Abstract
a. Objective
The objectives of this study were to develop a preclinical rodent model that produces migraine-like behaviors based on International Headache Society diagnostic criteria, to determine whether sex differences are present, and to determine whether expression of CGRP and the genes encoding its receptor in trigeminal ganglion or medulla correlates with those behaviors.
b. Background
Few animal studies of migraine have tested behaviors associated with migraine diagnostic criteria. In this study, changes in activity and in mechanical sensitivity of facial regions following application of inflammatory soup (IS) or vehicle (PBS) to the dura were measured to model changes in routine activity and allodynia. Calcitonin gene related peptide (CGRP), an important mediator of migraine pathogenesis, and the three components of its receptor, calcitonin-like receptor (CLR), receptor activity-modifying protein (RAMP1), and receptor component protein (RCP) mRNAs were quantified in the trigeminal ganglion and medulla to identify baseline sex differences and changes associated with application of IS or PBS to the dura.
c. Methods
Male and female Sprague-Dawley rats were implanted with a dural cannula. Groups of rats were treated with 10 or 20 microliter volumes of IS or PBS. Baseline behavioral testing was conducted prior to surgery and again at 7 days postsurgery, and dural application of IS or PBS was performed repeatedly for a total of 8 applications. Locomotor activity was assessed using force plate actimetry during and following application to provide information on distance traveled, bouts of low mobility, spatial confinement, and focused energy. Periorbital and perimasseter sensory testing was performed 20 min post-application to measure allodynia. The rats were sacrificed 30 minutes following the final dural treatment, tissue was dissected and total RNAs were isolated from ipsilateral trigeminal ganglia and ipsilateral medulla. Quantitative real-time polymerase chain reactions were used to measure the expression of amplified constructs using gene-specific primers for CGRP, RAMP1, CLR, and RCP.
d. Results
Both males and females showed behavioral effects of IS application, but there were pronounced sex differences. Females showed effects at the lower dose, and activity changes were present for a longer duration, but males required fewer applications of IS to exhibit behavioral changes. Females showed increased withdrawal responses for periorbital and perimasseter mechanical testing (10 µl IS groups), and males showed increased perimasseter withdrawal responses (20 µl IS group). In the trigeminal ganglion, there were no baseline sex differences in CGRP-encoding mRNA, but females had lower baseline expression of RAMP1, CLR, and RCP-encoding mRNAs. In the medulla, females had higher baseline levels of CGRP-encoding mRNAs and lower baseline levels of RAMP1, CLR, and RCP-encoding mRNAs than males. Both IS and PBS increased expression of mRNAs encoding CGRP, RAMP1, RCP and CLR in the trigeminal ganglion in males, but in females, only CLR and RCP were increased. In the medulla both IS and PBS increased expression of CGRP, CLR in males and CLR and RCP in females. Thus, expression of CGRP related genes did not mirror the behavioral differences between IS and PBS groups. Instead, CGRP related genes were upregulated by both IS and PBS applications.
e. Conclusions
This study demonstrates significant changes in locomotor activity and facial allodynia associated with application of IS to the dura as well as significant sex differences, demonstrating that International Headache Society diagnostic criteria can be used to design a rodent behavioral model of migraine. In addition, there were prominent baseline sex differences in expression of CGRP and its receptor in both the trigeminal ganglion and medulla, but the majority of changes in expression of CGRP and its receptor were present in both the IS and PBS treated rats. This suggests that the CGRP pathway responds to changes in intracranial pressure or meningeal stretch, while migraine-like behaviors occur after meningeal inflammation.
Keywords: Chronic migraine, inflammatory soup, rat, facial allodynia, trigeminal, locomotor, activity, sex differences, pain, inflammation, dura mater, CGRP
4. INTRODUCTION
Migraine is among the most common neurological diseases, reportedly occurring in at least 12% of the population.1, 2 The true burden of the disease is compounded by debilitation and losses in productivity.3 In addition, there is a pronounced sex difference as migraine is 3 times more prevalent in females than in males.1 Migraine pathophysiology is not well understood, in part because of the lack of established preclinical behavioral models, and the mechanistic basis of the female predominance is not understood.1, 4 The diagnosis of migraine is made using a set of clinical criteria established by the International Headache Society, International Classification of Headache Disorders (ICHD-II).5 All of these criteria are based on signs and symptoms that amount to behavioral changes (e.g. duration and intensity of pain, pulsating and unilateral nature of pain, and causing avoidance of routine physical activity). The goal of this study was to use those behavioral changes in combination with an established behavioral method to measure cutaneous allodynia6, 7 to assess the presence of sex differences in that model, and to determine whether behavioral changes are accompanied by changes in expression of the neuropeptide calcitonin gene-related peptide (CGRP) and its receptor in peripheral or central trigeminal structures.
Application of inflammatory soup (IS) to the dura has been widely used as a preclinical model of migraine in rodents because it shares several key features with migraine, including acute activation of trigeminal nociceptors resulting in peripheral and central sensitization.8–12 In this study, repeated delivery of IS or phosphate buffered saline (PBS) control over 21 days was used to cause trigeminal sensitization.13 Use of an implanted dural cannula to deliver IS and PBS allowed the activity of freely-moving rats to be assessed using a force plate actimeter both during and after the IS and PBS applications to provide data concurrent with both the onset and persistence phases of migraine.
The present study included both male and female rats to determine whether there are sex differences in the behavioral model. A consensus report of the Sex, Gender and Pain Special Interest group of the International Association for the Study of Pain has provided guidelines on the optimal experimental procedure to determine whether or not there are sex differences behavioral assay of pain.14 Their recommendation is that the first stage of any study should be to compare gonadally intact adult males and females, and that studies of cycling females, ovariectomized females, and hormone replacement should follow initial studies of gonadally intact males and females. That recommendation was followed in the present study.
There is strong evidence that CGRP and its receptor components are important in migraine pathophysiology. CGRP is a vasoactive neuropeptide, and increased expression of CGRP or its receptor could explain the vasodilatory component of migraine.15 CGRP peptide levels have been shown to be increased in models of inflammatory pain, and CGRP is present in nociceptors, including those in the trigeminal ganglion that innervate meninges and cerebral vasculature.16–18 The CRGP receptor consists of a G–protein coupled calcitonin-like receptor (CLR), a receptor activity-modifying protein (RAMP1), and a chaperone protein, receptor component protein (RCP).19 These receptor components are present and co-localized to varying degrees in the in the dura, the trigeminal ganglia, and the spinal trigeminal nucleus.20 In this study, gene expression levels of CGRP and its receptor components (CLR, RAMP1, and RCP) were evaluated for baseline sex differences and for changes in response to repeated application of IS or PBS to the dura in male and female rats.
5. METHODS
Animals
Animal care and use procedures were approved by and conducted according to University of Kansas Medical Center Institutional Animal Care and Use Committee and Institute of Laboratory Animal Research guidelines. Ten week old, sexually mature Sprague-Dawley rats were purchased from Harlan Sprague-Dawley Inc. (Indianapolis, IN). The animals were housed in the Kansas University Medical Center Laboratory Animal Resources Facility on 12 hour light-dark cycle in plastic cages and provided water and food ad libitum. Light-dark cycles were not reversed to allow behavioral testing under normal room lights during the normal light phase of the circadian cycle. Rats were divided into groups based on sex, application of IS or PBS, and volume of application, and all behavioral experiments were conducted between 9:00 and 14:00 h in a dedicated, temperature-controlled room. No estrous cycle staging was conducted because sampling vaginal cytology in females requires handling that could alter behavioral measurements14, and no appropriate control for this procedure has been devised for males.
