Abstract
Noxious stimuli cause pain and pain arises from noxious stimuli… usually. Exceptions to these apparent truisms are the basis for clinically important problems and provide valuable insight into the neural code for pain. In this Perspective, we will discuss how painful sensations are encoded. We will argue that although primary somatosensory afferents are specialized (i.e. tuned to specific stimulus features), natural stimuli often activate >1 type of afferent. Manipulating co-activation patterns can alter perception, which argues against each type of afferent acting independently (as expected for strictly labeled lines) and suggests instead that signals conveyed by different types of afferents interact. Deciphering the central circuits that mediate those interactions is critical for explaining the generation and modulation of neural signals ultimately perceived as pain. The advent of genetic and optical dissection techniques promise to dramatically accelerate progress towards this goal, which will facilitate the rational design of future pain therapeutics.
Pain alerts us to danger. Failure of this alarm system has dire consequences; for example, patients with congenital insensitivity to pain often succumb to medical problems because those problems go unnoticed (and untreated) in the absence of pain1. But the converse problem – pain in the absence of noxious sensory input – is far more common and debilitating. Each condition illustrates a different way in which the normal relationship between noxious stimulation and pain perception can break down. This prompts some important questions: How are noxious stimuli normally encoded so as to produce pain? More importantly from a clinical perspective, how does coding goes awry so that pain is perceived in the absence of noxious stimuli?
Pain Theories
For decades, peripheral and central specificity have been the focus of intense debate (for reviews, see 2, 3). Both issues boil down to tuning: Are certain neurons tuned so that they respond specifically, or at least preferentially, to noxious input? Tuning in primary afferent neurons (PANs) depends on their receptor expression and their association with specialized structures like Merkel’s disks, Pacinian corpuscles, etc. Tuning in central neurons depends on their synaptic input: A central neuron that receives input exclusively from only one type of PAN necessarily has the same tuning as that PAN, whereas any direct or indirect (polysynaptic) input from other PANs is liable to confer more complex tuning (see Fig 1). Strictly speaking, a labeled line is formed only in the former case. Labeled line connectivity will therefore give equivalent pre- and postsynaptic tuning, but equivalent pre- and postsynaptic tuning does not necessarily imply labeled line connectivity although this is how such connectivity is usually inferred (see below). Today, most everyone would agree that some degree of specialization exists both peripherally and centrally, but specialization does not actually prove that neurons tuned to noxious input are necessary and sufficient to cause pain, or more generally, that differently tuned neurons are not involved.
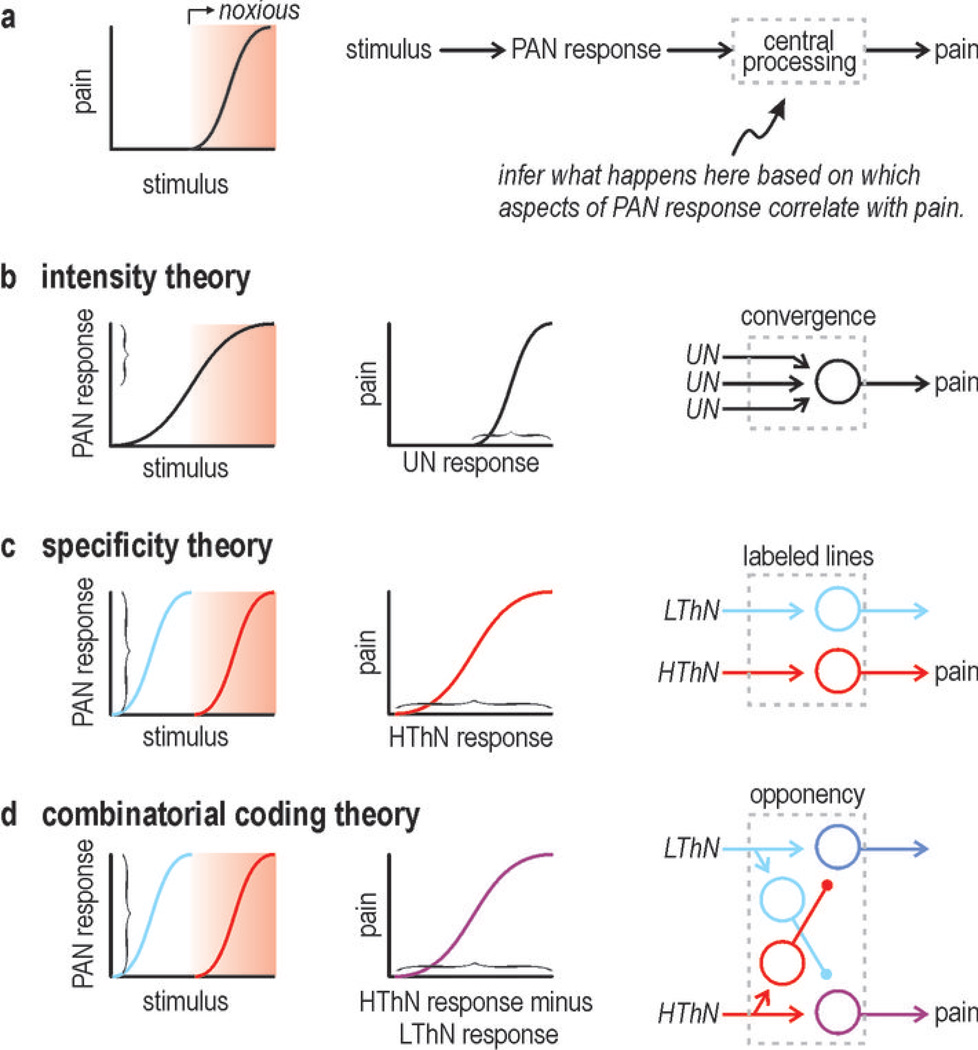
Figure 1. Inferring central processing steps.
(a) Psychometric curve shows typical relationship between noxious stimulation and pain. Adjacent flow chart shows intervening neural responses and processing steps that one could measure or deduce. (b) According to intensity theory, sufficiently strong activation of unspecialized neurons (UN) results in pain. (c) According to specificity theory, specialized high-threshold neurons (HThN) respond to noxious input and it is there activation that causes pain. (d) According to combinatorial coding theory, noxious stimulation activates HThN and their activation is involved in evoking pain, but the stimulus can also activate other PANs such as low-threshold neurons (LThN) and because the central pathways carrying these signals interact, pain will depend jointly on HThN and LThN activation levels. The nature of that joint dependence can take many forms; this example illustrates opponent processing.
Figure 1 illustrates the obvious normal psychometric relationship between stimulation and sensation (noxious stimulation → pain). It also shows how this relationship can be dissected into neurometric relationships that help identify what processing steps occur centrally. Pain theories, which essentially fall into three groups, predict differences in that processing.
Intensity theory holds that pain is elicited by strong activation of unspecialized PANs that converge onto central neurons. This theory has been ruled out by evidence for PAN specialization. Specificity theory holds that nociceptors are uniquely activated by noxious stimulation and that it is their activation that ultimately codes for pain; other PAN types respond to other stimuli and their activation is the basis for other percepts. The one-to-one relationship between stimulation and perception is consistent with signaling through labeled lines. Pattern theory holds that patterning of PAN activation forms the basis for any code. Gate control theory, which is a pattern-based theory, proposed that low- and high-threshold afferents converge on unspecialized central neurons and that sufficiently strong activation of those central neurons codes for pain; in this respect, the original gate control theory denied any form of central specialization, but other pattern-based theories do not and thus fall somewhere in between specificity theory and gate control theory.
