound to protein A–Sepharose. The immunoprecipitates were washed twice with the 20-mM Tris buffer and twice with buffer containing 25 mM HEPES (pH 7.4), 25 mM β-glycerophosphate, 25 mM MgCl2, 0.5 mM dithiothreitol, and 0.1 mM sodium orthovanadate. Protein kinase assays were then performed using 1 μg of myelin basic protein and 50 μM [γ-32P]ATP (10 Ci/mmol) in a final volume of 20 μl of the 25 mM HEPES buffer. The phosphorylation reaction was linear with time for at least 60 min. The reaction was terminated after 30 min at 30°C by the addition of Laemmli sample buffer. Phosphorylation of the substrate protein was examined after SDS-PAGE by autoradiography and PhosphorImager (Molecular Dynamics, Sunnyvale, California, USA) analysis.
Results
eNOS activation by E2.
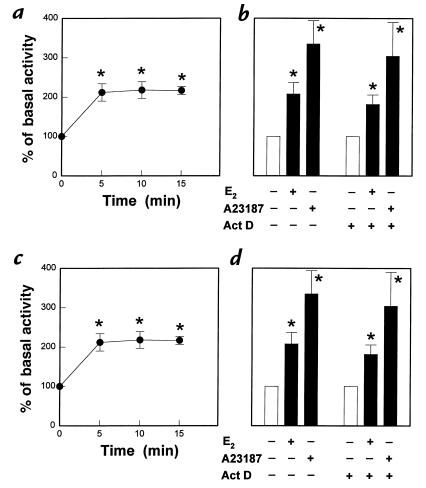
The time period over which physiologic concentrations of E2 cause changes in eNOS activity in PAEC is shown in Fig. 1a. E2 (10–8 M) stimulated an increase in eNOS activity, which reached maximal levels within 5 min of exposure to the hormone. The response was specific to E2, because 17α-estradiol (10–12 to 10–6 M) had no effect (data not shown). The responses to both E2 and the calcium ionophore A23187 were not altered by the inhibition of gene transcription with actinomycin D (Fig. 1b). To determine if this acute process involves rapid ER activation, the effect of concomitant treatment with the ER antagonist tamoxifen was determined (Fig. 1c). Tamoxifen caused no change in basal eNOS activity but fully inhibited the acute response to E2. In addition, simultaneous treatment with the pure ER antagonist ICI 182,780 also completely negated the rapid, E2-stimulated increase in eNOS activity but did not alter basal activity (Fig. 1d).
Figure 1.
Rapid activation of eNOS in endothelial cells. (a) Effect of E2 on eNOS activity in intact PAEC. [3H]L-arginine conversion to [3H]L-citrulline was measured over 5–15 min in the presence of 10–8 M E2. (b) Effect of actinomycin D (Act D) on the rapid activation of eNOS. After 120 min preincubation in the absence or presence of 25 μg/ml Act D, 15 min incubations were done with or without continued Act D and either 10–8 M E2 or the calcium ionophore A23187 (10–5 M). (c) Effect of tamoxifen on E2-stimulated eNOS activity. Fifteen-minute incubations were performed in the absence or presence of 10–8 M E2, with or without 10–6 M tamoxifen (Tam) added simultaneously. Partial inhibition (50%–70%) was also noted with 10–8 M Tam (13). (d) Effect of ICI 182,780 on E2-stimulated eNOS activity. Fifteen-minute incubations were performed in the absence or presence of 10–8 M E2, with or without 10–5 M ICI 182,780 added simultaneously. Full inhibition was also observed with 10–6 M ICI 182,780 (13). Values are mean ± SEM; n = 4–6. *P < 0.05 vs. basal. E2,estradiol-17β; eNOS, endothelial nitric oxide; PAEC, pulmonary artery endothelial cells.
Effect of ERα overexpression.