Cannula Implantation
A Plastics One (Roanoke, VA) cannula made of biocompatible material was used for dural delivery of IS or PBS. The cannula was implanted through the skull under isoflurane anesthesia (3.5% isoflurane for induction and 2.7% for maintenance in 30% oxygen in compressed air). During surgery, sterile ophthalmic ointment was used to protect the rat’s eyes, and absence of a corneal reflex confirmed the level of anesthesia. The rat was placed in a stereotactic apparatus, and the cannula was placed on the right side 5 mm lateral to midline and halfway between bregma and lambda to model unilateral migraine. The dura mater was exposed by first thinning a 1mm diameter region of the skull using a burr drill to remove the outer layer, taking care to irrigate the skull with room temperature sterile saline to prevent heating. A McCall currette was used to carefully remove the inner layer of bone while leaving the underlying dura intact. Sterile petrolatum was placed over the intact dura to prevent dural fixation to the cannula and calvarium. Single rostral and caudal drill holes were placed 3 mm from the center of the cannula, and stainless steel screws attached to the skull to provide anchors for dental cement (Ortho-Jet, Lang Dental Mfg. Co., Inc., Wheeling IL), which provided stability to the cannula. An internal obturator was placed within the cannula to maintain patency. The scalp was folded over the base of the cannula and sutured using #4 sterile silk. Following recovery from the isoflurane anesthesia, the rat was administered buprinorphine (0.05 mg/kg in 1 mL normal saline) by intraperitoneal injection for analgesia, and transferred to a clean cage with an external heat source. Breathing and movement were observed until the rat was fully awake and mobile, when it was returned to its home cage. Following implantation, the cannula and obturator cap projected less than one centimeter from the right side top of the head. In rare cases (2/43), rats that became ill following surgery were removed from the study. All animals were monitored regularly for health status by veterinary staff at the animal resources facility.
Treatment
The PBS control groups received repeated application to the dura of 10 or 20 µL of pyrogen-free phosphate buffered saline adjusted to a pH of 7.4. Groups selected for the inflammatory stimulus received repeated application to the dura of 10 or 20 µL of IS consisting of 1mM histamine, 1mmM 5-HT, 1mM bradykinin, and 0.1 mM PGE2, in PBS at a pH of 5.512. During the IS or PBS application, the obturator cap of the cannula was removed and an internal delivery cannula inserted and connected to PE50 tubing attached to an infusion pump (Cole Parmer) that delivered PBS or IS at a rate of 2 or 4 µl/min over 5 min.9, 13 Following application of IS or PBS and locomotor testing, the internal delivery cannula was removed and replaced with the obturator cap. Rats were weighed at the beginning of each behavioral testing session. All groups continued to gain weight across the course of the study, and there were no statistical differences between PBS and IS groups. A preliminary study showed that there were no behavioral differences between rats with cannulas implanted and non-surgical control animals, so all behavioral comparisons were made between IS and PBS groups.
Timeline
Prior to surgery the rats were trained for 10 days in the allodynia task, and presurgical baseline behavioral responses were measured for all tasks one day prior to surgery. Rats were allowed to recover for 7 days following surgery before resuming behavioral testing, a time when preliminary studies revealed the behavioral responses had returned to presurgical baseline values. Postsurgical behavioral measurements were obtained two days before delivering the first application of PBS or IS.
Behavioral testing was performed between 0 and 30 minutes following application of IS to the dura, when cutaneous allodynia is most pronounced.13 Because the temporal relationship between inflammation of the dura and onset of headache is not well understood, animals were tested during application of IS and during a second period 5 minutes later to determine the duration of the effects. The first five minutes (during IS application) was termed the “onset phase”, while the 10–15 minute time period after IS application was termed the “persistence phase”. Behavioral data were collected after application of IS on every third day for 7 total applications. The allodynia task was performed 20–30 minutes following application of IS or PBS. A final behavioral data point, termed “interictal” was taken 48 hours after the last application of IS or PBS. The post-surgical baseline data were subtracted from the Day 8 interictal results to calculate changes occurring over the 2.5 weeks of the experiment. For all behavioral testing, rats were coded and experimenters blind to treatment group.
Mechanical Allodynia Test
Rats were trained to hold their heads in a fixed position while withdrawal responses to mechanical stimulation of the face were tested using a procedure described previously.21 Threshold withdrawal was assessed using a 4g von Frey monofilament to test for mechanical allodynia of the ipsilateral periorbital and perimasseter regions. Previous studies using a 4g von Frey monofilament demonstrated this procedure is sensitive enough to detect changes in withdrawal responses due to inflammation or estrogen status.7, 22
Open-field Locomotor Activity
Activity was measured using a Force-Plate Actimeter (BASi, San Diego CA). The apparatus utilizes high sensitivity force transducers coupled to the four corners of a rigid, low-mass floor to monitor and track the rat’s locomotor activity. Software processes the force-location data and provides detailed analysis of locomotor behaviors including distance traveled, bouts of low mobility, spatial confinement, and stereotypy.23 Briefly, distance traveled was calculated by summing distance over a set time period (5 minutes) using the center of force reported according to the sampling rate (100 Hz). Bouts of low mobility were scored when the center of force defined did not move beyond a radius of 15 mm within a 10 second period. The spatial confinement score was designed to compare a test rat to a theoretical rat that visits every sector of the arena in a given time period. This value is essentially the standard deviation of the rat’s movement from an evenly distributed theoretical value. If the center of force did not move beyond a single sector, a maximum spatial confinement score of 100 was recorded. The aggregate score is the sum of individual scores over 5 minutes. Focused energy was calculated by computing the weighted average of the force variance over a 5 minute period. Taken together, these measures provide quantitative assessments of several aspects of routine animal behavior.
Statistical Analysis of behavioral data
Groups were compared using a two-way repeated-measures ANOVA with a Holm-Sidak post hoc comparison and confidence intervals after data were examined for non-normality and equal variance. Differences between groups were considered significant with a two-sided alpha level of 0.05. SigmaStat 3.5 (Systat Software Inc., Chicago, IL) was used for data analyses. Correlations were calculated using general linear regression with a goodness of fit analysis performed using ANOVA F-test.
Quantitative real-time polymerase chain reaction
Thirty minutes following the final dural application, the rats were sacrificed by decapitation following anesthesia with pentobarbital sodium (Beuthanasia,-D, Schering-Plough) at a dose of 60mg/kg IP, following recommendations of the Panel on Euthanasia, American Veterinary Medical Association. Brain and cervical spinal cord were removed and placed in RNALater (Ambion, Foster City, CA) and kept overnight at 4C. The next day, ipsilateral trigeminal ganglia and ipsilateral medulla extending from Bregma -9 to -19, were dissected and total RNA was extracted by homogenation with Polytron 2000 using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. RNA quantification was performed by spectrophotometry using a Nanodrop (Nanodrop Technologies, Wilmington, DE). Isolated RNA was treated with DNaseI (Ambion PCR Kit) to remove any contaminating genomic DNA according to manufacturer’s instruction, and RNA quantification was performed using a Nanodrop. RNA quality was assessed by measuring the RNA Integrity Number (RIN) with an Agilent 2100 Bioanalyzer (Agilant Technologies, Santa Clara, CA), and samples that did not meet quality standards were discarded.
