Abstract
Objective
To examine the association of common chronic disease and multimorbidity with subjective cognitive impairment (SCI) and cognitive impairment no-dementia (CIND), and to explore the contribution of genetic background and shared familial environment to these associations.
Design
Population-based twin study.
Setting
Nationwide Swedish twins.
Subjects
11,379 dementia-free individuals aged ≥ 65 from the Swedish Twin Registry.
Main Outcome Measures
SCI was defined as subjective complaint of cognitive change without objective cognitive impairment, and CIND was ascertained according to the standard definition. Chronic diseases were classified based on international criteria, and multimorbidity was assessed as the co-occurrence of at least two chronic diseases in the same individual.
Results
In unmatched, fully-adjusted regression models, musculoskeletal, respiratory, and urological diseases were significantly associated with increased odds ratios (ORs) of both SCI and CIND. Circulatory and gastrointestinal disorders were related to SCI, while endocrine diseases were associated with CIND. The adjusted ORs of multimorbidity were 2.1 [95% Confidence Intervals (95% CI): 1.8–2.3] for SCI and 1.5 for CIND (95% CI: 1.3–1.8). There was a significant dose-dependent relationship between number of chronic diseases and ORs for SCI but not for CIND. In co-twin control analyses, the chronic diseases-SCI association remained significant but the association with CIND was no longer statistically significant.
Conclusion
Chronic diseases are associated with both SCI and CIND and the association is stronger when there is multimorbidity. Genetic and shared environmental factors may partially explain the association of CIND but not that of SCI with chronic diseases.
Keywords: Cognitive impairment no-dementia (CIND), chronic diseases, multimorbidity, population-based, subjective cognitive impairment (SCI), twin-study
Introduction
In progressively aging populations, cognitive impairment and other chronic diseases have become one of the greatest challenges to health care systems, representing the major cause of loss of life and health worldwide.1 As chronic diseases may co-occur in the elderly person,2 the concept of multimorbidity has been proposed and is often defined as the co-existence of two or more chronic conditions. In addition to strongly influencing physical function,3 chronic diseases may also affect cognitive functioning leading to cognitive impairment.4–7 This intermediate stage of cognitive deterioration has gained clinical and public health relevance on the ground of its strong association with incident dementia.8 During the past two decades, different definitions of cognitive impairment have been proposed. In epidemiological studies the construct of cognitive impairment no dementia (CIND) has been frequently used.9,10 CIND includes people who have objective cognitive deficits but do not fulfill criteria for dementia. Recently, it has been suggested that objective cognitive impairment may be preceded by a stage when the cognitive changes are merely subjective.11 At this stage pathological brain changes may already be occurring but are not yet behaviorally manifest and cannot be captured by cognitive tests.12 It has been shown that people with subjective cognitive impairment (SCI) are between 3.5 and 4.5 times more likely to develop dementia after 1 to 7 years.13,14
Only few studies have explored the association between chronic diseases and subjective cognitive complaints15, 16 and, to date, the only study that excluded CIND from the definition of SCI, as we did, found no association between SCI and individual chronic disease.17 Other studies addressing the relation of CIND and chronic diseases were conducted on relatively small samples without the assessment of multimorbidity.4–7
Although a life-course basis for cognitive dysfunction and chronic disease has been suggested,18 the contribution of genetics and early-life environments to the association between multimorbidity and cognitive impairment has not been investigated. In the current investigation, conducted in the framework of the large, population-based, Swedish twin-study on aging and dementia (HARMONY), we aimed to: 1) examine the association of individual chronic disease and multimorbidity with SCI and CIND; and 2) explore the contribution of genetic background and early-life environments to these associations.
Methods
Study Population
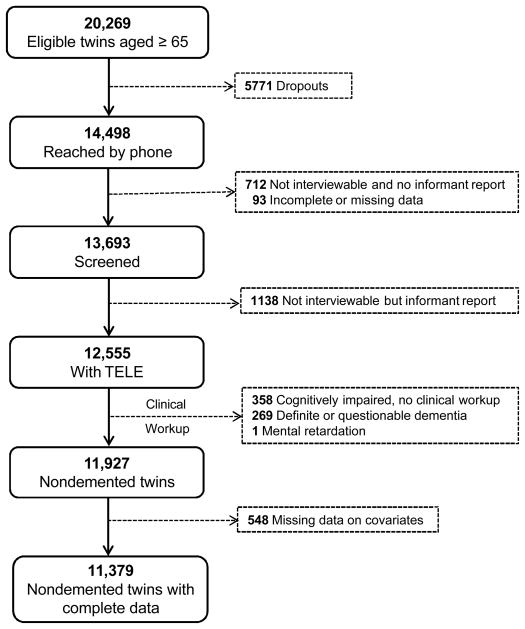
Participants were members of the nationwide Swedish Twin Registry. In 1998–2001, all living twins in the registry born in 1935 and earlier (aged ≥65 years) were invited to participate in HARMONY, a population-based twin study of aging and dementia.19 Of the 20,269 eligible twin individuals, 5,771 were not reached by phone and 712 were reached but unable to partake in the interview and had no available informant. For a further 93 subjects the information was incomplete or missing. Of the remaining 13,693 twin individuals, 1138 were unable to participate in the interview because of sickness or severe cognitive impairment but had an interviewable next of kin. Among the 12,555 participants with cognitive screening data, people with: dementia (n=144), questionable dementia (n=125), cognitive impairment and missing clinical workup (n=358), or mental retardation (n=1) were excluded. Of 11,927 dementia-free twin individuals, 548 subjects were excluded because of missing data, leaving 11,379 twin individuals with complete data for the current analysis.
