Abstract
Background
Swallowing physiology, diet, and patient-reported outcomes were evaluated after induction chemotherapy for oral tongue cancer.
Methods
Fifteen of 23 patients enrolled in a phase II clinical trial of induction chemotherapy followed by surgical resection for oral tongue cancer underwent instrumental and perceptual analysis of speech and swallowing. Oropharyngeal swallow efficiency (OPSE) was calculated. Patient-reported outcomes were collected. We compared pre- and post–chemotherapy results.
Results
OPSE scores were not significantly different (p > .05) after induction chemotherapy; however, patient-reported swallowing and diet levels were significantly higher (p < .001 and p = .015, respectively). Diet levels improved from soft-chewable to full diet in most patients. Speech intelligibility did not change (p = .328).
Conclusion
It appears that induction chemotherapy has a negligible effect on speech and swallowing physiology, but may provide symptomatic improvement of pain and swallowing after treatment. Further investigations are needed to corroborate these findings.
Keywords: induction chemotherapy, speech, swallowing, oral tongue cancer, head and neck cancer
INTRODUCTION
Traditionally, oral tongue cancer has been treated with surgical resection followed by postoperative radiation therapy often with severe functional consequences. Although data clearly demonstrate the deleterious effects of combined regimens on speech and swallowing function, the proportional contribution from each type of treatment is unknown. It has been postulated that induction chemotherapy might obviate postoperative radiation therapy in patients with oral tongue cancer, thereby improving preservation of speech and swallowing function.1-3 However, the independent effect of induction chemotherapy on functional outcomes has not been thoroughly investigated.
Recent experience at our institution and at other institutions has suggested that the addition of induction chemotherapy to standard regimens of radiation therapy does not result in further deterioration in speech and swallowing function caused by radiation treatment.4,5 However, the limitation of these studies is that function was evaluated after the completion of all treatments; none provided information about speech and swallowing function after induction chemotherapy alone—that is, before any further cancer treatment.
A recently published retrospective review showed that the addition of induction chemotherapy to surgical resection for squamous cell cancer of the oral tongue in young patients provided similar disease control and survival to patients treated with traditional modalities of surgery followed by postoperative radiotherapy. In addition, induction chemotherapy may have reduced the need for postoperative radiotherapy that has been associated with additional speech and swallowing dysfunction.3 These findings provided the foundation for a phase II clinical trial of induction chemotherapy followed by surgical resection for oral cavity cancer. As part of this prospective trial, we analyzed speech and swallowing in a subgroup of patients before and after induction chemotherapy to determine the independent effects of chemotherapy on functional outcomes.
MATERIALS AND METHODS
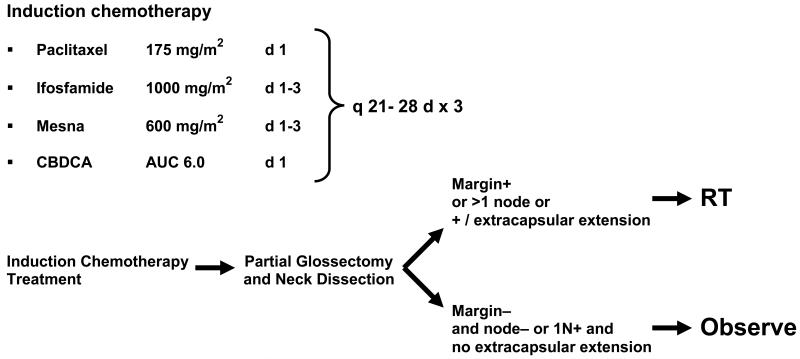
Twenty-three young patients (≤ 49 years old) with squamous cell carcinoma of the oral tongue and floor of the mouth were enrolled in a phase II clinical trial of induction chemotherapy followed by surgical resection with or without postoperative radiotherapy. The treatment schema is provided in Figure 1. Functional analysis of speech and swallowing was conducted at baseline; after induction chemotherapy but before surgery; and 6, 12, and 24 months after the completion of all treatment. In this study, we included only patients (N= 15) who completed swallowing evaluations at baseline and after induction chemotherapy. The clinical trial including functional analysis was approved by the Institutional Review Board of The University of Texas M. D. Anderson Cancer Center. Informed consent was obtained from all participants.
Figure 1. Treatment schema.
Abbreviations: mg, milligrams: AUC, area under curve: m2, meter squared: q, every: d, day: RT, radiation therapy: CBDCA, carboplatin
Pain scores were collected from medical records at baseline and after induction chemotherapy. Pain was rated on a scale of 0 to 10, with 0 indicating no pain and 10 indicating the most severe pain.