Complementary DNA (cDNA) was synthesized by using 10 µg total RNA from each sample and random hexamers in a Taqman reverse transcription reaction (Applied Biosystems, Foster City, CA, USA). A 2µg cDNA quantity of each sample was synthesized in reaction tubes using 2µg total RNA from each sample, random hexamers (10% by volume), and reverse transcriptase (1.25 U/µL) in a reaction volume 50µL. Reaction tubes were placed in a Hybaid PCR thermal cycler (Thermo Scientific) and run as follows: 10min at 25°C, 30min at 48°C, 5 min at 95°C. cDNA samples were stored for up to a month in a - 80C freezer before performing qRT-PCR. Gene-specific primers were selected based on literature review and in silico specificity screen with BLAST, and qPCR was performed in 96 well MicroAmp Optical reaction plates (Applied Biosystems, Foster City, CA). A sample of 20–40ng cDNA was added to a 20 µL reaction volume of SYBR Green PCR Master Mix (SYBR Green I Dye, AmpliTaqDNA polymerase, dNTPs mixture, dUTP, and optimal buffer components (Applied Biosystems), 1ul primer mixture (10 µg forward and 10 µg reverse primers in 150µl Sigma water) and Sigma water (SigmaAldrich, St. Louis, MO). Each plate was subjected to PCR amplification using an Applied Biosystems 7500 qPCR thermocycler. Thermocycling parameters were 40 cycles of 95C for 15 seconds for denaturation and 60C for 1 minute for annealing and extension. Thermocycling was followed by a melting curve stage of 60C for 20 seconds with a 1% temperature ramp to 95C. Parameters and reaction conditions were identical for all sets of primers. PCR reactions were conducted in duplicate with the exception of the internal control which was run in triplicate. The amplified transcripts were quantified with the comparative double delta Cq method using GAPDH and as internal control. Average Cq for GAPDH, CGRP, RAMP1, CLR and RCP were 22.74, 26.93, 30.51, 34.16, and 33.43 respectively. To validate the use of GAPDH as the housekeeping gene of choice, a pilot study compared GAPDH and β-actin expression. GAPDH was more stable than β-actin across the experimental conditions used in this study. Additionally the ratio of GAPDH expression to total RNA was calculated (delta Cq/ng/µl). This ratio was not significantly changed with behavioral manipulation or application of IS or PBS to the dura. Cq for non-template controls (NTC) were greater than 40 cycles. The amplicon qPCR products of each primer were visualized on agarose gels to check size, reaction specificity, and to confirm results. Applied Biosystems 7500 Software v 2.0.2 was used for outlier removal and qPCR analysis. Outliers were identified and removed if there were anomalous melting curves with multiple, wide, or blunted peaks (< 3:1 ratio) upon fluorescence derivative analysis. Cq of NTC below 40 for a given experiment was an additional criterion for disposition for the associated experimental values. SigmaStat 3.5 (Systat Software Inc., Chicago, IL) was used for all data analyses using a published method for statistical analysis of qRT-PCR data.24 Mean delta Cq and standard error are shown in Table 2. Data were examined for evidence of non-normality and equal variance, and Student’s t-tests were used for comparisons. All tests of hypotheses used a two-sided alpha level of 0.05.
Table 2.
Expression of CGRP-related genes in a rat model of chronic migraine.
| Trigeminal Ganglion | ||||||||
| Naïve | 10 µL PBS | 10 µL IS | 10 µL PBS vs IS | 20 µL PBS | 20 µL IS | 20 µL PBS vs IS | ||
| MALES | CGRP | 1.04 +/− 0.16 | 0.51 +/− 0.11** | 0.43 +/− 0.08** | NS | 0.43 +/− 0.02* | 0.62 +/− 0.07* | NS |
| RAMP1 | 1.01 +/− 0.06 | 0.41 +/− 0.04** | 0.47 +/− 0.14* | NS | 1.90 +/− 0.87 | 2.22 +/− 0.67 | NS | |
| CLR | 1.03 +/− 0.11 | 0.10 +/− 0.05** | 0.18 +/− 0.04** | NS | 3.08 +/− 0.54* | 2.55 +/− 0.46** | NS | |
| RCP | 1.06 +/− 0.14 | 0.48 +/− 0.10** | 1.18 +/− 0.24 | (P = 0.04) | 1.82 +/− 0.28* | 7.61 +/− 3.26** | NS | |
| FEALES | CGRP | 1.13 +/− 0.21 | 1.52 +/− 0.87 | 2.26 +/− 1.04 | NS | 0.80 +/− 0.02 | 0.87 +/− 0.16 | NS |
| RAMP1 | 1.05 +/− 0.16 | 1.68 +/− 0.60 | 3.20 +/− 1.39 | NS | 1.16 +/− 0.22 | 1.38 +/− 0.22 | NS | |
| CLR | 1.08 +/− 0.17 | 4.23 +/− 1.30 | 3.39 +/− 1.79 | NS | 4.14 +/− 0.45** | 4.26 +/− 0.36** | NS | |
| RCP | 1.14 +/− 0.24 | 2.75 +/− 0.59* | 3.48 +/− 0.78* | NS | 3.43 +/− 0.82* | 3.89 +/− 0.60** | NS | |
| Medulla | ||||||||
| Naïve | 10 µL vehicle | 10 µL IS | 10 µL PBS vs IS | 20 µL vehicle | 20 µL IS | 20 µL PBS vs IS | ||
| FEMALES | CGRP | 1.04 +/− 0.13 | 1.73 +/− 0.51 | 1.35 +/− 0.36 | NS | 0.48 +/− 0.08* | 0.52 +/− 0.05* | NS |
| RAMP1 | 1.01 +/− 0.05 | 1.07 +/− 0.25 | 0.60 +/− 0.16 | NS | 1.95 +/− 0.46 | 0.73 +/− 0.03** | NS | |
| CLR | 1.29 +/− 0.31 | 0.27 +/− 0.14* | 0.17 +/− 0.11** | NS | 0.54 +/− 0.10 | 2.93 +/− 1.73 | NS | |
| RCP | 1.05 +/− 0.13 | 1.34 +/− 0.38 | 0.76 +/− 0.21 | NS | 0.78 +/− 0.14 | 0.94 +/− 0.16 | NS | |
| FEMALES | CGRP | 1.08 +/− 0.18 | 0.71 +/− 0.15 | 0.99 +/− 0.28 | NS | 0.673 +/− 0.12 | 0.56 +/− 0.11* | NS |
| RAMP1 | 1.05 +/− 0.14 | 1.63 +/− 0.32 | 2.56 +/− 0.53* | NS | 1.49 +/− 0.13 | 0.99 +/− 0.12 | (P = 0.04) | |
| CLR | 1.07 +/− 0.23 | 18.78 +/− 10.21** | 11.46 +/− 3.56** | NS | 14.99 +/− 2.38* | 14.31 +/− 2.10** | NS | |
| RCP | 1.62 +/− 0.81 | 5.44 +/− 1.46* | 6.26 +/− 1.26** | NS | 7.11 +/− 0.10* | 7.51 +/− 1.27** | NS | |
Numbers indicate relative expression values for expression of CGRP, RAMP1, CLR, and RCP in the trigeminal ganglion and medulla in male and female rats in all experimental groups as measured by qRT-PCR using Δ ΔCt analysis. GAPDH was used as the internal control. Naïve sex-matched animals were used as subject control for normalization. Male naïve group, n=6; 10µl PBS group, n = 5; 10µl IS group, n = 5; 20µl PBS group, n = 3; 20µl IS group, n = 5; Female naïe group, n=6; 10µl PBS group, n = 9; 10µl IS group, n = 7; 20µl PBS group, n = 3, 20µl IS group, n = 5. Bolded, outlined cells marked with * indicate P <0.05 when compared with naïve control and ** indicate P <0.005 when compared with naïve control. PBS vs IS column compares IS treated groups with their equal volume controls. The P value is given when results are significant and NS given when not significant. Comparisons with significant differences from baseline are indicated by bold font and outline boxes.