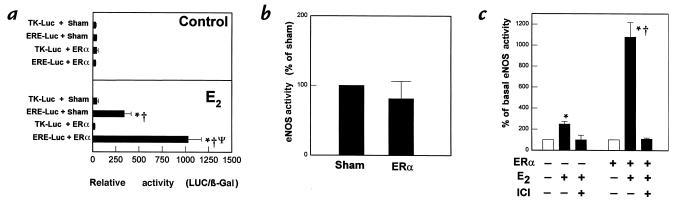
The potential role of ERα in acute eNOS activation was evaluated in overexpression studies. To first confirm the overexpression of functional ERα, estrogen-induced transcriptional transactivation was assessed by cotransfection of the estrogen-responsive reporter plasmid ERE-Luc or the control plasmid TK-Luc into PAEC with either ERα cDNA or the sham plasmid. In the absence of estrogen, reporter activity was not detectable in any paradigm (Fig. 2a, upper). In contrast, after prolonged treatment with 10–8 M E2, ERE-Luc reporter activity was eightfold greater than TK-Luc reporter activity in cells having a normal complement of ER (Fig. 2a, lower). Overexpression of ERα caused a further threefold augmentation of estrogen-induced transcriptional transactivation.
Figure 2.
Effect of ERα overexpression on transcriptional transactivation and on basal and E2-stimulated eNOS activity. (a) Effect of ERα overexpression on ERE-mediated gene transcription in PAEC. Transient transfections were performed with either the estrogen-responsive reporter plasmid ERE-Luc or the control plasmid TK-Luc, in combination with either sham plasmid or ERα cDNA. Reporter activity was then determined in control cells (upper panel) and cells exposed to 10–8 M E2 for 48 h (lower panel). Reporter activity is expressed as luciferase activity/β galactosidase activity (LUC/β-gal). *P < 0.05 vs. TK-Luc, †P < 0.05 vs. control cells, ΨP < 0.05 vs. sham. Similar findings were obtained in three independent experiments. (b) Effect of ERα overexpression on basal eNOS activity in PAEC. Cells were transfected with sham plasmid or ERα cDNA, and 72 h later [3H]L-arginine conversion to [3H]L-citrulline was measured over 15 min in nonstimulated, intact cells. (c). Effect of ERα overexpression on acute eNOS activation by E2. PAEC were transiently transfected with sham plasmid or ERα cDNA, and 72 h later [3H]L-arginine conversion to [3H]L-citrulline was measured in intact cells over 15 min in the absence or presence of 10–8 M E2, with or without 10–5 M ICI 182,780 added simultaneously. Values are mean ± SEM; n = 4–6. *P < 0.05 vs. basal, †P < 0.05 vs. sham. ERα, estrogen receptor α; ERE, estrogen response element.
The effect of ERα overexpression on basal eNOS activity in intact cells is depicted in Fig. 2b. Basal eNOS activity was unchanged by ERα overexpression. In addition, immunoblot analysis revealed that eNOS protein abundance was not affected by ERα overexpression (data not shown). Experiments were then performed to determine the effect of enhanced ERα expression on the acute response to E2 (Fig. 2c). It was found that the rapid response was augmented four- to fivefold in cells transfected with ERα compared to sham-transfected cells (Fig. 2c). Furthermore, the enhanced response was inhibited completely by concomitant acute treatment with ICI 182,780.
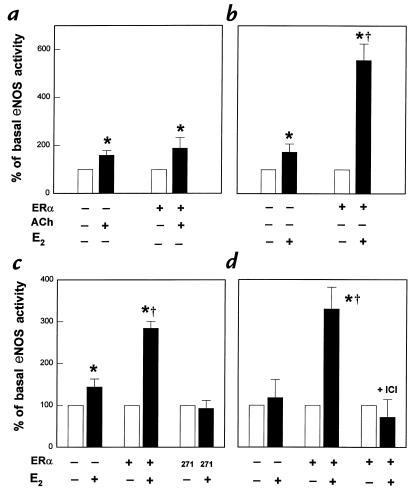
To determine the specificity of the augmentation in eNOS activity in response to E2, experiments were done comparing the rapid effects of acetylcholine and E2 in PAEC overexpressing ERα. With acetylcholine, there were comparable increases in eNOS activity in sham and ERα-transfected cells (Fig. 3a). This contrasted with the augmented response to E2 after ERα transfection (Fig. 3b). To determine the role of the ERα HBD, additional studies were performed in PAEC transfected with a truncation mutant of ERα lacking coding sequence distal to amino acid 271 (Fig. 3c). In contrast to the enhanced E2-mediated response observed after wild-type ERα overexpression, E2-mediated eNOS activity was not augmented in cells expressing the truncation mutant lacking the HBD. To further define the specific role of ERα, studies were performed to reconstitute the phenomenon in COS-7 cells that do not constitutively express either ER or eNOS and that are not estrogen responsive. In COS-7 cells transfected with eNOS alone, E2 had no effect on eNOS activity (Fig. 3d). However, in cells transfected with both eNOS and ERα, there was a more than threefold increase in eNOS activity upon acute stimulation with E2. In contrast, ERα expression had no effect on basal eNOS activity (102 ± 33% of activity in sham-transfected cells, or ERα–). In addition, activity stimulated by A23187 was unchanged, being 376 ± 105% and 357 ± 73% of basal levels in ERα– and ERα+ cells, respectively. The rapid stimulation of eNOS by E2 in the COS-7 cells transfected with eNOS and ERα was completely inhibited by concomitant ICI 182,780 treatment.