Informed consent was requried from all participants during the telephone interview and again in the clinical phase. The data collection precedures were reviewed and approved by the Regional Ethic Committee at Karolinska Institutet, Stockholm, Sweden; and by the institutional review board of the University of Southern California, California, USA.
Cognitive assessment
The cognitive status of the participants was first assessed by administrating a previously validated telephonic interview (TELE).20, 21 Performance on the TELE is summarized in a total score that ranges from 0 (worst performance) to 19 (best performance). TELE interview examines the following four cognitive areas: 1) orientation, assessed by 10 items of the mental status questionnaire (MSQ); 2) attention, measured by counting backwards in threes; 3) reasoning, tapped with questions about similarities and differences between pairs of nouns; and 4) episodic memory, evaluated using a three-item free recall task. In case of failure to recall all the items in the free recall condition, the subjects were administered a recognition task, in which they were required to identify the correct word or words within a list of distractors. TELE includes also a section investigating cognitive complaints, with a general question asscertaining subjective memory change “Have you noticed any change in your memory during the last three years?”, followed by more specific questions focusing on different cognitive problems such as forgetting errands, forgetting people’s name, forgetting appointments, forgetting familiar places, and forgetting words. Further questions addressed whether respondents were living independently, their employment status, their recent visits for medical care, their eyesight and hearing, assistance with practical tasks in daily life, and their mood.
When people did not perform optimally or could not perform TELE, an informant was interviewed with questions regarding the subject’s health, functional status, activities of daily living, and employment status. If cognitive problems were indicated, follow-up questions were used to obtain a history of the impairment and the pattern of decline, as well as a description of any contacts with the healthcare system concerning health problems. The eleven items of the Blessed Dementia Rating Scale (BDRS) were also included in the informant interview to assess cognitive functioning in everyday activities. The BDRS ranges from 0 to 17, with higher score indicating greater frequency of problems.22
Finally, people who were suspected of cognitive dysfunction according to TELE and BDRS combined underwent a comprehensive in-person dementia workup. The dementia workup included a physical and neurological examination, a review of medical history, informant interview, and a neuropsychological assessment as described in the protocol of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD).
Definition of dementia, SCI and CIND
Clinical diagnoses of dementia followed DSM-IV criteria.23 Preliminary diagnoses made by the interviewing team were reviewed by a diagnostic board consisting of a neurologist and a psychologist. When DSM-IV criteria were completely fulfilled, subjects were diagnosed as having “dementia” in contrast with a category of “questionable dementia” which was used for individuals who did not fulfill one of the first three DSM-IV diagnostic criteria but did exhibit either cognitive impairment or functional disability.19
SCI was defined as any perceived cognitive change in the last three years in otherwise cognitively intact people. The operationalization criteria of SCI included: 1) presence of subjective cognitive complaint was defined as self-reported memory change within the last three years that was asked to the subject as part of TELE; 2) absence of objective cognitive impairment defined as CIND, as described below; 3) absence of dementia defined on the basis of the clinical diagnosis, as described above.
CIND was defined according to current criteria9, 10 as cognitive impairment in the absence of dementia including: 1) presence of cognitive impairment defined as a performance at least two standard deviations below the age- and education-specific mean at any of the four cognitive tasks of TELE; and 2) absence of dementia defined on the basis of a clinical diagnosis following the DSM-IV criteria, as described above. The age- and education-specific means of TELE’s cognitive tasks were based on the average performance of the dementia-free population classified in eight age- and education-specific groups. Due to the operational definitions adopted in this study, SCI and CIND were mutually exclusive, although CIND included people with and without subjective cognitive complaints.
A further category was created for people with no cognitive impairment (NCI), subjective or objective.
Covariates collection and definition
Sociodemographics and zygosity
Information on age, sex and zygosity status was obtained from the Swedish Twin Registry. Information on education was gathered during the subject’s telephonic interview and verified by an informant when the subject was unable to be interviewed or did not perform well on the TELE. The validity of ascertainment of zygosity based on self-reports of similarity has been tested against blood markers and found valid in 99% of the cases.24
Age in years was categorized in four groups of five years each, plus a fifth group of people aged 85 years and over. Education was categorized into three groups based on the years of attained formal education, ranging from low (0 to 7 years), to average (8 to 10 years) and high (more than 10 years) education. There were four possible categories of the variable zygosity: monozygotic, in case of identical twins; same-sex dizygotic; unlike-sex dizygotic; and undetermined. This latter category included twin individuals for whom zygosity could not be ascertained.
