Abstract
BACKGROUND
Non–small-cell lung cancer (NSCLC) harboring the anaplastic lymphoma kinase gene (ALK) rearrangement is sensitive to the ALK inhibitor crizotinib, but resistance invariably develops. Ceritinib (LDK378) is a new ALK inhibitor that has shown greater antitumor potency than crizotinib in preclinical studies.
METHODS
In this phase 1 study, we administered oral ceritinib in doses of 50 to 750 mg once daily to patients with advanced cancers harboring genetic alterations in ALK. In an expansion phase of the study, patients received the maximum tolerated dose. Patients were assessed to determine the safety, pharmacokinetic properties, and antitumor activity of ceritinib. Tumor biopsies were performed before ceritinib treatment to identify resistance mutations in ALK in a group of patients with NSCLC who had had disease progression during treatment with crizotinib.
RESULTS
A total of 59 patients were enrolled in the dose-escalation phase. The maximum tolerated dose of ceritinib was 750 mg once daily; dose-limiting toxic events included diarrhea, vomiting, dehydration, elevated aminotransferase levels, and hypophosphatemia. This phase was followed by an expansion phase, in which an additional 71 patients were treated, for a total of 130 patients overall. Among 114 patients with NSCLC who received at least 400 mg of ceritinib per day, the overall response rate was 58% (95% confidence interval [CI], 48 to 67). Among 80 patients who had received crizotinib previously, the response rate was 56% (95% CI, 45 to 67). Responses were observed in patients with various resistance mutations in ALK and in patients without detectable mutations. Among patients with NSCLC who received at least 400 mg of ceritinib per day, the median progression-free survival was 7.0 months (95% CI, 5.6 to 9.5).
CONCLUSIONS
Ceritinib was highly active in patients with advanced, ALK-rearranged NSCLC, including those who had had disease progression during crizotinib treatment, regardless of the presence of resistance mutations in ALK. (Funded by Novartis Pharmaceuticals and others; ClinicalTrials.gov number, NCT01283516.)
Genetic alterations in the anaplastic lymphoma kinase gene (ALK) are implicated in the pathogenesis of several human cancers.1 ALK can be aberrantly activated by mutation, gene amplification, or chromosomal rearrangement, leading to the expression of a potent oncogenic driver. In non–small-cell lung cancer (NSCLC), ALK rearrangement occurs in approximately 5% of cases.2–8 ALK-rearranged tumors depend on ALK for growth and survival and show marked sensitivity to ALK inhibitors such as crizotinib. Among patients with advanced, ALK-rearranged NSCLC, crizotinib has been associated with response rates of approximately 60% across multiple studies and a median progression-free survival of 8 to 10 months.9–11
Despite initial responses to crizotinib, the majority of patients have a relapse within 12 months, owing to the development of resistance.12,13 Acquired resistance has been observed in other oncogene-dependent tumors, including epidermal growth factor receptor (EGFR)–mutated NSCLC treated with EGFR inhibitors14–16 and chronic myeloid leukemia treated with ABL inhibitors.17,18 Approximately one third of patients with ALK-rearranged NSCLC have a relapse owing to an acquired mutation within the ALK tyrosine kinase domain or amplification of the ALK fusion gene.12,13 In the remaining resistant cases, the ALK fusion gene is unchanged, and a variety of resistance mechanisms have been reported.12,13,19 Treatment options after the failure of crizotinib are limited and include cytotoxic chemotherapy, palliative radiotherapy, or supportive care.20
Ceritinib (LDK378, Novartis Pharmaceuticals) is an oral, small-molecule, ATP-competitive, tyrosine kinase inhibitor of ALK.21 In enzymatic assays, ceritinib is 20 times as potent as crizotinib against ALK.22 In contrast to crizotinib, ceritinib does not inhibit the kinase activity of MET; however, it does inhibit the insulin-like growth factor 1 (IGF-1) receptor, although the inhibition of the IGF-1 receptor is less potent than the inhibition of ALK by a factor of 50.23 In xenograft models of ALK-rearranged NSCLC, ceritinib showed marked antitumor activity against both crizotinib-sensitive and crizotinib-resistant tumors.21,22 These preclinical studies suggest that ceritinib may be active in patients with NSCLC who have not received crizotinib previously, as well as in patients who have had disease progression during crizotinib treatment.
We conducted a phase 1 study of ceritinib to determine the safety, maximum tolerated dose (MTD), pharmacokinetic properties, and antitumor activity of this drug in patients with advanced, ALK-rearranged NSCLC and other cancers harboring ALK alterations.