Modified barium swallow (MBS) studies were conducted using a standard technique described elsewhere.6 Oropharyngeal swallowing efficiency (OPSE), is defined as the percentage of the bolus swallowed [(100 - (% residue - % aspirated)] divided by the bolus transit time, from the oral cavity through the cricopharyngeus. OPSE has been used to quantify the safety and efficiency of bolus transit through the oral cavity and pharynx into the esophagus7 and was calculated for each patient’s swallows of three bolus types: 10 mL thin liquid, 1 tsp. pudding, and ¼ cracker with barium paste, and averaged to provide an overall global swallowing efficiency score for each patient at baseline and after induction chemotherapy. The modified barium swallows were analyzed by five trained clinicians blinded to subject and condition (pre or post chemotherapy). All clinicians met standard target levels of reliability for temporal analysis published by Logemann, 1993.8
The M. D. Anderson Dysphagia Inventory (MDADI) and the Performance Status Scale for Head and Neck Cancer Patients (PSS-HN) were administered to each patient. The MDADI measures a patient’s global, physical, emotional, and functional perceptions of swallowing dysfunction. This instrument has been validated at our institution in patients with head and neck cancers.9 In addition, diet levels were recorded using the PSS-HN Normalcy of Diet subscale, in which 0 indicates non-oral nutrition and 100 represents full oral nutrition without restrictions. Speech intelligibility was measured using the PSS-HN Understandability of Speech subscale.10 The descriptions of PSS-HN scores for those two subscales are listed in Table 1. The Assessment of Intelligibility of Dysarthric Speech Sentence Level11 was also used to measure the percent of speech intelligibility in many patients. The method for audio recording and transcription was performed similar to that described by Yorkston.11,12 Speech samples were recorded on a high-speed digital audiorecorder (TASCAM DA-30 Digital Audio Tape recorder; Tokoyo, Japan) in a quiet environment using a hand held microphone (Shure SM-48; Niles, IL) placed eight-inches in front of the speaker’s mouth. Each recording was transcribed by an unfamiliar listener comprised of speech pathology graduate trainees listening to the digitally recorded audio samples using head phones (Sennheiser HD 457; Wedemark, Wennebostel – Germany). Judges were instructed to listen to the entire sentence; judges were allowed to listen as many times as needed. The number of words understood was divided by the total number of words to obtain the percentage of speech intelligibility.
Table 1.
Performance status scale for head and neck cancer patients subscale scores and descriptions
| Subscale score | Description |
|---|---|
| Normalcy of diet | |
| 100 | Full diet (no restriction) |
| 90 | Full diet (liquid assist) |
| 80 | All meat |
| 70 | Raw carrots, celery |
| 60 | Dry bread and crackers |
| 50 | Soft chewable foods (e.g., macaroni, canned/soft fruits, cooked vegetables, fish, hamburger, small pieces of meat) |
| 40 | Soft foods requiring no chewing (e.g., mashed potatoes, applesauce, pudding) |
| 30 | Pureed foods (in blender) |
| 20 | Warm liquids |
| 10 | Cold liquids |
| 0 | Non-oral feeding (tube fed) |
| Understandability of speech | |
| 100 | Always understandable |
| 75 | Understandable most of the time; occasional repetition necessary |
| 50 | Usually understandable; face-to-face contact necessary |
| 25 | Difficult to understand |
| 0 | Never understandable; may use written communication |
Comparisons between baseline and post–induction chemotherapy speech and swallowing measures were assessed using the two-tailed t test for dependent samples. t tests and calculations of descriptive statistics were performed using the STATISTICA data analysis software system, version 8.0 (StatSoft, Inc.; www.statsoft.com; Tulsa, OK). A p value of less than .05 was considered to be significant.
RESULTS
Population and Treatment
Twenty-three patients underwent induction chemotherapy followed by surgical resection with or without postoperative radiation therapy for cancer of the oral tongue or floor of the mouth. An MBS study was performed at baseline for 22 of the 23 patients. Fifteen of these 22 patients underwent another MBS study after the completion of induction chemotherapy but before surgical resection and were included in our analysis. Mean age was 37 years (range: 22-47). Of the 15 patients with oral tongue cancer, one had extension to the floor of mouth and one had extension to the floor of mouth and base of tongue.
Pre- and post induction chemotherapy pain scores were available for 12 patients. After chemotherapy, pain scores improved for 8 patients and worsened for 2 patients. Two patients did not report pain at baseline or after chemotherapy. The median pain score was 5.5 (range: 0 – 10) at baseline and 0 (range: 0 – 6) after induction chemotherapy. Table 2 summarizes patient, tumor, and treatment characteristics.