Chronic diseases
Medical history was obtained from two sources: a) the inpatient register system, and b) self- and informant reports. The inpatient register encompasses all hospital admissions in Sweden from 1969 onwards. The International Classification of Disease, 8th revision (ICD-8) was used in the register system until 1986; from 1987, the International Classification of Disease, 9th revision (ICD-9) was adopted. Diagnoses regarding common chronic diseases25 were grouped according to the International Classification of Disease, 9th revision (ICD-9) as described in Table 2. More specifically, psychosis (dementia excluded) and affective disorders included ICD-8, 9 codes: 291–299. Ischemic heart disease included ICD-8, 9 codes: 410–414. Cardiac dysrhythmia, heart failure or other myocardial insufficiency included ICD-8, 9 codes: 427 and 428. Hypertension included ICD-8 codes: 400–404; and ICD-9 codes: 401–405. Stroke included ICD-8, 9 codes: 430–438. Articular diseases included ICD-8 codes: 712–718; and ICD-9 codes: 710–719. Osteoporosis included ICD-8 code: 723; and ICD-9 code: 733. Hip fracture included ICD-8 code: N820; and ICD-9 code: 821. Chronic obstructive pulmonary disease (COPD) included ICD-8, 9 codes: 491. Emphysema included ICD-8, 9 codes: 492. Asthma included ICD-8, 9 codes: 493. Diabetes included ICD-8, 9 codes: 250. Thyroid dysfunction included ICD-8, 9 codes: 240–246. Intestinal diverticula included ICD-8, 9 codes: 562. Ulcerous colitis included ICD-8 code: 563, and ICD-9 code: 556. Crohn’s disease included ICD-8 code: 563; and ICD-9 code 555. Liver cirrhosis included ICD-8, 9 codes: 571. Cholelithiasis included ICD-8, 9 codes: 574. Renal failure included ICD-8 code: 582; and ICD-9 code 585. Renal calculosis included ICD-8, 9 codes: 592. Prostate hypertrophy included ICD-8, 9 codes: 600. Recurrent cystitis included ICD-8, 9 codes: 595. Malignant tumors included ICD-8 codes 140–209; and ICD-9 codes: 140–208.
Table 2.
Chronic diseases’ groups according to ICD-9 (WHO).
| Disease groups | Diseases in the group |
|---|---|
| Mental | Psychosis and affective disorders and anxiety?? |
| Circulatory | Ischemic heart disease, cardiac dysrhythmia, heart failure or other myocardial insufficiency, hypertension, stroke |
| Musculoskeletal | Articular diseases, osteoporosis, hip fracture |
| Respiratory | Chronic obstructive pulmonary disease, emphysema, asthma |
| Endocrine | Diabetes, thyroid dysfunction |
| Gastrointestinal | Intestinal diverticula, ulcerous colitis, Crohn’s disease, liver cirrhosis, cholelithiasis |
| Urological | Renal failure, renal calculosis, prostate hypertrophy, recurrent cystitis |
| Malignancy | Malignant tumors |
According to previous research, multimorbidity was defined as having at least two chronic diseases co-occurring in the same individual.25
Current affective symptoms
Depressive symptoms were assessed during the telephone screening using the short form of the Center for Epidemiologic Studies Depression Scale (CES-D).26, 27 The short CES-D consists of 11 items scored on a 0 to 3 scale and related to the frequency of symptoms (from 0=rarely/none to 3=most/all of the time). Two items referring to feeling happy and enjoying life are reverse scored. The other nine items refer to poor appetite, feeling depressed, feeling like everything is an effort, restless sleep, feeling lonely, feeling that people are unfriendly, feeling sad, feeling disliked, and feeling of not being able to get “going”. CES-D total score was dichotomized using a cut-off of 9.26
Anxiety symptoms were assessed during the telephone screening by using the Composite International Diagnostic Interview – Short Form (CIDI-SF).28 People were classified as having current anxiety symptoms if they reported any episode of worry or anxiety and answered positively to the question “are you still anxious?”.
Data analysis
The characteristics of participants with SCI, CIND and NCI were compared using chi-square tests for categorical variables, and one-way ANOVA or Mann-Whitney test for continuous variables. The association of chronic diseases with SCI or CIND was performed following two strategies: 1) case-control analysis comparing individuals with SCI/CIND with those classified as NCI, using Generalized Estimating Equation (GEE) models; and 2) matched case-control analysis comparing twin pairs discordant for cognitive status (e.g. twin a=SCI/CIND, twin b=NCI) using Conditional Logistic Regression. GEE analysis is conceptually equivalent to logistic regression, but controls for the clustering of twins within a pair. Conditional Logistic Regression allows matching for unmeasured familial factors, such as genetic background and shared familial environment. If the association found with GEE models becomes attenuated in Conditional Logistic Regression models, familial factors are likely to play a role in the association. In contrast, if the association remains significant, the influence of genetic background and early environmental factors are likely to be marginal.24 In basic adjusted models, age, sex, education, and zygotic status were considered as potential confounders. In addition to this, all other chronic diseases, current anxiety and current depressive symptoms were added in fully adjusted models.
To assess if there was a dose-dependent effect in the association between chronic diseases with SCI and CIND, the total number of chronic diseases co-occurring in the same individual was computed. Two summary variables were created based on the number of chronic diseases, one variable on 3-level ordinal scale (0, 1, 2+) and one variable on a 4-level ordinal scale (0, 1, 2, 3+).
Missing values were imputed using Multiple Imputation based on available information on covariates.29
All statistical analyses were performed with the statistical software packages PASW 18.0 and STATA 10.0.
Results
Characteristics of the study participants by cognitive status are reported in Table 1. Compared to NCI, both SCI and CIND were older (SCI: F=83.1, p <0.001; CIND: F=63.2, p<0.001), and people with CIND were less educated (F=110.1, p<0.0001). There was no difference in gender and zygosity among the three groups. SCI and CIND subjects had higher prevalence of all diseases, with the exception of cancer, compared with NCI.
Table 1.