METHODS
PATIENTS
Eligible patients had a locally advanced or metastatic cancer harboring genetic alterations in ALK. In patients with NSCLC, the demonstration of ALK rearrangement was required in at least 15% of tumor cells by means of a fluorescence in situ hybridization (FISH) assay, with the use of break-apart probes. ALK FISH testing at a central laboratory was not required.
Other eligibility criteria included an age of 18 years or older, an Eastern Cooperative Oncology Group performance status score of 0, 1, or 2 (on a scale from 0 to 5, with 0 indicating that the patient is fully active and higher numbers indicating greater disability), and adequate end-organ function. One patient with an ECOG performance status score of 3 was enrolled with an eligibility waiver because the score had changed from 2 to 3 during screening, after the patient had provided consent for the study (Table 1). Patients with asymptomatic untreated or treated central nervous system metastases were eligible, as were patients who had received prior treatment with one or more ALK inhibitors.
Table 1.
Characteristics of the Patients at Baseline.
| Characteristic | All Patients (N = 130) |
|---|---|
| Age — yr | |
| Median | 53 |
| Range | 22–80 |
| Female sex — no. (%) | 78 (60) |
| Race — no. (%)* | |
| White | 97 (75) |
| Asian | 29 (22) |
| Other | 4 (3) |
| Smoking status — no. (%) | |
| Never smoked | 81 (62) |
| Former smoker | 44 (34) |
| Current smoker | 5 (4) |
| ECOG performance status score — no. (%)† | |
| 0 | 25 (19) |
| 1 | 89 (68) |
| 2 | 15 (12) |
| 3 | 1 (1) |
| Tumor type — no. (%) | |
| Non–small-cell lung cancer | 122 (94) |
| Breast cancer | 4 (3) |
| Alveolar rhabdomyosarcoma | 1 (1) |
| Inflammatory myofibroblastic tumor | 1 (1) |
| Anaplastic large-cell lymphoma | 1 (1) |
| Rectal adenocarcinoma | 1 (1) |
| Select sites of metastases — no. (%) | |
| Lung | 101 (78) |
| Thoracic lymph nodes | 73 (56) |
| Brain | 64 (49) |
| Liver | 51 (39) |
| Bone | 49 (38) |
| Meninges | 1 (1) |
| Prior crizotinib therapy for non–small-cell lung cancer — no./total no. (%) | |
| Yes | 83/122 (68) |
| No | 39/122 (32) |
Race was determined by the investigator.
Eastern Cooperative Oncology Group (ECOG) performance status scores range from 0 to 5, with 0 indicating that the patient is fully active and higher numbers indicating greater disability. One patient with an ECOG performance status score of 3 was enrolled with an eligibility waiver because the score had changed from 2 to 3 during screening, after the patient had provided consent for the study.
STUDY OVERSIGHT
This study was conducted in accordance with the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonisation. The protocol, which is available with the full text of this article at NEJM.org, was approved by the local human investigations committee at each participating site. Written informed consent was obtained from all the patients before screening.
The study was designed by the sponsor (Novartis Pharmaceuticals) together with the study investigators. The sponsor collected the data and analyzed them in conjunction with the authors. The first author wrote the first draft of the manuscript. Editorial support was provided by Articulate Science and funded by the sponsor. All the authors made the decision to submit the manuscript for publication and vouch for the accuracy of the data and analyses reported and for the fidelity of the study to the protocol.
STUDY DESIGN
The primary objective was to determine the MTD of ceritinib in adult patients with tumors harboring a genetic alteration in ALK. Key secondary objectives were to characterize the safety and side-effect profile, pharmacokinetic profile, and antitumor activity of ceritinib. The study included a dose-escalation phase, followed by an expansion phase in which all the patients received treatment at the maximum dose established in the dose-escalation phase. In the dose-escalation phase, treatment included a single ceritinib dose, followed by a 3-day pharmacokinetic evaluation period, and subsequent daily oral dosing for the remainder of the cycle. Daily dosing of ceritinib was continued in 21-day cycles. The starting dose was 50 mg daily, on the basis of preclinical safety data.
Dose escalation was guided by means of a two-parameter Bayesian logistic-regression model with the use of the principle of escalation with overdose control.24,25 For the expansion phase, patients with ALK-rearranged NSCLC (regardless of whether the patient had received an ALK inhibitor previously) and patients with other ALK-activated tumors were enrolled and received treatment with the MTD of ceritinib that had been established in the dose-escalation phase. Patients continued treatment with ceritinib until the disease progressed, an unacceptable level of toxic events developed, or the patient withdrew consent. Treatment after disease progression was not permitted, unless the sole site of progression was the central nervous system.