6. RESULTS
Behavioral responses during the onset phase of application of IS or PBS (0–5 minute period)
The study included 24 male and 30 female Sprague-Dawley rats. Behavioral studies were conducted over a period of 37 days for each group. Activity measurements during IS or PBS application modeled changes in routine activity at the onset of the headache (onset phase). Measurements of total distance traveled provided a quantitative measure of ambulation (Figure 1A). In male 10µl groups, there was no difference in the distance traveled between IS and PBS groups, while in females, the 10µl IS group traveled less distance than the 10µl PBS group (P < .001). Both the male and female 20µl IS groups traveled less distance than PBS controls in the same period (male 20µl P < .05, female 20µl P < .01). The female 20µl group that received IS were less active than female 10µl IS group (P < .05), demonstrating a dose effect. These data demonstrate that females are sensitive to the effects of IS in a dose-dependent manner, and that females respond to the IS at a lower dose than males. An additional sex difference was that the change in activity required more IS applications in females than in males. In females, reductions in activity occurred following 2–3 applications of IS, while in males, reduced activity was apparent following 1–2 applications of IS (Figure 1A).
Figure 1. Inflammatory soup decreases locomotor activity in onset phase.
A) Distance traveled measurement: millimeters of distance traveled between t=0 and t=5 minutes. B) Bouts of low mobility: number of ten-second periods of inactivity between t=0 and t=5 minutes. C) Spatial confinement score: unit measure with inverse relation to area explored between t=0 and t=5 28 minutes. D) Focused energy: unit measure of activity while animal remains stationary between t=0 and t= 5 minutes. X-axes show number of treatments. For each animal, 10µl or 20µ PBS (closed circles) or IS (open circles) was delivered to the surface of intact dura via cannula. Male 10µl PBS group, n = 5; male 10µl IS group, n=5; female 10µl PBS group, n=9; female 10µl IS group, n=7; male 20µl PBS group, n= 3; male 20µl IS group, n=5; female 20µl PBS group, n = 3; female 20µl IS group, n=5. Standard error is shown in error bars. # indicates a statistical significance with P < .05, * indicates P < .005.
Quantification of bouts of low mobility assessed the temporal component of inactivity in response to IS treatment, i.e. time spent remaining inactive. The male 10µl IS groups showed no change in time spent inactive when compared to 10µl PBS males during onset phase (Figure 1B), whereas the female 10µl IS group spent more time inactive than did the female 10µl PBS group (P < .01). There was no significant difference between the male 20µl IS and female 20µl IS groups and the 20 µl male and female PBS groups. A sex difference was also noted in this behavioral measurement. The female 10µL IS group showed more bouts of low mobility than males, indicating that IS causes greater inactivity in females than males.
Spatial confinement measurements quantify the area explored; i.e., with a high spatial confinement measure, rats stayed in their immediate vicinity and did not explore the arena. Normally, rats spend much of their time exploring a new environment and relatively little time remaining in a confined area. Thus, an increase in the spatial confinement score represents a change in routine activity. Sex differences were also observed in this measurement. The male 10µl IS group was not different from the male 10µl PBS group, while the female 10µl IS group demonstrated higher spatial confinement scores than the female 10µl PBS group (Figure 1C, P < .001). Both the male and females 20µl IS groups explored less area than did male and female 20µl PBS groups (P < .05 and P < .01 respectively).
Focused energy was used to quantify the amount of restless activity that occurs while the animal is stationary (Figure 1D). This could occur if they are grooming or scratching, but not moving. Male and female 10µl IS groups were not different from 10µl PBS groups, but male and female 20µl IS groups displayed lower focused energy scores than the male and female 20µl PBS groups (P < .005 and P < .05 respectively). It is also noteworthy that the PBS groups increased their focused energy scores over time, while the IS groups remained relatively immobile.
Behavioral responses during the persistence phase following application of IS or PBS (10–15 minutes)
Locomotor activity measurements in the period starting 10 minutes after the infusion of IS or PBS were designed to measure the persistence of changes in routine activity. There were also sex differences in activity during this period in all three measurements of locomotor activity. The male 10µl IS group showed no change in distance travelled when compared with PBS controls (Figure 2A), while the female 10µl IS group traveled less distance than PBS controls (P < .001). Neither the male 20µl nor the female 20µl IS groups showed any statistical difference in distance traveled as compared to the PBS treated groups. For the bouts of low mobility measurement, there was no difference between IS and PBS treatment in the male 10µl groups (Figure 2B), while the female 10µl IS group showed a higher bouts of low mobility score than the 10µl female PBS group (P < .001). There were significant interactions between Day and Treatment in the bouts of low mobility score for the 10µl males (P <.05) and in the 10µl females (P<0.05). Again, male and female 20µL IS groups showed no statistical difference from the 10µl PBS groups. For the spatial confinement measurement, the male 10µl IS group showed no changes when compared to the male 10µl PBS group (Figure 2C), while the female 10µl IS group remained confined to a small space when compared with the female 10µl PBS group (P < .0005). As was observed with the other locomotor measurements, the male 20µl and female 20µl IS and PBS groups were not different.
Figure 2. Inflammatory soup decreases locomotor activity in persistence phase.
A) Distance traveled measurement: millimeters of distance traveled between t=10 and t=15 minutes. B) Bouts of low mobility: number of ten-second periods of inactivity between t=10 and t=15 minutes. C) Spatial confinement score: unit measure with inverse relation to area explored between t=10 30 and t= 15 minutes. D) Focused energy: unit measure of activity while animal remains stationary between t =10 and t=15 minutes. X-axes show number of treatments. For each animal, 10µl or 20 µl PBS (closed circles) or IS (open circles) was delivered to the surface of intact dura via cannula. Male 10µl PBS group, n=5; male 10µl IS group, n= ; female 10µl PBS group, n=9 ; female 10µl IS group, n=7; male 20µl PBS group, n=3; male 20µl IS group, n=5; female 20µl PBS group, n=3, female 20µl IS group, n=5. Standard error is shown in error bars. # indicates a statistical significance with P < .05, * indicates P < .005.
There were also sex differences in the focused energy measurements. The male 10µl IS group was no different with regard to its focused energy score as compared to the male 10µl PBS group (Figure 2D), while the female 10µl IS group displayed a lower focused energy score than the female 10µl PBS group (P < .005). Both the male 20µl and female 20µl IS groups showed higher focused energy scores than the male 20µl and female 20µl PBS groups (P < .05 and P < .05 respectively). The behavior outcome of this measurement differed from the locomotor activity measurements. Both male and female 20µl groups showed persistent changes in focused energy, and the 10µl female group showed changes in focused energy in the persistence phase that were not present in the onset phase.
Facial allodynia following IS treatment
Sex differences in facial allodynia were also apparent. Mechanical testing of the periorbital region measured sensitization of the first division of the trigeminal nerve (Figure 3A). The male 10µl IS group group showed significant allodynia of the periorbital region only one testing day (day 5, 5 (P < .05) as compared to the PBS group, while the male 20µl IS group showing significant periorbital allodynia on 4 testing days (days 3, 4, 5 and 7, P < .05), when compared with the male 20µl PBS group. The female 10µl IS group showed significant periorbital allodynia 4 testing days (days 4, 5, 6 and 7, P < .05) when compared with the female 10µl PBS group. The female 20µl IS and PBS groups were not significantly different from each other due to high variability in this measurement. Thus, for the 10µl IS groups, females showed more allodynia than males, but in the 20µl groups, males showed more allodynia than females because of the high variability in the females. Females also showed a delay in development of allodynia in comparison to males, i.e. they require more IS applications than males before allodynia appeared (Figure 3).