Characteristics of the study subjects (n=11,379) by cognitive status
| NCIa n=3,890 |
SCIb n=4,602 |
CINDc n=2,887 |
|
|---|---|---|---|
| Age, yrs | 71.9 (3.2) | 73.1 (5.9) | 73.1 (6.3) |
| Women | 2,133 (55) | 2,526 (55) | 1,649 (57) |
| Education | |||
| Low | 1,852 (48) | 2,275 (49) | 1,557 (54) |
| Average | 1,166 (30) | 1,372 (30) | 983 (34) |
| High | 872 (22) | 955 (21) | 347 (12) |
| Zygosity | |||
| Monozygotic | 925 (24) | 1,116 (24) | 647 (22) |
| Heterozygotic | 1,577 (41) | 1,885 (41) | 1,181 (41) |
| Opposite sex | 1,332 (34) | 1,532 (33) | 1,007 (35) |
| Undetermined | 56 (1) | 69 (2) | 52 (2) |
| Chronic diseases | |||
| Mental | 214 (6) | 425 (9) | 262 (9) |
| Circulatory | 1,387 (36) | 1,952 (42) | 1,184 (41) |
| Musculoskeletal | 1,413 (36) | 2,109 (46) | 1,284 (45) |
| Respiratory | 412 (11) | 602 (14) | 396 (14) |
| Endocrine | 574 (15) | 799 (17) | 554 (19) |
| Gastrointestinal | 840 (22) | 1,195 (26) | 702 (24) |
| Urological | 1,122 (29) | 1,751 (38) | 992 (34) |
| Malignancy | 368 (10) | 533 (12) | 285 (10) |
NCI=No Cognitive Impairment;
SCI=Subjective Cognitive Impairment;
CIND=Cognitive Impairment No Dementia.
Data are n (%) or mean (SD).
In GEE models for all participants (n=11,379), mental, circulatory, musculoskeletal, respiratory, endocrine, gastrointestinal, urological diseases and malignancy were significantly associated with increased odds of SCI in basic adjusted models. Similarly, mental, circulatory, musculoskeletal, respiratory, endocrine, and urological diseases were associated with increased odds of CIND. In fully adjusted models, the associations of endocrine diseases and cancer with SCI and that of circulatory disease with CIND were no longer significant (Table 3).
Table 3.
Odds ratios (ORs) and 95% confidence intervals (CIs) of subjective cognitive impairment (SCI) and cognitive impairment no dementia (CIND) in relation to chronic diseases: results from Generalized Estimating Equation (GEE) models for all participants (N=11,379).
| SCI | CIND | |||
|---|---|---|---|---|
|
| ||||
| Model 1a OR (95% CI) |
Model 2b OR (95% CI) |
Model 1a OR (95% CI) |
Model 2b OR (95% CI) |
|
| Mental | 1.8 (1.2–1.6)** | 1.5 (1.2–1.8)** | 1.7 (1.4–2.0)** | 1.4 (1.2–1.8)** |
| Circulatory | 1.3 (1.2–1.4)** | 1.2 (1.1–1.3)** | 1.2 (1.1–1.3)** | 1.1 (1.0–1.2) |
| Musculoskeletal | 1.5 (1.3–1.6)** | 1.4 (1.2–1.5)** | 1.3 (1.2–1.5)** | 1.2 (1.1–1.4)** |
| Respiratory | 1.3 (1.2–1.5)** | 1.2 (1.0.–1.4)* | 1.3 (1.1–1.5)** | 1.2 (1.1–1.4)* |
| Endocrine | 1.2 (1.1–1.3)** | 1.1 (0.9–1.2) | 1.2 (1.1–1.3)** | 1.2 (1.1–1.4)** |
| Gastrointestinal | 1.3 (1.2–1.4)** | 1.2 (1.0–1.3)* | 1.1 (1.0–1.3) | 1.0 (0.9–1.1) |
| Urological | 1.5 (1.3–1.6)** | 1.3 (1.2–1.5)** | 1.3 (1.1–1.4)** | 1.2 (1.0–1.3)* |
| Malignancy | 1.2 (1.1–1.4)** | 1.1 (1.0–1.3) | 1.1 (0.9–1.3) | 1.0 (0.8–1.2) |
GEE model adjusted for age, gender, education, and zygosity;
GEE model adjusted for age, gender, education, zygosity, all chronic diseases, current depressive symptoms and current anxiety;
p<0.05;
p<0.01.
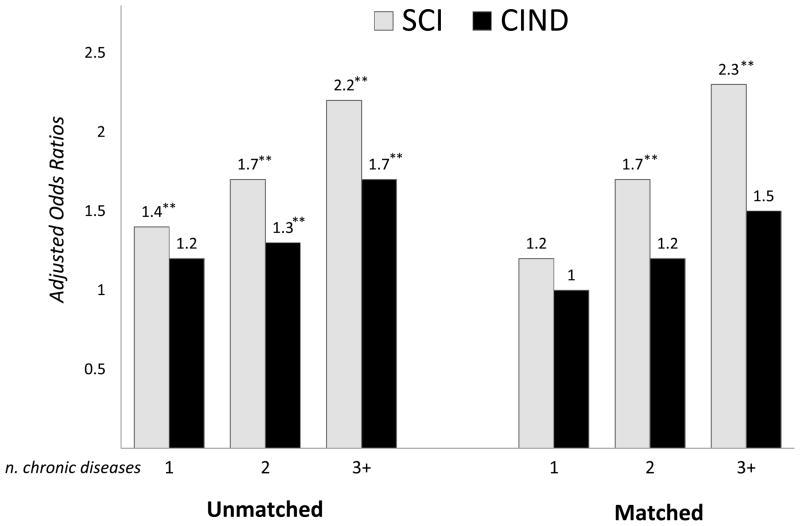
There was a significant dose-dependent relationship between number of chronic diseases and odds of SCI, while in CIND this effect was less clearly linear (Figure 2). Multimorbidity, defined as the co-occurrence of two or more chronic diseases in the same individual, was present among 61% (n=2,817) of people with SCI, compared to 58% (n=1,616) of CIND and 49% (1,895) of NCI people. In unmatched case-control GEE models, multimorbidity was associated with SCI and CIND, both after basic (SCI: OR 2.0, 95% CI 1.8–2.3; CIND: OR 1.5, 95% CI 1.3–1.8) and full adjustment (SCI: OR 1.9, 95% CI 1.7–2.2; CIND: OR 1.4, 95% CI 1.3–1.7).