STUDY ASSESSMENTS
Adverse events were graded according to the Common Terminology Criteria for Adverse Events, version 4.0 (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf). Blood samples for pharmacokinetic assessments were obtained in both the dose-escalation and expansion phases of the study.
All the patients underwent tumor imaging at baseline, including computed tomography of the chest and abdomen, as well as brain imaging. Restaging scans were obtained at 6-week intervals during treatment and were assessed by investigators according to the Response Evaluation Criteria in Solid Tumors, version 1.0 (www.iconplc.com/services/imaging/RECIST-1.0-Criteria-ICON-Medical-Imaging.pdf).
MOLECULAR ANALYSIS OF TUMOR SAMPLES
At a single center, patients with NSCLC who had had disease progression during crizotinib treatment underwent tumor biopsy before starting ceritinib therapy. All the samples were evaluated for ALK rearrangement and ALK gene amplification with the use of FISH. Resistance mutations in ALK were identified as described previously.12
STATISTICAL ANALYSIS
For the dose-escalation study, the Bayesian logistic-regression model was used to estimate the posterior distributions for the probabilities of dose-limiting toxic events at various dose levels after each cohort of patients (Table S1 in the Supplementary Appendix, available at NEJM.org). The MTD was defined as the dose associated with the highest probability that dose-limiting toxic events would occur in 16% to less than 33% of patients and as the dose that did not exceed the overdose criterion (<25% probability that dose-limiting toxic events would occur in ≥33% of patients).
For the secondary efficacy and safety end points, data from patients in the dose-escalation and expansion phases who received the MTD were pooled. Safety data are summarized for all the patients who received at least one dose of ceritinib. Efficacy data are summarized for all the patients with NSCLC who received at least one dose of ceritinib. Pharmacokinetic analyses were based on data from patients in the dose-escalation phase. The current analysis includes all the patients who received ceritinib by October 19, 2012. The data-cutoff date was August 2, 2013. Enrollment in the expansion phase continued through July 2013.
RESULTS
PATIENTS
As of October 19, 2012, a total of 130 patients had been treated: 59 patients in the dose-escalation phase and 71 in the expansion phase. The majority of patients (122 of 130 patients [94%]) had advanced NSCLC (Table 1) and had received cytotoxic chemotherapy previously (Table S2 in the Supplementary Appendix). A total of 83 of 122 patients with NSCLC (68%) had received crizotinib previously. Of the 8 patients with cancers other than NSCLC, 4 had breast cancer and 1 patient each had alveolar rhabdomyosarcoma, rectal adenocarcinoma, anaplastic large-cell lymphoma, and inflammatory myofibroblastic tumor (Table 1).
ADVERSE EVENTS
Patients in the dose-escalation phase of the study were treated at dose levels of 50 to 750 mg daily. Dose-limiting toxic events occurred in six patients during cycle 1, at daily doses of 400 mg or more (Table S3 in the Supplementary Appendix). Dose-limiting toxic events included diarrhea (at a daily dose of ≥600 mg), vomiting (at 750 mg daily), nausea (at 750 mg daily), dehydration (at 600 mg daily), elevated alanine aminotransferase level (at 400 mg daily), and hypophosphatemia (at 400 mg daily). All dose-limiting toxic events resolved on discontinuation of treatment. Treatment was resumed in all patients except one, who had simultaneous disease progression. In all the patients, the study drug was interrupted for 8 days or less. On the basis of the dose-limiting toxic events that occurred during cycle 1, the Bayesian logistic-regression model permitted escalation to the 900-mg daily dose. However, owing to persistent gastrointestinal adverse events of grade 2 and aminotransferase elevations of grade 3 in later cycles, the MTD was determined to be 750 mg daily.
Table S4 in the Supplementary Appendix summarizes the adverse events (those occurring in ≥5% of patients) of any grade and any cause. The most common adverse events included nausea (in 82% of patients), diarrhea (in 75%), vomiting (in 65%), fatigue (in 47%), and increased alanine aminotransferase levels (in 35%). The most common adverse events of grade 3 or 4 that were considered to be related to the study drug were increased alanine aminotransferase levels (in 21% of patients), increased aspartate aminotransferase levels (in 11%), diarrhea (in 7%), and increased lipase levels (in 7%) (Table 2), all of which were reversible on discontinuation of treatment.