Table 2.
Patient, tumor, and treatment characteristics (n = 15)
| Characteristic | No. (%) of patients |
|---|---|
| Sex | |
| Male | 6 (40) |
| Female | 9 (60) |
| Tumor characteristics | |
| Clinical stage | |
| Tx | 2 (13) |
| T2 | 7 (47) |
| T3 | 6 (40) |
| N0 | 7 (47) |
| N1 | 3 (20) |
| N2b | 4 (27) |
| N2c | 1 (7) |
| AJCC II | 3 (20) |
| AJCC III | 7 (47) |
| AJCC IV | 5 (33) |
| Site of lesion | |
| OT | 13 (87) |
| OT + FOM | 1 (7) |
| OT + FOM + BOT | 1 (7) |
| Induction cycles | |
| 3 | 12 (80) |
| 2 | 3 (20) |
| Pain Scores * | |
| Improved | 8 (67) |
| No change | 2 (17) |
| Worsened | 2 (17) |
Abbreviations: AJCC, American Joint Committee on Cancer; BOT, base of the tongue; FOM, floor of the mouth; OT, oral tongue.
12 patients had pain scores available
OPSE
Figure 2 shows the mean OPSE scores at baseline and after induction chemotherapy for each bolus type liquid, pudding, and cracker. The overall average scores for all consistencies combined were 73.03 at baseline and 72.95 after induction chemotherapy (p = .991). Baseline OPSE scores were 82.2, 73.2, and 63.7 for liquid, pudding, and cracker, respectively. Mean OPSE scores after induction chemotherapy did not differ significantly from baseline scores for any bolus type (liquid, p = .664; pudding, p =0 .442; cracker, p = .421). One patient aspirated during a single thin liquid swallow at baseline due to reduced oral control resulting in aspiration prior to the swallow; the same patient aspirated during thin liquid swallows after induction chemotherapy as a result of both oral and pharyngeal phase dysfunction including reduced hyolaryngeal elevation and weak pharyngeal contraction. Aspiration was not detected for any other patient during baseline or post–induction chemotherapy MBS studies.
Figure 2.
OPSE for each bolus consistency before and after induction chemotherapy.
Quality of Life and Diet Level
MDADI scores and diet levels were significantly higher after induction chemotherapy than at baseline (Figure 3). The mean composite scores from the MDADI (emotional, physical and functional subscales) increased significantly, from 74.0 to 87.3, after induction chemotherapy (p < .001). The individual mean scores for the global, emotional, and physical subscale scores also significantly improved after induction chemotherapy (p < .001). Although the mean score for the functional subscale improved, the difference was not statistically significant (p = .142). Diet scores increased from soft-chewable consistencies at baseline (PSS-HN score, 50) to full diet/all consistencies (PSS-HN score, 100) after induction chemotherapy in most patients, 10/15 and 11/15, respectively (p = .015).
Figure 3.
MDADI responses and PSS-HN scores for patient-reported diet levels.
Speech Intelligibility
Figure 4 shows the comparison of speech intelligibility ratings at baseline and after induction chemotherapy. Speech intelligibility data from the PSS-HN were available for 14 of the 15 patients. Eleven of the 14 patients at baseline and 13 of the 14 patients after induction chemotherapy received a score of 100 (“always understandable”) on the Understandability of Speech subscale. Only 10 of the total 15 patients underwent speech intelligibility testing using the Assessment of Intelligibility of Dysarthric Speech. Average intelligibility was 98.6% at baseline and 92.2% after induction chemotherapy (p = .328). Average speech intelligibility for all patients except one was 99% before and after induction chemotherapy. Excluding a single patient who showed a decrease of 62% in intelligibility between baseline and post–induction chemotherapy (95% to 33%), no clinically meaningful difference was found in speech intelligibility before and after chemotherapy.
Figure 4.

Speech intelligibility ratings at baseline and after induction chemotherapy.
DISCUSSION
To our knowledge, our findings are the first to report the independent effects of induction chemotherapy on speech and swallowing outcomes in patients with tumors of the oral tongue. Although other investigators have reported on functional outcomes in head and neck cancer patients treated with chemotherapy, outcomes have generally been reported after the completion all treatment modalities including chemotherapy, surgery, and radiation therapy.4,5,13,14 Our findings suggest that chemotherapy, independent of other modalities, does not negatively affect speech and swallowing. However, we acknowledge that these are preliminary findings based on a small cohort of patients and that these findings need confirmation in a larger, prospective study.