Figure 2. The dose-dependent association between number of chronic disease and subjective cognitive impairment (SCI) or cognitive impairment no dementia (CIND).
Unmatched models describe results from unmatched case-control analysis for all participants (n=11,379) using generalized estimating equation (GEE), adjusted for age, gender, education, zygosity, current anxiety, and current depressive symptoms; matched models describe results from co-twin matched case-control analysis for SCI- or CIND-discordant twin pairs (n=1,720 and n=926) using conditional logistic regression, adjusted for age education; *p<0.05; **p<0.01.
In Conditional logistic regression models with SCI- or CIND-discordant twin pairs (n=1,720 and n=926) (Table 4), the associations of chronic diseases with CIND were no longer significant, with the exception of cancer. In contrast, the associations of SCI with most chronic diseases, including musculoskeletal, respiratory, gastrointestinal and urological diseases were unchanged. The dose-dependent association with number of chronic diseases was still observable for SCI but not as prominently for CIND (Figure 2). Similarly, in conditional logistic regression models, multimorbidity was still associated with SCI (OR 1.9, 95% CI 1.4–2.6) but no longer with CIND (OR 1.3, 95% CI 0.9–2.0).
Table 4.
Odds ratios (ORs) and 95% confidence intervals (CIs) of subjective cognitive impairment (SCI) and cognitive impairment no dementia (CIND) in relation to chronic diseases: results from co-twin matched case-control analysis for SCI- or CIND-discordant twin pairs (N=1,720 and N=926) using Conditional Logistic Regression.
| SCI | CIND | |||
|---|---|---|---|---|
|
| ||||
| Model 1a OR (95% CI) |
Model 2b OR (95% CI) |
Model 1a OR (95% CI) |
Model 2b OR (95% CI) |
|
| Mental | 1.5 (1.0–2.2) | 1.1 (0.7–1.7) | 1.5 (0.9–2.5) | 1.2 (0.7–2.2) |
| Circulatory | 1.3 (1.1–1.7)* | 1.7 (0.9–1.5) | 1.2 (0.9–1.6) | 1.2 (0.9–1.7) |
| Musculoskeletal | 1.5 (1.2–1.8)** | 1.4 (1.1–1.7)** | 1.2 (0.9–1.6) | 1.0 (0.7–1.3) |
| Respiratory | 1.5 (1.1–2.1)* | 1.5 (1.1–2.1)* | 1.1 (0.7–1.6) | 1.0 (0.7–1.6) |
| Endocrine | 1.3 (0.9–1.7) | 1.2 (0.9–1.6) | 1.4 (1.0–2.0) | 1.3 (0.9–1.9) |
| Gastrointestinal | 1.8 (1.4–2.3)** | 1.6 (1.2–2.2)** | 1.0 (0.7–1.5) | 1.0 (0.7–1.5) |
| Urological | 1.4 (1.1–1.7)** | 1.4 (1.1–1.7)** | 1.0 (0.7–1.3) | 0.9 (0.7–1.5) |
| Malignancy | 0.9 (0.7–1.3) | 0.9 (0.6–1.2) | 1.7 (1.1–2.7)* | 1.7 (1.0–2.8)* |
Conditional Logistic Regression model adjusted for education and zygosity;
Conditional Logistic Regression model adjusted for education, zygosity, all chronic diseases, current depressive symptoms and current anxiety;
p<0.05;
p<0.01.
Discussion
In this population-based study of Swedish twins, chronic diseases were associated to increased odds of SCI and CIND. Both SCI and CIND were related to mental, musculoskeletal, respiratory, and urological diseases. Although groups of diseases have been investigated sparsely in relation to subjective or objective cognitive impairment, available data support our findings. In particular, mental diseases have been associated with cognitive complaints and CIND, and there is agreement about the complex relationship of psychiatric conditions with subjective and objective cognitive functioning.5, 11, 30, 31 Regarding musculoskeletal diseases, the relationship between osteoporosis, hip fracture and cognitive decline has previously been observed,5, 32 while articular diseases are associated with chronic pain that, in turn, have been related to cognitive complaints33 and slower cognitive processing speed.34 Our findings of an association of SCI and CIND with respiratory and urological diseases are in agreement with previous reports linking asthma and COPD to cognitive impairment35, 36 and with the growing evidence of an association between chronic kidney disease and reduced cognitive performance.37, 38
SCI, but not CIND, was associated with circulatory and gastrointestinal diseases. Our results regarding circulatory diseases and SCI are at odds with a study that did not find an association between SCI and specific circulatory diseases17 but are in agreement with a report on improved subjective cognitive functioning after successful coronary bypass surgery.39 The present finding of a lack of association of circulatory diseases with CIND is in line with some5, 7, 40 but in disagreement with other sets of evidence.5,7, 41 Discrepancies can be explained by alternative operationalizations of CIND, differential severity and types of circulatory disease, and heterogeneity in study populations. On the other hand, gastrointestinal diseases have generally been neglected in relation to cognition. One exception is the known association of impaired cognition with severe liver disease and, recently, also with mild biliary cirrhosis.42 Our finding of an association with SCI can imply that gastrointestinal diseases have an impact on sub-clinical forms of cognitive impairment.