Table 2.
Adverse Events of Grade 3 or 4 That Were Suspected to Be Related to Ceritinib Therapy.*
| Dose | |||||||
|---|---|---|---|---|---|---|---|
| Event | 50–300 mg/day (N = 10) |
400 mg/day (N = 14) |
500 mg/day (N = 10) |
600 mg/day (N = 10) |
700 mg/day (N = 5) |
750 mg/day (N = 81) |
Total (N = 130) |
| number of patients with event (percent) | |||||||
| Any event | 2 (20) | 4 (29) | 3 (30) | 5 (50) | 4 (80) | 46 (57) | 64 (49) |
| Elevated alanine aminotransferase level | 1 (10) | 1 (7) | 2 (20) | 0 | 4 (80) | 19 (23) | 27 (21) |
| Elevated aspartate aminotransferase level | 0 | 1 (7) | 0 | 0 | 3 (60) | 10 (12) | 14 (11) |
| Diarrhea | 0 | 1 (7) | 1 (10) | 1 (10) | 0 | 6 (7) | 9 (7) |
| Elevated lipase level | 0 | 0 | 0 | 1 (10) | 0 | 8 (10) | 9 (7) |
| Nausea | 0 | 0 | 1 (10) | 0 | 0 | 6 (7) | 7 (5) |
| Fatigue | 0 | 0 | 1 (10) | 0 | 0 | 5 (6) | 6 (5) |
| Vomiting | 0 | 0 | 0 | 1 (10) | 0 | 5 (6) | 6 (5) |
| Hypophosphatemia | 0 | 1 (7) | 0 | 1 (10) | 0 | 2 (2) | 4 (3) |
| Elevated amylase level | 0 | 0 | 0 | 1 (10) | 0 | 2 (2) | 3 (2) |
| Elevated blood alkaline phosphatase level | 0 | 0 | 0 | 0 | 1 (20) | 2 (2) | 3 (2) |
| Hyperglycemia | 0 | 0 | 0 | 0 | 0 | 3 (4) | 3 (2) |
Adverse events listed here are those that were reported in at least 2% of patients and that were suspected to be related to the study drug. Events were graded according to the Common Terminology Criteria for Adverse Events, version 4.0. Patients who had more than one occurrence of the same event were only counted once within each category. Patients were categorized according to the initial dose received.
We noted four cases of interstitial lung disease that were possibly related to ceritinib therapy; all resolved with the discontinuation of ceritinib and the administration of standard treatments. We also observed one case of asymptomatic grade 3 prolongation of the corrected QT inter-Ceritinib in Non–Small-Cell Lung Cancer val that was possibly related to ceritinib. A total of 66 of 130 patients (51%) required at least one dose reduction, and the median duration of treatment interruption was 7.3 days. In 8 of 130 patients (6%), the study drug was permanently discontinued owing to an adverse event. At the 750-mg dose level, 50 of 81 patients (62%) required at least one dose reduction; in 32 patients, this reduction occurred in cycle 3 or later. No treatment-related deaths occurred.
PHARMACOKINETICS
Pharmacokinetic analyses showed that exposure to ceritinib increased with the dose. The increase in the maximal plasma concentration (Cmax) was slightly greater than proportional to the dose across the daily dose range of 50 mg through 750 mg (Fig. S1A in the Supplementary Appendix). During the 3-day pharmacokinetic evaluation period after the administration of the first dose of ceritinib at the established MTD, Cmax of ceritinib was achieved approximately 6 hours after receipt of the dose (Fig. S1B in the Supplementary Appendix), and the mean terminal half-life of ceritinib was approximately 40 hours. The mean (±SD) area under the plasma concentration–time curve over a 24-hour period on day 8 was 16,500±4750 ng per milliliter per hour. The mean Cmax was 800±205 ng per milliliter. On the basis of trough concentrations after repeated daily dosing, steady-state levels of ceritinib were achieved by approximately day 15.
EFFICACY
Tumor Response
Among the 8 patients with NSCLC treated with ceritinib at a dose of 50 to 300 mg daily, 2 had a confirmed partial response: 1 who had not received crizotinib previously (daily ceritinib dose, 200 mg) and 1 who had received crizotinib previously (daily ceritinib dose, 300 mg). A total of 114 patients with NSCLC received at least 400 mg of ceritinib daily, of whom 1 (1%) had a confirmed complete response, 65 (57%) had a confirmed partial response, and 25 (22%) had stable disease; 12 patients (11%) had progressive disease on the first restaging scans, and for 11 patients (10%), the response was not known owing to early withdrawal from the study (Fig. 1A, and Table S5 in the Supplementary Appendix). The overall response rate was 58% (95% confidence interval [CI], 48 to 67). Among the 78 patients with NSCLC who received 750 mg daily, 46 had a confirmed partial response, for an overall response rate of 59% (95% CI, 47 to 70).