Figure 3.
Specificity of the augmentation of eNOS activation by ERα overexpression. (a and b) Comparison of the effects of ERα overexpression on acute eNOS activation by acetylcholine (ACh) and E2. PAEC were transiently transfected with sham plasmid or with ERα cDNA. After 72 h, [3H]L-arginine conversion to [3H]L-citrulline was measured in intact cells over 15 min in the absence or presence of 10–6 M ACh (a), or in the absence or presence of 10–8 M E2 (b). (c) Role of HBD in acute eNOS activation by E2. PAEC were transfected with either sham plasmid, wild-type ERα cDNA, or an ERα mutant lacking coding sequence distal to amino acid 271 (labeled 271), which excludes the HBD. The eNOS activity in the absence or presence of 10–8 M E2 (15 min) was evaluated after 72 h. (d) Reconstitution of acute E2 response in COS-7 cells. Transfections were performed with human eNOS cDNA and either sham plasmid or ERα cDNA, and eNOS activation was measured 72 h later over 15 min in the absence or presence of 10–8 M E2. Additional studies were done in ERα-transfected cells with 10–5 M ICI 182,780 added simultaneously. Values are mean ± SEM; n = 4–6. *P < 0.05 vs. basal, †P < 0.05 vs. sham. HBD, hormone-binding domain.
Role of tyrosine kinase–MAP kinase pathway.
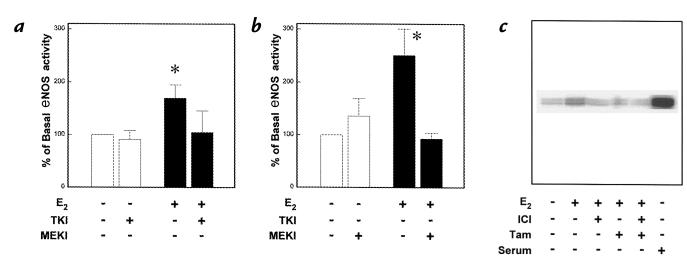
The role of the tyrosine kinase–MAP kinase signaling pathway in the endothelial cell response to E2 was assessed by both pharmacologic intervention and measurements of MAP kinase activity. The tyrosine kinase inhibitor genistein did not alter basal eNOS activity in the PAEC but completely inhibited the response to E2 (Fig. 4a). Similar results were obtained with herbimycin A (data not shown). In addition, basal eNOS activity was not modified by the specific MEK inhibitor PD98059, but the stimulatory effect of E2 was fully negated by PD98059 (Fig. 4b). Furthermore, a five-minute exposure to 10–8 M E2 caused an increase in MAP kinase activity (Fig. 4c), and this effect was completely inhibited by the ER antagonists ICI 182,780 and tamoxifen.
Figure 4.
Role of tyrosine kinase–MAP kinase signaling pathway. (a) Role of tyrosine kinase in acute eNOS activation by E2. [3H]L-arginine conversion to [3H]L-citrulline was measured over 15 min in intact PAEC in the absence or presence of 10–8 M E2, with or without treatment with the tyrosine kinase inhibitor (TKI) genistein (50 μM). (b) Role of MEK in acute eNOS activation by E2. eNOS activity was measured in the absence or presence of 10–8 M E2, with or without treatment with the MEK inhibitor (MEKI) PD98059 (50 μM). Values are mean ± SEM; n = 4–6. *P < 0.05 vs. basal. (c) Effect of E2 on MAP kinase activity in PAEC. Cells were treated for 5 min with 10–8 M E2 in the absence or presence of 10–5 M tamoxifen (TAM) or 10–5 M ICI 182,780, or with serum to serve as a positive control. Endogenous kinase was immunoprecipitated with anti-Erk2 antibody, and protein kinase activity was measured by evaluating the capacity to phosphorylate myelin basic protein. Quantification by PhosphorImager yielded values of 1, 2.6, 1, 1, 1, and 7.5, respectively, relative to untreated cells. Results shown are representative of five independent experiments. MAP, mitogen-activated protein; MEK, mitogen-activated protein kinase.