Figure 3. Inflammatory soup induces facial allodynia.
A) Primary division allodynia: Withdrawal scores in response to monofilament (4g) stimulation of the periorbital region. B) Secondary division allodynia. Withdrawal scores in response to monofilament (4g) stimulation of the perimasseter region. Over the course of testing the withdrawal scale measures the maximum withdrawal as a score of 15 and no response as a score of 0. X-axis shows number of treatments. For each animal, 10µl or 20µl PBS (closed circles) or IS (open circles) was delivered to the surface of intact dura via cannula. Male 10µl PBS group, n=5; male 10µl IS group, n=5; female 10µl PBS group, n= 9; female 10µl IS group, n=7; male 20µl PBS group, n=3; male 20µl IS group, n= ; female 20µl PBS group, n=3, female 20µl IS group, n=5. Standard error is shown in error bars. # indicates a statistical significance with P < .05, * indicates P < .005.
Sex differences were also apparent following mechanical testing of the perimasseter region. This measurement assesses sensitization of the third division (mandibular branch) of the trigeminal nerve, and allodynia in this region may represent central sensitization (Figure 3B). The male 10µl IS group showed no allodynia in this region. The male 20 µl IS group showed significant allodynia in this region on 4 testing days (days 1, 3, 4, and 5, P < .05). In contrast, the female 10µl IS group experienced allodynia during mechanical stimulation of the perimasseter region only on testing days 5 and 7 (P < .05), and the female 20µl group showed high variability. Thus, the male 20µl IS group showed the greatest allodynia in the perimasseter region under these conditions, and again females showed a delay in onset of perimasseter allodynia. There were significant interactions between Day and Treatment for the periorbital withdrawal measurements in the low dose female group (P = 0.003).
Interictal behavioral response (48 hours)
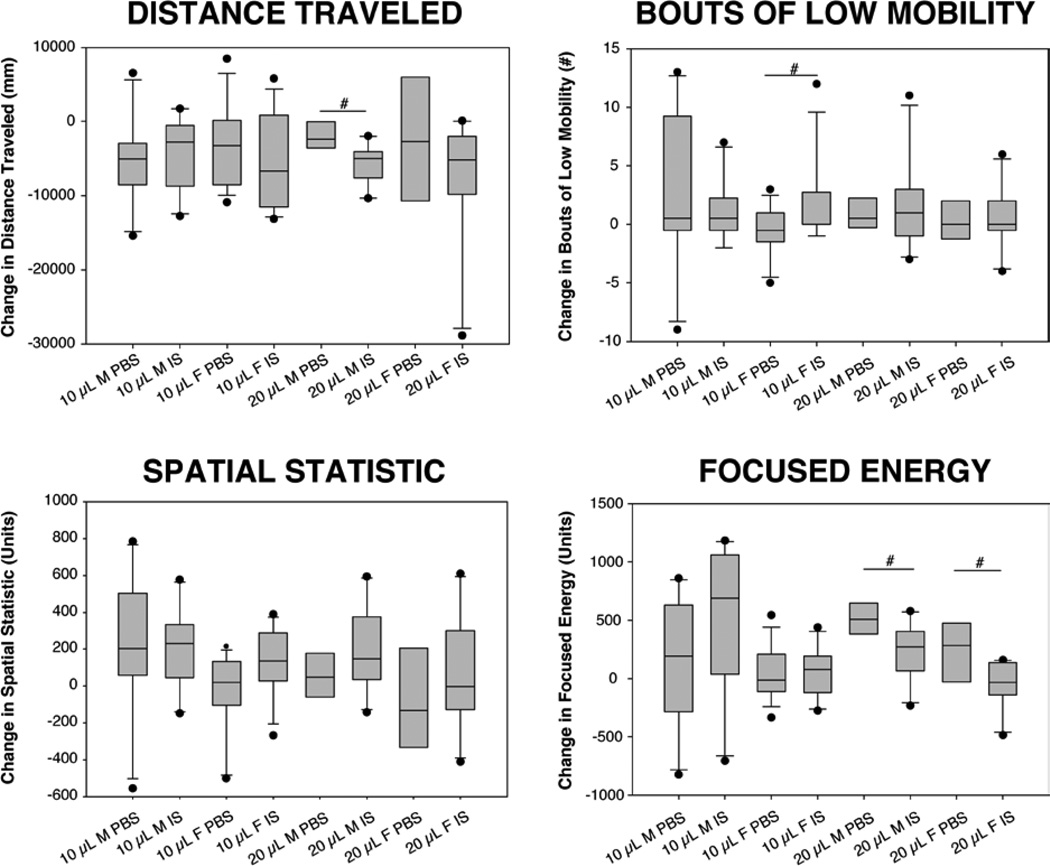
Locomotor activity measurements 48 hours after the final infusion of vehicle or IS were designed to determine whether the behavioral changes were persistent. The male and female 10µl IS groups displayed no statistically significant difference in distance traveled in the interictal period over the course of the experiment as compared to the 10µl PBS groups (Figure 4A). The male 20 µL IS group showed a decrease in distance traveled in the interictal period as compared to the male 20 µl PBS group (P < .05) over the course of the experiment, while the 20 µl female groups were not different. The male 10µL IS group showed no statistical difference in changes in bouts of low mobility from the male 10µl PBS group (Figure 4B), while the female 10µl IS group showed statistically significantly higher numbers of bouts of low mobility than the female 10µl PBS group (P < .05). Neither the male nor female 20µl IS groups demonstrated any statistical differences in bouts of low mobility in the interictal period from the 20µl PBS groups (Figure 4B). Neither the male nor the female 10µl or 20µl IS groups showed any difference in spatial confinement when compared to PBS groups (Figure 4C).The male and female 10µl IS groups did not show any difference in the focused energy score when compared to PBS groups (Figure 4D), but there were significant differences in both the male and female 20 µl IS groups from the male and female 20 µl PBS groups (both P < .05).
Figure 4. Inflammatory soup decreases routine activity interictally.
A) Distance traveled measurement: difference in millimeters of distance traveled between post-surgical baseline and treatment day 8. B) Bouts of low mobility: difference in number of 10 second periods of inactivity between post-surgical baseline and treatment day 8. C) Change in spatial confinement score between post-surgical baseline and treatment day 8. D) Change in focused energy score between post-surgical baseline and treatment day 8. For each animal, 10µl or 20µl PBS (closed circles) or IS (open circles) was delivered to the surface of intact dura via cannula. Male 10µl PBS group, n=5; male 10µl IS group, n=5; female 10µl PBS group, n=9; female 10µl IS group, n = 7; male 20µl PBS group, n=3; male 20µl IS group, n=5; female 20µl PBS group, n= 3, female 20 µl IS group, n=5. Data are shown as a box plot with a line within the box representing the 50th percentile or median, while the boundary of the box closest to zero indicates the 25th percentile and the boundary of the box farthest from zero indicates the 75th percentile. The whiskers mark the 10th and 90th percentiles. Outlying data points are shown.