Many different patterns are conceivable but evidence points toward PAN co-activation patterns forming the basis for what has been referred to as a population code4, 5 or, more specifically, a combinatorial code6. Combinatorial coding involves differential activation of different PAN types and, therefore, meaningful co-activation patterns cannot occur without PAN specialization. On the other hand, a combinatorial code cannot be decoded unless central neurons receive input from >1 type of co-activated PAN, which argues against labeled lines in the strictest sense (i.e. exclusive synaptic connectivity) but is not inconsistent with pre- and postsynaptic neurons being equivalently tuned under most conditions and thus often operating as if they were labeled lines under most conditions.
To summarize, specificity theory posits that perception depends on which one PAN subtype is activated and how much. Combinatorial coding posits that perception depends on what combination of PAN subtypes are activated and in what proportion. Both coding strategies therefore require PAN specialization, which, as an aside, does not exclude co-activation of different PANs: PAN specialization means that a neuron responds preferentially to a certain stimulus feature, not that a stimulus preferentially activates a certain type of PAN. Co-activation patterns can hold information beyond what is available from individual PAN activation levels but that extra information is irrelevant unless central circuits can decode it. This is where specificity theory and combinatorial coding theory disagree: Combinatorial coding requires some degree of interaction between otherwise labeled lines, or what has been called crosstalk5. Crosstalk is a design fault according to specificity theory whereas it is a necessary design feature according to combinatorial coding theory insofar as it enables information carried by co-activation patterns to be used. We will argue that this “design” has computational benefits and works so well as to be inapparent unless the system is tricked (as during an illusion) or perturbed (as in neuropathic pain conditions).
Comparison with other sensory systems
Before proceeding, it is helpful to compare the somatosensory system with other sensory systems as some of the concepts proposed here may seem foreign to the pain field but are well established in other fields.
Within the retina, cone photoreceptors are tuned to long, medium, or short wavelengths of light (roughly red, green, or blue) and yet we can perceive an entire rainbow of colors. We perceive this range of colors not based on which one type of photoreceptor is activated but, instead, based on the relative activation of differently tuned photoreceptors – this is referred to as trichromacy7. This “design” has numerous benefits. For one, only three variants of opsin are required and their tuning can be relatively broad; if color vision relied on labeled lines, many more variants with much narrower tuning would be required. In that scenario, spatial acuity would necessarily suffer from trying to pack more differently-tuned photoreceptors into the same surface area of retina. Instead, the retina implements opponent processing wherein downstream neurons receive convergent input from multiple photoreceptor types and calculate the ratio or difference in activation across those photoreceptors. This convergence is not indiscriminant; on the contrary, opponent cells receive certain patterns of excitation and inhibition that redefine color tuning along new, derivative dimensions. In addition, opponent processing helps disambiguate the color and intensity of light: Increased light intensity produces stronger absolute activation of all photoreceptors but the relative activation of differently tuned photoreceptors remains constant, thus preventing a change in light intensity from being misperceived as a change in color. Overall, this processing works well, but as revealed by numerous color illusions, the resulting percept is not always an accurate reflection of the initial stimulus8. Such illusions can be deliberately constructed or reverse engineered because of our thorough understanding of the stimulus space (i.e. how to quantify color) and retinal circuitry.
Within the olfactory system, olfactory receptor neurons typically express only one type of odorant receptor out of several hundred9 and all neurons expressing a certain receptor converge onto one or two glomeruli within the olfactory bulb10. On the surface, this constitutes amazing specificity. However, an odorant can bind to >1 receptor type and a receptor can bind to >1 odorant, meaning the odorant can only be identified on the basis of which combination of olfactory receptor neurons are activated11. In psychophysical terms, difficulty identifying component odorants within a complex olfactory stimulus argues in favor of configural odor perception as opposed to elemental odor perception12. The opposite would be expected if the olfactory system was organized according to labeled lines.
These observations suggest certain tests that we might apply to the pain system. For example, could a pain signal be synthesized from sensory inputs that are innocuous, akin to the color illusions mentioned above? Such illusions could be used to unmask aspects of processing that point toward one or another theory, as per the strategy explained in Figure 1. Similarly, might certain enigmatic features of neuropathic pain be explained by the disruption of such processing?
Burning pain without burning heat, and vice versa
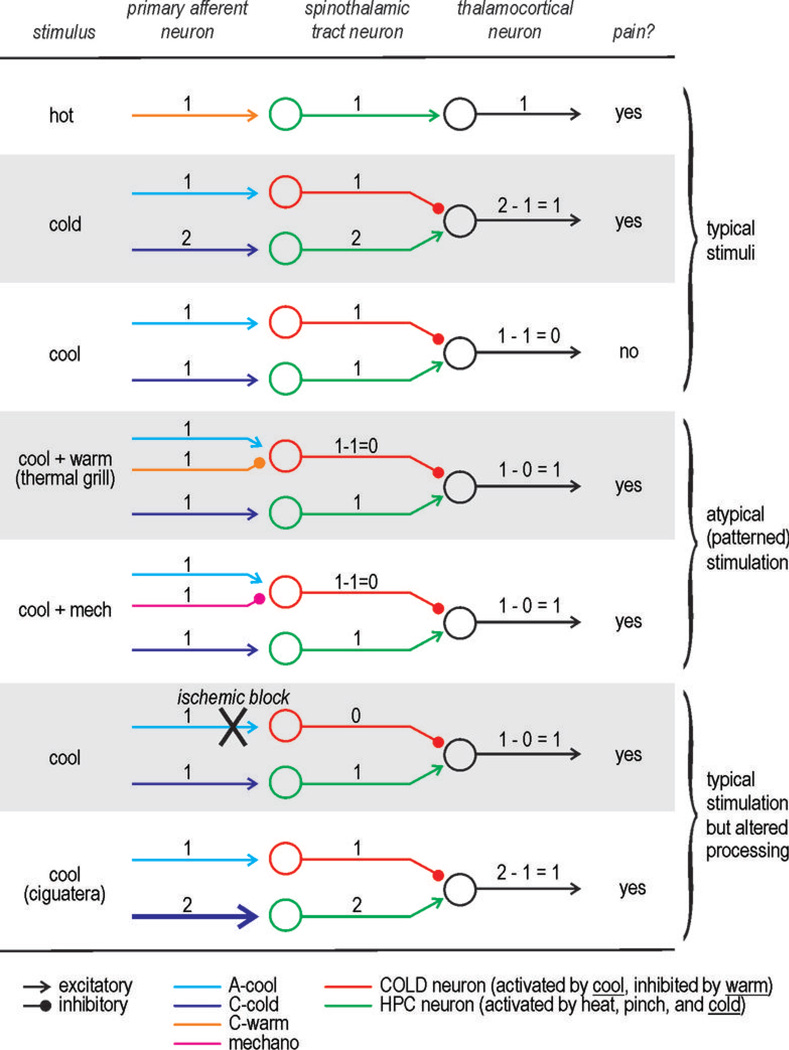
Burning pain can be evoked by many different stimuli (Fig. 2). Hot stimuli can obviously produce burning pain. A cold stimulus, such as immersing one’s hand in ice water, can also evoke a burning sensation. More surprisingly, warm and cool stimuli, neither of which are painful when experienced in isolation, can evoke burning pain when presented together in the right spatial pattern. This was discovered over a century ago and is known as Thunberg’s thermal grill illusion. The neural basis for this illusion has been shown to involve an opponent process13 not unlike that described above for the retina. In brief, the interleaved warm and cool stimuli co-activate respectively tuned PANs; moreover, the cool stimulus activates both Aδ fibers tuned to cool temperatures (A-cool fibers) as well C fibers tuned to cold temperatures but partially activated at cool temperatures (C-cold fibers). A-cool fibers inhibit C-cold fibers centrally and thereby prevent cool temperatures from being perceived as cold. But the same neurons activated by A-cool input are inhibited by input from warm-sensitive fibres (when the “correct” spatial patterning of warm and cool stimuli occurs), thereby disinhibiting the C-cold fibers and unmasking a burning sensation. Note that this phenomenon requires PANs that are differentially tuned to temperature and that those PANs form pathways that interact rather than remain independent. Related to this, ~20% of neuropathic pain patients (compared with ~2% of normal controls) experience paradoxical heat sensations when warming and cooling ramp stimulation is alternatively applied to a limb14; the likelihood of this phenomenon in normal subjects can be increased with carefully chosen stimulus parameters15. The mechanism for this remains uncertain but one might reasonably postulate that it involves the same sort of unmasking mechanism but now implemented through temporal patterning of stimulation rather than spatial patterning.