Discussion
In this study we have demonstrated that E2 causes acute activation of eNOS in cultured endothelial cells (within five minutes). The response is specific to E2 because 17α-estradiol had no effect. When the rapidity of the response is considered along with the observation that it was not altered by the inhibition of gene transcription with actinomycin D, this suggests that the process does not require the classical nuclear effects of the hormone. However, the acute response was fully inhibited by concomitant acute treatment with the ER antagonists tamoxifen and ICI 182,780, suggesting that this occurs via rapid ER activation. The specific level of E2 that was studied is readily achieved during pregnancy (39, 40), and we have previously shown that the response observed in this model is also evident at concentrations that are well below those found in normal cycling women (21, 41, 42). These cumulative observations are consistent with a novel, nongenomic physiologic role for ER in endothelial cells.
To specifically define the role of ERα in the rapid response to E2, overexpression studies were performed. Overexpression of ERα resulted in augmented estrogen-induced transcriptional transactivation as expected, and it had no effect on the level of basal eNOS activity or eNOS protein abundance in the intact cells. However, the acute response to E2 was augmented four- to fivefold in cells transfected with ERα compared with sham-transfected cells, and the enhanced rapid response was inhibited completely by concomitant acute treatment with ICI 182,780. These findings suggest that ERα is capable of mediating the acute response.
To determine the specificity of the augmentation in eNOS activity in response to E2, further experiments were done comparing the rapid effects of acetylcholine and E2 in PAEC-overexpressing ERα. With acetylcholine, there were comparable increases in eNOS activity in sham- and ERα-transfected cells, which contrasted with the augmented response to E2 after ERα transfection. As such, the enhanced acute response to E2 in the presence of increased ERα is specific to the hormone, and it is not due to changes in the expression or function of other components of the eNOS signaling cascade. In contrast to the findings with wild-type ERα overexpression, E2-mediated eNOS activity was not augmented in cells expressing a truncation mutant of ERα lacking the HBD. This is consistent with the inhibition of the acute eNOS response to E2 in native endothelial cells by tamoxifen and ICI 182,780, both of which cause ER antagonism via interaction with the ER HBD (10), supporting the conclusion that the HBD of ERα is required for the acute activation of eNOS by E2. Moreover, we have recently found that physiologic concentrations of E2 cause activation of eNOS in isolated plasma membranes from PAEC that is fully inhibited by ICI 182,780 and that ERα protein is readily detectable in the plasma membrane by immunoblot analysis (43). These observations suggest that a specific subpopulation of cell-surface receptors may be involved in the rapid, nongenomic response.
To generalize the findings in the native endothelial cells, experiments were performed to reconstitute the ERα-mediated nongenomic activation of eNOS in a cell type that does not constitutively express either ER or eNOS and that is not estrogen responsive. In COS-7 cells transfected with both eNOS and ERα, there was a more than threefold increase in eNOS activity upon acute stimulation with E2, whereas there was no response in cells transfected with eNOS alone. As in native endothelial cells, the rapid activation of eNOS by E2 in the COS-7 cells transfected with eNOS and ERα was completely inhibited by concomitant ICI 182,780 treatment, indicating that the response is mediated by acute ERα activation. Thus, the cotransfection of ERα into cells expressing only eNOS confers the ability of E2 to rapidly activate eNOS, confirming the role of ERα in this process.