CIND, but not SCI, was associated with endocrine diseases. This is in agreement with previous studies, which reported that diabetes and thyroid dysfunction were positively associated with cognitive impairment.43, 44 The lack of association of endocrine diseases with SCI may be explained with the severity of the cognitive deficits linked to this type of somatic condition, which may make more likely for subjects with endocrine diseases to be in the CIND rather than in the SCI category.
Finally, we did not find an association with malignancy in either SCI or CIND, when considering unmatched analysis conducted on the whole cohort. This is in line with some reports45 but is at odds with other investigations that focused on the relationship between specific cognitive functions and types of cancer,45 aspects not investigated in the present study.
We further observed a dose-dependent effect in the relationship of chronic diseases with SCI and CIND. This effect was linear in SCI but not in CIND. In particular, multimorbidity defined as having at least two chronic diseases25 was associated with 100% increased odds of SCI and to 50% increased odds of CIND, after adjustment for sociodemographic variables and zygosity. Further adjustment for current affective symptoms did not alter the association. Our findings are in agreement with previous studies that reported a positive association between cognitive complaints and CIND with poor health and multimorbidity.4–6, 15, 16
Both general and specific mechanisms may lie behind the association of SCI and CIND with chronic diseases. Examples of specific mechanisms are low oxygen levels in chronic respiratory diseases, insulin imbalance in diabetes and thyroid hormone deficiency or excess in thyroid dysfunction, all conditions that can directly affect the brain. At a more general level, both physical and mental co-morbidities can generate tiredness and lack of concentration, possibly resulting in the subjective feeling of “not being as sharp as before” and in eventual impaired cognitive performance. Another mechanism that may be involved is the reduction in functional independence associated with several chronic diseases and the consequent reduction in leisure and social activities, known protective factors for cognitive decline.46 Moreover, several chronic conditions can reduce the amount or the quality of sleep, with known reduced cognitive efficiency47 and long-term increased risk of cognitive impairment.48 In addition, chronic stress may accompany chronic diseases and affect the brain through the imbalance of the adrenocortico axis.49 It has also been suggested that both circulatory and kidney diseases might affect the brain through microvascular damage,45 while a possible cytokine activated immune system dysregulation in the brain may be a common response to inflammation or injury in any organ system in the body.45 Finally, most chronic diseases are pharmacologically treated and possible effects of medications on the brain or on cognitive performance cannot be ruled out50 especially in the case of multimorbidity, which often leads to polypharmacy.51,5
In co-twin control analysis, the association of CIND with malignancy was strengthened and reached significance. This suggests that the effect of cancer on cognition may be mediated by environmental factors related to adult life and confirms the results of a previous study on a smaller sample of Swedish twin pairs that reported an association between cancer and cognitive dysfunction.52 We observed that confounding by familial factors attenuated the associations of CIND with all other disease clusters, including mental, musculoskeletal, respiratory, endocrine, gastrointestinal and urological diseases. This indicates a role for genetic background and shared familial environment in determining the relationship of CIND with most chronic diseases. On the other hand the association of SCI with musculoskeletal, respiratory, gastrointestinal and urological diseases was not influenced by familial factors. This implies that, at least at the level of SCI, adult life environment plays the major role in determining the association with most chronic diseases. Considering that several aspects related to adult environment are potentially modifiable, the present findings have important implications for possible preventive interventions.
Our study has many strengths, including the nation-wide, population-based design; the inclusion of several groups of chronic diseases; the assessment of the association of chronic diseases with both SCI and CIND; the control for major confounders, such as current depressive and anxiety symptoms; the evaluation of the contribution of genetic background and shared familial environment to the association of chronic diseases with SCI and CIND. There are also some limitations to be considered. First, SCI and CIND were assessed with a relatively synthetic, although well validated, telephone interview, therefore, the risk of misclassification cannot be ruled out. However, the cognitive screening represented only the first of a three-step assessment of cognitive functioning, which involved reports from close informants and direct extensive clinical examination of subjects with suspected cognitive impairment. Secondly, because of the cross-sectional case-finding strategy for both SCI and CIND, reverse causation cannot be ruled out. People with SCI, for instance, might have been more likely to report somatic diseases, while people with CIND, because of their cognitive deficits, might have been less attentive to their health and therefore more liable to somatic diseases. Even so, our definitions of chronic disease involved information coming from multiple sources, including the national inpatient register and self- and informant reports. Regarding CIND, the great majority (78%) of the cases was mild; as a consequence, long lasting effects on organ systems is unlikely. Thirdly, duration of disease was measured neither for SCI and CIND, nor for chronic diseases. Further investigations are thus warranted to confirm and expand the present findings.
In conclusion, chronic diseases are associated with both SCI and CIND and the association is stronger when chronic diseases co-occur. Alternative mechanisms can underlie these associations. Genetic and early-life environmental factors may partially explain the association of CIND but not that of SCI with chronic diseases and multimorbidity.
Figure 1.
Flow-chart of the study population.