Figure 2. Diverse ways to produce burning pain.
Cartoon depicts known cell types at different levels of the neuraxis although local interneurons are omitted. Level of activation is represented numerically: 0 – no activation, 1 – modest activation, 2 – strong activation.
The sense of burning can also be modulated by the co-occurrence of mechanical stimulation. For instance, a cool stimulus was judged as 10× more burning when a thermode already applied to the skin was cooled (static condition) compared to when a pre-cooled thermode was applied to the skin (dynamic condition)16. It would appear that mechanically evoked inputs, if not already adapted, can mask the thermally evoked inputs sensed as burning17.
Beyond constructing stimuli designed to co-activate PANs in atypical patterns, there are other ways to make innocuous stimuli seem painfully hot that involve manipulating which PAN signals reach the spinal cord. Because A-cool fibers are myelinated whereas C-cold fibers are not, the former can be preferentially blocked by applying a blood pressure cuff around the limb. Applying a cool stimulus while preventing the signal carried by A-cool fibers from reaching the spinal cord leads to the same disinhibition caused by co-stimulation with warm, thereby unmasking burning pain18, 19. One may have experienced this phenomenon when washing numb hands (e.g. after coming in from the cold) in cool water.
Independent of any contrived stimulus pattern or other manipulation, innocuous cooling evokes intense burning pain in certain neuropathic pain syndromes. Cold allodynia is especially common in people suffering from ciguatera. This food borne illness is caused by ingestion of reef fish contaminated with certain toxins, most notably ciguatoxin20. Ciguatoxin is a sodium channel activator and could therefore be expected to cause hyperexcitability, but it causes neither mechanical allodynia nor heat allodynia21. For reasons that remain unclear, TRPA1-expressing PANs seem to be preferentially affected21. TRPA1 is expressed in only a subset of C fibers22 and so an increase in C-cold fiber excitability without a concomitant increase in A-cool fiber excitability could allow cool temperatures to evoke the same relative activity as cold temperatures, namely a C-cold fiber response that is too large to be masked by the A-cool fiber response.
Microneurographic studies in humans support the proposed interaction between C and A-delta fibers23. Furthermore, the co-occurrence of cold allodynia and cold hypoesthesia in neuropathic pain patients – a larger temperature drop is needed to detect the temperature change, but a smaller drop is needed to elicit pain – is consistent with altered inter-PAN interactions that could compromise masking24. Notably, alteration of detection thresholds and pain thresholds in opposite directions is common in neuropathic pain syndromes14 and may provide valuable insights into the pathophysiological process.
The examples described above have focused on burning pain in the absence of noxious thermal stimulation, but it is also possible for noxious heat to elicit sensations other than burning pain. For instance, in spinal cord injury patients, noxious hot and cold stimulation of skins areas without thermal sensibility evokes pricking pain25. Similarly, capsaicin can evoke burning or pricking pain depending on how it is applied to the skin26. These examples suggest that activating TRPV1-expressing C fibers is not sufficient to cause burning pain and, instead, that central processing steps involving crosstalk – wherein an output signal is constructed from >1 input signals – are the norm. Importantly, such processing is only apparent when something goes wrong with it.
Tactile allodynia: crosstalk between pathways rather than intra-pathway modulation
Mixing information across pathways such that innocuous stimuli evoke pain is not unique to thermoception. Another example of such crosstalk is illustrated by tactile allodynia characteristic of neuropathic pain. In both spinal and trigeminal lamina I relay pathways, the majority of neurons (>80%) are nociceptive specific27, 28 and they do not receive direct input from nonnociceptive PANs29. Yet after nerve injury, the majority respond to innocuous touch27. This can be replicated simply by impairing glycine- and/or GABAA-mediated inhibition (notably via disrupted Cl− homeostasis), thus showing that central disinhibition unmasks existing interconnections between separate sensory pathways27, 28. This crosstalk also appears to provide a substrate by which central inflammatory processes (e.g., microglial activation) can regulate pain processing by spinal neurons, by modulating the strength of Cl− mediated inhibition30.
Besides nociceptive specific (NS) neurons, the spinal dorsal horn (especially deeper laminae) also contains wide dynamic range (WDR) neurons that respond to both innocuous and noxious stimuli. Given that WDR neurons already receive some low-threshold input, they could contribute to allodynia on the basis of exaggerated low-threshold input, i.e. low-threshold input need not arise de novo from crosstalk as is required for NS neurons to contribute to allodynia. The mixed population of NS and WDR neurons that comprise the spinothalamic tract (STT) thus offers an opportunity to test whether crosstalk between specific pathways or plasticity within a non-specific pathway is responsible for allodynia. A recent quantitative neurometric study in rats showed that only normally NS-STT neurons exhibited altered response properties after nerve injury or pharmacological disinbibition; WDR-STT neurons exhibited no change in threshold or input-output profile (e.g., maximal response to noxious stimulation). Restoring central inhibition by rescuing KCC2 function31 restored the specificity of NS-STT neurons, thus indicating that disinhibition was both necessary and sufficient to explain the observed crosstalk and arguing that crosstalk was indeed responsible for the allodynia32,
The lack of plasticity in WDR-STT neurons is nevertheless surprising, but not unprecedented. In other systems, invariant neurons with inhibition-independent input-output curves have been described (e.g., visual system) and it has been speculated that they serve as strict encoder of intensity33. This arrangement may provide for another level of combination occurring at supraspinal levels wherein NS-STT neurons provide information about stimulus context whereas WDR-STT neurons provide information about stimulus intensity.
How to crack the neural code for pain
The above examples illustrate that certain PAN co-activation patterns, whether induced peripherally or misprocessed centrally, can lead to misperception of the stimulus. In many respects, this is consistent with configural rather than elemental sensation, to use the terminology applied to olfactory sensation. Furthermore, these observations argue against strictly labeled lines as well as totally convergent pathways, and instead suggest that pathways interact centrally.