Because E2 can cause the rapid activation of signaling pathways involving c-src–related tyrosine kinases and MAP kinases in nonendothelial cells (7, 8), the potential role of tyrosine kinase–MAP kinase signaling in acute ERα-mediated eNOS activation was evaluated. The tyrosine kinase inhibitors genistein and herbimycin A completely inhibited the response to E2. In addition, the specific MEK inhibitor PD98059 also fully negated the stimulatory effect of E2. Furthermore, E2 caused a rapid increase in MAP kinase activity in the PAEC, and this effect was prevented by both tamoxifen and ICI 182,780. These data indicate that the acute stimulation of eNOS by E2 and ERα entails the activation of tyrosine kinase/MAP kinase. It has been shown that calcium signaling in endothelial cells in response to agonists such as bradykinin involves tyrosine kinase/MAP kinase activation (44). We therefore postulate that the rapid stimulation of eNOS by E2 is due to an increase in intracellular calcium that is mediated by tyrosine kinase/MAP kinase activation.
There is strong evidence from both human and animal studies that estrogen is protective against vascular injury and atherosclerosis. This occurs both indirectly by an effect on lipoprotein metabolism and directly through effects on the vessel wall, including alterations in vascular cell gene expression, mediated at least in part by ERα acting as a ligand-activated transcription factor. However, the beneficial effects of estrogen also have an important rapid component in the vasculature, which includes the acute activation of endothelial NO production (10, 11, 16–19). The present observations indicate that ERα may mediate the latter process, revealing for the first time that this protein regulates physiologic responses in a nongenomic fashion independent of its known ability to control transcription. As such, our current conceptualization of steroid hormone receptor function may be too narrow.
Along with the implications regarding estrogen and vascular endothelial function, the present findings are important to the mechanisms underlying the rapid effects of estrogen in a variety of other cell types. For example, the effects of opioids on hypothalamic neurons are acutely blunted by E2, and this response is attenuated by ER antagonism (5). In addition, E2 rapidly inhibits acid production and alters cell shape in osteoclasts in a receptor-dependent manner (45). Similarly, there are rapid, nongenomic, receptor-mediated effects of the hormone that ultimately regulate the growth of oncogenic cells (7, 8). Thus, the acute activation of ERα is likely to be important in a myriad of cellular responses to estrogen. Further studies of the rapid activation of ERα in endothelium, using models such as the ERα knockout mouse, will enhance both our specific knowledge of the role of estrogen in the vasculature and in other tissues and our general understanding of the nongenomic functions of steroid hormone receptors.
Acknowledgments
We are indebted to Marilyn Dixon for preparing this manuscript. This work was supported by National Institutes of Health grants HL58888, HL53546, and HD30276 (to P.W. Shaul), HL30386 (to R.H. Karas), and HL56069 and HL59953 (to M.E. Mendelsohn). The project was supported, in part, by the Lowe Foundation and was done during the tenure of Established Investigatorships of the American Heart Association (P.W. Shaul and M.E. Mendelsohn).
References
- 1.Kumar V, et al. Functional domains of the human estrogen receptor. Cell. 1987;51:941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- 2.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carson-Jurica MA, Shrader WT, O’Malley BW. Steroid receptor family: structure and functions. Endocr Rev. 1990;11:201–220. doi: 10.1210/edrv-11-2-201. [DOI] [PubMed] [Google Scholar]
- 4.Wehling M. Specific nongenomic actions of steroid hormones. Annu Rev Physiol. 1997;59:365–393. doi: 10.1146/annurev.physiol.59.1.365. [DOI] [PubMed] [Google Scholar]