Interictal facial allodynia (48 hours)
Mechanical testing of the periorbital region measured sensitization of the first division (ophthalmic branch) of the trigeminal nerve during the interictal time period. There was no significant allodynia in any of the groups in this facial region (Figure 5A). Mechanical testing of the perimasseter region measured sensitization of the third division (mandibular branch) of the trigeminal nerve (Figure 5B). Again, there was no significant allodynia in any of the groups.
Figure 5. Inflammatory soup does not illicit interictal allodynia.
A) Primary division allodynia: Withdrawal scores in response to monofilament (4g) stimulation of the periorbital region. B) Secondary division allodynia. Withdrawal scores in response to monofilament (4g) stimulation of the perimasseter region. Change calculated as difference between post-surgical baseline and treatment day 8. Over the course of testing the withdrawal scale measures the maximum withdrawal as a score of 15 and no response as a score of 0. For each animal, 10µl or 20µl PBS (closed circles) or IS (open circles) was delivered to the surface of intact dura via cannula. Male 10µL PBS group, n=5; male 10µL IS group, n=5 ; female 10µl PBS group, n=9 ; female 10µl IS group, n=7 ; male 20µl PBS group, n=3 ; male 20µl IS group, n=5; female 20µl PBS group, n=3, female 20µl IS group, n=5. Standard error is shown in error bars. # indicates a statistical significance with P < .05, * indicates P < .005. Data are shown as a box plot with a line within the box representing the 50th percentile or median, while the boundary of the box closest to zero indicates the 25th percentile and the boundary of the box farthest from zero indicates the 75th percentile. The whiskers mark the 10th and 90th percentiles. Outlying data points are shown.
Correlation of locomotor and allodynia data
To determine whether these behavior changes occur in the same rats and to the same extent, a comparison was made between the distance traveled and allodynia data by performing a goodness of fit analysis following a general linear regression followed by an ANOVA F-test. In female rats, distance traveled during the onset phase is highly correlated with interictal periorbital mechanical facial allodynia (Figure 6, p<0.0005). This correlation was not present in males.
Figure 6. In female rats, onset phase distance traveled is highly correlated with interictal periorbital mechanical facial allodynia.
X-axis shows interictal periorbital withdrawal scores in response to monofilament (4g) stimulation of the periorbital region during the interictal period on Day 8. Y-axis shows distance traveled on Day 7. Female 10µl PBS group, n = 5; female 10µl IS group, n = 6; female 20µl PBS group, n = 3, female 20µl IS group, n = 5. The data are shown with a general linear regression with a goodness of fit analysis performed using ANOVA F-test. The correlation is statistically significant (P = .0003).
Gene expression
Sex Differences in Trigeminal Ganglia and Medulla
Expression of genes encoding CGRP and its receptor in the trigeminal ganglion and medulla was examined in naïve rats. Sex differences in the expression of specific genes in the trigeminal ganglia included significantly lower mRNA levels of RAMP1, CLR, and RCP genes (P=0.008, P<0.001, and P=0.003 respectively) in female rats. In the medulla, sex differences included significantly lower expression levels of RAMP1, CLR, and RCP genes (P<0.01, P<0.005, and P<0.001 respectively) in female subjects. Expression levels of the CGRP-encoding gene in the medulla was higher in female rats (P<0.05).
Gene Expression in IS and PBS treated rats
Significant gene expression differences from baseline were observed in all four genes studied (Table 2), but most of these differences were present when comparing either IS or PBS groups to their baseline values. In the trigeminal ganglion, the males showed significant differences from baseline in CGRP, RAMP1, CLR and RCP in both the PBS and IS groups, while only RCP in the 10µl group was significantly different between PBS and IS treated rats. By contrast, in females, only RCP in both 10 and 20µl groups and CLR in the 20µl group were different from baseline in the trigeminal ganglion, and there were no significant differences between PBS and IS treated female rats. In the medulla, CLR was different from baseline in both the 10µl male IS and PBS groups, while CGRP was different from baseline in both 20µl IS and PBS groups, and RAMP1 was different from baseline in the 20µl IS group. Overall, there were more significant differences from baseline levels in the females. CLR and RCP were different from baseline in all female groups, and RAMP1 was different from baseline in the 10µl IS female group. RAMP1 was significantly different between PBS and IS treated rats in the 20µl group. Thus, males show more differences from baseline in the trigeminal ganglion, while females show more differences from baseline in the medulla, but in males and females these differences were present in both the IS and PBS treated groups.
7. DISCUSSION
This study demonstrated that changes in locomotor activity occur while trigeminal nociceptors are activated by inflammatory mediators. This finding suggests that this reduction in activity may be similar to the avoidance of routine activity that occurs during migraine attacks.25, 26 Previous studies demonstrated that IS application to the dura activates and sensitizes the trigeminal system.8–12, 27–29 The present findings suggest trigeminal activation produces changes in routine activity, and that these changes are accompanied by allodynia, providing face validity for this animal model as useful tool for studying the behavioral and physiological changes associated with trigeminal sensitization that occurs during migraine. This study also adds to a current goal of developing animal models that demonstrate behaviors (i.e.symptoms) typical of migraine that can be used to discover new therapies.
Sex differences in behavioral responses
The results of this study demonstrate significant sex differences in behavioral responses to IS application to the dura. A 10 µL application of IS decreased activity, as measured by decreased distance traveled, increased bouts of low mobility, and increased spatial confinement, in female but not male rats. This finding is of particular interest because, in addition to the increased prevalence of migraine in females,1, 30 they may also show greater avoidance of routine activity as judged by the number of work days missed.31 This study also demonstrated a significant dose effect in the behavioral measurements. Others have shown dose related increases in sensitivity of neurons innervating the dura in response to the inflammatory mediators in IS, 5-HT, histamine, and PGI2.11 These sex differences in dose-response effects may be explained by the finding that sensitization in the trigeminal ganglia is regulated by estrogen.7, 22, 32, 33 An additional mechanism that should be considered is that the frequency and propagation speeds of cortical spreading depression, a condition thought to be linked to migraine with aura, is greater in females than males in a mouse model of familial hemiplegic migraine.34
Changes in locomotor activity were not as robust in the interictal period as they were during and minutes after IS application, but some of the same changes present in the acute phase were still present 48 hours after the last IS application. The general trend was that groups treated with IS tended to ambulate less and display less head and body movement even while standing still (measured by focused activity), even in the interictal period. Thus, in this animal model, some effects of chronic IS application are apparent as long as 48 hours following the final application of inflammatory soup.
In the present study, primary facial mechanical allodynia was measured in the periorbital region (i.e. the same sensory dermatome as dura) and secondary allodynia was measured in the perimasseter region (different trigeminal dermatome from dura) as a measure of the spread of sensitization in the trigeminal pathway. There was a sex difference in the location of facial allodynia. Periorbital region allodynia, as measured by increased withdrawal scores, was significantly increased in females receiving inflammatory soup application, while males showed significant allodynia in response to stimulation of the perimasseter region. Previous studies have shown allodynia of the periorbital region in males, but those studies did not test other regions of the face or compare males to females,13 or they conducted behavioral studies hours to days after application of IS, but did not examine allodynia within minutes of administration of the IS.13, 35, 36 None of the groups examined in this work showed interictal cutaneous perimasseter allodynia, indicating that allodynia in this region, innervated by a separate division of the trigeminal nerve, is present acutely and subsides in the interictal period. This finding is consistent with clinical research showing that facial mechanical pain thresholds decrease 63% by 4 hours following onset of headache from the interictal values.37
Female rats required more dural applications of IS than male rats before they showed behavioral changes in activity and allodynia. This difference may reflect a sex difference in the mechanism of sensitization of the trigeminal system. One hypothesis that is consistent with those findings is that sensitization occurs more centrally in females and more peripherally in males, and that central sensitization requires repeated episodes of IS application to develop. Another hypothesis is that these differences are related to the estrous cycle in females. This is unlikely, however, because in female rats, estrogen levels are low and constant except during the estrogen surge, which begins in the afternoon of proestrus and lasts for 8–12 hours38. In the present study, each rat was studied over a period of a month, and responses were stable in the baseline period, in post surgery testing, and when allodynia and reductions in activity developed, there was no evidence of an underlying cycle. In addition, in the PBS female groups, responses were stable across the study.