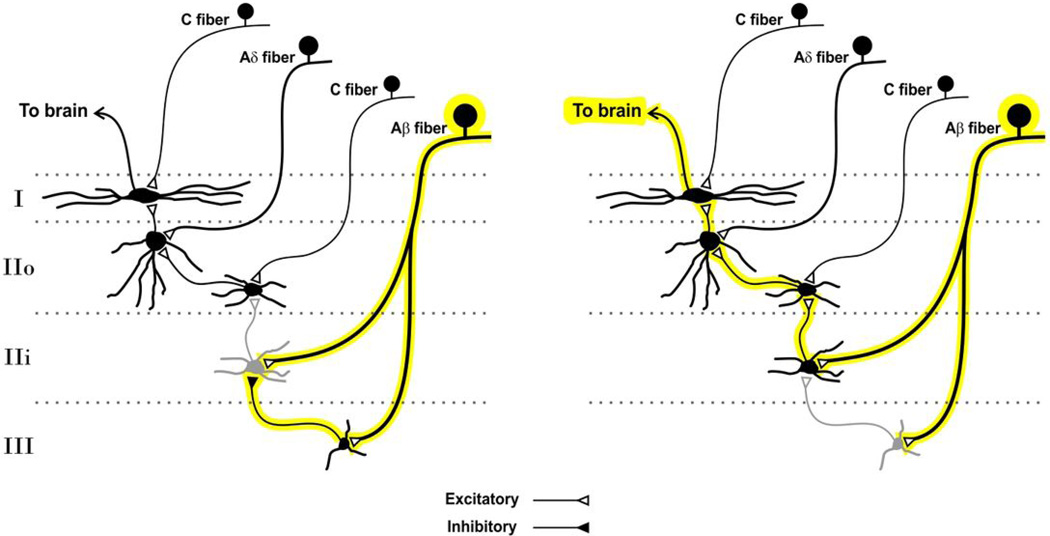
It follows that tackling pathological pain hinges on an appropriate grasp of the underlying microcircuitry. Yet, in general, our understanding of the functional organization of the dorsal horn remains much too incomplete. The best understood example is the microcircuitry involved in the crosstalk associated with tactile allodynia. Structurally speaking, spinal lamina I output neurons are organized not to receive direct input from low-threshold afferents, as their dendrites are restricted to lamina I whereas low-threshold afferents terminate in deeper laminae29. Retrograde trans-synaptic labeling revealed that, in contrast, the neurons directly presynaptic to lamina I projection neurons (stalked or ventral cells) have ventrally directed dendrites which enables them to sample input from deeper layers34. Several converging studies have identified a polysynaptic pathway, normally repressed by inhibition, linking low-threshold mechanosensitive afferents to lamina I projection neurons27, 28, 35–37. Direct identification of the different components of one such polysynaptic pathway was revealed by paired patch clamp recordings in spinal slices where a feedforward glycinergic interneuron represses the relay of innocuous input to lamina I by a PKCγ excitatory interneuron (Fig. 3)37.
Figure 3. Spinal microcicuitry underlying tactile allodynia associated with neuropathic pain.
Spinal lamina I output neurons do not receive direct input from low-threshold (Aβ) afferents. Yet a polysynaptic pathway indirectly links Aβ fibers to lamina I neurons. The link is normally repressed by inhibitory interneurons (left). After nerve injury, impaired inhibition unmasks the interconnection thus enabling low-threshold inputs to drive lamina I projection neurons (right).
These studies, along with morphological characterizations of specific subpopulations of interneurons and trans-synaptic tracing studies, provide clues to start unraveling the wiring relationships between different cells populations in the spinal dorsal horn. Classification schemes drawing correlation between morphology, transmitter phenotype, intrinsic membrane properties and some aspects of functional responses are also emerging (for review see 29), but remain incomplete and progress is relatively slow. Indeed, progress on this front in the dorsal horn has lagged behind compared to what has been achieved to date in other areas of the nervous system. This is likely due in part to the lack of local stereotypical organization of dorsal horn circuits, in contrast to cerebellar or cortical circuits for example, as well as challenges posed for functional studies of spinal dorsal horn (see below). What is also largely missing are quantitative means to measure and manipulate the inputs, the outputs, and the different subcomponents of the dorsal horn, in both normal and pathological conditions, in order to isolate the local transformation. For this, there is an urgent need to develop new tools to label, silence, activate and otherwise probe specific populations of dorsal horn neurons. Clearly, major efforts are necessary to enhance the throughput of such experiments. Fortunately, several technological advances promise to enable a significant acceleration of efforts on this front, as outlined below.
It should also be noted that the exploration of spinal microcircuits stands to benefit from the pre-existing knowledge of microcircuits in other brain areas. For instance, if we know or suspect that certain computations are performed, we should expect to find certain microcircuit motifs and not others6. Knowing what to look for can certainly help looking for it.
Developmental ontogeny of spinal neurons
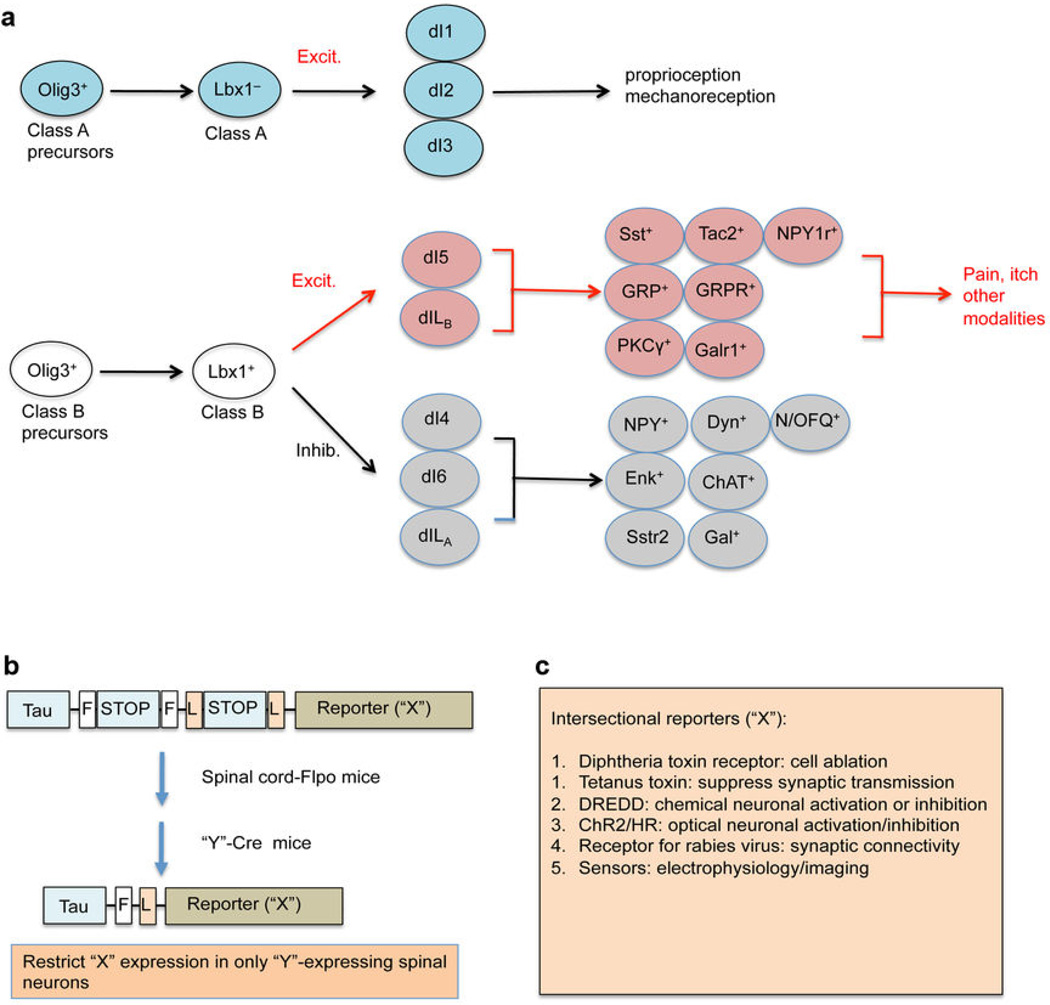
Perhaps more than in other systems (see above), the development of genetic tools to target components of the dorsal horn network is crucial for deciphering the microcircuitry. Towards this end, exploiting the ontogeny of dorsal horn neurons is liable to be key. The development of the spinal cord is subject to both spatial and temporal control, leading to modular and hierarchical organization of dorsal horn neurons (Fig. 4a). Within the dorsal neural tube, the neural precursors are divided into two classes, A and B. Class A precursors express the Olig3 transcription factor and their specification relies on signals from the roof plate38. During E10.5–E12.5, they produce three types of class A dorsal horn neurons (dI1, dI2 and dI3), each of which is defined by a unique combination of transcription factors39, 40. Olig3-negative Class B precursors are located in the ventral half of the dorsal neural tube and their specification is independent of roof plate signals. These precursors give rise to five groups of class B neurons, including dI4, dI5, and dI6 neurons formed during E10.5–E11.5, and dILA and dILB neurons formed during E11.5–E13.539–41. All class B neurons initially express the Lbx1 homeobox protein, thereby distinguishing them from class A neurons that lack Lbx139, 40. Regarding neurotransmitter phenotypes, class A neurons belong to glutamatergic excitatory neurons, and Class B neurons are divided into glutamatergic excitatory neurons (dI5 and dILB) marked by the expression of the homeobox proteins Lmx1b and Tlx3, and GABAergic/glycinergic inhibitory neurons (dI4, dI6, and dILA) marked by the expression of Ptf1a and Pax2 39, 40, 42. Both class B excitatory and inhibitory neurons can be further subdivided into molecularly distinct subtypes marked by the expression of peptides, transmitter receptors, and signaling molecules (summarized in Fig. 4a)43–49. Developmentally, Tlx3 acts as a selective gene that is required to specify most known features associated with class B excitatory neurons, including glutamatergic and peptidergic transmitters42, 44, 45. Conversely, Ptf1a and Pax2 specify glycinergic/GABAergic as well as peptidergic transmitter phenotypes in inhibitory neurons46, 47, 50, 51. Thus, the ontogeny of dorsal horn neurons shows a modular organization, as indicated by the division of class A versus class B neurons, and their further subdivision into various dIL subtypes. It also reveals a hierarchical developmental control, such that the same Tlx3 homeobox protein coordinates the development of a large cohort of class B excitatory neurons.