Figure 1. Response to Ceritinib in ALK-Rearranged Non–Small-Cell Lung Cancer (NSCLC).
Panel A shows the change in tumor size after patients received ceritinib at doses of 400 to 750 mg per day. The bars indicate the largest percentage change in target lesions from baseline. The dashed line indicates a 30% reduction from baseline. Dots below individual bars indicate patients with disease progression or death at the time of data cutoff. Panel B shows positron- emission tomographic scans taken at baseline (left) and after 3.5 weeks of ceritinib treatment (right) in a patient with crizotinib-resistant disease. Subsequent computed tomographic scans after 6 weeks of ceritinib treatment showed a 52% reduction in tumor burden in this patient.
The majority of patients with NSCLC who were treated with ceritinib had received crizotinib previously (83 of 122 patients [68%]) (Table 1). Among patients previously treated with crizotinib, the overall response rate was 56% (95% CI, 45 to 67) among those who received ceritinib at a dose of 400 mg or more daily (45 of 80 patients) and 56% (95% CI, 41 to 70) among those treated with ceritinib at a dose of 750 mg daily (28 of 50 patients) (Table S5 in the Supplementary Appendix). Some responses were rapid and dramatic (Fig. 1B). In addition, responses were seen in untreated lesions in the central nervous system in patients who had received crizotinib previously (Fig. S2 in the Supplementary Appendix). Similar tumor responses were observed in the group of patients who had not received crizotinib previously. Among the 34 patients who had not received crizotinib previously and who were treated with at least 400 mg of ceritinib daily, 21 had a partial response, for an overall response rate of 62% (95% CI, 44 to 78).
Among the eight patients with advanced cancers other than NSCLC, two had a response to ceritinib: one patient with anaplastic large-cell lymphoma, and one with inflammatory myofibroblastic tumor. The remaining six patients, including four with advanced breast cancer and increased ALK copy number, did not have a response to ceritinib (Table S6 in the Supplementary Appendix).
Duration of Response and Progression-free Survival
Among the 66 patients with NSCLC who had a response and who had been treated with at least 400 mg of ceritinib daily, 64% (95% CI, 50 to 74) had a duration of response of 6 months or longer. The median duration of response was 8.2 months (95% CI, 6.9 to 11.4); however, data for 47% of the patients with a response (31 of 66 patients) were censored at the time of data cutoff. Among 114 patients with NSCLC who received at least 400 mg of ceritinib daily, the median follow-up was 9.5 months (range, 0.3 to 24.4), and the median progression-free survival was 7.0 months (95% CI, 5.6 to 9.5), with data for 43 patients (38%) censored.
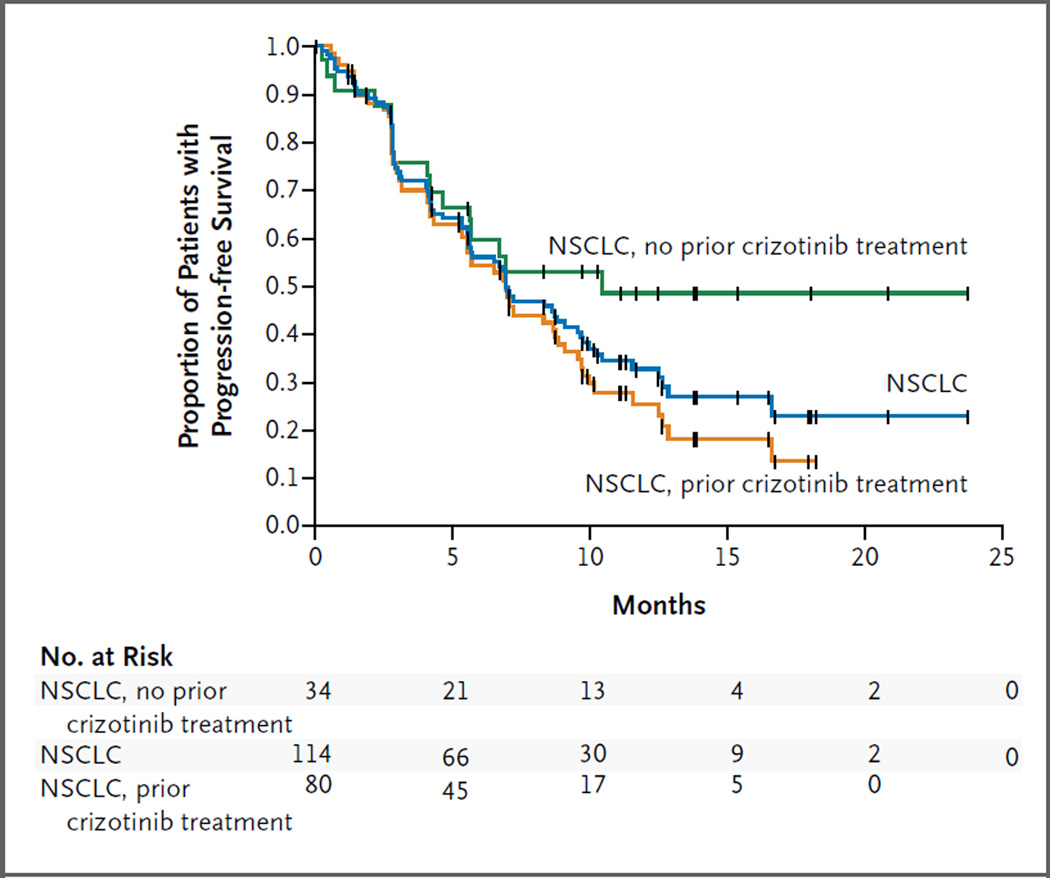