After induction chemotherapy, our patients did not demonstrate changes in swallowing physiology nor did they demonstrate the functional deterioration commonly reported after definitive treatment regimens for head and neck cancer.4,5,13,14 In fact, after induction chemotherapy, our patients’ perception of their ability to swallow actually improved, as did their diet levels, despite there being no change in swallowing physiology.
Studies have shown that pain is an important component in a patient’s ability to eat, the perception of his or her swallowing potential, and food choices.15,16 We postulate that for some patients, a reduction in tumor size or a decrease in pain after induction chemotherapy may facilitate an improved perception of swallowing ability and the choice of diet. In fact, the majority of patients had an improvement in their pain scores after induction chemotherapy. Further studies are needed to clarify the exact relationship between chemotherapy, pain management, and swallowing function, as well as the effects of perception and the ability to eat.
Global, emotional, and physical subscale scores on the MDADI showed significant improvement after induction chemotherapy (p < .001), but the improvement in functional subscale scores did not reach significance (p = .142). This finding is not surprising, given that the functional subscale questions are generally representative of a return to normal routines in the areas of cooking, income, and social activities. Our patients were still receiving treatment and had not yet returned to normal patterns of work, social relationships, and activities of daily living. Thus, the functional subscale of the MDADI may not provide as much information as the other domains for patients who remain hospitalized or are still receiving treatment.
Quality of life and performance status questionnaires have been used independent of instrumental examinations as indicators of swallowing function.17,18 Despite significant improvements in MDADI and PSS-HN scores in our study, the OPSE scores did not significantly change after induction chemotherapy. These findings reinforce the need for both instrumental examinations, such as the MBS study, in combination with patient-reported measures to comprehensively evaluate swallowing function, because perception does not always reflect actual physiology and vice versa. We find that both measures are useful to explain swallowing behavior.
Another outcome of our study was that induction chemotherapy did not significantly change speech intelligibility. Speech intelligibility remained high at baseline (98.6%) as well as after induction chemotherapy (92.2%), despite the fact that not all patients had tumor regression after induction chemotherapy. This is not an uncommon finding, patients who have articulation problems often remain intelligible because they compensate by slowing their rate of speech or by using more deliberate articulatory placements when tongue mobility is impaired because of a tumor.
One of the strengths of our study is that we prospectively obtained speech and swallowing function data before and after induction chemotherapy, but before any further cancer treatment was provided. Our findings suggest that chemotherapy alone does not significantly affect swallowing physiology in patients with oral tongue cancer. We agree with other investigators 5,6,13,14,15,17 who have reported that the deleterious effects on functional outcomes are likely a product of radiation and surgical intervention, either alone or in combination. Radiation-induced fibrosis and structural alterations after surgery have both been shown to result in dysphagia. It is likely that chemotherapy intensifies the effects of radiation therapy when they are administered concurrently15 but when administered before radiation or surgery may not intensify the functional deterioration.
Overall, the patients in this study showed little change from baseline in swallowing function after induction chemotherapy. One patient who had base of tongue extension of the oral tongue tumor aspirated.. No other patient in this study demonstrated aspiration at baseline or after treatment. Therefore, pharyngeal phase disorders were not characteristic of the dysphagia observed in all but one patient in this study. This is not surprising as most of these patients had tumor involvement of the oral tongue and not the tongue base. Data have shown that tongue base involvement generally results in more severe swallowing dysfunction and aspiration than the dysphagia associated with oral tongue tumors.19 Our patients showed little change in swallowing after chemotherapy; however, the results of this study may have been different in patients with cancers of the oropharynx or larynx who are more likely to have pharyngeal phase swallowing problems at baseline.
In conclusion, we believe that the findings of our study have important implications for quality-of-life outcomes in patients who will be treated using protocols that include induction chemotherapy. While it appears that the addition of induction chemotherapy has a negligible effect on speech and swallowing physiology, our findings provide evidence for symptomatic improvement of swallowing and pain after induction chemotherapy. Further investigations are needed to corroborate our findings.
Footnotes
CS2002-00007045RM (ID01-062) Bristol-Myers Squibb, Phase II Study of Induction Chemotherapy Followed by Surgical Resection for Young Tongue Patients with Squamous Cell Carcinoma of the Oral Tongue and Floor of Mouth; 08/02/02-05/31/2007; $108.750.00.