- 5.Lagrange AH, Ronnekleiv OK, Kelly MJ. Modulation of G protein coupled receptors by an estrogen receptor that activates protein kinase A. Mol Pharmacol. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- 6.Endoh H, et al. Rapid activation of MAP kinase by estrogen in the bone cell line. Biochem Biophys Res Commun. 1997;235:99–102. doi: 10.1006/bbrc.1997.6746. [DOI] [PubMed] [Google Scholar]
- 7.Migliaccio A, et al. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 8.Di Domenico M, Castoria G, Bilancio A, Migliacio A, Auricchio F. Estradiol activation of human colon carcinoma-derived Caco-2 cell growth. Cancer Res. 1996;56:4516–4521. [PubMed] [Google Scholar]
- 9.Meizel S, Turner KO. Progesterone acts at the plasma membrane of human sperm. Mol Cell Endocrinol. 1991;77:R1–R5. doi: 10.1016/0303-7207(91)90080-c. [DOI] [PubMed] [Google Scholar]
- 10.Mendelsohn ME, Karas RH. Estrogen and the blood vessel wall. Curr Opinion Cardiol. 1994;9:619–626. doi: 10.1097/00001573-199409000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. FASEB J. 1996;10:615–624. [PubMed] [Google Scholar]
- 12.Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. JAMA. 1991;265:1861–1867. [PubMed] [Google Scholar]
- 13.Grady D, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016–1037. doi: 10.7326/0003-4819-117-12-1016. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson JC, Crook D, Godsland IF, Collins P, Whitehead MI. Hormone replacement therapy and the cardiovascular system. Nonlipid effects. Drugs. 1994;47:35–41. doi: 10.2165/00003495-199400472-00007. [DOI] [PubMed] [Google Scholar]
- 15.Guetta V, Cannon RO., III Cardiovascular effects of estrogen and lipid-lowering therapies in postmenopausal women. Circulation. 1996;93:1928–1937. doi: 10.1161/01.cir.93.10.1928. [DOI] [PubMed] [Google Scholar]
- 16.Williams JK, Adams MR, Herrington DM, Clarkson TB. Short-term administration of estrogen and vascular responses of atherosclerotic coronary arteries. J Am Coll Cardiol. 1992;20:452–457. doi: 10.1016/0735-1097(92)90116-5. [DOI] [PubMed] [Google Scholar]
- 17.Reis SE, et al. Ethinyl estradiol acutely attenuates abnormal coronary vasomotor responses to acetylcholine in postmenopausal women. Circulation. 1994;89:52–60. doi: 10.1161/01.cir.89.1.52. [DOI] [PubMed] [Google Scholar]
- 18.Gilligan DM, Badar DM, Panza JA, Quyyumi AA, Cannon RO. Acute vascular effects of estrogen in postmenopausal women. Circulation. 1994;90:786–791. doi: 10.1161/01.cir.90.2.786. [DOI] [PubMed] [Google Scholar]
- 19.Guetta V, et al. The role of nitric oxide in coronary vascular effects of estrogen in postmenopausal women. Circulation. 1997;96:2795–2801. doi: 10.1161/01.cir.96.9.2795. [DOI] [PubMed] [Google Scholar]
- 20.Moncada S, Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 21.Lantin-Hermoso RL, et al. Estrogen acutely stimulates nitric oxide synthase activity in fetal pulmonary artery endothelium. Am J Physiol. 1997;273:L119–L126. doi: 10.1152/ajplung.1997.273.1.L119. [DOI] [PubMed] [Google Scholar]
- 22.Caulin-Glaser T, Watson CA, Bender JR. 17α-estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ Res. 1997;81:885–892. doi: 10.1161/01.res.81.5.885. [DOI] [PubMed] [Google Scholar]
- 23.Venkov CD, Rankin AB, Vaughn DE. Identification of authentic estrogen receptors in cultured endothelial cells. A potential mechanism for steroid hormone regulation of endothelial function. Circulation. 1996;94:727–733. doi: 10.1161/01.cir.94.4.727. [DOI] [PubMed] [Google Scholar]
- 24.Kim-Schulze S, et al. Expression of an estrogen receptor by human coronary artery and umbilical vein endothelial cells. Circulation. 1996;94:1402–1407. doi: 10.1161/01.cir.94.6.1402. [DOI] [PubMed] [Google Scholar]
- 25.Kuiper GGJM, Gustafsson J-Å. The novel estrogen receptor-β subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Lett. 1997;410:87–90. doi: 10.1016/s0014-5793(97)00413-4. [DOI] [PubMed] [Google Scholar]
- 26.Shaul PW, Wells LB. Oxygen modulates nitric oxide production selectively in fetal pulmonary endothelial cells. Am J Respir Cell Mol Biol. 1994;11:432–438. doi: 10.1165/ajrcmb.11.4.7522486. [DOI] [PubMed] [Google Scholar]