Sex differences in expression of CGRP related genes in naïve rats
One goal of this study was to determine whether or not there are sex difference in mRNA levels of CGRP related genes in naïve rats and after applications of IS or PBS to the dura. Major sex differences were observed in mRNA levels of CGRP related genes in naïve rats in both the trigeminal ganglion and medulla. In the trigeminal ganglia, CGRP mRNA levels did not show a sex difference in naïve rats, while mRNA levels of the genes encoding CGRP receptor components RAMP1, CLR, and RCP were all higher in males than females. In the medulla, naïve females had higher mRNA levels of the gene encoding CGRP than males, while males had higher mRNA levels of the genes encoding RAMP1, CLR and RCP than females.
Changes in gene expression after IS and PBS applications to the dura
An unexpected finding of this study was that both IS and PBS caused changes in CGRP-related genes that were, for the most part, unrelated to the behavioral changes. Increased expression of CGRPrelated genes by PBS applications suggests they may be induced by mechanical stimulation including stretch or increased intracranial pressure. That would suggest a multi step model linking CGRP to allodynia and reduced activity, viewed in this study as “migraine-like” behaviors. In the first step dural stretch would increase expression genes in the CGRP pathway, and in the second step, CGRP release would activate other cells, such as dural mast cells, to release inflammatory mediators, which, in turn would excite trigeminal nociceptors to produce “ migraine-like” behaviors. That speculation is supported by recent studies reporting that mast cell mediators sensitize meningeal nociceptors,11 and that CGRP itself does not activate meningeal nociceptors39, but, instead, causes mast cell degranulation.40
Greater dynamic range of CGRP receptor induction in female rats
The mRNA levels of the RAMP1, CLR, and RCP-encoding genes in the trigeminal ganglia and medulla were lower in females than males, and in most cases expression of these receptor components was increased following both IS and PBS applications in both sexes. Because of the lower baseline levels, gene expression increases after IS and PBS applications were much larger relative in females than in males. For example, female CLR-encoding gene expression was increased 12.6-fold in the 20µl IS group compared to the naïve control group, while the comparable change in males was only 2.2-fold. This high dynamic range of mRNA induction in females may lead to greater relative differences in peptide or protein levels after stimulation in females.
Sex differences in location of changes in gene expression in the trigeminal system
The data reported in this study also demonstrate a sex difference in the location of the largest changes in gene expression evoked by dural stimulation. Males showed larger changes in the trigeminal ganglia, while females showed larger changes in the medulla. This sex difference in the location of the maximal effects suggests that trigeminal sensitization following dural inflammation may occur more peripherally in males and more centrally in females, and that central sensitization may be more likely to occur in females.
Conclusion
The present study has demonstrated that application of inflammatory mediators to the dura results in allodynia and reduced activity behaviors in rats, and that there are pronounced sex differences in these behaviors. In addition, this study demonstrates that the dynamic range of induction of the CGRP pathway is greater in females, and that CGRP-related genes are upregulated in conditions that reflect increased intracranial pressure or mechanical deformation of the dura rather than those that directly result in behaviors in rats that are similar to those typical of migraine attacks.
Figure 7. Sex Differences in baseline expression of CGRP-related Genes in Rat Trigeminal Ganglion and Medulla.
Relative expression of CGRP-related genes in the trigeminal ganglia and medulla as measured by qRT-PCR using ΔΔCt analysis to calculate relative expression. GAPDH was used as the internal control. Average male expression levels were used as comparison controls. Animals are naïve control male or naïve control female Sprague-Dawley rats. Male group, n=6, female group, n=6. Error bars indicate standard error. # indicates a statistically significant difference from male naïve with P < .05, * indicates P < .005.
Table 1. RT-PCR primers.
Information on primers used in this study. Gene name: CGRP (Calca calcitonin-related polypeptide alpha); GAPDH (glyceraldehyde-3-phosphate dehydrogenase) ; RAMP1 (receptor G protein-coupled activity modifying protein 1); CLR (calcitonin receptor-like); RCP (CGRP receptor component) Primers: Forward (first row) and Reverse (second row) primer sequences. Length: amplicon size. Location in DNA: Location of primer sequence in genomic DNA.
| Gene name |
Primers | Location in mRNA |
Accession Number |
Length | Location in DNA |
|---|---|---|---|---|---|
| CGRP | GTGTCACTGCCCAGAAGAGATC | 358–379 | NM_001033955.1 | 101 | 3772–3793 |
| CAAAGTTGTCCTTCACCACACC | 438–459 | 3852–3873 | |||
| GAPDH | ATGACATCAAGAAGGTGGTG | 837–856 | NM_017008.3 | 176 | 3325–3344 |
| CATACCAGGAAATGAGCTTC | 994–1013 | 3482–3501 | |||
| RAMP1 | ACTGGGGAAAGACCATAGGGAG | 212–233 | NM_031645.1 | 229 | 20481–20500 |
| AGTCATGAGCAGTGTGACCGTA | 420–441 | 46326–46347 | |||
| CLR | ATTGTGGTGGCTGTGTTTGCA GAG | 1126–1149 | NM_012717.1 | 141 | 78843–78866 |
| TGTCTGAGCTGATCCAGCAGTT GT | 1244–1267 | 80720–80739 | |||
| RCP | GAACTTGAACGCCATCACCT | 134–153 | NM_053670.3 | 172 | 12773–12792 |
| GGATCTCAACAGCGGTCATT | 287–306 | 24220–24239 |
Acknowledgements
This study was funded by grants from the American Headache Society, Migraine Research Foundation, the University of Kansas Medical Center Biomedical Research Training Program, NICHD HD02528, NIEHS 5 T32 ES007079, P20 RR016475, and by a research grant from the Investigator-Initiated Studies Program of Merck & Co., Inc. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck & Co., Inc.
Abbreviations
- IS
inflammatory soup
- PBS
phosphate-buffered saline
- CGRP
calcitonin gene-related peptide
- CLR
calcitonin-like receptor
- RAMP1
receptor activity-modifying protein (RAMP1)
- RCP
receptor component protein.
Footnotes
Conflict of interest statement: NLS: No Conflict., EG: No Conflict., MKW: No Conflict., YH: No Conflict., KM’C: No Conflict., NEJB: Conflict of Interest: Consultant for MAP Pharmaceuticals, financial interest less than $10,000; grant support from Merck and Co., Inc, honoraria from National Headache Foundation, financial interest less than $10,000.