REFERENCES
- 1.Monnerat C, Faivre S, Temam S, Bourhis J, Raymond E. End points for new agents in induction chemotherapy for locally advanced head and neck cancers. Ann Oncol. 2002;13:995–1006. doi: 10.1093/annonc/mdf172. [DOI] [PubMed] [Google Scholar]
- 2.Licitra L, Vermorken JB. Is there still a role for neoadjuvant chemotherapy in head and neck cancer? Ann Oncol. 2004;15:7–11. doi: 10.1093/annonc/mdh001. [DOI] [PubMed] [Google Scholar]
- 3.Sturgis EM, Moore BA, Glisson BS, Kies MS, Shin DM, Byers RM. Neoadjuvant chemotherapy for squamous cell carcinoma of the oral tongue in young adults: a case series. Head Neck. 2005;27:748–756. doi: 10.1002/hed.20240. [DOI] [PubMed] [Google Scholar]
- 4.Logemann JA. Evaluation and treatment of swallowing disorders. 2nd ed. Pro-Ed; Austin, TX: 1998. pp. 168–180. [Google Scholar]
- 5.Hutcheson KA, Barringer DA, Rosenthal DI, et al. Swallowing outcomes after radiotherapy for laryngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2008;134(2):1–7. doi: 10.1001/archoto.2007.33. [DOI] [PubMed] [Google Scholar]
- 6.Logemann JA, Rademaker AW, Pauloski BR, et al. Site of disease and treatment protocol as correlates of swallowing function in patients with head and neck cancer treated with chemoradiation. Head Neck. 2006;28:64–73. doi: 10.1002/hed.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rademaker AW, Pauloski BR, Logemann JA, et al. Oropharyngeal swallow efficiency as a representative measure of swallowing function. J Speech Hearing Res. 1994;66:314–325. doi: 10.1044/jshr.3702.314. [DOI] [PubMed] [Google Scholar]
- 8.Logemann JA. Manual for the Videofluorgraphic Study of Swallowing. 2nd ed. Pro-Ed; Austin, Texas: 1993. p. 123. [Google Scholar]
- 9.Chen AY, Frankowski R, Bishop-Leone J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson Dysphagia Inventory. Arch Otolaryngol Head Neck Surgery. 2001;127:870–876. [PubMed] [Google Scholar]
- 10.List MA, Ritter-Sterr C, Lansky SB. A performance status scale for head and neck cancer patients. Cancer. 1990;66:564–569. doi: 10.1002/1097-0142(19900801)66:3<564::aid-cncr2820660326>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 11.Yorkston KM, Beukelman DR. Pro-Ed; Austin, TX: 1984. Assessment of intelligibility of dysarthric speech. [Google Scholar]
- 12.Yorkston KM, Beukelman DR. Communication Efficiency of Dysarthric Speakers As Measured By Sentence Intelligibility and Speaker Rate. Journal of Speech and Hearing Disorders. 1981:296–301. doi: 10.1044/jshd.4603.296. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen NP, Frank C, Moltz CC, et al. Aspiration rate following chemoradiation for head and neck cancer: an underreported occurrence. Radiother Oncol. 2006;80:302–306. doi: 10.1016/j.radonc.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 14.Gillespie MB, Brodsky MB, Day TA, et al. Laryngeal penetration and aspiration during swallowing after the treatment of advanced oropharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2005;131:615–619. doi: 10.1001/archotol.131.7.615. [DOI] [PubMed] [Google Scholar]
- 15.Murphy BA, Lewin JS, Ridner S, et al. Mechanisms of weight loss in patients treated with head and neck cancer who were treated with chemoradiation. American Society of Clinical Oncology Education Book. 2006 Spring:340–344. [Google Scholar]
- 16.Wong PC, Dodd MJ, Miaskowski C, et al. Mucositis pain induced by radiation therapy: prevalence, severity, and use of self-care behaviors. J Pain Sympt Mgt. 2006;32:27–37. doi: 10.1016/j.jpainsymman.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Gillespie MB, Brodsky MB, Day TA, et al. Swallowing-related quality of life after head and neck cancer treatment. Laryngoscope. 2004;114:1362–1367. doi: 10.1097/00005537-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Kulbersh BD, Rosenthal EL, McGrew BM, et al. Pretreatment, preoperative swallowing exercises may improve dysphagia quality of life. Laryngoscope. 2006;116:883–886. doi: 10.1097/01.mlg.0000217278.96901.fc. [DOI] [PubMed] [Google Scholar]
- 19.Pauloski BR, Rademaker AW, Logemann JA, et al. Surgical variables affecting swallowing in patients treated for oal/oropharyngeal cancer. Head Neck. 2004;26:625–636. doi: 10.1002/hed.20013. [DOI] [PubMed] [Google Scholar]