Figure 4. Developmental ontogeny and genetic dissection of spinal microcircuits.
(a) Ontogeny of spinal neurons. “Excit.”: excitatory neurons. “Inhib.”: inhibitory neurons. “Sst”: somatostatin; “Tac2”:tachykinin 2; “NPY1r”: neuropeptide Y receptor 1; “GRP”: gastrin-releasing peptide; “GRPR”: GRP receptor; “Galr1”: galanin receptor 1; “Dyn”: dynorphin; “N/OFQ”: nociceptin/orphanin FQ; “Enk”: enkephalin; “ChAT”: choline acetyl transferase; “Sstr2”: somatostatin receptor 2; “Gal”: galanin. (b) Schematic depicting the intersectional genetic manipulation. The top is the intersectional reporter mice: Tau-FSTOPF-LSTOPL-X. “F”: the Flpo recominase recognition sequence; “L”: the Cre recombinase recognition sequence. (c) A list of intersectional reporters. “DREDD”: “designer receptor exclusively activated by a designer drug”; “ChR2”: channelrhodopsin; “HR”: halorhodopsin.
The subdivision of class A versus class B excitatory neurons appears to be correlated with distinct sensory information processed by these neurons. Class A neurons migrate ventrally and settle in deep dorsal horn (laminae III–V), a region receiving inputs mainly from Aβ low threshold mechanoreceptors and Aα proprioceptors. Consistently, two class A neurons are involved with sensory-motor coordination, with dI1 neurons necessary for proprioception52 and dI3 neurons involved with hand grasp performance53. Class B excitatory neurons are enriched in laminae I and II, but also settle in deeper laminae41. Mice with developmental impairment of class B excitatory neurons, as recently created by a conditional knockout of Tlx3 in dI5 and dILB neurons, do not exhibit nocifensive behaviors evoked by a range of pain-related and itch-related stimuli48. Development of spinal neurons processing chronic mechanical versus chronic thermal pain might be differentially controlled by the TR4 transcription factor54. Notably, PKC-γ+ neurons, which link Aβ mechanoreceptors to lamina I pain output neurons to mediate injury induced mechanical allodynia, belong to Tlx3-dependent class B excitatory neurons48. Thus, while class A excitatory neurons process information related to touch and body positions, class B excitatory neurons are implicated in pain, itch and other modalities (Fig. 4a). The ontogeny of spinal excitatory processing innocuous cold or warm, however, remains unknown48. Meanwhile, several studies have revealed distinct roles of different class B inhibitory neurons. dI6 inhibitory neurons, whose development is dependent on the DMRT3 transcription factor, are required to generate specific locomotion patterns55. Bhlhb5-dependent inhibitory neurons is involved with itch inhibition, whose developmental impairment leads to itch sensitization and excessive spontaneous scratching56.
An intersectional genetic strategy for dissecting spinal microcircuits
Isolating sensory processing at the spinal level is complicated by the fact that many important molecular markers are not restricted to the dorsal horn. Based on developmental ontogeny shown in Fig. 4a, here we suggest how an intersectional genetic manipulation, pioneered by the Dymecki group57, can be used to label, silence, and activate these molecularly defined dorsal horn neurons, thereby opening new avenues by which to carefully dissect spinal microcircuits (Fig. 4b). The basic idea is to drive a gene (referred to as “X”) into a specific population of spinal neurons distinguished by their expression of a specific gene (referred to as “Y”). The intersectional genetic manipulation is designed to selectively introduce “X” into “Y”-expressing neurons located in the dorsal spinal cord or in the hindbrain, but not into “Y”-expressing neurons located in other parts of the nervous system.
To achieve this, we need three sets of mouse lines (Fig. 4b). The first one is the intersectional reporter mouse lines, such as TauLSL-FSF-X, which drives the “X” reporter from the pan-neuronal Tau promoter. However, “X” expression is activated only after removal of two stop cassettes (“L-STOP-L” and “F-STOP-F”) mediated by the Cre and Flpo DNA recombinases. The second set includes Flpo mice, in which Flpo will be driven by a gene selectively expressed in the dorsal spinal cord. As described in Fig. 4a, Lbx1 is expressed in spinal class B excitatory neurons processing pain and itch as well as all of inhibitory neurons in the dorsal horn. Importantly, Lbx1 is not expressed in peripheral neurons, cerebellum, midbrain or forebrain39, 40. Thus, Lbx1 could be a candidate for driving Flpo expression in the dorsal spinal cord (and dorsal hindbrain). The third set is the Cre lines that drive reporter expression in specific subsets of dorsal spinal neurons, such as those distinguishing class B excitatory or inhibitory neuron subtypes (Fig. 4a). Many such Cre lines have are already been made by GENSAT (http://www.gensat.org/CrePipeline.jsp). By crossing these Cre lines with Lbx1FlpO mice and TauLSL-FSF-X mice, one will be able to specifically label and manipulate specific subsets of dorsal spinal/hindbrain neurons, without affecting any other parts of the nervous system.
Selective manipulation of spinal components
Based on the nature of gene “X” and in which specific cell type it is expressed, spinal microcircuits can be analyzed or manipulated in different ways (Fig. 4c). For example, neurons can be ablated or silenced chemically in numerous ways: the human diphtheria toxin (DTX) receptor (DTR) enables neuronal ablation upon DTX injection58, 59, the tetanus toxin (Toc) enables silencing of synaptic transmission60, and the G-protein coupled receptor Di, a DREADD, enables suppression of action potential firing by injection of the ligand clozapine-N-oxide (CNO)61, 62. Experiments might include ablating or silencing (i) a subpopulation of output neurons to assess their contribution to specific subtypes of pain, (ii) a subpopulation of excitatory interneurons to test their involvement in mediating crosstalk between certain pathways, or (iii) a subpopulation of inhibitory interneurons to test whether this unmasks normally silenced interconnections. Intersectional reporters can also be used for trans-synaptic tracing to map presynaptic inputs and postsynaptic outputs, such as the molecules used for rabies virus infection and monosynaptic retrograde labeling63. They can also be used to monitor and manipulate neuronal activity on a much finer timescale using optical methods (see below).