In the subgroup of 80 patients with NSCLC who had previously received crizotinib, the median progression-free survival was similar, at 6.9 months (95% CI, 5.3 to 8.8) (Fig. 2). In the subgroup of 34 patients with NSCLC who had not received crizotinib previously, the median progression- free survival was 10.4 months (95% CI, 4.6 to could not be estimated), with data for 18 patients (53%) censored and a median follow-up of 9.5 months (Fig. 2). Among 64 patients with central nervous system metastases at baseline, the median progression-free survival (with a daily ceritinib dose of ≥400 mg) was similar to that among 50 patients without central nervous system metastases (6.9 and 7.0 months, respectively; P = 0.37 by the log-rank test).
Figure 2. Progression-free Survival.
Shown are Kaplan–Meier estimates of progression-free survival among patients with advanced, ALK-rearranged non–small-cell lung cancer (NSCLC) who received ceritinib at doses of 400 to 750 mg daily. In these 114 patients, the median progression-free survival was 7.0 months (blue). In the subgroup of 80 patients who had received crizotinib previously, the median progression-free survival was 6.9 months (orange). In the subgroup of 34 patients who had not received crizotinib previously, the median progression-free survival was not reached (green). Vertical lines on the survival curves indicate censoring of data.
The durations of treatment are shown in Fig. S3 in the Supplementary Appendix. At the time of data cutoff, the overall survival data were immature, with data for 72% of the patients having been censored. The overall survival rate at 12 months was 65%, and the median overall survival had not been reached.
MOLECULAR DETERMINANTS OF RESPONSE
A total of 19 patients with NSCLC who had had disease progression during crizotinib treatment underwent repeat tumor biopsy at one institution before study treatment. All the samples were positive for ALK rearrangement according to FISH; 2 of the 19 samples showed ALK gene amplification, and 5 were found to harbor secondary resistance mutations in the ALK tyrosine kinase domain. In the remaining 12 cases, no genetic alteration of ALK other than the original rearrangement was identified.
Tumor responses in this subgroup of patients classified according to molecular status are summarized in Figure 3. Tumor regression was observed in all the patients, regardless of molecular status. Confirmed responses were seen in 6 of 7 patients with ALK gene amplification or mutation and in 7 of 12 patients without ALK alteration. These findings suggest that the activity of ceritinib in patients whose tumors had progressed during crizotinib treatment may be independent of the underlying mechanism of acquired resistance.
Figure 3. Correlation of Response to Ceritinib with ALK Gene Alteration among Patients with Crizotinib Resistance.