Acknowledgments
This study was supported by grants from the Swedish Council for Working Life and Social Research, NIH grant R01 AG08724, Regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, and the Swedish Brain Power Initiative. Private funding from Stiftelsen Gamla Tjänarinnor and Gun and Bertil Stohnes Foundation has also been provided.
Footnotes
Conflict of interest: The Authors of this study have no conflict of interest to declare.
References
- 1.Division DoEaSAP. World Population Ageing 2009. New York: United Nations; 2010. [Google Scholar]
- 2.Batstra L, Bos EH, Neeleman J. Quantifying psychiatric comorbidity--lessions from chronic disease epidemiology. Soc Psychiatry Psychiatr Epidemiol. 2002;37:105–111. doi: 10.1007/s001270200001. [DOI] [PubMed] [Google Scholar]
- 3.Marengoni A, von Strauss E, Rizzuto D, Winblad B, Fratiglioni L. The impact of chronic multimorbidity and disability on functional decline and survival in elderly persons. A community-based, longitudinal study. J Intern Med. 2009;265:288–295. doi: 10.1111/j.1365-2796.2008.02017.x. [DOI] [PubMed] [Google Scholar]
- 4.Lyketsos CG, Toone L, Tschanz J, et al. Population-based study of medical comorbidity in early dementia and “cognitive impairment, no dementia (CIND)”: association with functional and cognitive impairment: The Cache County Study. Am J Geriatr Psychiatry. 2005;13:656–664. doi: 10.1176/appi.ajgp.13.8.656. [DOI] [PubMed] [Google Scholar]
- 5.Monastero R, Palmer K, Qiu C, Winblad B, Fratiglioni L. Heterogeneity in risk factors for cognitive impairment, no dementia: population-based longitudinal study from the Kungsholmen Project. Am J Geriatr Psychiatry. 2007;15:60–69. doi: 10.1097/01.JGP.0000229667.98607.34. [DOI] [PubMed] [Google Scholar]
- 6.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010;58:248–255. doi: 10.1111/j.1532-5415.2009.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atti AR, Forlani C, De Ronchi D, et al. Cognitive Impairment after Age 60: Clinical and Social Correlates in the “Faenza Project”. J Alzheimers Dis. 2010;21:1325–1334. doi: 10.3233/jad-2010-091618. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 9.Ebly EM, Hogan DB, Parhad IM. Cognitive impairment in the nondemented elderly. Results from the Canadian Study of Health and Aging. Arch Neurol. 1995;52:612–619. doi: 10.1001/archneur.1995.00540300086018. [DOI] [PubMed] [Google Scholar]
- 10.Caracciolo B, Palmer K, Monastero R, Winblad B, Backman L, Fratiglioni L. Occurrence of cognitive impairment and dementia in the community: a 9-year-long prospective study. Neurology. 2008;70:1778–1785. doi: 10.1212/01.wnl.0000288180.21984.cb. [DOI] [PubMed] [Google Scholar]
- 11.Reisberg B, Prichep L, Mosconi L, et al. The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimers Dement. 2008;4:S98–S108. doi: 10.1016/j.jalz.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Barnes LL, Schneider JA, Boyle PA, Bienias JL, Bennett DA. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology. 2006;67:1581–1585. doi: 10.1212/01.wnl.0000242734.16663.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6:11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jessen F, Wiese B, Bachmann C, et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67:414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 15.Comijs HC, Deeg DJ, Dik MG, Twisk JW, Jonker C. Memory complaints; the association with psycho-affective and health problems and the role of personality characteristics. A 6-year follow-up study. J Affect Disord. 2002;72:157–165. doi: 10.1016/s0165-0327(01)00453-0. [DOI] [PubMed] [Google Scholar]
- 16.Aarts S, van den Akker M, Hajema KJ, et al. Multimorbidity and its relation to subjective memory complaints in a large general population of older adults. Int Psychogeriatr. 2011;23:616–624. doi: 10.1017/S1041610210002024. [DOI] [PubMed] [Google Scholar]
- 17.Benito-Leon J, Mitchell AJ, Vega S, Bermejo-Pareja F. A population-based study of cognitive function in older people with subjective memory complaints. J Alzheimers Dis. 2010;22:159–170. doi: 10.3233/JAD-2010-100972. [DOI] [PubMed] [Google Scholar]
- 18.Whalley LJ, Dick FD, McNeill G. A life-course approach to the aetiology of late-onset dementias. Lancet Neurol. 2006;5:87–96. doi: 10.1016/S1474-4422(05)70286-6. [DOI] [PubMed] [Google Scholar]
- 19.Gatz M, Fratiglioni L, Johansson B, et al. Complete ascertainment of dementia in the Swedish Twin Registry: the HARMONY study. Neurobiol Aging. 2005;26:439–447. doi: 10.1016/j.neurobiolaging.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Gatz M, Reynolds C, Nikolic J, Lowe B, Karel M, Pedersen N. An empirical test of telephone screening to identify potential dementia cases. Int Psychogeriatr. 1995;7:429–438. doi: 10.1017/s1041610295002171. [DOI] [PubMed] [Google Scholar]
- 21.Gatz M, Reynolds CA, John R, Johansson B, Mortimer JA, Pedersen NL. Telephone screening to identify potential dementia cases in a population-based sample of older adults. Int Psychogeriatr. 2002;14:273–289. doi: 10.1017/s1041610202008475. [DOI] [PubMed] [Google Scholar]