Non-genetic approaches to dissect components of the circuitry have also been used successfully. Most notable is the use of saporin-congugated peptides to ablate spinal neurons expressing peptide receptors (Fig. 5). For example, ablation of neurons which express the GRP or Nppb receptors with intrathecal injection of their agonists conjugated with saporin toxin abolishes scratching responses evoked by a range of itch-provoking compounds64. This has been combined with ablation of neurons expressing the NK1 receptor with substance P-saporin injections in an effort to understand the respective contribution of these two systems to pain and itch65, 66. One advantage of such non-genetic, toxin-based ablation approach is that it is more amenable to use in species for which transgenic models are not readily available; on the other hand, a single receptor phenotype is often not sufficient to target only one subpopulation of cells.
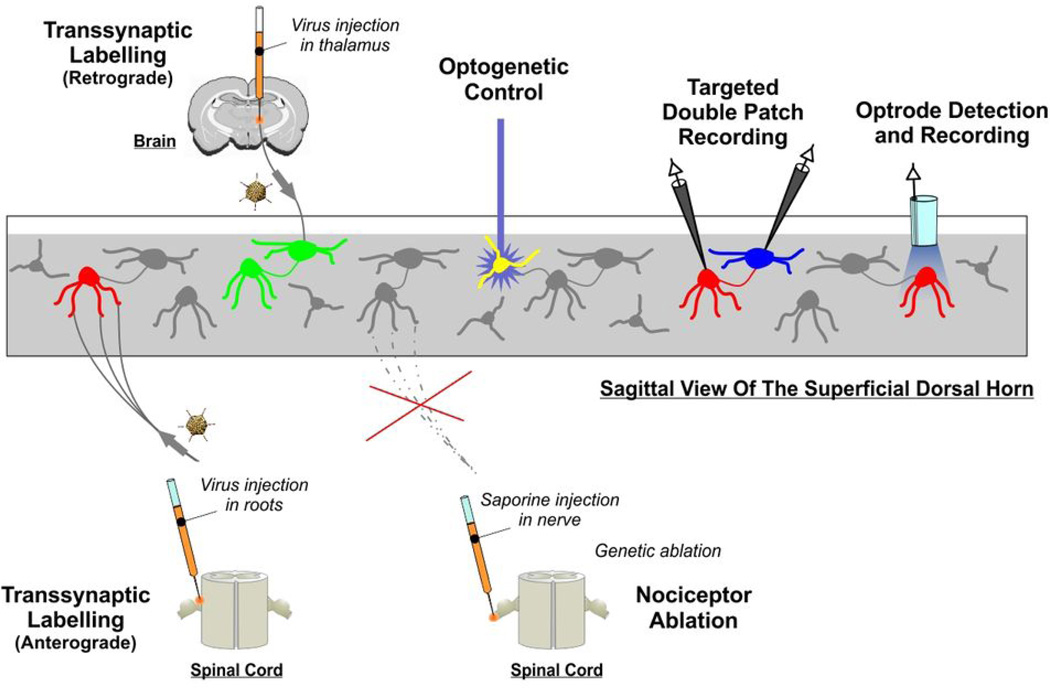
Figure 5. Technological arsenal to manipulate spinal microcircuitry.
Diagrammatic summary of the different techniques presented in the Perspective that can be exploited to decipher microcircuit structure and function within the dorsal horn.
The optical revolution
Many of the classical techniques such as tract-tracing, ultrastructural analysis of synaptic contact and electrophysiology remain essential to a proper dissection of the neural circuits. Electrophysiological approaches, for example, are not only key to demonstrate that interconnections are functional, but also to delve into the functional dynamics and plasticity of the synaptic connections (e.g. 67, 68). What the optogenetic revolution (which in the broadest sense involves both observing and controlling with light) offers in addition is the possibility, on the one hand, to dramatically enhance the throughput of microcircuit dissection, enabling the activity of thousands of cells to be monitored simultaneously69. Combined with the development of multispectral activity sensors, one can envisage deciphering the interaction between separate populations of cells or even separate signaling mechanism concurrently70. On the other hand, optogenetics also offers the possibility to scale across preparations such that the same cellular and molecular processes studied in isolated ex vivo preparations can be equivalently tracked and manipulated in intact preparations.
As an adjunct to electrophysiology, for example, optogenetics enables targeted patch clamp recordings in slices with dramatically enhanced throughput by allowing visual identification of subpopulation of neurons, especially when these represent a small minority of the overall population71, 72. But the ability to combine single-cell electrophysiology with single-cell optical sensing and activation in vivo using novel micro-optrodes will be particularly instrumental to determine the role of specific subpopulation of cells within the intact circuit and in contextual environment73. Another example stems from selective optogenetic activation of pathways, which can allow comparison of synaptic plasticity in slices with sensitization at the behavioral level via activation of the exact same input with the same temporal characteristics 74.
Challenges posed by spinal microcircuits
The high level of parallelization enabled by all-optical approaches is particularly powerful for dissection of microcircuits in the intact brain. It allows one to not only identify correlations between activity in different brain areas, but also to perform concurrent microstimulation with unprecedented spatial and temporal precision. These capabilities will be instrumental for identifying the computations performed by spinal microcircuits. Application of these approaches to the spinal cord or brainstem present several challenges however, including movement correction and light penetration into tissue. The spinal cord remains more prone to movement than many brain preparations, even in anesthetized animals. High performance adaptive movement compensation is a promising avenue on this front75. More challenging is the high level of myelination, as myelin is particularly bad for scattering light76, which dramatically degrades our ability to perform high resolution imaging in deep tissue, i.e. beyond the first 200 µm75. Microendoscopy is a promising avenue but, based on its invasiveness, has limited applicability to mouse spinal cord77. But notably, high resolution imaging is not always essential if sensors and actuators are targeted to specific cell populations, and even to specific subcellular compartments70; consequently, diffuse optical techniques may circumvent certain limitations78. Progress on this front will hinge on the further development of fiber optics technologies. In any case, cracking the neural code for pain will not be achieved on the basis of any one approach, and will instead involve several complementary approaches. In this context, optical methods are particularly attractive because they are easily adaptable at relatively low costs and are amenable to combination with other techniques, thus enabling multimodal interrogation of spinal tissue.
Conclusion and outlook
Intense basic research efforts have not yet translated into clinical breakthroughs when it comes to treating neuropathic pain. Should efforts simply be redoubled, or should we focus on identifying and closing the biggest gaps in our current understanding? The microcircuitry involved in pain processing represents a huge gap, especially when one compares against advances made in other sensory systems. If, as we have argued, the neural code for pain is a combinatorial code, then spinal microcircuits will be crucial for decoding the PAN co-activation patterns that carry important information. Altering co-activation patterns or altering how they are decoded (i.e. by altering microcircuit function) is liable to alter the output signal and that, in turn, will alter how the original stimulus is perceived. That is ultimately the problem with neuropathic pain – that pain is perceived in response to the wrong stimuli, or without any stimulus at all. And so deciphering the neural code for pain is not an esoteric endeavor; on the contrary, such efforts may be crucial for capitalizing on the molecular knowledge and know-how that has amassed in recent years to achieve translational breakthroughs that have been so frustratingly elusive.