A total of 19 patients with crizotinib-resistant, ALK-rearranged non–small-cell lung cancer underwent biopsy at one study site before the initiation of ceritinib. Shown here is the largest percentage decrease in target lesions in these 19 patients. All the tumors were positive for ALK rearrangement, on the basis of the standard fluorescence in situ hybridization (FISH) assay with the use of break-apart probes. ALK genotypes are shown above the bars. Amp denotes amplification of the ALK fusion gene as determined by means of FISH, and NM no ALK mutation or amplification. Data are shown for patients who had received crizotinib as the last therapy before ceritinib treatment (dark blue bars) and for patients who received any intervening systemic therapy between crizotinib and ceritinib (light blue bars). Dots below individual bars indicate patients with disease progression or death at the time of data cutoff.
DISCUSSION
Ceritinib showed clinical activity in patients with advanced, ALK-rearranged NSCLC. Ceritinib at a dose of 400 mg or more daily was similarly effective in patients who had received prior crizotinib treatment and in those who had not received crizotinib previously. Adverse events were primarily gastrointestinal and were of grade 1 or 2. Approximately half the patients required some dose modification.
In NSCLC, the benefit of targeted therapies in subgroups classified according to molecular status has been limited by the development of drug resistance. In the case of EGFR-mutated NSCLC, irreversible pan-ERBB inhibitors have been developed to overcome resistance. Despite promising activity in resistant models in preclinical studies, the response rates reported with these agents among patients who have had a relapse during treatment with first-generation EGFR inhibitors are less than 10%.26–28 In contrast, ceritinib was active in the majority of patients with ALK-rearranged NSCLC who received crizotinib previously. Among these patients, the overall response rate and median progression-free survival observed with ceritinib were similar to those seen after initial crizotinib treatment. This suggests that in patients with ALK-rearranged NSCLC, a more potent and specific ALK inhibitor can effectively treat the majority of patients in whom resistance to crizotinib develops.
Several distinct mechanisms of resistance to crizotinib have been described, including ALK gene amplification and a variety of secondary resistance mutations in the ALK tyrosine kinase domain.12,13,19 In the absence of ALK alterations, cell growth and survival in preclinical models are often driven by alternative signaling pathways, and resistant cells are presumed to be resistant to ALK inhibition. In this study, marked antitumor activity was observed in patients treated with ceritinib, with responses occurring in the majority of patients who had been treated with crizotinib, including those without an ALK mutation or amplification. These findings suggest that the large majority of crizotinib-resistant tumors may remain ALK-dependent and that an important factor contributing to crizotinib resistance may be subtherapeutic inhibition of the target, which may be overcome by more potent and structurally distinct ALK inhibitors such as ceritinib. Alternatively, ceritinib may inhibit an unknown kinase that has not yet been found to play a role in the biology of these tumors.
Overall, the safety profile of ceritinib was similar but not identical to that of crizotinib. Gastrointestinal adverse events have been seen frequently with both drugs. However, drug-related diarrhea of grade 3 or 4 was reported in 9 of 130 patients (7%) treated with ceritinib, as compared with 0 of 321 patients treated with crizotinib.9,11 Similarly, ceritinib had a higher incidence of grade 3 or 4 nausea than crizotinib (5% vs. 1%).9,11 Like crizotinib, ceritinib was associated with liver-function abnormalities, most commonly an elevated level of alanine aminotransferase (in 21% of patients). These abnormalities were not associated with an elevated bilirubin level and resolved with temporary discontinuation of the study drug.
Among patients with ALK-rearranged NSCLC for whom crizotinib is no longer effective, more potent inhibition of the target by a structurally distinct ALK kinase inhibitor such as ceritinib can induce substantial and durable responses in the majority of cases. Confirmatory trials of the clinical activity of ceritinib in NSCLC are needed, involving patients who have received prior crizotinib treatment and those who have not.
Supplementary Material
Acknowledgments
Supported by Novartis Pharmaceuticals, by a grant from the National Cancer Institute (5R01CA164273, to Drs. Shaw and Engelman), by a V Foundation Translational Research Grant (to Drs. Shaw and Engelman), by Be a Piece of the Solution, and by the Evan Spirito Memorial Foundation.