- 22.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 23.Association AP, editor. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 24.Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med. 2002;252:184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- 25.Marengoni A, Winblad B, Karp A, Fratiglioni L. Prevalence of chronic diseases and multimorbidity among the elderly population in Sweden. Am J Public Health. 2008;98:1198–1200. doi: 10.2105/AJPH.2007.121137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 28.Kessler RC, Ustun TB. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int J Methods Psychiatr Res. 2004;13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 30.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15:983–991. doi: 10.1002/1099-1166(200011)15:11<983::aid-gps238>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 31.Monastero R, Mangialasche F, Camarda C, Ercolani S, Camarda R. A systematic review of neuropsychiatric symptoms in mild cognitive impairment. J Alzheimers Dis. 2009;18:11–30. doi: 10.3233/JAD-2009-1120. [DOI] [PubMed] [Google Scholar]
- 32.Rolland Y, Abellan van Kan G, Benetos A, et al. Frailty, osteoporosis and hip fracture: causes, consequences and therapeutic perspectives. J Nutr Health Aging. 2008;12:335–346. doi: 10.1007/BF02982665. [DOI] [PubMed] [Google Scholar]
- 33.Westoby CJ, Mallen CD, Thomas E. Cognitive complaints in a general population of older adults: prevalence, association with pain and the influence of concurrent affective disorders. Eur J Pain. 2009;13:970–976. doi: 10.1016/j.ejpain.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Lee DM, Pendleton N, Tajar A, et al. Chronic widespread pain is associated with slower cognitive processing speed in middle-aged and older European men. Pain. 2010;151:30–36. doi: 10.1016/j.pain.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 35.Moss M, Franks M, Briggs P, Kennedy D, Scholey A. Compromised arterial oxygen saturation in elderly asthma sufferers results in selective cognitive impairment. J Clin Exp Neuropsychol. 2005;27:139–150. doi: 10.1080/13803390490515450. [DOI] [PubMed] [Google Scholar]
- 36.Antonelli-Incalzi R, Corsonello A, Trojano L, et al. Correlation between cognitive impairment and dependence in hypoxemic COPD. J Clin Exp Neuropsychol. 2008;30:141–150. doi: 10.1080/13803390701287390. [DOI] [PubMed] [Google Scholar]
- 37.Tsai CF, Wang SJ, Fuh JL. Moderate chronic kidney disease is associated with reduced cognitive performance in midlife women. Kidney Int. 2010;78:605–610. doi: 10.1038/ki.2010.185. [DOI] [PubMed] [Google Scholar]
- 38.Thornton WL, Shapiro RJ, Deria S, Gelb S, Hill A. Differential impact of age on verbal memory and executive functioning in chronic kidney disease. J Int Neuropsychol Soc. 2007;13:344–353. doi: 10.1017/S1355617707070361. [DOI] [PubMed] [Google Scholar]
- 39.Sandau KE, Lindquist RA, Treat-Jacobson D, Savik K. Health-related quality of life and subjective neurocognitive function three months after coronary artery bypass graft surgery. Heart Lung. 2008;37:161–172. doi: 10.1016/j.hrtlng.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Anttila T, Helkala EL, Viitanen M, et al. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. Bmj. 2004;329:539. doi: 10.1136/bmj.38181.418958.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nunes B, Silva RD, Cruz VT, Roriz JM, Pais J, Silva MC. Prevalence and pattern of cognitive impairment in rural and urban populations from Northern Portugal. BMC Neurol. 2010;10:42. doi: 10.1186/1471-2377-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newton JL, Hollingsworth KG, Taylor R, et al. Cognitive impairment in primary biliary cirrhosis: symptom impact and potential etiology. Hepatology. 2008;48:541–549. doi: 10.1002/hep.22371. [DOI] [PubMed] [Google Scholar]
- 43.Atti AR, Forlani C, De Ronchi D, et al. Cognitive Impairment after Age 60: Clinical and Social Correlates in the “Faenza Project”. J Alzheimers Dis. 2010 doi: 10.3233/jad-2010-091618. [DOI] [PubMed] [Google Scholar]
- 44.Hogervorst E, Huppert F, Matthews FE, Brayne C. Thyroid function and cognitive decline in the MRC Cognitive Function and Ageing Study. Psychoneuroendocrinology. 2008;33:1013–1022. doi: 10.1016/j.psyneuen.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Gasquoine PG. Cognitive impairment in common, noncentral nervous system medical conditions of adults and the elderly. J Clin Exp Neuropsychol. 2011:1–11. doi: 10.1080/13803395.2010.536759. [DOI] [PubMed] [Google Scholar]
- 46.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 47.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 48.Malhotra RK, Desai AK. Healthy brain aging: what has sleep got to do with it? Clin Geriatr Med. 2010;26:45–56. doi: 10.1016/j.cger.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Peavy GM, Salmon DP, Jacobson MW, et al. Effects of chronic stress on memory decline in cognitively normal and mildly impaired older adults. Am J Psychiatry. 2009;166:1384–1391. doi: 10.1176/appi.ajp.2009.09040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore AR, O’Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs Aging. 1999;15:15–28. doi: 10.2165/00002512-199915010-00002. [DOI] [PubMed] [Google Scholar]
- 51.Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. Am J Geriatr Pharmacother. 2007;5:345–351. doi: 10.1016/j.amjopharm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Heflin LH, Meyerowitz BE, Hall P, et al. Cancer as a risk factor for long-term cognitive deficits and dementia. J Natl Cancer Inst. 2005;97:854–856. doi: 10.1093/jnci/dji137. [DOI] [PubMed] [Google Scholar]