But now, more than ever before, molecular breakthroughs have enabled experiments that were unimaginable a mere decade ago. Using techniques discussed in this Perspective, one can visualize, ablate, reversibly silence, or activate select subsets of neurons. In some cases, these new techniques can be used to facilitate classical techniques such as paired recording. In other cases, these new techniques can replace older techniques. But as experiments get more sophisticated and data more difficult to interpret, so too will it be important to capitalize on computer modeling to make sense of it all. Furthermore, it would be grossly naïve to think that pain processing occurs entirely within the spinal dorsal horn; on the contrary, such processing occurs throughout the neuraxis and things are liable to get increasingly complicated the deeper into the system we get. But these are exactly the issues that we must come to terms with in order to understand how painful sensations are normally encoded, and how that coding goes awry in neuropathic conditions.
Acknowledgement
This work was supported by NIH grants R01NS047710, P01 NS0272040 and R01NS086372 to Q.M., NIH grants R01 NS076706 and R21 NS074146 and a New Investigator Award from the Canadian Institutes of Health Research (CIHR) to S.A.P, and CIHR grant MOP 12942 to Y.D.K. and the CIHR Neurophysics program. The idea of using intersectional genetic manipulations to dissect spinal pain circuits is jointly developed by Dr. Martyn Goulding at the Salk Institute and by Q.M. We thank Sylvain Côté for expert assistance with artwork.
References
- 1.Baxter DW, Olszewski J. Congenital universal insensitivity to pain. Brain. 1960;83:381–393. doi: 10.1093/brain/83.3.381. [DOI] [PubMed] [Google Scholar]
- 2.Bonica JJ. History of pain concepts and therapies. In: Bonica JJ, editor. The Management of Pain. Philadelphia: Lea & Febiger; 1990. pp. 2–17. [Google Scholar]
- 3.Perl ER. Ideas about pain, a historical view. Nat Rev Neurosci. 2007;8:71–80. doi: 10.1038/nrn2042. [DOI] [PubMed] [Google Scholar]
- 4.Ma Q. Population coding of somatic sensations. Neurosci Bull. 2012;28:91–99. doi: 10.1007/s12264-012-1201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma Q. Labeled lines meet and talk: population coding of somatic sensations. J Clin Invest. 2010;120:3773–3778. doi: 10.1172/JCI43426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prescott SA, Ratté S. Pain processing by spinal microcircuits: afferent combinatorics. Curr Opin Neurobiol. 2012;22:631–639. doi: 10.1016/j.conb.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon SG, Lennie P. The machinery of colour vision. Nat Rev Neurosci. 2007;8:276–286. doi: 10.1038/nrn2094. [DOI] [PubMed] [Google Scholar]
- 8.Conway BR. Color vision, cones, and color-coding in the cortex. Neuroscientist. 2009;15:274–290. doi: 10.1177/1073858408331369. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan SL, Ressler KJ, Buck LB. Odorant receptor diversity and patterned gene expression in the mammalian olfactory epithelium. Prog Clin Biol Res. 1994;390:75–84. [PubMed] [Google Scholar]
- 10.Vassar R, et al. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 11.Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- 12.Gottfried JA. Central mechanisms of odour object perception. Nat Rev Neurosci. 2010;11:628–641. doi: 10.1038/nrn2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig AD, Bushnell MC. The thermal grill illusion: unmasking the burn of cold pain. Science. 1994;265:252–255. doi: 10.1126/science.8023144. [DOI] [PubMed] [Google Scholar]
- 14.Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150:439–450. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Hamalainen H, Vartiainen M, Karvanen L, Jarvilehto T. Paradoxical heat sensations during moderate cooling of the skin. Brain Res. 1982;251:77–81. doi: 10.1016/0006-8993(82)91275-6. [DOI] [PubMed] [Google Scholar]
- 16.Green BG, Pope JV. Innocuous cooling can produce nociceptive sensations that are inhibited during dynamic mechanical contact. Exp Brain Res. 2003;148:290–299. doi: 10.1007/s00221-002-1280-9. [DOI] [PubMed] [Google Scholar]
- 17.Green BG, Schoen KL. Evidence that tactile stimulation inhibits nociceptive sensations produced by innocuous contact cooling. Behav Brain Res. 2005;162:90–98. doi: 10.1016/j.bbr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Yarnitsky D, Ochoa JL. Release of cold-induced burning pain by block of cold-specific afferent input. Brain. 1990;113:893–902. doi: 10.1093/brain/113.4.893. [DOI] [PubMed] [Google Scholar]
- 19.Mackenzie RA, Burke D, Skuse NF, Lethlean AK. Fibre function and perception during cutaneous nerve block. J Neurol Neurosurg Psychiatry. 1975;38:865–873. doi: 10.1136/jnnp.38.9.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isbister GK, Kiernan MC. Neurotoxic marine poisoning. Lancet Neurol. 2005;4:219–228. doi: 10.1016/S1474-4422(05)70041-7. [DOI] [PubMed] [Google Scholar]
- 21.Vetter I, et al. Ciguatoxins activate specific cold pain pathways to elicit burning pain from cooling. EMBO J. 2012;31:3795–3808. doi: 10.1038/emboj.2012.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi K, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 23.Campero M, Baumann TK, Bostock H, Ochoa JL. Human cutaneous C fibres activated by cooling, heating and menthol. J Physiol. 2009;587:5633–5652. doi: 10.1113/jphysiol.2009.176040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochoa JL, Yarnitsky D. The triple cold syndrome. Cold hyperalgesia, cold hypoaesthesia and cold skin in peripheral nerve disease. Brain. 1994;117:185–197. doi: 10.1093/brain/117.1.185. [DOI] [PubMed] [Google Scholar]
- 25.Defrin R, Ohry A, Blumen N, Urca G. Sensory determinants of thermal pain. Brain. 2002;125:501–510. doi: 10.1093/brain/awf055. [DOI] [PubMed] [Google Scholar]
- 26.Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66–75. doi: 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keller AF, Beggs S, Salter MW, De Koninck Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol Pain. 2007;3:27. doi: 10.1186/1744-8069-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miraucourt LS, Dallel R, Voisin DL. Glycine inhibitory dysfunction turns touch into pain through PKCgamma interneurons. PLoS One. 2007;2:e1116. doi: 10.1371/journal.pone.0001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribeiro-da-Silva A, De Koninck Y. Morphological and Neurochemical Organization of the Spinal Dorsal Horn. In: Basbaum AI, Bushnell MC, editors. The Science of Pain. New York: Academic Press; 2008. pp. 279–310. [Google Scholar]
- 30.Ferrini F, De Koninck Y. Microglia Control Neuronal Network Excitability via BDNF Signalling. Neural Plast. 2013;2013:429815. doi: 10.1155/2013/429815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gagnon M, et al. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat Med. doi: 10.1038/nm.3356. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavertu G, Côté S, De Koninck Y. Enhancing K + -Cl − cotransport restores normal encoding within the spinothalamic tract in a model of neuropathic pain. Brain. doi: 10.1093/brain/awt334. In press. [DOI] [PubMed] [Google Scholar]
- 33.Kayama Y, Riso RR, Bartlett JR, Doty RW. Luxotonic responses of units in macaque striate cortex. J Neurophysiol. 1979;42:1495–1517. doi: 10.1152/jn.1979.42.6.1495. [DOI] [PubMed] [Google Scholar]
- 34.Cordero-Erausquin M, et al. Dorsal horn neurons presynaptic to lamina I spinoparabrachial neurons revealed by transynaptic labeling. J Comp Neurol. 2009;517:601–615. doi: 10.1002/cne.22179. [DOI] [PubMed] [Google Scholar]