We thank the participating patients, their families, research coordinators, and nurses; and Matthew Naylor (Articulate Science) for providing editorial assistance with an earlier version of the manuscript.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Barreca A, Lasorsa E, Riera L, et al. Anaplastic lymphoma kinase in human cancer. J Mol Endocrinol. 2011;47:R11–R23. doi: 10.1530/JME-11-0004. [DOI] [PubMed] [Google Scholar]
- 2.Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14:4275–4283. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol. 2008;3:13–17. doi: 10.1097/JTO.0b013e31815e8b60. [DOI] [PubMed] [Google Scholar]
- 4.Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115:1723–1733. doi: 10.1002/cncr.24181. [DOI] [PubMed] [Google Scholar]
- 5.Boland JM, Erdogan S, Vasmatzis G, et al. Anaplastic lymphoma kinase immunoreactivity correlates with ALK gene rearrangement and transcriptional upregulation in non-small cell lung carcinomas. Hum Pathol. 2009;40:1152–1158. doi: 10.1016/j.humpath.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Martelli MP, Sozzi G, Hernandez L, et al. EML4-ALK rearrangement in non-small cell lung cancer and non-tumor lung tissues. Am J Pathol. 2009;174:661–670. doi: 10.2353/ajpath.2009.080755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res. 2009;15:5216–5223. doi: 10.1158/1078-0432.CCR-09-0802. [Erratum, Clin Cancer Res 2009; 15:7110.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi T, Sonobe M, Kobayashi M, et al. Clinicopathologic features of nonsmall- cell lung cancer with EML4-ALK fusion gene. Ann Surg Oncol. 2010;17:889–897. doi: 10.1245/s10434-009-0808-7. [DOI] [PubMed] [Google Scholar]
- 9.Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–1019. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DW, Ahn MJ, Shi Y, et al. Updated results of a global phase II study with crizotinib in advanced ALK-positive nonsmall cell lung cancer; Presented at the European Society for Medical Oncology Annual Congress, Vienna, September 28– October 2, 2012. abstract. [Google Scholar]
- 11.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 12.Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4:120ra17. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18:1472–1482. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer. 2009;10:281–289. doi: 10.3816/CLC.2009.n.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non–small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 17.Roumiantsev S, Shah NP, Gorre ME, et al. Clinical resistance to the kinase inhibitor STI-571 in chronic myeloid leukemia by mutation of Tyr-253 in the Abl kinase domain P-loop. Proc Natl Acad Sci U S A. 2002;99:10700–10705. doi: 10.1073/pnas.162140299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 19.Shaw AT, Engelman JA. ALK in lung cancer: past, present, and future. J Clin Oncol. 2013;31:1105–1111. doi: 10.1200/JCO.2012.44.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network. NCCN guidelines: non-small-cell lung cancer, v2. 2013 ( http://www.nccn.org/professionals/physician_gls/recently_updated.asp)
- 21.Marsilje TH, Pei W, Chen B, et al. Synthesis, structure-activity relationships and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor LDK378 currently in phase 1 and 2 clinical trials. J Med Chem. 2013;56:5675–5690. doi: 10.1021/jm400402q. [DOI] [PubMed] [Google Scholar]
- 22.Li N, Michellys PY, Kim S, et al. Activity of a potent and selective phase I ALK inhibitor LDK378 in naive and crizotinibresistant preclinical tumor models; Presented at the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics, San Francisco, November 12–16, 2011. abstract. [Google Scholar]
- 23.Shaw AT, Mehra R, Kim D-W, et al. Clinical activity of the ALK Inhibitor LDK378 in advanced, ALK-positive NSCL; Presented at the Annual Meeting of the American Society of Clinical Oncology, Chicago, May 31–June 4, 2013. abstract. [Google Scholar]
- 24.Babb J, Rogatko A, Zacks S. Cancer phase I clinical trials: efficient dose escalation with overdose control. Stat Med. 1998;17:1103–1120. doi: 10.1002/(sici)1097-0258(19980530)17:10<1103::aid-sim793>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Neuenschwander B, Branson M, Gsponer T. Critical aspects of the Bayesian approach to phase I cancer trials. Stat Med. 2008;27:2420–2439. doi: 10.1002/sim.3230. [DOI] [PubMed] [Google Scholar]
- 26.Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012;13:528–538. doi: 10.1016/S1470-2045(12)70087-6. [Erratum, Lancet Oncol 2012;13(5):e186. [DOI] [PubMed] [Google Scholar]
- 27.Sequist LV, Besse B, Lynch TJ, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: results of a phase II trial in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:3076–3083. doi: 10.1200/JCO.2009.27.9414. [DOI] [PubMed] [Google Scholar]
- 28.Campbell A, Reckamp KL, Camidge DR, et al. PF-00299804 (PF299) patient (pt)-reported outcomes (PROs) and efficacy in adenocarcinoma (adeno) and nonadeno non-small cell lung cancer (NSCLC): a phase (P) II trial in advanced NSCLC after failure of chemotherapy (CT) and erlotinib (E); Presented at the Annual Meeting of the American Society of Clinical Oncology, Chicago, June 4–8, 2010. abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.