- 27.Davda RK, Chandler LJ, Crews FT, Guzman NJ. Ethanol enhances the endothelial nitric oxide synthase response to agonists. Hypertension. 1993;21:939–943. doi: 10.1161/01.hyp.21.6.939. [DOI] [PubMed] [Google Scholar]
- 28.Shaul PW, Farrar MA, Zellers TM. Oxygen modulates endothelium-derived relaxing factor production in fetal pulmonary arteries. Am J Physiol. 1992;262:H355–H364. doi: 10.1152/ajpheart.1992.262.2.H355. [DOI] [PubMed] [Google Scholar]
- 29.Guo JP, Murohara T, Buerke M, Scalia R, Lefer AM. Direct measurement of nitric oxide release from vascular endothelial cells. J Appl Physiol. 1996;81:774–779. doi: 10.1152/jappl.1996.81.2.774. [DOI] [PubMed] [Google Scholar]
- 30.Liu LH, et al. Defective endothelium-dependent relaxation of vascular smooth muscle and endothelial cell Ca2+ signaling in mice lacking sarco(endo)plasmic reticulum Ca2+-ATPase isoform 3. J Biol Chem. 1997;272:30538–30545. doi: 10.1074/jbc.272.48.30538. [DOI] [PubMed] [Google Scholar]
- 31.Weih MK, Weikert S, Freyer D, Dirnagl U. Chemiluminescence detection of nitric oxide production from rat cerebral cortical endothelial cells in culture. Brain Res Brain Res Protoc. 1998;2:175–182. doi: 10.1016/s1385-299x(97)00037-8. [DOI] [PubMed] [Google Scholar]
- 32.Jun, S.S., Chen, Z., Pace, M.C., and Shaul, P.W. Glucocorticoids downregulate cyclooxygenase-1 gene expression and prostacyclin synthesis in fetal pulmonary artery endothelium. Circ. Res. In press. [DOI] [PubMed]
- 33.Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–3873. [PubMed] [Google Scholar]
- 34.MacRitchie AN, et al. Estrogen upregulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ Res. 1997;81:355–362. doi: 10.1161/01.res.81.3.355. [DOI] [PubMed] [Google Scholar]
- 35.Ponglikitmongkol M, Green S, Chambon P. Genomic organization of the human estrogen receptor gene. EMBO J. 1988;7:3385–3388. doi: 10.1002/j.1460-2075.1988.tb03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaul PW, et al. Acylation targets endothelial nitric oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 37.Marsen TA, Simonson MS, Dunn MJ. Thrombin induces the preproendothelin-1 gene in endothelial cells by a protein tyrosine kinase-linked mechanism. Circ Res. 1995;76:987–995. doi: 10.1161/01.res.76.6.987. [DOI] [PubMed] [Google Scholar]
- 38.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 39.Turnbull AC, et al. Significant fall in progesterone and rise in oestradiol levels in human peripheral plasma before onset of labour. Lancet. 1974;1:101–103. doi: 10.1016/s0140-6736(74)92337-x. [DOI] [PubMed] [Google Scholar]
- 40.Cousins LM, Hobel CJ, Chang RJ, Okada DM, Marshall JR. Serum progesterone and estradiol-17beta levels in premature and term labor. Am J Obstet Gynecol. 1977;127:612–615. doi: 10.1016/0002-9378(77)90359-3. [DOI] [PubMed] [Google Scholar]
- 41.Rosselli M, Imthurn B, Macas E, Keller PJ, Dubuy RK. Circulating nitrite/nitrate levels increase with follicular development: indirect evidence for estradiol mediated NO release. Biochem Biophys Res Commun. 1994;202:1543–1552. doi: 10.1006/bbrc.1994.2107. [DOI] [PubMed] [Google Scholar]
- 42.Hashimoto M, et al. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation. 1995;92:3431–3435. doi: 10.1161/01.cir.92.12.3431. [DOI] [PubMed] [Google Scholar]
- 43.Wyckoff MH, Yuhanna IS, Pace MC, Mendelsohn ME, Shaul PW. Plasma membrane-associated estrogen receptors mediate the acute activation of eNOS by estrogen. Circulation. 1998;98:I312. (Abstr.) [Google Scholar]
- 44.Fleming I, Fisslthaler B, Busse R. Calcium signaling in endothelial cells involves activation of tyrosine kinases and leads to activation of mitogen-activated protein kinases. Circ Res. 1995;76:522–529. doi: 10.1161/01.res.76.4.522. [DOI] [PubMed] [Google Scholar]
- 45.Brubaker K, Gay CV. Specific binding of estrogen to osteoclast surface. Biochem Biophys Res Commun. 1994;200:899–907. doi: 10.1006/bbrc.1994.1535. [DOI] [PubMed] [Google Scholar]