REFERENCES
- 1.Bigal ME, Lipton RB. The epidemiology, burden, and comorbidities of migraine. Neurol Clin. 2009;27:321–334. doi: 10.1016/j.ncl.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Stovner L, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 3.Buse DC, Rupnow MF, Lipton RB. Assessing and managing all aspects of migraine:migraine attacks, migraine-related functional impairment, common comorbidities, and quality of life. Mayo Clin Proc. 2009;84:422–435. doi: 10.1016/S0025-6196(11)60561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen BK. Migraine and tension-type headache in a general population: precipitating factors, female hormones, sleep pattern and relation to lifestyle. Pain. 1993;53:65–72. doi: 10.1016/0304-3959(93)90057-V. [DOI] [PubMed] [Google Scholar]
- 5.The International Classification of Headache Disorders. Cephalalgia. (2nd edition) 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 6.Oshinsky ML, Ginibcgareibsurum S, Luo J. Recurrent dural stimulation in awake rats chronically reduces periorbital pressure thresholds and potentiates the response to glyceryl trinitrate. Headache. 2007;47:741. [Google Scholar]
- 7.Liverman CS, Brown JW, Sandhir R, Klein RM, McCarson K, Berman NE. Oestrogen increases nociception through ERK activation in the trigeminal ganglion: evidence for a peripheral mechanism of allodynia. Cephalalgia. 2009;29:520–531. doi: 10.1111/j.1468-2982.2008.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steen KH, Reeh PW, Anton F, Handwerker HO. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004;55:27–36. doi: 10.1002/ana.10785. [DOI] [PubMed] [Google Scholar]
- 10.Jakubowski M, Levy D, Kainz V, Zhang XC, Kosaras B, Burstein R. Sensitization of central trigeminovascular neurons: Blockade by intravenous naproxen infusion. Neuroscience. 2007 doi: 10.1016/j.neuroscience.2007.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang XC, Strassman AM, Burstein R, Levy D. Sensitization and activation of intracranial meningeal nociceptors by mast cell mediators. J Pharmacol Exp Ther. 2007;322:806–812. doi: 10.1124/jpet.107.123745. [DOI] [PubMed] [Google Scholar]
- 12.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 13.Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache. 2007;47:1026–1036. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenspan JD, Craft RM, LeResche L, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26–S45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCulloch J, Uddman R, Kingman TA, Edvinsson L. Calcitonin gene-related peptide: functional role in cerebrovascular regulation. Proc Natl Acad Sci U S A. 1986;83:5731–5735. doi: 10.1073/pnas.83.15.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- 17.Nakakita K. Peptidergic innervation in the cerebral blood vessels of the guinea pig: an immunohistochemical study. J Cereb Blood Flow Metab. 1990;10:819–826. doi: 10.1038/jcbfm.1990.138. [DOI] [PubMed] [Google Scholar]
- 18.Keller JT, Marfurt CF. Peptidergic and serotoninergic innervation of the rat dura mater. J Comp Neurol. 1991;309:515–534. doi: 10.1002/cne.903090408. [DOI] [PubMed] [Google Scholar]
- 19.Arulmani U, Maassenvandenbrink A, Villalon CM, Saxena PR. Calcitonin gene-related peptide and its role in migraine pathophysiology. Eur J Pharmacol. 2004;500:315–330. doi: 10.1016/j.ejphar.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 20.Lennerz JK, Ruhle V, Ceppa EP, et al. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J Comp Neurol. 2008;507:1277–1299. doi: 10.1002/cne.21607. [DOI] [PubMed] [Google Scholar]
- 21.Liverman CS, Brown JW, Sandhir R, McCarson KE, Berman NE. Role of the oestrogen receptors GPR30 and ERalpha in peripheral sensitization: relevance to trigeminal pain disorders in women. Cephalalgia. 2009 doi: 10.1111/j.1468-2982.2008.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liverman CS, Brown JW, Sandhir R, McCarson KE, Berman NE. Role of the oestrogen receptors GPR30 and ERalpha in peripheral sensitization: relevance to trigeminal pain disorders in women. Cephalalgia. 2009;29:729–741. doi: 10.1111/j.1468-2982.2008.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fowler SC, Birkestrand BR, Chen R, et al. A force-plate actometer for quantitating rodent behaviors: illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. J Neurosci Methods. 2001;107:107–124. doi: 10.1016/s0165-0270(01)00359-4. [DOI] [PubMed] [Google Scholar]
- 24.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelman L. Pain characteristics of the acute migraine attack. Headache. 2006;46:942–953. doi: 10.1111/j.1526-4610.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 26.Martins IP, Gouveia RG, Parreira E. Kinesiophobia in migraine. J Pain. 2006;7:445–451. doi: 10.1016/j.jpain.2006.01.449. [DOI] [PubMed] [Google Scholar]
- 27.Kessler W, Kirchhoff C, Reeh PW, Handwerker HO. Excitation of cutaneous afferent nerve endings in vitro by a combination of inflammatory mediators and conditioning effect of substance P. Exp Brain Res. 1992;91:467–476. doi: 10.1007/BF00227842. [DOI] [PubMed] [Google Scholar]
- 28.Ter Horst GJ, Meijler WJ, Korf J, Kemper RH. Trigeminal nociception-induced cerebral Fos expression in the conscious rat. Cephalalgia. 2001;21:963–975. doi: 10.1046/j.1468-2982.2001.00285.x. [DOI] [PubMed] [Google Scholar]
- 29.Knyihar-Csillik E, Tajti J, Csillik AE, Chadaide Z, Mihaly A, Vecsei L. Effects of eletriptan on the peptidergic innervation of the cerebral dura mater and trigeminal ganglion, and on the expression of c-fos and c-jun in the trigeminal complex of the rat in an experimental migraine model. Eur J Neurosci. 2000;12:3991–4002. doi: 10.1046/j.1460-9568.2000.00299.x. [DOI] [PubMed] [Google Scholar]
- 30.Greenspan JD, Craft RM, LeResche L, et al. Studying sex and gender differences in pain and analgesia: A consensus report. Pain. 2007 doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stang PE, Osterhaus JT. Impact of migraine in the United States: data from the National Health Interview Survey. Headache. 1993;33:29–35. doi: 10.1111/j.1526-4610.1993.hed3301029.x. [DOI] [PubMed] [Google Scholar]
- 32.Puri V, Cui L, Liverman CS, et al. Ovarian steroids regulate neuropeptides in the trigeminal ganglion. Neuropeptides. 2005;39:409–417. doi: 10.1016/j.npep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Flake NM, Bonebreak DB, Gold MS. Estrogen and inflammation increase the excitability of rat temporomandibular joint afferent neurons. J Neurophysiol. 2005;93:1585–1597. doi: 10.1152/jn.00269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eikermann-Haerter K, Dilekoz E, Kudo C, et al. Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J Clin Invest. 2009;119:99–109. doi: 10.1172/JCI36059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edelmayer RM, Vanderah TW, Majuta L, et al. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol. 2009;65:184–193. doi: 10.1002/ana.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wieseler J, Ellis A, Sprunger D, et al. A novel method for modeling facial allodynia associated with migraine in awake and freely moving rats. J Neurosci Methods. 2009;185:236–245. doi: 10.1016/j.jneumeth.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123(Pt 8):1703–1709. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- 38.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 39.Levy D, Burstein R, Strassman AM. Calcitonin gene-related peptide does not excite or sensitize meningeal nociceptors: implications for the pathophysiology of migraine. Ann Neurol. 2005;58:698–705. doi: 10.1002/ana.20619. [DOI] [PubMed] [Google Scholar]
- 40.Ottosson A, Edvinsson L. Release of histamine from dural mast cells by substance P and calcitonin gene-related peptide. Cephalalgia. 1997;17:166–174. doi: 10.1046/j.1468-2982.1997.1703166.x. [DOI] [PubMed] [Google Scholar]