- 35.Baba H, et al. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24:818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- 36.Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2006;26:1833–1843. doi: 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu Y, et al. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest. 2013;123:4050–4062. doi: 10.1172/JCI70026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muller T, et al. The bHLH factor Olig3 coordinates the specification of dorsal neurons in the spinal cord. Genes Dev. 2005;19:733–743. doi: 10.1101/gad.326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gross MK, Dottori M, Goulding M. Lbx1 specifies somatosensory association interneurons in the dorsal spinal cord. Neuron. 2002;34:535–549. doi: 10.1016/s0896-6273(02)00690-6. [DOI] [PubMed] [Google Scholar]
- 40.Müller T, et al. The homeodomain factor Lbx1 distinguishes two major programs of neuronal differentiation in the dorsal spinal cord. Neuron. 2002;34:551–562. doi: 10.1016/s0896-6273(02)00689-x. [DOI] [PubMed] [Google Scholar]
- 41.Helms AW, Johnson JE. Specification of dorsal spinal cord interneurons. Curr Opin Neurobiol. 2003;13:42–49. doi: 10.1016/s0959-4388(03)00010-2. [DOI] [PubMed] [Google Scholar]
- 42.Cheng L, et al. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat Neurosci. 2004;7:510–517. doi: 10.1038/nn1221. [DOI] [PubMed] [Google Scholar]
- 43.Todd AJ, Spike RC. The localization of classical transmitters and neuropeptides within neurons in laminae I–III of the mammalian spinal dorsal horn. Prog Neurobiol. 1993;41:609–645. doi: 10.1016/0301-0082(93)90045-t. [DOI] [PubMed] [Google Scholar]
- 44.Xu Y, et al. Tlx1 and Tlx3 coordinate specification of dorsal horn pain-modulatory peptidergic neurons. J Neurosci. 2008;28:4037–4046. doi: 10.1523/JNEUROSCI.4126-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo Z, et al. Tlx1/3 and Ptf1a control the expression of distinct sets of transmitter and peptide receptor genes in the developing dorsal spinal cord. J Neurosci. 2012;32:8509–8520. doi: 10.1523/JNEUROSCI.6301-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang M, et al. Ptf1a, Lbx1 and Pax2 coordinate glycinergic and peptidergic transmitter phenotypes in dorsal spinal inhibitory neurons. Dev Biol. 2008;322:394–405. doi: 10.1016/j.ydbio.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 47.Bröhl D, et al. A transcriptional network coordinately determines transmitter and peptidergic fate in the dorsal spinal cord. Dev Biol. 2008;322:381–393. doi: 10.1016/j.ydbio.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Xu Y, et al. Ontogeny of excitatory spinal neurons processing distinct somatic sensory modalities. J Neurosci. 2013;33:14738–14748. doi: 10.1523/JNEUROSCI.5512-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polgár E, et al. Functional differences between neurochemically defined populations of inhibitory interneurons in the rat spinal dorsal horn. Pain. 2013 doi: 10.1016/j.pain.2013.05.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng L, et al. Lbx1 and Tlx3 are opposing switches in determining GABAergic versus glutamatergic transmitter phenotypes. Nat Neurosci. 2005;8:1510–1515. doi: 10.1038/nn1569. [DOI] [PubMed] [Google Scholar]
- 51.Glasgow SM, Henke RM, Macdonald RJ, Wright CV, Johnson JE. Ptf1a determines GABAergic over glutamatergic neuronal cell fate in the spinal cord dorsal horn. Development. 2005;132:5461–5469. doi: 10.1242/dev.02167. [DOI] [PubMed] [Google Scholar]
- 52.Bermingham NA, et al. Proprioceptor pathway development is dependent on Math1. Neuron. 2001;30:411–422. doi: 10.1016/s0896-6273(01)00305-1. [DOI] [PubMed] [Google Scholar]
- 53.Bui TV, et al. Circuits for grasping: spinal dI3 interneurons mediate cutaneous control of motor behavior. Neuron. 2013;78:191–204. doi: 10.1016/j.neuron.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, et al. Excitatory superficial dorsal horn interneurons are functionally heterogeneous and required for the full behavioral expression of pain and itch. Neuron. 2013;78:312–324. doi: 10.1016/j.neuron.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andersson LS, et al. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature. 2012;488:642–646. doi: 10.1038/nature11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ross SE, et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:65. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dymecki SM, Kim JC. Molecular neuroanatomy's "Three Gs": a primer. Neuron. 2007;54:17–34. doi: 10.1016/j.neuron.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saito M, et al. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol. 2001;19:746–750. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- 59.Buch T, et al. A Cre inducible diphtheria toxin receptor mediates cell lineage ablation alter toxin administration. Nat Methods. 2005;2:419–426. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- 60.Kim JC, et al. Linking genetically defined neurons to behavior through a broadly applicable silencing allele. Neuron. 2009;63:305–315. doi: 10.1016/j.neuron.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5638. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ray RS, et al. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wickersham IR, et al. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun YG, et al. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mantyh PW, et al. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- 66.Carstens EE, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport. 2010;21:303–308. doi: 10.1097/WNR.0b013e328337310a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu Y, Perl ER. Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II) J Neurosci. 2005;25:3900–3907. doi: 10.1523/JNEUROSCI.0102-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Labrakakis C, Lorenzo LE, Bories C, Ribeiro-da-Silva A, De Koninck Y. Inhibitory coupling between inhibitory interneurons in the spinal cord dorsal horn. Mol Pain. 2009;5:24. doi: 10.1186/1744-8069-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahrens MB, Orger MB, Robson DN, Li JM, Keller PJ. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat Methods. 2013;10:413–420. doi: 10.1038/nmeth.2434. [DOI] [PubMed] [Google Scholar]
- 70.Zhao Y, et al. An expanded palette of genetically encoded Ca(2)(+) indicators. Science. 2011;333:1888–1891. doi: 10.1126/science.1208592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci. 2003;23:8752–8758. doi: 10.1523/JNEUROSCI.23-25-08752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mesnage B, et al. Morphological and functional characterization of cholinergic interneurons in the dorsal horn of the mouse spinal cord. J Comp Neurol. 2011;519:3139–3158. doi: 10.1002/cne.22668. [DOI] [PubMed] [Google Scholar]
- 73.LeChasseur Y, et al. A microprobe for parallel optical and electrical recordings from single neurons in vivo. Nat Methods. 2011;8:319–325. doi: 10.1038/nmeth.1572. [DOI] [PubMed] [Google Scholar]
- 74.Daou I, et al. Remote optogenetic activation and sensitization of pain pathways in freely moving mice. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.2424-13.2013. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laffray S, et al. Adaptive movement compensation for in vivo imaging of fast cellular dynamics within a moving tissue. PLoS ONE. 2011;6:e19928. doi: 10.1371/journal.pone.0019928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Belanger E, et al. Live animal myelin histomorphometry of the spinal cord with video-rate multimodal nonlinear microendoscopy. J Biomed Opt. 2012;17:021107. doi: 10.1117/1.JBO.17.2.021107. [DOI] [PubMed] [Google Scholar]
- 77.Ghosh KK, et al. Miniaturized integration of a fluorescence microscope. Nat Methods. 2011;8:871–878. doi: 10.1038/nmeth.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ouakli N, Guevara E, Dubeau S, Beaumont E, Lesage F. Laminar optical tomography of the hemodynamic response in the lumbar spinal cord of rats. Opt Express. 2010;18:10068–10077. doi: 10.1364/OE.18.010068. [DOI] [PubMed] [Google Scholar]